Mechanically ventilated patients comprise a uniquely vulnerable population, and these patients frequently experience both morbidity and mortality. Best practice “bundles” have been proposed to manage this excess risk associated with ventilators, particularly ventilator-associated pneumonia (VAP). 1 The mechanically ventilated bundle includes measures to ensure that deep vein thrombosis (DVT) prophylaxis and stress ulcer prophylaxis are initiated, that oral hygiene is accomplished with chlorhexidine, that daily sedation breaks are taken, and that head-of-bed elevation is provided. This list addresses several preventable causes of healthcare-associated injury. Compliance with VAE bundles is effective in reducing VAP.Reference Berenholtz, Pham and Thompson 2 , Reference Eom, Lee and Chun 3 VAP is associated with prolonged hospitalization, intensive care unit (ICU) stay, ventilator requirements, and, possibly, increased mortality.Reference Kollef, Hamilton and Ernst 4 – Reference Warren, Shukla and Olsen 6
However, detection of VAP is challenging. Initial symptoms are nonspecific, and there is no clinical gold standard for making the diagnosis. Existing definitions are complex and prone to low inter-rater reliability and reproducibility. In 2011, the Centers for Disease Control/National Healthcare Safety Network (CDC/NHSN) proposed a new definition for surveillance purposes, termed ventilator-associated events (VAEs).Reference Magill, Klompas and Balk 7 In the VAE schema, a patient who experiences decompensation of any sort, infectious or otherwise while on the ventilator, has had a “ventilator-associated complication” (VAC). With clinical suspicion of infection, evidenced by fever, antibiotic use or white count, the event is an “infectious VAC” (IVAC). With further clinical and microbiological evidence, it can progress to the categories of “possible” and “probable” VAP (Figure 1). There is not a strong correlation between VAE and the older definitions of VAP,Reference Klein Klouwenberg, van Mourik and Ong 8 , Reference McMullen, Boyer, Schoenberg, Babcock, Micek and Kollef 9 but VAE is closely associated with patient outcomes, such as length of stay and mortality.Reference Klompas, Kleinman and Khan 10 , Reference Bouadma, Sonneville and Garrouste-Orgeas 11 We sought to determine whether compliance with ventilator bundle practices affects the risk of developing a VAE.
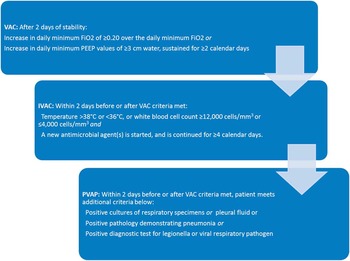
FIGURE 1 Simplified VAE criteria. Abbreviations: C, Celsius; cm, centimeters; FiO2, fraction of inspired oxygen; IVAC, infectious ventilator-associated complication; mm, millimeters; PEEP, positive end expiratory pressure; VAP, ventilator-associated pneumonia; VAC, ventilator-associated complication.
METHODS
We constructed a retrospective cohort study of all adult ventilated patients in the ICUs at Mayo Clinic Rochester between January 2012 and August 2014. The Mayo Clinic Institutional Review Board approved this investigation as a minimal risk study.
We analyzed each episode of ICU admission separately. We excluded patients age <18 years, those who did not have a research authorization on file, those dismissed from the ICU <24 hours after arriving, and those who died within 4 days after ventilator initiation. We excluded the last group because the VAE definition requires the patient be ventilated for ≥2 calendar days to establish a baseline as well as at least 2 days of worsening oxygenation. Thus, the earliest a patient can qualify for a VAE diagnosis is day 4, and records for those expiring before that time are insufficient to classify the occurrence as a VAE versus a non-VAE. 12
We identified the VAE cases using a database maintained by infection control (IC) personnel for ongoing monitoring activity that uses the CDC/NHSN VAE surveillance algorithm. The IC database used an electronic rule to flag patients who met the criteria for the first tier of VAE, VAC. Infection preventionists reviewed the medical records of all patients who met the VAC criteria to identify the next tiers of VAE and to classify each IVAC case as probable or possible VAE. We considered VAE our primary end point; thus, we removed a patient from the study when an event occurred.
We separately assessed compliance with ventilator bundle elements using an electronic search algorithm to query a database of patient variables in ICUs at our institution.Reference Herasevich, Pickering, Dong, Peters and Gajic 13 Elements assessed included sedation break, DVT prophylaxis, stress ulcer prophylaxis, and oral care. We elected not to evaluate head-of-bed elevation because chart data were inconsistent.Reference Lan, Thongprayoon and Ahmed 14
We assessed compliance with each element in 24-hour windows. An eligible patient was considered to have been “exposed” to a failure the first time when that patient did not have a documented intervention in place. For example, a patient who was on sedation for at least 24 hours was eligible for a sedation break, and if charting failed to note a sedation holiday, we considered this a “failure.” For univariate analysis, we carried this onward through the rest of the hospital stay.
We considered DVT prophylaxis compliant if a patient was administered an anticoagulant during that window. This included both therapeutic and prophylactic medications as determined from the medication administration record (MAR). We did not allow for exceptions because this was an efficacy study. Although it would be clinically valid to withhold chemoprophylaxis from a patient at risk for bleeding (eg, from a major procedure), the risk of DVT is increased from the day without prophylaxis. We considered peptic ulcer prophylaxis compliant if the patient had any acid inhibitory drug or sulfalcrate on that day charted in the MAR. We evaluated chlorhexidine via the presence of a chlorhexidine in the MAR. Sedation break was assessed only for patients with a continuous IV sedative or opioid present during the window; compliance was considered to have been achieved if that patient had a charted period of at least 15 minutes with all sedatives stopped in the IV fluid administration table. The methods for deriving and validating these definitions are discussed at length elsewhere.Reference Lan, Thongprayoon and Ahmed 14
We conducted statistical analyses using JMP 10.0 software (SAS Institute, Cary, NC) and R 3.1.1 (Mavericks, Vienna, Austria). We conducted survival analysis using the R survival package version 2.38.Reference Therneau and Grambsch 15 We undertook univariate analysis for compliance with each element by considering VAEs occurring within a 2-day window of failure to meet any ventilator bundle element. We assessed differences in variables between groups using Wilcoxon rank sum, Kruskal-Wallis, or χ2 test. We used Cox proportional hazard models to assess the effect of stress ulcer prophylaxis, DVT prophylaxis, oral care, and sedation breaks on VAEs. Patients were censored at discharge or death. We adjusted models for gender, age, and Acute Physiology and Chronic Health Evaluation (APACHE) III scores. Because age is a factor in the APACHE III score and, thus, is collinear, we constructed this adjustment with 2 separate models, an age-gender model and a gender-APACHE model. We considered a 2-sided P value <.05 statistically significant.
RESULTS
A total of 2,660 patients, comprising 16,858 days at risk, met the inclusion criteria for our study; 77 patients had at least 1 VAE in that period. The median duration of ventilation was 4 days (interquartile range [IQR], 3–7). Median age was 63.9 years (IQR, 53–74 years). The population was predominantly male (61%) (Table 1).
TABLE 1 Characteristics of Those With and Without VAE
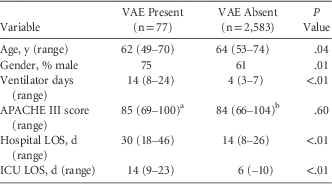
NOTE. LOS, length of stay; ICU, intensive care unit.
a Missing 2.
b Missing 115.
In Table 2, we summarize unadjusted hazard ratios (HRs) for exposure to a bundle element failure. Neither stress ulcer prophylaxis nor DVT prophylaxis nor sedation break were associated with a significant reduction of risk of a VAE. Oral care, however, was statistically significant. Estimating the HR using a multivariate Cox proportional hazard models for all deficiencies in bundle practice for patients at risk of a VAE, the stress ulcer prophylaxis did not attain statistical significance for increased risk of developing VAE. Oral care was again associated with a reduction in VAEs (Table 2).
TABLE 2 Risk of VAE With Bundle Elements, Unadjusted

NOTE. CI, confidence interval; DVT, deep vein thrombosis; HR, hazard ratio.
Constructing a multivariate model and adjusting the significant baseline factors age and gender, we again noted borderline increased risk of VAE from compliance with stress ulcer prophylaxis, significant reductions in VAEs with consistent oral care, and no statistically significant impact of DVT prophylaxis nor sedation breaks. Adjustment for the APACHE score and gender yielded similar results. We excluded 117 patients that did not have an APACHE score calculated from this analysis. The age-gender model and APACHE gender model are provided in Table 3.
TABLE 3 Adjusted Hazard Ratio for VAE With Bundle Elements

NOTE. CI, confidence interval; DVT, deep vein thrombosis; HR, hazard ratio.
DISCUSSION
Overall, our findings suggest that while oral care with chlorhexidine appears to be associated with a reduction of VAE, the other bundle elements were not significantly preventive. Sedation breaks and DVT prophylaxis appeared to have no overall effect, and stress ulcer prophylaxis may have been trending toward harm.
The value of oral chlorhexidine in preventing VAP is well studied, but results are inconsistent as to who benefits. A recent meta-analysis demonstrated a consistent benefit to oral care with chlorhexidine across 17 published studies,Reference Li, Ai, Zheng and Jie 16 while another found the benefit to be limited only to the cardiac surgery population.Reference Klompas, Speck, Howell, Greene and Berenholtz 17 In our study, there was a clear benefit, but the study was too small to determine whether these benefits were specific to certain population subsets. The mechanism of oral chlorhexidine for VAP prevention is intuitive. Many VAP organisms are oral flora and may represent some degree of aspiration; thus, reducing the oral bacterial burden should prevent VAP.Reference Par, Badovinac and Plancak 18 Of the VAP prevention elements, only head-of-bed elevation and oral care are specifically geared toward infection prevention. The fact that chlorhexidine alone was beneficial in reducing VAEs suggests that infectious events and VAP are still drivers of VAE.
We observed a consistent trend toward harm with stress ulcer prophylaxis. This was not nearly as strong as the benefit seen with chlorhexidine, and it did not ultimately reach significance. This is broadly consistent with other reports questioning the utility of stress ulcer prophylaxis,Reference Krag, Perner and Wetterslev 19 and the data that stress ulcer prophylaxis may increase the risk of complications, such as C. difficile Reference Buendgens, Bruensing and Matthes 20 and, depending on the pharmacologic choice, pneumonia.Reference Khorvash, Abbasi, Meidani, Dehdashti and Ataei 21 Based on our results, we cannot say that stress ulcer prophylaxis causes harm, but we observed no benefit.
The DVT prophylaxis was not particularly beneficial. This may be because of the relatively short duration of hospitalization (ie, not sufficient time to develop DVT). However, we did not adjust for other known risk factors for DVT (eg, trauma, oncology). It may be that mechanical ventilation alone is not as great a risk factor as other confounders prevalent in this population.
Sedation break trended slightly toward beneficial, but it did not reach statistical significance in any analysis. It is not clear whether this finding is related to the power of our study, though Mehta et alReference Mehta, Burry and Cook 22 have recently brought the sedation break into question. This finding may reflect study power.
The only nonmodifiable risk factor that was significant was gender. This finding is consistent with prior studiesReference Sharpe, Magnotti and Weinberg 23 indicating a higher risk of VAP in men. Our study’s primary limitation is the low baseline rate of VAE at our institution. With only 77 VAEs for evaluation in this cohort, the strength of any conclusion reached using these data is limited. This low rate further limits generalizability: we did not track other interventions in this study, which may have been responsible for the low rate of VAE and/or may have diluted the impact of traditional bundle elements for prevention.
A specific intervention we could not track was head-of-bed elevation as a bundle element. Our previous validation study did not find reliable enough charting to assess the impact of this intervention. Our definition of noncompliance may also be a limitation; the exact “dose” and window of effect for each intervention are unknown, and our consideration of missing any element for 1 day within a 2-day window of VAE may be too sensitive or specific.
Nonetheless, our study supports the importance of oral care as part of a bundle. Future research should continue to evaluate the role of DVT prophylaxis, sedation breaks, and the potential for harm from stress ulcer prophylaxis. Moreover, other modifiable risk factors such as low tidal volume strategies, glucose control, and early physical therapy should be studied. Non-modifiable risk factors to allow for better targeting of prevention strategies should be further investigated, such as underlying comorbidities and specific ICU population types.
In conclusion, the VAP bundle elements are not well targeted for VAE prevention. Although oral chlorhexidine appears beneficial, other elements had no discernable effect or trend toward increased risk of VAE. If there is a mortality benefit from these interventions, it is not in VAE prevention. Further research is needed to develop a new bundle for the VAE era.
ACKNOWLEDGMENTS
Financial support: This study was funded, in part, by departmental funds from the Critical Care Research Committee at Mayo Clinic, Rochester, Minnesota. This report was made possible, in part, by funding from the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/ice.2016.207.






