Carbapenem-resistant Enterobactereaceae (CRE) are a growing problem in hospitals throughout the world; they pose a major public health threat and are associated with considerable morbidity and mortality.Reference Tangden and Giske1-Reference Albiger, Glasner and Struelens3 CRE by definition are resistant to imipenem, meropenem, doripenem, or ertapenem; alternatively, however, documentation shows that the isolate possesses a carbapenemase.4 CRE encompasses both carbapenemase-producing (CP) and non–carbapenemase-producing (non-CP) Enterobacteriaceae.
Non–CP-CRE emerge as a result of heterogeneous mechanisms such as production of extended-spectrum β-lactamases (ESBLs) and/or AmpC cephalosporinases combined with reduced outer membrane permeability.Reference Tamma, Huang, Opene and Simner5,Reference Doi and Paterson6 Some studies have revealed an association with a loss of organism fitness and reduced transmissibility.Reference Tamma, Huang, Opene and Simner5,Reference Chiotos, Han and Tamma7
The carbapenem-resistant phenotype of CP-CRE is easily transmissible to other gram-negative organisms because the genes encoding carbapenemases are located on mobile genetic elements such as plasmids, transposons, and insertion sequences, which can spread easily from patient to patient in healthcare settings.Reference Tangden and Giske1,Reference Tamma, Huang, Opene and Simner5 CP-CRE colonization can persist for >12 months.Reference Vink, Otter and Edgeworth8 The most common carbapenemase genes in the Unites States and in Israel are bla KPC; however, other carbapenemase genes, including bla NDM, bla VIM, bla IMP, and bla OXA, have been increasingly observed.Reference Kang, Yi, Ko, Lee, Lee and Kim9-Reference Ling, Tee and Tan11
These resistance patterns have important infection control implications. CP-CRE seems to be primarily responsible for the increasing spread of CRE in the United States and therefore has been targeted for aggressive prevention.4 CP-CRE screening via rectal swab at hospital admission is usually performed on high-risk patients with admission risk factors.Reference Vink, Otter and Edgeworth8,Reference Saidel-Odes and Borer12-Reference Jamal, Gracia-Jeldes and Baqi14 Following the Center for Disease Control and Prevention recommendations, we apply cohorting of patients with CP-CRE colonization (with or without infection) in a dedicated unit in one of our internal medicine departments (CP-CRE cohort). When a patient is found during hospitalization to be newly colonized with CP-CRE, we perform screening cultures of the patient’s room contacts. A patient colonized with CP-CRE is moved to the CP-CRE cohort. A patient colonized with non–CP-CRE remains in their current department is placed on strict contact precautions including hand hygiene protocols followed by healthcare personnel.
Many countries have implemented an extensive infection control program to contain these virulent, almost untreatable pathogens.Reference Tangden and Giske1-Reference Albiger, Glasner and Struelens3,Reference Doi and Paterson6,Reference Schwaber and Carmeli13,Reference Jamal, Gracia-Jeldes and Baqi14 This being said, does the CRE resistance mechanism have a profound effect on patient outcome? A study by Tamma et alReference Tamma, Goodman, Harris, Tekle, Roberts, Taiwo and Simner15 compared the clinical outcomes of patients with CP-CRE and non–CP-CRE bacteremia, suggesting that CP-CRE may be more virulent than non–CP-CRE and may be associated with poorer outcomes. A study by Dautzenberg et alReference Dautzenberg, Wekesa and Gniadkowski16 showed that patients colonized with CP-CRE have a higher hazard of dying in the intensive care unit (ICU) than noncolonized patients. Mathers et alReference Mathers, Vegesana and German-Mesner17 found that healthcare exposures, antimicrobials, and invasive procedures increased the risk of Klebsiella pneumonia CP colonization or infection.Reference Mathers, Vegesana and German-Mesner17
To the best of our knowledge, no studies have compared the risk factors and outcomes between patients colonized with one of the CRE groups, comparing CP-CRE–colonized and non–CP-CRE–colonized patients. In the present work, we address these issues.
Materials and methods
Study design
We performed a comparative historical study of CP-CRE–colonized and non–CP-CRE–colonized adult patients hospitalized in Soroka University Medical Center (1,000 beds, ~80,000 admissions per year).
Study population
Screening with rectal swab cultures for CRE is performed in our hospital for the following high-risk patient populations: patients admitted from other hospitals or from nursing homes, patients hospitalized in other healthcare facilities in the previous 6 months, patients transferred between departments in our hospital on admission to the new department, and contacts of a patient who was found positive for CP-CRE during hospitalization.
All rectal swab cultures for CRE collected from the bacteriology laboratory from July 2016 through June 2018 were reviewed. Culture results from hospitalized patients aged ≥18 years were included for analysis. The rectal cultures identified were divided into 3 subgroups depending on the culture result: (1) patients with a rectal culture positive for CP-CRE, (2) patients with a rectal culture positive for non–CP-CRE, and (3) patients with a rectal culture negative for CRE (non-CRE). The sample size of each group was limited to the number of rectal cultures positive for CP-CRE. Patients colonized with non–CP-CRE and those with a non-CRE culture result were randomly selected throughout the entire study period. Only 1 CP-CRE or non–CP-CRE culture (first) per patient was included. Patients infected with CP-CRE and/or non–CP-CRE were excluded.
Measures
Data were collected using a predesigned structured questionnaire covering demographic background including age, gender, ethnicity, origin, the department in which hospitalized, and Charlson comorbidity index. Prior antibiotic therapy and/or immunosuppressive therapy was included when administered ≤3 months preceding the index admission. Previous hospital admissions as well as nursing home residency during the 6 months prior to admission were identified and included in the analysis. ICU admission, mechanical ventilation, the presence of a permanent urinary catheter, and a decubitus ulcer were also recorded. Antibiotic therapy, length of hospital stay, and in-hospital mortality rate were included.
Microbiological analysis
Rectal swabs were inoculated on CHROMagar mSuperCARBA (HyLabs Rehovot, Israel), for isolation and detection of suspected carbapenemase-resistant Enterobacteriaceae (CRE). Isolates were identified by VITEK-MS (bioMérieux, Marcy-l’Étoile, France), and tested against meropenem using disc diffusion (Oxoid, Basingstoke, UK) and E-test methods (bioMérieux). Resistance was determined according to the Clinical and Laboratory Standard Institute (CLSI) definition. For meropenem-resistant strains, carbapenemase production was confirmed using Gene Xpert Carba-R assay (Cepheid, Sunnyvale, CA) for detection and differentiation of KPC, NDM, VIM, OXA-48, and IMP. A CP-CRE case was defined as a meropenem-resistant strain and Gene Xpert positive result. A non–CP-CRE case was defined as a meropenem-resistant strain and Gene Xpert negative result. A non-CRE case was defined as no growth on CHROMagar mSuperCARBA medium or suspected strain on CHROMagar mSuperCARBA medium, which was susceptible to meropenem.
Statistical analysis
All data were analyzed with SPSS version 23.0 software (SPSS, Chicago, IL) and R version 3.5.3 software (The R Foundation for Statistical Computing, www.r-project.org). Categorical variables were analyzed using a χ2 test or the Fisher exact test, and continuous variables were analyzed using independent samples t test or a Mann-Whitney test. Variables that were significantly associated with the different outcomes (P < .05) during univariate analyses were gradually added to stepwise selection multivariable models. Odds ratios (ORs) and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of each individual association. The association between the significant independent variables and the dichotomous outcomes were analyzed using multivariable logistic regressions, and the association between the different variables and mortality during hospitalization was examined using Cox regression.
Results
We identified and entered into the study 447 hospitalized adult patients for whom a rectal swab for CRE had been obtained during 24 months from July 2016 to June 2018. Among them, 147 were positive for CP-CRE, 147 were positive for non–CP-CRE, and 147 were negative for both (non-CRE).
Patient demographic and epidemiological characteristics are presented in Table 1. The 3 groups were similar regarding patient age, gender, and ethnicity (80% Jewish and 20% Bedouin Arab). Patients colonized with CP-CRE or with non–CP-CRE versus those not colonized (ie, negative rectal swab indicates no CRE) resided in nursing homes, received antibiotics 3 months prior to admission, and received corticosteroids 3 months prior to admission. Patients colonized with non–CP-CRE versus those colonized with CP-CRE had higher Charlson index scores and were more likely to be transferred between hospital departments. Patients colonized with CP-CRE versus those colonized with non–CP-CRE were more likely to have been admitted from nursing care facilities (Table 1).
Table 1. Demographic and Epidemiological Characteristics of Patient Population: CP-CRE–Colonized Patients Versus Patients Not Colonized with CRE (negative), non–CP-CRE–Colonized Patients Versus Patients Not Colonized With CRE (negative), and CP-CRE–Colonized Patients Versus Non–CP-CRE–Colonized Patients
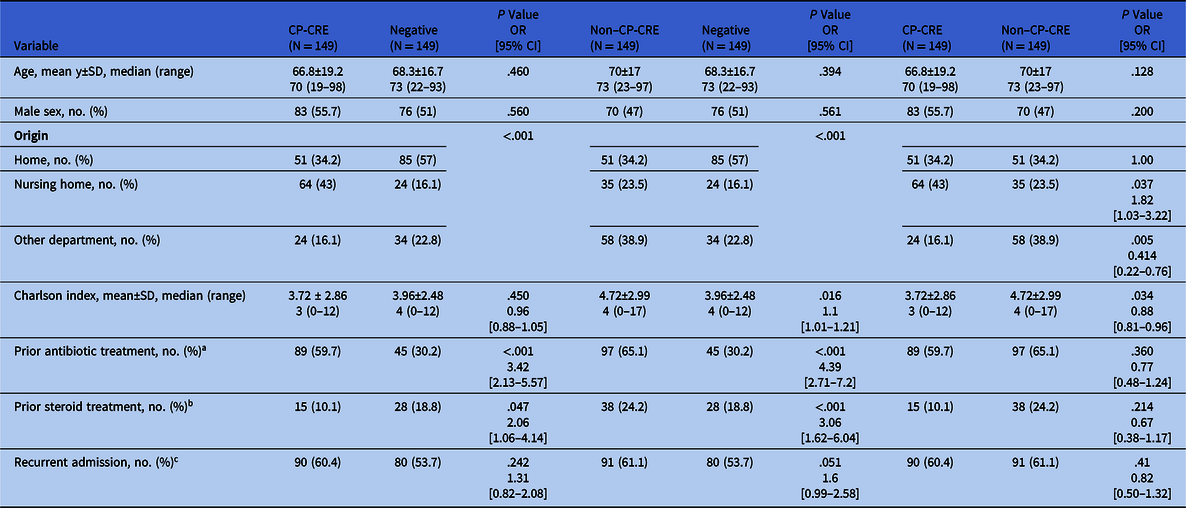
Note. CP-CRE, carbapenemase-producing carbapenem-resistant Enterobacteriaceae; OR, odds ratio; CI, confidence interval; SD, standard deviation.
a Antibiotic treatment 3 mo before admission.
b Glucocorticosteroids treatment 3 mo before admission.
c Recurrent admission within 3 mo.
Patient clinical characteristics during hospitalization and outcomes are presented in Table 2. Patients colonized with CP-CRE or with non–CP-CRE versus those not colonized with CRE were more likely to undergo mechanical ventilation, to have a urinary catheter, and to have a decubitus ulcer. They were prescribed a higher number of different antibiotic treatments during hospitalization: 2.6 times more for CP-CRE patients and 2.1 times more for non–CP-CRE patients. Before and after colonization detection, penicillins, carbapenems, and aminoglycosides were prescribed more frequently to patients colonized with CP-CRE or with non–CP-CRE than to those not colonized with CRE. Patients colonized with CP-CRE were treated with cephalosporins 1.99 times more than those not colonized with CRE: 63 (42.3%) vs 40 (26.8%), respectively (P = .005). Patients colonized with non–CP-CRE had a longer length of hospital stay compared to those not colonized with CRE (P < .001). Both patients colonized with CP-CRE or with non–CP-CRE had a higher in-hospital mortality rate, 2–3.9 times higher than patients not colonized with CRE (Table 2 and Fig. 1).
Table 2. Clinical Characteristics During Hospitalization and Outcome of Patient Population: CP-CRE Colonized Patients Versus Patients Not Colonized With CRE (negative), Non–CP-CRE Colonized Patients Versus Patients Not Colonized With CRE (negative), and CP-CRE Colonized Patients Versus Non–CP-CRE Colonized Patients
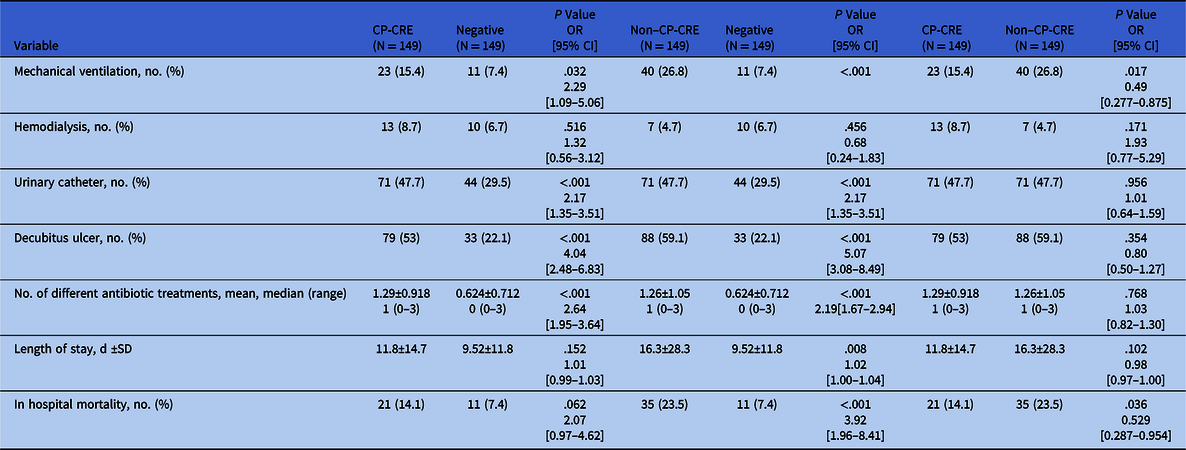
Note. CP-CRE, carbapenemase-producing carbapenem-resistant Enterobacteriaceae; OR, odds ratio; CI, confidence interval; SD, standard deviation.

Fig. 1. Kaplan-Meier survival curve; CP-CRE versus non–CP-CRE versus non-CRE colonized patients. Cumulative survival during hospitalization shows the following findings: (1) Patients colonized with non–CP-CRE had a significantly higher in-hospital mortality rate than patients not colonized with CRE: 35 (23.5%) versus 11 (7.4%) (P < .001). (2) Patients colonized with CP-CRE showed a higher in-hospital mortality rate than patients not colonized with CRE: 21 (14.1%) versus 11 (7.4%) (P = .062). (3) Patients colonized with non–CP-CRE had a significantly higher in-hospital mortality rate than patients colonized with CP-CRE: 35 (23.5%) versus 21 (14.1%) (P = .036).
Univariate analysis revealed that patients colonized with non–CP-CRE had a higher rate of mechanical ventilation and in-hospital mortality than patients colonized with CP-CRE (Table 2). Regarding different antibiotic treatments prescribed during hospitalization, patients colonized with CP-CRE received cephalosporins 1.74 times more than patients colonized with non–CP-CRE (P = .023).
A Cox multivariate regression for mortality prediction showed that age, Charlson index, and treatment with glucocorticosteroids within 3 months before admission all influenced mortality, whereas colonization with CP-CRE or with non–CP-CRE, antibiotic treatment within 3 months before admission and having a decubitus ulcer did not (Table 3).
Table 3. Cox Multivariable Regression Independent Risk Factors for In-Hospital Mortality

Note. OR, odds ratio; CI, confidence interval; CP-CRE, carbapenemase-producing carbapenem-resistant Enterobacteriaceae.
a Antibiotic treatment 3 mo before admission.
b Glucocorticosteroid treatment 3 mo before admission.
Multivariate analyses revealed that colonization with non–CP-CRE was a significant predictor for intubation, which occurred 2.5 times more than with colonization with CP-CRE.
On multivariate regression, variables predicting non–CP-CRE colonization versus no CRE colonization included admission from a nursing home, antibiotic treatment within 3 months prior to admission, having a decubitus ulcer, mechanical ventilation, and movement between departments within the hospital. Variables predicting CP-CRE colonization versus no CRE colonization included admission from a nursing home, antibiotic treatment within 3 months prior to admission, and having a decubitus ulcer (Table 4).
Table 4. Multivariate Regression of Independent Risk Factors Predicting CP-CRE Colonization and Non–CP-CRE Colonization

Note. OR, odds ratio; CI, confidence interval; CP-CRE, carbapenemase-producing carbapenem-resistant Enterobacteriaceae; SE standard error.
Discussion
The emergence and rapid spread of CRE with limited antimicrobial options for treatment is a growing problem in hospitals throughout the world. It poses a major public health threat and is associated with considerable morbidity and mortality. CRE resistance can be divided into 2 major groups: CP-CRE and non–CP-CRE. Previous studiesReference Kang, Yi, Ko, Lee, Lee and Kim9-Reference Saidel-Odes and Borer12,Reference Mathers, Vegesana and German-Mesner17-Reference Predic, Delano, Tremblay, Iovine, Brown and Prins24 have addressed the issue of risk factors for CRE colonization/infection as a whole (ie, 1 group), showing that the following factors were associated with CRE acquisition: male sex, nursing home residency before hospital admission, history of admission within 1 year, poor functional status, Charlson index score ≥3, urinary catheter, decubitus ulcer, mechanical ventilation, intensive care unit stay, undergoing an invasive procedure with a scope device, and prior antibiotic exposure including penicillins, cephalosporins, carbapenems, vancomycin and fluoroquinolones.
Our protocol addressed risk factors for CP-CRE and non–CP-CRE colonization as 2 separate entities. We aimed to define risk factors and outcomes that may be unique to 1 of these groups, comparing each to patients not colonized with CRE and comparing them to each other.
On univariate analysis, as in previous studies,Reference Kang, Yi, Ko, Lee, Lee and Kim9-Reference Ling, Tee and Tan11,Reference Mathers, Vegesana and German-Mesner17,Reference Nicolas-Chanoine, Vigan, Laouénan and Robert21-Reference Eser, Yılmaz and Güner23 we found that risk factors for both CP-CRE colonization and non–CP-CRE colonization included nursing home residency before hospital admission, antibiotic treatment 3 months before admission, urinary catheter, decubitus ulcer, and mechanical ventilation. We did not find men to be at an increased risk for CP-CRE or non–CP-CRE colonization in comparison to other studies,Reference Kang, Yi, Ko, Lee, Lee and Kim9,Reference Marimuthu, Ng and Cherng10 which may have been due to an older patient population in our study and the longer life expectancy of women in Israel. Additionally, we showed that glucocorticosteroid treatment within 3 months prior to admission was also a risk factor for both CP-CRE colonization and non–CP-CRE colonization, which has not been described previously. Glucocorticosteroids are immunosuppressive agents that may lead to downregulation of the immune system, which might be followed by a higher incidence of infectious complications in patients receiving glucocorticosteroids.Reference Stuck, Minder and Frey25
A previous study from FranceReference Nicolas-Chanoine, Vigan, Laouénan and Robert21 addressing risk factors for CP-CRE compared to non–CP-CRE infections found that only CP-CRE cases were associated with previous travel and hospitalization abroad; other risk factors were the same for both groups. We did not evaluate previous travel and hospitalization abroad in our patient population because both CP-CRE and non–CP-CRE are prevalent in our region.
On multivariate analysis, as in previous studies,Reference Ling, Tee and Tan11,Reference Eser, Yılmaz and Güner23 we demonstrated that predictors for CP-CRE colonization and for non–CP-CRE colonization included nursing home residency before hospital admission, antibiotic treatment 3 months before admission, and having a decubitus ulcer. In our study, risk factors unique for non–CP-CRE colonization (and not for CP-CRE colonization) included mechanical ventilation and patient movement between various hospital departments. Notably, patients colonized with CP-CRE in our hospital are cohorted in a dedicated unit with minimal movement to other departments, whereas patients colonized with non–CP-CRE are hospitalized throughout the hospital departments and strict contact precautions and hand hygiene protocols are followed. Non–CP-CRE colonization was a predictor for mechanical ventilation, with a risk 2.5 times higher than CP-CRE colonization. Patients colonized with non–CP-CRE also had a longer length of in-hospital stay than those not colonized with CRE. These findings were expected because patients colonized with non–CP-CRE had a higher Charlson index on admission than CP-CRE–colonized patients and than patients not colonized with CRE, indicating poorer prognosis compared to the 2 other groups.
During hospitalization, patients colonized with CP-CRE or with non–CP-CRE versus those not colonized with CRE were prescribed a higher number of different antibiotic treatments in total. Antibiotic selective pressure is the main cause of colonization with a multidrug-resistant strain. Patients colonized with CP-CRE were treated with cephalosporins 1.74 times more than those colonized with non–CP-CRE and 1.99 times more than those not colonized with CRE. This finding has not been described previously. At our hospital, prescription of first- and second-generation cephalosporins is not restricted; therefore, their prescription rate is high. However, the reason that the rate was highest among CP-CRE colonized patients remains unclear.
In a 2010 study, we showed that the crude and attributable mortality rates associated with carbapenem-resistant Klebsiella pneumoniae bacteremia were striking.Reference Borer, Saidel-Odes and Riesenberg2 In our current study, we investigated CP-CRE and non–CP-CRE colonization and not infection. On univariate analysis, in-hospital mortality was significantly higher in CRE-colonized patients versus patients not colonized with CRE. Furthermore, in-hospital mortality was the highest in non–CP-CRE–colonized patients. The Cox multivariate regression for independent risk factors predicting in-hospital mortality showed that age, Charlson index, and treatment with glucocorticosteroids within 3 months before admission influenced mortality in our patient population, but not CP-CRE or non–CP-CRE colonization themselves. CP and non–CP-CPE colonization most probably serve as markers for poorer prognosis in this patient population.
In summary, we present data from a large cohort of CP-CRE colonized patients versus non–CP-CRE colonized patients in a single hospital. We assessed the risk factors for colonization and outcome. Furthermore, we emphasized both the risk factors that overlap and those unique to each patient group. An unanticipated outcome in our study was the tendency for a higher mortality rate in non–CP-CPE–colonized patients compared to CP-CRE–colonized patients. A previous study had suggested that CP-CRE bacteremia may be more virulent than non–CP-CRE bacteremia and is associated with poorer outcomes.Reference Tamma, Goodman, Harris, Tekle, Roberts, Taiwo and Simner15 In addressing colonization without infection, we identified a trend for poorer outcomes in non–CP-CRE–colonized patients, including a higher number of patients requiring mechanical ventilation, a longer length of hospital stay, and a higher rate of in-hospital mortality.
Our study has several limitations. First, it was retrospective study in a single medical center, and these results may not be generalizable in all respects to other medical centers. Second, we excluded patients infected with CP-CRE and/or non–CP-CRE; therefore, we did not account for their influence on outcome, but the number of these patients was very small. Third, antibiotic therapy reflects the entire hospitalization period, not limited to the time before detection of colonization due to limitation in outpatient data availability. Unfortunately, this method did not help to elucidate differences in outcomes. Fourth, although patient isolation is an established and important aspect of infection control, it may also negatively influence direct patient care.Reference Abad, Fearday and Safdar26 Patients colonized with CP-CRE were placed in regular departments in a dedicated cohort whereas those colonized with non–CR-CRE were placed in strict contact precautions with hand hygiene protocol. These 2 different isolation conditions might have influenced patient outcomes.
In conclusion, this is the first study to compare risk factors and outcomes of patients colonized with CP-CRE and non–CP-CRE. We have demonstrated that there are both overlapping risk factors associated with CP-CRE and non–CP-CRE colonization and unique risk factors associated with non–CP-CRE colonization. These risk factors, together with other known risk factors, can assist in predicting on admission which patients should be screened for CRE colonization, flagging them for strict contact precautions pending rectal culture results. Patients colonized with non–CP-CRE had a longer length of stay and a higher in-hospital mortality rate. Further studies comparing risk factors and outcomes of patients infected with CP-CRE versus non–CP-CRE are needed.
Acknowledgments
We appreciate the contributions of Seeda Eskira, RN, MPH, Batia Shterer RN, and Ronit Nativ RN, MPH of the Infection Control Unit at Soroka University Medical Center in their assistance with providing the study team a database of eligible participants.
Financial support
All authors report no financial support related to this study.
Conflict of interest
All authors report no conflicts of interest relevant to this article.








