Antimicrobial resistance (AMR) is a critical patient safety and public health crisis emphasized by the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).1, 2 Calls for a coordinated approach to antibiotic stewardship emerged in the literature more than 40 years ago.Reference Counts3 The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) first recommended antibiotic stewardship in acute-care hospitals (ACH) in 1997 then updated guidelines for implementation in 2007.Reference Dellit, Owens and McGowan4, Reference Shlaes, Gerding and John5 In 2012, SHEA, IDSA, and the Pediatric Infectious Diseases Society (PIDS) urged for antibiotic stewardship programs (ASPs) to be required through regulatory mechanisms.6
In 2014, the Presidential Executive Order—Combating Antibiotic-Resistant Bacteria (CARB)—called for a comprehensive antibiotic stewardship plan, and the CDC’s “7 Core Elements” for a successful hospital ASP.7, Reference Pollack and Srinivasan8 The following year, the National Action Plan directed all ACHs to establish ASPs by 2020 and to expand antibiotic stewardship across the healthcare continuum.9 The National Quality Forum and the Joint Commission’s standards incorporated the CDC core elements, 3 of which refer directly to resource allocation: dedicated human, financial, and information technology resources.Reference Pollack and Srinivasan8, 10, 11 However, the degree of resources required for a successful ASP at a given institution is not standardized and is influenced by numerous variables including bed size, case-mix index, healthcare delivery model, level of training, and number of support pharmacists. These factors were specifically acknowledged in a recent multisociety white paper.Reference Ostrowsky, Banerjee and Bonomo12
Mounting evidence demonstrates that ASPs can optimize individual patient outcomes, improve the quality of care, and provide critical patient safety processes while reducing antimicrobial-associated adverse events (eg, acute kidney injury and C. difficile infection rates), length of stay, and AMR development.Reference Pollack and Srinivasan8, Reference Davey, Marwick and Scott13, Reference Barlam, Cosgrove and Abbo14 Antibiotic stewardship strategies can be implemented in any healthcare setting, and they are often cost-saving for institutions. Multidisciplinary engagement and myriad interventions from allergy management to rapid diagnostic review have demonstrated profound success.
Researchers have studied optimal provider staffing, including physicians, nurses, and pharmacy and quality personnel, in diverse healthcare settings, often demonstrating improved patient outcomes with appropriate staffing standards, particularly in intensive care units (ICUs).Reference Pronovost, Angus, Dorman, Robinson, Dremsizov and Young15–Reference Harrington, Schnelle, McGregor and Simmons22 This review describes the existing literature on antibiotic stewardship staffing, builds on the historical parallel of infection prevention staffing standardization, and concludes with a call to action for formal antibiotic stewardship staffing standards.
The infection prevention parallel
Infection prevention programs serve as an important model for leveraging ASP infrastructure and implementation resources.Reference Manning, Septimus and Ashley23 Reviewing the timeline reveals similar struggles with establishing formal staffing guidelines and appropriate funding mechanisms (Fig. 1). One of the first infection prevention studies addressing staffing was an 18-month evaluation of time required to “carry out a surveillance program of at least intermediate effectiveness (p 314)” in 6 community hospitals from 1965 to 1966.Reference Eickhoff, Brachman, Bennett and Brown24 The outcomes informed the initial CDC infection prevention staffing recommendation of 1 infection preventionist full-time equivalent (FTE) per 250 occupied beds.Reference Hughes25 The CDC’s landmark Study on the Efficacy of Nosocomial Infection Control (SENIC) project demonstrated that several foundational infection prevention activities and a ratio of 1 infection preventionist for every 250 beds yielded a 32% reduction in nosocomial infection (Table 1).Reference Haley, Culver and White26 Further analysis to explore a more “lenient” staffing ratio confirmed these findings: infection reductions “declined sharply” as the number of occupied beds per infection preventionist rose above 250.

Fig.’1. Recommended Infection Prevention Staffing Resources. IP, Infection Prevention; FTE, full-time equivalent; SENIC, Study on the Efficacy of Nosocomial Control; NNIS, National Nosocomial Infections Surveillance; APIC, Association for Professionals in Infection Control and Epidemiology; CICA, Canadian Infection Control Alliance; EIC, European Infection Control; SIGHT, Systematic Review and Evidence-Based Guidance on Organization of Hospital Infection Control Programmes; PH&S, Providence Health and Services.
Table 2. Selected Surveys Reporting Antimicrobial Stewardship Program Resources

Note. ASP, antimicrobial stewardship program; EIN, Emerging Infections Network; SHEA, Society for Healthcare and Epidemiology of America; ACH, acute-care hospital; NHSN, National Healthcare Safety Network; SHARPS, Sharing Antimicrobial Reports for Pediatric Stewardship; USNWR, United States News and World Report; FTE, full-time equivalent.
a Survey sent to freestanding children’s hospitals that are members of Children’s Hospital Assocation.
Participation in the CDC’s National Nosocomial Infections Surveillance (NNIS) system is limited to hospitals with a minimum of 1 infection preventionist FTE for the first 100 occupied beds (and 1 FTE for each additional 250 beds).Reference Richards, Emori, Edwards, Fridkin, Tolson and Gaynes27 The increasing volume and complexity of infection prevention activities prompted the Association for Professionals in Infection Control and Epidemiology (APIC) to re-evaluate infection preventionist staffing ratios. Using the Delphi method, a panel of 45 infection preventionists reported 40 of the 46 “essential” tasks identified were not regularly completed, citing many barriers foreshadowing antibiotic stewardship concerns, namely “competing responsibilities” and “access to resources (p 998).” Reference O’Boyle, Jackson and Henly28 The APIC then recommended 1 infection preventionist FTE per 100 occupied beds, nearly double that of the existing SENIC guidelines and similar to NNIS staffing directives.
The 2011 Prevention of Nosocomial Infections and Cost Effectiveness Refined (P-NICER) study of 975 hospitals and 1,534 ICUs provided the most comprehensive evaluation of infection prevention program structure and support in the United States after SENIC; it reported an average of 1.2 infection preventionists per 100 beds.Reference Stone, Pogorzelska-Maziarz and Herzig29 The authors concluded that the current recommendation of “0.8 to 1 infection preventionist per 100 hospital beds … are most likely out of date due to the complexity and responsibilities of infection prevention in hospitals today (p 97)” and staffing was “not consistent with published guidelines (p 98).”
Infection preventionist staffing standards are coming into focus throughout the globe; recent data suggesting that current recommendations may still be below actual labor needs.Reference Morrison30–Reference Dickstein, Nir-Paz and Pulcini34 Recently, Providence Health and Services, a large healthcare organization comprising 34 hospitals, performed a multifaceted evaluation including literature review, current infection prevention time allocation assessment, regional meetings with key stakeholders, and a quantitative needs assessment. These measures resulted in a staffing model developed to address priorities and gaps individualized to regions and hospitals. They concluded that the ideal benchmark should be 1 infection preventionist per 69 occupied beds if outpatient and long-term care (LTC) settings are included.Reference Bartles, Dickson and Babade35 Unfortunately, hospital surveys often demonstrate poor “real world” adherence to staffing recommendations despite consensus regarding their impact on patient safety—a phenomenon also evident in the antibiotic stewardship literature. The barriers and progress in infection preventionist staffing and resource allocation serve as an ideal framework through which to view ASP development.
Surveys describing stewardship staffing and financial needs and barriers
A recent white paper on behalf of IDSA, SHEA, and PIDS recommends that compensation for ASPs be distinct from funds dedicated to infection prevention, with protected time afforded to antibiotic stewardship physicians and staff “appropriately scaled to facility size (p 998).”Reference Ostrowsky, Banerjee and Bonomo12 Unfortunately, surveys of budding and active ASPs routinely cite insufficient financial resources, time, and staff as barriers to program success.Reference Doernberg, Abbo and Burdette36–Reference Pakyz, Moczygemba, VanderWielen, Edmond, Stevens and Kuzel38 A 1999 survey of Emerging Infections Network (EIN) members—a network of US infectious diseases providers established by the CDC—found that 50% of respondents performed antimicrobial prior authorization but only 18% received remuneration for this effort (Table 2).Reference Sunenshine, Liedtke, Jernigan and Strausbaugh39 An accompanying commentary emphasized the value of this monitoring despite “little or no pay” and suggested that antibiotic stewardship physicians receive a global fee for “non–patient-care activities,” which can be justified by an annual report to hospital administration.Reference McGowan40, Reference McQuillen, Petrak, Wasserman, Nahass, Scull and Martinelli41
Table 1. Selected Studies of Infection Prevention Resources

Note. IP, infection prevention; FTE, full-time equivalent; SENIC, Study on the Efficacy of Nosocomial Control; NNIS, National Nosocomial Infections Surveillance; APIC, Association for Professionals in Infection Control and Epidemiology; P-NICER, Prevention of Nosocomial Infections and Cost Effectiveness Refined; SIGHT, Systematic Review and Evidence-Based Guidance on Organization of Hospital Infection Control Programmes; PROHIBIT, Prevention of Hospital Infections by Intervention and Training; ESCMID, European Society of Clinical Microbiology and Infectious Diseases.
Following the 2007 IDSA/SHEA antibiotic stewardship guidelines, 52% of health professionals surveyed lacked an ASP; personnel shortages (55%) and financial considerations (35%) were cited as the top 2 barriers to program implementation,Reference Pope, Dellit, Owens and Hooton42 which was confirmed in a separate 2009 survey with similar results.Reference Doron, Nadkarni and Lyn Price43 A follow-up EIN survey in 2009 demonstrated only a modest increase ASP presence, with 25% of ASPs lacking physician involvement and only 52% of physicians receiving compensation for antibiotic stewardship activities.Reference Johannsson, Beekmann, Srinivasan, Hersh, Laxminarayan and Polgreen44
Pediatric ASPs have faced similar resource challenges. In a 2008 EIN survey, only 33% of pediatric facilities featured an ASP, and >50% of respondents cited funding and personnel insufficiencies as barriers to ASP implementation.Reference Hersh, Beekmann, Polgreen, Zaoutis and Newland45 A subsequent 2011 survey of freestanding children’s hospitals demonstrated similar results: 38% had a formal ASP and 36% were planning implementation. Identical support barriers were voiced among those without an ASP.Reference Newland, Gerber and Weissman46 For existing ASPs, the median number of total FTE support was only 0.63 (median bed size, 295) even though total FTE support, particularly pharmacist FTE, correlated with the number of monitored antibiotics. More recently, the Sharing Antimicrobial Reports for Pediatrics Stewardship (SHARPS) collaborative reported data on their 36 participating hospitals with an overall antibiotic stewardship FTE of only 0.75 (median bed size, 284).Reference Newland, Gerber and Kronman47
In 2011, 5 years after California Senate Bill 739 mandated all state ACHs develop an ASP,48 only 50% of facilities had complied with only 73% of physicians and 80% of pharmacists receiving any dedicated antibiotic stewardship FTE support.Reference Trivedi and Rosenberg49 Subsequent California legislation in 2014 (Senate Bill 1311) went further, requiring inpatient ASPs to have at least 1 physician or pharmacist leader.50 Missouri passed a similar legislative mandate (Senate Bill 579), also requiring National Healthcare Safety Network (NHSN) antimicrobial use reporting though staffing and funding mechanisms were not clarified.51
The first NHSN survey accounting for antibiotic stewardship practices in the United States was conducted in 2014. Only 32% of the 4,184 ACHs surveyed provided antibiotic stewardship salary support despite both the 2014 and 2015 NHSN surveys demonstrating salary support to be an independent predictor for achieving all 7 CDC core elements.Reference Pollack, van Santen, Weiner, Dudeck, Edwards and Srinivasan52, Reference O’Leary, van Santen, Webb, Pollock, Edwards and Srinivasan53 The theme of limited resources for antibiotic stewardship continues to ripple through the movement’s timeline, with particular impact on smaller, community hospitals.Reference Johannsson, Beekmann, Srinivasan, Hersh, Laxminarayan and Polgreen44, Reference Stenehjem, Hyun and Septimus54 Small community hospitals (<200 beds) represent 72% of US nonfederal hospitals, but only 31% of hospitals with <50 beds and 26% of critical-access hospitals (<25 beds) have an ASP featuring all 7 CDC core elements.Reference Srinivasan55–Reference Buckel, Veillette, Vento and Stenehjem57 Despite these substantial barriers, successes have been demonstrated in the community setting by optimizing available resources.Reference Stenehjem, Hyun and Septimus54, Reference Buckel, Veillette, Vento and Stenehjem57–Reference Ohl and Dodds Ashley60
Even among US News and World Reports (USNWR) highest-ranking hospitals, a recently published 2016 survey reported that fewer than half of institutions (48%) have a dedicated ASP budget.Reference Nhan, Lentz, Steinberg, Bell and Morris61 Most of these hospitals (65%) have ≤0.5 physician FTE, and 48% of programs feature only 0.51–1.0 pharmacist FTE. For surveyed ASPs with a budget, most fell within the range of $50,000–$250,000 per year. However, as an example, pediatric hospital ASP budgets ranged from $17,000 to $388,500 annually, without correlation to hospital size, which demonstrates the inconsistencies in hospital ASP funding.
International antimicrobial stewardship staffing
Much of the concrete guidelines for antibiotic stewardship resources have been provided by stewardship colleagues abroad (Table 3). Nevertheless, international ASPs still struggle to meet policy recommendations. The French Ministry of Health has mandated public reporting of each hospital’s antibiotic policy since 2007.Reference Hajjar62, Reference Wang, Pulcini, Rabaud, Boivin and Birge63 Data from the 2007 antibiotic policy questionnaire produced a composite index (ICATB) to assess appropriate antimicrobial use.Reference Amadeo, Dumartin, Parneix, Fourrier-Reglat and Rogues64 In 2015, using the previously developed ICATB indices, a French AMR task force surveyed 65 French facilities to assess the human resources required to implement recommended ASP activities. Ultimately, they recommended 3.6 antibiotic supervisor FTE, 2.5 pharmacist FTE, and 0.6 microbiologist FTE per 1,000 acute-care beds—a dramatic increase from prior staffing targets.Reference Le Coz, Carlet, Roblot and Pulcini65
Table 3. Selected Antimicrobial Stewardship Program Staffing Proposals
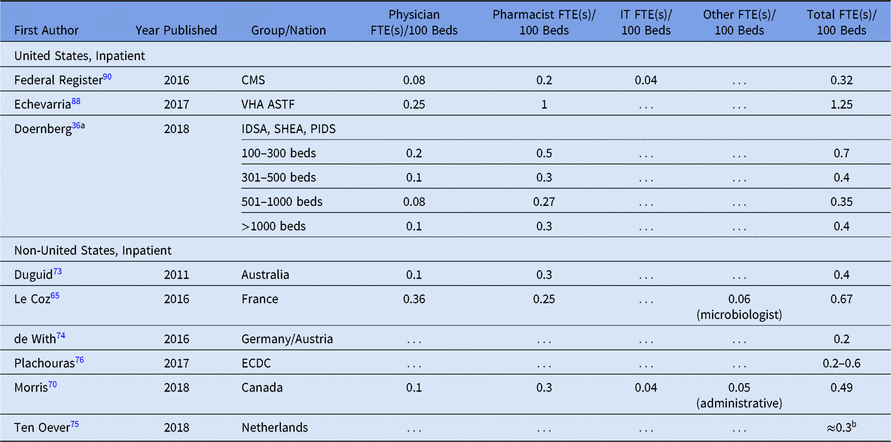
Note. FTE, full-time equivalent; IT, information technology; CMS, Centers for Medicare and Medicaid Services; VHA, Veterans’ Health Administration; ASTF, antimicrobial stewardship task force; IDSA, Infectious Diseases Society of America; SHEA, Society for Healthcare Epidemiology of America; PIDS, Pediatric Infectious Diseases Society; ECDC, European Centre for Disease Prevention and Control.
a Physician, pharmacist and total FTE/100 beds calculated from average bed size per given range (eg, 200 for 100–300 range, 400 for 301–500, 750 for 501–1,000) except for >1,000 beds, which was calcuated per 1,000 beds.
b Approximated from recommended range for “optimal staffing standards during the first few years of implementing an ASP” of 1.25 FTE per 300 beds to 3.18 FTE per 1,200 beds (ie, 0.27–0.42 FTE per 100 beds).
In 2011, the Australian Commission on Safety and Quality in Health Care required all hospitals to implement an ASP by 2013.66 However, surveys in 2012 demonstrated that implementation was lagging with only 5% of hospitals in Victoria and 19% of Queensland hospitals reporting a dedicated ASP.Reference James, McIntosh and Luu67, Reference Avent, Hall and Davis68 Lack of educational training in antimicrobial use and insufficient pharmacy resources were leading barriers.
An internet-based survey distributed to 660 hospitals in 67 countries by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in 2012 sought to characterize global AS.Reference Howard, Pulcini and Levy Hara69 Respondents were mostly European from tertiary teaching hospitals (48%) with >500 beds (52%). National antibiotic stewardship standards existed in 52% of countries, dominated by Europe (81%), but formal ASPs were present in only 58% of hospitals, ranging from 67% in North America to 14% in Africa. The number of resource hours per week varied dramatically between countries; lack of funding and personnel were reported as the major barriers to implementation by all respondents.
In Canada, antibiotic stewardship has been required in ACHs since 2013.Reference Morris, Rennert-May and Dalton70 Given the lack of clarity around necessary human resources required and the complexity of petitioning hospital administration, the Association of Medical Microbiology and Infectious Diseases Canada (AMMI) recently published a “business case” for ACH ASPs through expert consensus. They proposed 1 physician FTE, 3 pharmacist FTE, 0.5 administrative staff FTE, and 0.4 data analyst FTE per 1,000 ACH beds, with a minimum requirement of 0.1 physician FTE and 0.3 pharmacist FTE regardless of institutional size. Nevertheless, a recent survey of 97 organizations in Ontario found that only 50% of hospitals had designated antibiotic stewardship resources; teaching hospitals reported 0.57 physician FTE and 2.16 pharmacist FTE per 1,000 beds. Small community hospitals averaged only 0.006 pharmacist FTE and 0 physician FTE.Reference Leung, Wu, Langford and Garber71
In 2017, PulciniReference Pulcini, Morel and Tacconelli72 et al summarized the proposed minimum staffing standards by countries with mandatory hospital antimicrobial stewardship: Australia (4 FTE per 1,000 acute-care beds), Austria and Germany (2 FTE per 1,000 beds), Canada (4.9 FTE per 1,000 acute-care beds), France (“optimal” goal of 6.7 FTE per 1,000 acute-care beds), and The Netherlands (3 FTE for bed size >750).Reference Le Coz, Carlet, Roblot and Pulcini65, Reference Morris, Rennert-May and Dalton70, Reference Pulcini, Morel and Tacconelli72–Reference Ten Oever, Harmsen and Schouten75 This summary also commented that ASPs remain understaffed or nonexistent in most countries, with almost exclusive inpatient focus despite the fact that most global antimicrobial use originates in the outpatient environment. The 2017 European Centre for Disease Prevention and Control (ECDC) technical report proposed 0.5–1.5 FTE for antibiotic stewardship activities per 250 acute-care beds, citing the French and German recommendations.76
Comparing ASP FTE between individual countries is complicated by varying expectations and definitions of antibiotic stewardship activity in a given nation and by differing funding streams (eg, private vs national health system).Reference Pulcini, Morel and Tacconelli72, Reference Charani, Smith and Skodvin77, 78 Importantly, much of the world’s antimicrobial overuse occurs in low- and middle-income countries with scant resources for antibiotic stewardship.79, Reference Walia, Ohri and Mathai80 The United Nations General Assembly high-level AMR meeting in 2016 inspired calls for a “Global Antimicrobial Conservation Fund” to provide transitional financial and technical support to build ASP capacity in the developing world.Reference Mendelson, Dar, Hoffman, Laxminarayan, Mpundu and Rottingen81, Reference Plachouras and Hopkins82
Unfortunately, most international stewardship literature—regardless of nation—does not comment on ASP team composition nor provide FTE data, leading some to propose that human resources be added to the reporting checklist for epidemiologic studies on AMR (STROBE-AMS).Reference Tacconelli, Cataldo and Paul83, Reference Harder, Eckmanns, Schmidt, Kern and Sin84
Proposed staffing ratios in the United States
Within the United States, the Veterans’ Health Administration (VHA) has led the way in promoting antibiotic stewardship implementation and staffing requirements, creating the antibiotic stewardship initiative in 2010, followed by the National Antibiotic Stewardship Task Force (ASTF) in 2011.Reference Srinivasan55, Reference Suda, Livorsi and Goto85 In 2012, the VHA Healthcare Analysis and Information Group (HAIG) surveyed all 130 VHA facilities to characterize antibiotic stewardship structure and practices.Reference Chou, Graber and Jones86 At the time, 38% of hospitals had an ASP defined as at least a physician and clinical pharmacist. In 2014, VHA Directive 1031 mandated every VHA facility implement antibiotic stewardship paired with annual ASP evaluations.Reference Suda, Livorsi and Goto85, Reference Kelly, Jones and Echevarria87 Following this directive, 89% of facilities had a defined ASP by 2015 (compared to 41% in 2011), with a 12% decrease in inpatient antimicrobial use compared to 2010.Reference Kelly, Jones and Echevarria87
Next, the VHA ASTF partnered with the Clinical Pharmacy Practice Office, a national program that previously developed standardized clinical pharmacy staffing models, to create a staffing calculator based on time-in-motion tracking studies from 12 facilities in 2014 for both clinical interventions and program management activities.Reference Echevarria, Groppi, Kelly, Morreale, Neuhauser and Roselle88 The ASTF found that a median of 2.62 FTE (1.01 FTE per 100 occupied beds) were required. After excluding outliers, the group proposed 1 pharmacist FTE per 100 occupied beds (Fig. 2). Though not extrapolated from this study, the group also proposed 0.25 physician FTE per 100 occupied beds. They concluded that a minimum of 0.25 physician FTE and 0.5 pharmacist FTE should be allotted for hospitals with <100 beds. In 2017, VHA Directive 1131 required minimum physician and pharmacist FTE staffing in keeping with this study’s findings based on facility complexity.89

Fig. 2. Selected Antimicrobial Stewardship Program Staffing Proposals. ASP, Antimicrobial Stewardship Program; FTE, full-time equivalent; ACH, Acute Care Hospital; US, United States; ECDC, European Centre for Disease Prevention and Control.
A 2016 cross-sectional electronic survey of 244 members of IDSA, SHEA, and PIDS actively involved in antibiotic stewardship reported on “self-reported effectiveness” in relation to staffing levels, defined as demonstrating ≥1 of the following: cost savings, decreased antimicrobial use or decreased rate of multidrug-resistant organisms in the prior 2 years.Reference Doernberg, Abbo and Burdette36 Multivariate analysis accounting for bed size showed a 1.48-fold increase in program effectiveness for every additional combined 0.5 FTE support. These authors proposed minimum combined FTE support of 1.4 FTE for hospitals with 100–300 beds, 1.6 FTE for 301–500 beds, 2.6 FTE for 501–1,000 beds, and 4 FTE for settings with >1,000 beds. Furthermore, they proposed a physician-to-pharmacy ratio of 1:3 for the “highest-value use of resources.”
In 2016, the Department of Health and Human Services Centers for Medicare and Medicaid Services (CMS) stated, “(However, we believe that) the burden of implementing and maintaining an AS (program) includes the salaries of the qualified personnel needed to establish and manage such a (CAH) program (p 39474).” They suggested 0.1 physician FTE (preferably trained in infectious diseases), 0.25 pharmacist FTE, and 0.05 data analyst FTE for an average-sized hospital of ~124 beds.90
Antimicrobial stewardship team composition: Who “counts?”
Numerous studies and reviews have evaluated different permutations of a successful antibiotic stewardship team, including varied approaches to leadership, team composition, and antibiotic stewardship–specific training.Reference Kapadia, Abramson and Carter37, Reference Buckel, Veillette, Vento and Stenehjem57, Reference Trivedi and Kuper58, 91–Reference Olans, Olans and DeMaria94 The 2012 SHEA, IDSA, and PIDS policy statement recommended that an ASP should include a physician, a pharmacist, a clinical microbiologist, and an infection preventionist.6 The Joint Commission suggests a multidisciplinary ASP include an infectious disease physician, pharmacist(s), and infection preventionist(s) when available, They allow part-time, consulting, and even telehealth staff.11 Most proposed FTE metrics refer to either physician or pharmacy personnel; minimal comment or data pertain to information technology (IT) and administrative support. In the previously mentioned 2016 resources survey, only 16% of surveyed programs had data analytics support (average FTE, 0.25) and only 13% featured administrative support (mean FTE, 0.16).Reference Doernberg, Abbo and Burdette36 AMMI Canada formally recommended designated administrative and data analytic support, though a follow-up survey demonstrated that only 11% of established ASPs had such funding.Reference Leung, Wu, Langford and Garber71 Despite the potential effectiveness and efficiency of antibiotic stewardship IT systems, resource allocation is often lacking, as is analytic support for the data generated.Reference Kapadia, Abramson and Carter37, Reference Calloway, Akilo and Bierman95–Reference Kuper, Nagel, Kile, May and Lee97
The open question of “who counts” when evaluating antibiotic stewardship staffing is especially important for smaller medical facilities.Reference Stenehjem, Hyun and Septimus54, Reference Buckel, Veillette, Vento and Stenehjem57 The VHA and other authors have called for future studies to facilitate the recruitment of less “traditional” ASP personnel (including hospitalists, nursing staff and tele-ASPs), particularly for institutions where infectious disease specialists are simply not available, including many long-term care (LTC) settings.Reference Ostrowsky, Banerjee and Bonomo12, Reference Suda, Livorsi and Goto85, Reference Childs-Kean, Briggs and Cho92, Reference Ohl and Luther98 Although a variety of staffing models exist, the importance of dedicated support for AS-specific activities cannot be overstated.
Stewardship staffing outside the hospital
The 2012 SHEA, IDSA and PIDS policy statement asks for antibiotic stewardship to be a “fiduciary responsibility for all healthcare institutions across the continuum of care (p 322).” 6 In 2015, the CDC published its Core Elements of Antibiotic Stewardship for Nursing Homes then the Core Elements of Outpatient Antibiotic Stewardship in 2016.99, 100 Long-term care ASPs have been required by CMS since November 2017.101 Most antimicrobial use and expenditure occurs outside the hospital (eg, clinics, emergency departments (ED), hemodialysis units and LTC facilities), and only one-third of outpatient prescriptions are appropriate.Reference Suda, Hicks, Roberts, Hunkler and Danziger102–Reference King, Fleming-Dutra and Hicks104 Data on the prevalence of outpatient antibiotic stewardship activity are scant, clouding our understanding of true staffing needs.Reference Suda, Livorsi and Goto85, Reference Drekonja, Filice and Greer105–Reference D’Agata, Lindberg and Lindberg108
Outpatient stewardship staffing
Several reviews of evidence-based outpatient antibiotic stewardship interventions exist, but they do not provide guidance on funding mechanisms.Reference Drekonja, Filice and Greer105, Reference Dobson, Klepser and Pogue109, Reference Klepser, Dobson and Pogue110 According to one reviewer, compared to inpatient strategy, it is difficult “to justify funding based on reductions in antibiotics expenditures or decreased length of stay (p 458).”Reference Klepser, Dobson and Pogue110 Although resource-intensive approaches such as provider feedback demonstrate impact and support outpatient ASP expansions, interventions often focus on educational awareness and IT decision support tools.Reference Drekonja, Filice and Greer105, Reference Klepser, Dobson and Pogue110–Reference Meeker, Linder and Fox118 Various personnel models for outpatient antibiotic stewardship infrastructure have been suggested, including engaging, training, and incentivizing community pharmacists and public health department personnel and leveraging community collaborations and health systems.Reference Suda, Livorsi and Goto85, Reference Klepser, Dobson and Pogue110, Reference Blanchette, Gauthier and Heil119 Experts continue to call for research into outpatient ASPs with varying resources as well as “potential policies or incentives” to promote outpatient antibiotic stewardship.Reference Zetts, Stoesz, Smith and Hyun120
Long-term care stewardship staffing
A comprehensive 2016 review of LTC antibiotic stewardship found that <20% of nursing homes employ full-time physicians and that most medical directors spend only 8–12 hours per week providing direct patient care.Reference Morrill, Caffrey, Jump and Dosa121 An early survey of Nebraska LTC facilities found that 60% had an ASP, though more recent surveys revealed only 23% in Michigan and 28% in Rhode Island where a paltry 15% received budgeted support with mean FTE allocations for physicians and infectious disease pharmacists of 0.02 and 0.01, respectively.Reference Malani, Brennan, Collins, Finks, Pogue and Kaye122–Reference Van Schooneveld, Miller, Sayles, Watkins and Smith124 A variety of antibiotic stewardship approaches have been employed in LTC facilities to leverage limited available resources, including sharing antibiotic stewardship personnel.Reference Morrill, Caffrey, Jump and Dosa121, Reference Peron, Hirsch, Jury, Jump and Donskey125–Reference Seo, Lo and Abbo129 Despite some success, staffing limitations often prohibit more reliable but resource-heavy interventions.Reference Wu, Langford, Daneman, Friedrich and Garber127
Other considerations
Beyond setting size, location, and team composition, additional variables affecting appropriate stewardship staffing are worth considering but are rarely discussed.Reference Ostrowsky, Banerjee and Bonomo12 Care complexity influences resource allocation for high-risk patient populations (eg, transplant recipients or burn patients) who are especially prone to prolonged antibiotic exposure and complications.Reference Aitken, Palmer, Topal, Gabardi and Tichy128 A 2015 survey of 71 solid-organ hematopoietic stem cell transplant centers in 32 states cited staffing challenges as a barrier for transplant antibiotic stewardship.Reference Seo, Lo and Abbo129 ASPs presumably require more resources in the “initiation” phase (particularly for IT support) compared to an established program in the “maintenance” phase of program development.Reference Kuper, Nagel, Kile, May and Lee97 Data evaluating how complexity and intensity of care as well as the presence of specialty services are limited, but the effect of these variables on antimicrobial use and need for risk adjustment have been examined previously.Reference Polk, Hohmann, Medvedev and Ibrahim130–Reference van Santen, Edwards and Webb132 It follows that staffing ratios would similarly require calibration to reflect differing needs. Whether minimum requirements are tied only to occupied bed count or some other measure warrants further study. Elements of the NHSN’s pioneering standardized antimicrobial administration ratio (SAAR) (eg, academic affiliation and ICU bed count) could be utilized for adjusting expected ASP staffing needs.Reference van Santen, Edwards and Webb132, Reference Fridkin and Srinivasan133
Yet another call to action
The recurring theme in antibiotic stewardship staffing literature is insufficient financial and human resources. Spellberg et alReference Spellberg, Bartlett and Gilbert134 point out the temptation for institutions to “check the box” in response to regulatory requirements yet still understaff the true needs of a robust multidisciplinary ASP. The literature is replete with “real world” examples of this phenomenon in California, Canada, Australia, and beyond. As stated bluntly by Pulcini et al,Reference Pulcini, Morel and Tacconelli72 formal antibiotic stewardship staffing standards are needed and should be linked to sustainable funding mechanisms.
The general movement away from “fee for service” models toward reimbursement for quality of care presents an opportunity for a productive partnership between antibiotic stewardship and hospital administration.Reference Nagel, Stevenson, Eiland and Kaye135, Reference Pedersen, Schneider and Scheckelhoff136 Conditions of participation in Medicare were recently approved and include language to regulate and incentivize ASP development and references prior 2016 CMS staffing proposals.137 Specific quality and staffing metrics (some with direct monetary incentives) are emerging in visible national organizations, including the Leapfrog Group, Agency for Healthcare Research and Quality and USNWR.Reference Morris, Brener and Dresser138 Leapfrog now relies on information collected from the NHSN survey, and the USNWR pediatric survey includes a minimum threshold of 0.4 FTE for pharmacy support, 0.3 FTE for medical director, and 0.2 FTE for analyst support dedicated to ASP.139, 140 Such incentives are likely to help ASPs “compete” for resource allocation in a given institution.Reference Fridkin and Srinivasan133
Most inpatient ASP staffing proposals recommend a combined physician and pharmacist FTE of roughly 1 to every 100–250 occupied beds, with a suggested physician-to-pharmacist ratio of 1:3.Reference Doernberg, Abbo and Burdette36, Reference Le Coz, Carlet, Roblot and Pulcini65, Reference Morris, Rennert-May and Dalton70, Reference Pulcini, Morel and Tacconelli72, Reference Echevarria, Groppi, Kelly, Morreale, Neuhauser and Roselle88 Therefore, a formal recommendation establishing a total of 1 FTE ASP support for every 250 beds, optimally with ~1 physician for every 3 pharmacists, offers a bare minimum expectation for inpatient facilities. Relying on even the most up-to-date staffing recommendations is fraught with limitations because the optimal stewardship FTE-to-bed ratio remains a “moving target.” The minimum inpatient recommendation should evolve over time and with facility complexity, just as infection preventionist staffing expectations have matured since SENIC.
Stewardship resource standards are desperately needed for outpatient and LTC settings as well as for accompanying analytic and administrative support. Technology support is required to integrate ASP tools to enhance the vital human components of ASPs, and resources for software as well as support likewise deserve attention.Reference Kuper, Nagel, Kile, May and Lee97 Further studies are needed to characterize human resource parameters for antibiotic stewardship across the healthcare continuum, which should both further refine inpatient standards and prompt yet another call to action for outpatient stewardship staffing benchmarks.Reference Buckel, Veillette, Vento and Stenehjem57, 141, Reference Hulscher and Prins142
Zahn et alReference Zahn, Adalja and Auwaerter143 could not be more right in stating, “Physicians performing infection control and antimicrobial stewardship work should be compensated for these activities (p 355).” Stewardship staffing standards, analogous to evolving infection prevention recommendations, are necessary to provide appropriate resources for ASPs. Just as medical centers, providers, patients, and their families expect robust infection prevention activity to optimize safe and quality care, healthcare entities should sufficiently staff and fund antibiotic stewardship for both inpatients and outpatients to decrease the public threat of antibiotic resistance and adverse antibiotic exposure outcomes.
Acknowledgments
None
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.







