Prior hospitalization and antibacterial exposure are 2 important risk factors for multidrug-resistant organism (MDRO) carriage at the time of hospital admission.Reference Morgan, Day and Furuno 1 – Reference Papadimitriou-Olivgeris, Marangos and Fligou 6 Regional hospital discharge data, such as those collected by public health departments, are a potential source of data for healthcare facilities to determine whether an admitted patient is at high risk for MDRO carriage.Reference Lin, Rezny, Ray, Jovanov, Weinstein and Trick 7 Such discharge databases contain detailed information about a patient’s history of healthcare exposure (including number of hospital visits, lengths of stay, and associated diagnosis codes), but they typically lack information about antibacterial exposure.
We hypothesized that a patient’s antibacterial exposure history could be inferred through information contained in administrative billing codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) from prior hospitalizations. Specifically, hospital encounters billed with ICD-9-CM codes representing infections likely treated as bacterial (eg, urinary tract infection or community acquired pneumonia) would more likely be associated with antibacterial receipt, with greater antimicrobial spectrum, and with longer duration of treatment, compared with hospital encounters without such infection codes. If true, ICD-9-CM codes could be used as a surrogate for antibacterial use, thereby augmenting the power of healthcare discharge databases to predict a patient’s risk of MDRO carriage at the time of admission.
METHODS
Data Sources
We obtained and merged encounter-level hospital inpatient data, including diagnostic billing codes and medication administration records, from validated clinical data warehouses of 3 hospitals: Rush University Medical Center (RUMC), a 676-bed urban academic hospital; Rush Oak Park Hospital (ROPH), a 176-bed suburban community hospital (January 1, 2014 through December 31, 2014); and John H. Stroger, Jr, Hospital (JSH), a 464-bed urban public safety net hospital in the Cook County Health and Hospitals System (March 2010 through March 2015). With the approval of our institutional review board, we merged records based on a unique, deidentified encounter identification number. We aggregated data containing antimicrobial receipt using standardized antibacterial names and characteristics: broad spectrum (yes/no) or surgical prophylaxis (yes/no) (Appendix A). All diagnostic codes were grouped into larger categories using the Agency for Healthcare Research and Quality’s (AHRQ) Clinical Classification Software (CCS) designations, developed as part of the Health Care Cost Utilization Project (HCUP) designed to cluster patient diagnoses and procedure codes into manageable categories. 8 We removed duplicate records at the encounter level and excluded patients <18 years of age.
Variables
We classified an ICD-9-CM-CM code as an “infection code” if it represented a disease definitely or potentially caused by bacteria because such infections would typically warrant definitive antibacterial treatment (Appendix B). The classification procedure was performed by 2 investigators (M.Y.L. and W.E.T.). To illustrate the classification scheme, both ICD-9-CM codes 322.9 (meningitis not otherwise specified) and 320.9 (bacterial meningitis not otherwise specified) were considered “infection codes” because both infections are either potentially or definitely caused by bacteria. However, code 054.72 (herpes simplex meningitis) was not an “infection code” because the disease is caused by a virus and does not require definitive antibacterial treatment, even though patients with herpes meningitis often receive empiric antibacterial treatment. A given hospital encounter was categorized as having an infection code if any of its associated billing ICD-9-CM codes contained an infection code. We classified all medications in the medication administration record as “any antibacterial” or “not antibacterial.” We further subcategorized antibacterial agents as “broad-spectrum antibacterials,” and “surgical prophylaxis antibacterials” according to CDC’s Antimicrobial Use and Resistance (AUR) module 9 (Appendix C). We calculated the total number of antibacterial days for each encounter based on CDC AUR definitions. We recorded length of stay, age, and sex as additional covariates.
Analytic Methods
We generated pooled and hospital-specific descriptive statistics. We then examined the bivariate associations using the Pearson χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous variables. We used contingency table analysis to generate unadjusted risk differences and risk ratios describing the association between infection ICD-9-CM codes and antibacterial receipt. We then built a multivariable model adjusting for length of stay, age, sex, and multiple hospital visits by the same patient. We used modified Poisson regression with robust error variance to estimate adjusted risk ratios for models with categorical antibacterial exposure outcomes and negative binomial regression for models with the continuous outcome of antibacterial days. Finally, we assessed the presence of an infection code as a classification test by calculating sensitivity, specificity, and positive and negative predictive values for categorical antibacterial outcomes, stratified by hospital. We used Stata version 14.2 software (College Station, TX) to perform all analyses.
RESULTS
We analyzed 121,916 hospital encounters representing 78,094 patients. Of all hospital encounters, whereas ~50% of patients received an antibacterial, only ~25% of encounters were associated with an infection code, and broad-spectrum antibacterial administration was less common (Table 1). Including admissions in which no antibacterial was administered, hospital encounters had an overall mean of 3.3 antibacterial days (Table 1).
TABLE 1 Characteristics of Patient Encounters Across 3 Hospitals

NOTE. RUMC, Rush University Medical Center; ROPH, Rush Oak Park Hospital; JSH, John H. Stroger, Jr, Hospital; SD, standard deviation; ICD-9-CM, International Classification of Disease, Ninth Revision, Clinical Modification.
a January through December 2014.
b March 2010 through March 2015.
c ICD-9-CM codes that represented infections expected to result in antibacterial receipt were selected.
d Receipt of any antibacterial agent during their hospitalization.
Hospital encounters with infection codes, compared to those without infection codes, were more likely to be associated with the following: any antibacterial therapy (absolute risk difference 47%; 95% confidence interval [CI], 46%–48%), broad-spectrum antibacterial therapy (absolute risk difference 30%; 95% CI, 29%–31%), and greater antibacterial days of therapy (5 vs 0 median days; P<.001) (Figures 1 and 2). Antibacterial recipients had a longer median length of stay (4 vs 3 days; P<.001). Overall, hospital encounters with infection codes accounted for 60% of all antibacterial therapy days and 71% of all broad-spectrum antibacterial therapy days. When analyzing only hospital encounters with antibacterial therapy, the presence of infection codes was still associated with greater antibacterial therapy days and a higher proportion of broad-spectrum antibacterial receipt (Figure 2).
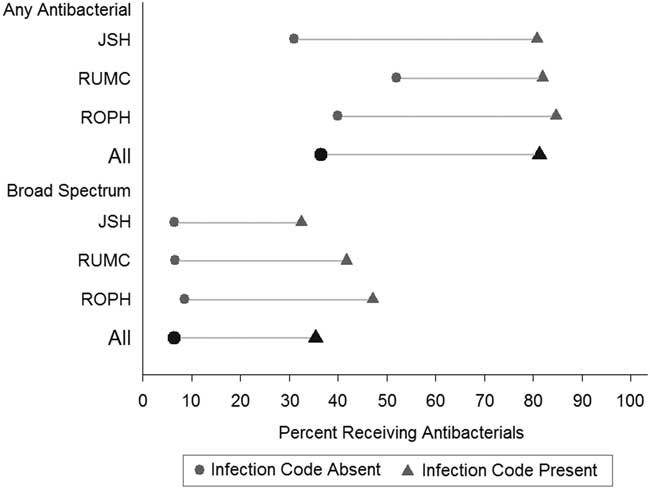
FIGURE 1 Risk differences for receiving antibacterial agents by infection code status, stratified by hospital and in aggregate. NOTE: Broad-spectrum antibacterial was defined by the Center for Disease Control and Prevention’s (CDC’s) Antimicrobial Use and Resistance Module. 9
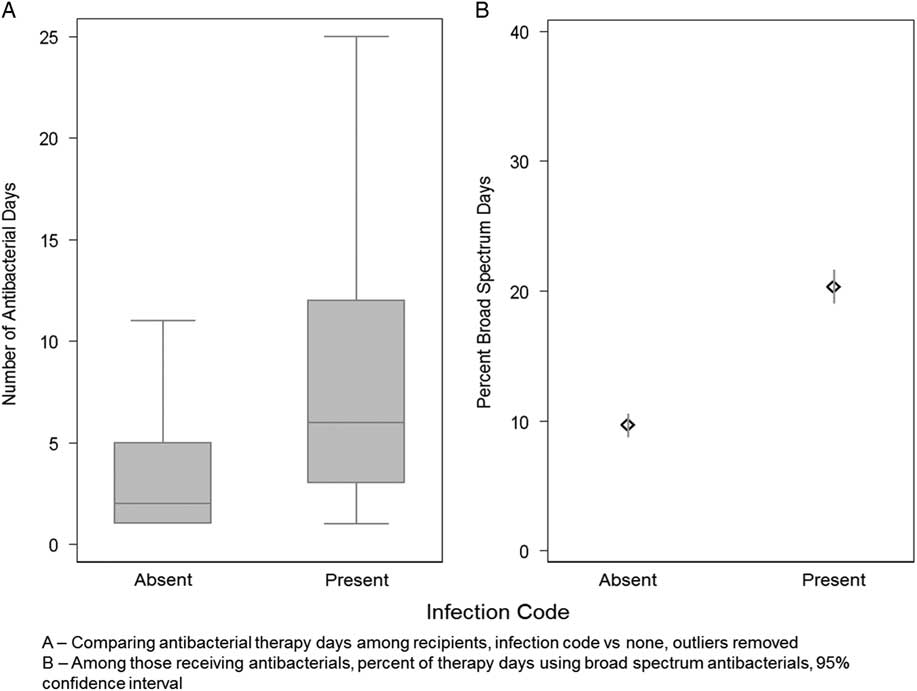
FIGURE 2 Number and intensity of antibacterial therapy days by infection code among encounters with antibacterial receipt. NOTE. 95% confidence intervals are represented by whiskers in A and lines in B.
Infection codes were associated with a 2-fold increased risk of any antibacterial receipt compared to no infection code (unadjusted relative risk [RR], 2.29; 95% CI, 2.27–2.31) (Table 2). Additionally, infection codes were associated with a 5-fold increased risk in broad-spectrum antibacterial receipt compared to no infection code (RR, 5.52; 95% CI, 5.37–5.67). The presence of infection codes increased the count of antibacterial therapy days by a factor of 3 (RR, 3.49; 95% CI, 3.44–3.55). After adjusting for age, sex, length of stay, and multiple hospital encounters per patient, we found that the adjusted associations were similar in magnitude to the crude measures.
TABLE 2 Associations Between Infection Codes and Antibacterial Receipt

NOTE. RR, relative risk; CI, confidence interval; RUMC, Rush University Medical Center; ROPH, Rush Oak Park Hospital; JSH, John H. Stroger, Jr, Hospital.
Antibacterial agents were administered in 36% of hospital encounters that did not have an infection code. Surgical prophylaxis antibacterials were exclusively administered among 28% of such hospital encounters without an infection code, compared to 2% for hospital encounters with an infection code (prevalence ratio, 15.1; 95% CI, 13.8–16.4).
When viewed as a diagnostic test, infection codes performed with a sensitivity of 40.2 percent (95% CI, 39.8%–40.6%) and a specificity of 92.9% (95% CI, 92.7%–93.1%). The positive predictive value of an infection code for any antibacterial receipt was 83.4% (95% CI, 83.0%–83.9%) and negative predictive value was 63.5% (95% CI, 63.2%–63.9%).
Each patient was assigned, on average, 9 ICD-9-CM codes per encounter (range, 1–75). Of the 1,118,080 assigned codes (8,745 distinct) across all encounters, 5% (438 distinct) were infection codes. Of all infection codes found in the study cohort, the most common diagnosis code was urinary tract infection (12.3%), followed by cellulitis of the leg (5.0%) and unspecified septicemia (5.0%) (Table 3). The 20 most common infection codes made up 62 percent of all infection codes among antibacterial recipients.
TABLE 3 The 20 Most Common Infection Diagnosis Codes, Among Those Receiving Antibacterials Among Inpatients at 3 Separate Hospitals, January 2013 through December 2014

NOTE. UTI, urinary tract infection; NOS, not otherwise specified; NEC, not elsewhere classified.
a The top 20 infection codes represent 62% of the 404 distinct infection codes among antibacterial recipients.
b CCS Level-1 descriptions: 1, infectious and parasitic diseases; 8, diseases of the respiratory system; 9, diseases of the digestive system; 10, diseases of the genitourinary system; 12, diseases of the skin and subcutaneous tissue.
DISCUSSION
Prior antibacterial exposure is an important risk factor for assessing a patient’s risk of MDRO carriage at the time of hospital admission, yet this information often is unavailable, particularly when a patient’s medical history includes care at an outside institution. To address this gap, we assessed whether historical diagnostic billing codes, which are more readily available and shared between institutions via regional healthcare discharge databases, could serve as a surrogate for antibacterial exposure.
We found that historical infection codes (diagnostic billing codes that represented possible or probable bacterial infection) were strongly associated with an increased likelihood of prior antibiotic receipt; specifically, having an infection code increased the likelihood of antibacterial receipt more than 2-fold, from 36% to 83%. Not only were patients who had an infection code more likely to have received antibacterial therapy, such patients were also more likely to be treated with broad-spectrum antibacterials and for a longer duration of therapy. Although only 1 of every 4 hospitalized patients had an infection-related code, these patients accounted for >50% of antibiotic days and close to 75% of broad-spectrum antibiotic days. Consistent with prior publications, antibiotic use was common such that ~50% of all hospitalized patients received at least 1 antibacterial agent.Reference Magill, Edwards and Beldavs 10
Our findings have potential applications in infection control and antimicrobial stewardship. First, infection-related diagnosis codes can be used in multivariable prediction models to identify patients at high risk for carriage of MDROs. Our current analysis, which validates use of infection diagnostic billing codes as a surrogate for antibacterial exposure, is an important initial step for including such information in prediction models. In a separate analysis, we found that a multivariable model that included healthcare exposure parameters and infection codes performed well at discriminating patients with carbapenem-resistant Enterobacteriaceae on admission.Reference Lin, Rezny, Ray, Jovanov, Weinstein and Trick 7 Early identification of patients harboring an MDRO supports the “Detect and Protect” strategy promoted by CDC, 11 and MDRO risk prediction models could focus active surveillance and prevention efforts on high-risk patients as well as guide judicious use of antimicrobial therapy based on MDRO risk rather than on the basis of healthcare exposure alone.Reference Chalmers, Rother, Salih and Ewig 12 , Reference Yap, Datta and Metersky 13
A separate application of our findings is the potential use of diagnostic codes to improve risk adjustment of antimicrobial use between facilities. Currently, the CDC assesses whether hospitals use a standardized antimicrobial administration ratio (SAAR), which compares observed antimicrobial usage against a predicted rate of antimicrobial usage that accounts for patient care location (eg, intensive care unit versus medical ward). 9 Infection codes could enhance the antimicrobial usage prediction model by adjusting for the differential burden of bacterial infections at facility locations. Further validation of infection codes for antimicrobial use risk adjustment would be needed, including identifying other noninfectious billing codes that routinely trigger appropriate antibacterial therapy (eg, prophylaxis for surgery or chemotherapy-induced neutropenia).
The use of diagnostic billing codes to identify antibacterial use has inherent limitations because billing codes are not designed to document medical history; rather, the primary purpose of billing codes is for financial remuneration.Reference Jhung and Banerjee 14 Depending on the context, discharge diagnosis codes can underestimate or overestimate hospital-acquired infections, in part due to variability in clinician documentation and coder interpretation of the medical record. Importantly, unlike many other datasets,Reference Jhung and Banerjee 14 2 hospitals in our dataset did not cap the number of diagnosis codes per hospital visit and the third hospital had a liberal cap of 50 codes. We found that ~33% of hospital encounters without any infection code were associated with antibacterial administration. The use of antibacterials for surgical prophylaxis explains a large proportion of such discrepant encounters. Other possible explanations include undercoding (a bacterial infection was present but not coded), the empiric use of antibacterials for nonbacterial conditions (eg, initial antibacterial treatment for Herpes simplex virus meningitis prior to confirmation or exacerbation of chronic obstructive pulmonary disease), or the lack of precision of some ICD-9-CM codes (eg, fever) to differentiate bacterial infection from other (eg, viral or noninfectious) processes. The limitations of ICD-9-CM codes can lead to misclassification of disease states, potentially biasing our results toward the null. To maximize the specificity, we chose a priori ICD-9-CM codes associated with definitive (ie, final, often prolonged) antibacterial treatment rather than codes clinically associated with only empirical (ie, initial) antibacterial treatment.
We used manual ICD-9-CM categorization methods because, to our knowledge, no publicly available categorization of ICD-9-CM codes for infection currently exists. The Healthcare Cost and Utilization Project’s Clinical Classifications Software (CCS) tool 8 categorizes ICD-9-CM codes into 4 hierarchical levels based on clinically meaningful groupings. An “infectious and parasitic diseases” category is 1 of 18 Level-I CCS categories, but it does not effectively capture all infections. For example, urinary tract infections are found in the separate “genitourinary system” CCS Level-I category, and pneumonia is found in the “respiratory system” CCS Level-I category. Our list of infection codes is available in the Appendix. Additional mapping and validation would be required to apply our current ICD-9-CM infection code categorization to the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM).
In summary, we found that infection-related hospital diagnostic billing codes were associated with inpatient antibacterial exposure and that this exposure is often prolonged and with broad-spectrum agents. Until detailed antibacterial administration becomes more widely shareable, diagnostic billing codes may serve as a useful proxy for assessing patient-level and institutional-level antibacterial usage.
ACKNOWLEDGMENTS
We acknowledge Bala Hota, MD, MPH, Ekta B. Kishen, MPH, and Michael Carrasquilla, BS, of Rush University Medical Center and Huiyuan Zhang, MS, of Cook County Health and Hospitals System for their help with data acquisition, extraction, and validation. We acknowledge Robert A. Weinstein for his review of an earlier version of the manuscript.
Financial support: This study was funded by the Centers for Disease Control and Prevention (grant no. RFA-CK-11-0010501SUPP15: Antimicrobial Resistance and Adverse Events—A Multicenter Program Expansion [U54]), and Robert A. Weinstein was the principal investigator.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.23







