Candida auris is an emerging multidrug-resistant fungal pathogen of increasing global concern. Like other pathogenic yeast, C. auris can cause invasive infections with high mortality rates.Reference Forsberg, Woodworth and Walters1 However, C. auris is distinct in its ability to cause transmission-mediated outbreaks in healthcare settings that are difficult to control. Previous studies have found extensive and persistent environmental contamination in healthcare settings; C. auris has been isolated from surfaces including but not limited to windows, doorknobs, nursing carts, television remotes, soap dispensers, chairs, and patient beds, as well as diverse medical equipment such as temperature probes, glucometers, blood pressure cuffs, and more.Reference Adams, Quinn and Tsay2 Effective environmental disinfection is essential to infection control efforts. However, some commonly used disinfectants with US Environmental Protection Agency (EPA)–registered claims for fungi and Candida albicans are not effective against C. auris.Reference Cadnum, Shaikh and Piedrahita3 To prevent the use of ineffective products, the EPA and the Centers for Disease Control and Prevention (CDC) collaboratively implemented conservative interim guidance in 2017 recommending that healthcare facilities with C. auris cases use disinfectants on the EPA’s List K, a collection of sporicidal agents known to kill Clostridioides difficile.4 The EPA has since released EPA MLB SOP MB-35-00, a quantitative disk carrier method designed to help generate the C. auris–specific efficacy data needed to inform guidance.5 However, at this writing, limited data have been reported using this method. In this study, we compare the response of 2 C. auris isolates, AR 0381 and AR 0385, to reagent grade sodium hypochlorite (NaOCl). We then evaluated the efficacy of 9 commercially available disinfectants against C. auris AR 0385 using MB-35-00.
Methods
9 disinfectants were selected based on reported usage in healthcare facilities with C. auris cases and reports of commonly used products from infection control subject-matter experts. Among them, 6 disinfectants included quaternary ammonium compounds (QACs), including 3 disinfectants that also contained alcohols, and three included hydrogen peroxide (Table 1). Testing was performed in accordance with EPA MLB SOP MB-35-00: “OECD Quantitative Method for Evaluating the Efficacy of Liquid Antimicrobials against Candida auris on Hard, Nonporous Surfaces.”5 Briefly, 50 µL of test substance was applied to 5–6 log10 colony-forming units (CFU) of C. auris cells dried on AISI type 430 stainless-steel carrier disks (n = 5). In accordance with MB-35-00, inocula were prepared with a composite soil load that included bovine serum albumin, yeast extract, and mucin. When applicable, test substances were diluted in 375 ppm hard water. Test substance contact time was chosen based on existing product-label instructions for C. albicans or, if unavailable, as instructed for fungicidal claims (Table 1). All test substances were neutralized with 10 mL Dey-Engley neutralization solution (Sigma catalog no. D3435). Complete neutralization was verified for each test substance using EPA MLB MB-37-00: “Neutralization Confirmation for Evaluating the Efficacy of Liquid Antimicrobials using the OECD Quantitative Method against Candida auris on Hard, Nonporous Surfaces.”6 Cells from neutralized reactions and relevant dilutions were collected on 0.45 µM polyethersulfone filter membranes and transferred to Sabouraud dextrose Emmon agar. The CFU were counted after incubating for 72 hours at 30°C. Log10 reduction in CFU was calculated relative to mean phosphate-buffered saline (PBS) control carrier counts performed on the same day. Testing was performed on C. auris isolates AR 0381 (Clade II, East Asian) and AR 0385 (Clade IV, South American).7
Table 1. Efficacy of Disinfectants Against C. auris AR 0385 According to EPA MLB SOP MB-35: “OECD Quantitative Method for Evaluating the Efficacy of Liquid Antimicrobials against Candida auris on Hard, Nonporous Surfaces”
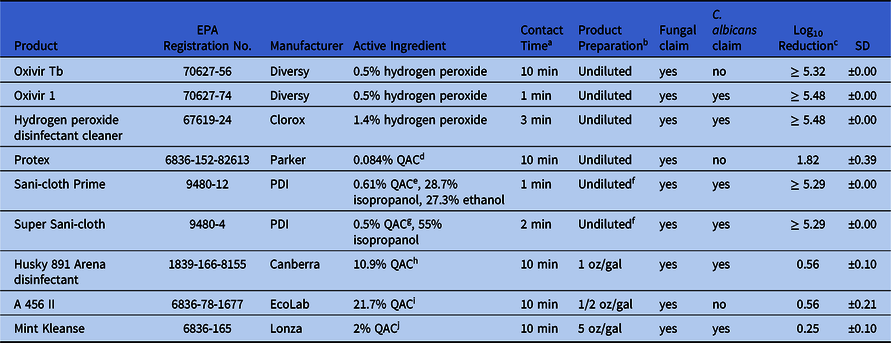
Note. EPA, US Environmental Protection Agency; SD, standard deviation.
a Contact time reflects registration claim of product for C. albicans or if not applicable, the contact time associated with fungal claim was used.
b Dilutions prepared in 375 ppm hardwater.
c Mean across 5 replicate disks (n = 5).
d QAC: 0.025 % octyl decyl dimethyl ammonium chloride; 0.010 % dioctyl dimethyl ammonium chloride; 0.015 % didecyl dimethyl ammonium chloride; 0.034 % alkyl (C14, 50 %; C12, 40 %; C16, 10 %) dimethyl benzyl ammonium chloride.
e QAC: 0.61% Didecyl dimethyl ammonium chloride.
f Extracted from cloth.
g QAC: 0.25% n-alkyl (68% C12, 32% C14) dimethyl ethylbenzyl ammonium chlorides, 0.25% n-alkyl (60% C12, 30% C14, 5% C12, 5% C18) dimethyl benzyl ammonium chlorides.
h QAC: 0.033% octyl decyl dimethyl ammonium chloride, 0.016% dioctyl dimethyl ammonium chloride, 0.016% didecyl dimethyl ammonium chloride, 0.043 alkyl (C14, 50%; C12, 40%; C16, 10%) dimethyl benzyl ammonium chloride.
i QAC: 6.51% octyl decyl dimethyl ammonium chloride, 2.60% dioctyl dimethyl ammonium chloride, 3.91% didecyl dimethyl ammonium chloride, 8.68 % alkyl (C14, 50%; C12, 40%; C16, 10%) dimethyl benzyl ammonium chloride.
j QAC: 2.0% alkyl (C14 58%, C16 28%, C12 14%) dimethyl benzyl ammonium chloride.
Results
Testing with reagent grade NaOCl (Sigma catalog no. 239305-500mL) revealed isolate-specific differences in the log10 reduction observed. Specifically, complete kill (ie, ≥5.05 log10 CFU reduction) was observed when the type strain isolate AR 0381 (clade II, East Asian) was challenged with 100 and 200 ppm NaOCl (Fig. 1). In contrast, intermediate kills were observed with South American (clade IV) isolate C. auris AR 0385, which was reduced by 1.12 log10 CFU at 100 ppm and by 2.39 log10 CFU at 200 ppm (Fig. 1). Based on these results, we decided to proceed with disinfectant efficacy testing using C. auris AR 0385, which was more resistant and more closely related to strains causing healthcare-associated outbreaks in the United States. Using C. auris AR 0385, we observed complete kill (ie, ≥5 log10 reduction) when challenged with the 3 products with hydrogen peroxide (Oxivir TB, Oxivir 1, and hydrogen peroxide disinfectant cleaner) and the 2 products that included QACs with alcohols (Sani-cloth Prime and Super Sani-cloth). In contrast, the 4 QAC-only products (Protex, Husky 891 Arena disinfectant, A 456 II, and Mint Kleanse) did not meet the 5 log10 CFU reduction required to demonstrate efficacy.

Fig. 1. Efficacy of reagent grade sodium hypochlorite (NaOCl) against AR 0381 (clade II), the original C. auris type strain from Japan (black bars) and AR 0385 (clade IV), a strain originally isolated from South America (gray bars). Values show mean log10 CFU reduction after 5 minutes of contact time (n = 3).
Discussion
Environmental disinfection remains a critical challenge for healthcare facilities working to control C. auris.Reference Adams, Quinn and Tsay2 Given the recent and rapid emergence of C. auris, limited species-specific data are available to inform environmental disinfection guidance. Here, 5 products with accelerated hydrogen peroxide or alcohol-based chemistries were highly effective against C. auris, achieving ≥5 log10 reduction. In contrast, all 4 products dependent on QAC-based chemistries alone were not effective.
Our results corroborate a growing body of evidence that QAC-dependent products are not effective against C. auris,Reference Cadnum, Shaikh and Piedrahita3,Reference Rutala, Kanamori and Gergen8 which is concerning because QAC-dependent disinfectants are widely used in healthcare settings and many have fungicidal and C. albicans label claims (eg, Husky 891 Arena disinfectant and Mint Kleanse). However, C. albicans label-claim requests are evaluated using a semiquantitative AOAC “use dilution” type approach that is fundamentally different from the quantitative disk-carrier method used in our study. Although differences between C. auris and C. albicans might explain this difference, the poor performance of QAC products with C. albicans claims might be attributed to differences in the test method. A recent study that compared the effect of Virex II 256, a QAC-based product, against several Candida spp using both use-dilution and quantitative-disk methods.Reference Cadnum, Shaikh and Piedrahita3 The following log10 reductions were observed: 3.3 for C. albicans, 3.8 for C. glabrata, and 2.2 for C. auris by the use-dilution method. However, <1 log10 reduction was observed for all 3 species when the quantitative disk carrier method was used, suggesting that differences in test methods best explain why some products with C. albicans claims were not effective against C. auris in this study.
Further research is needed to understand why AR 0381 was more sensitive to NaOCl than AR 0385. The original type strain from Japan, AR 0381, belongs to clade II, whose isolates are primarily cause ear infections and have not been implicated in outbreaks.Reference Welsh, Sexton and Forsberg9 A recent study also demonstrates that clade II isolates have large chromosomal rearrangements and are missing a number of genes present in the other 2 C. auris clades.Reference Munoz, Welsh and Shea10 In contrast, AR 0385 from clade IV is very closely related to isolates causing outbreaks across the Americas and has chromosomal and genetic structure similar to those of clades I and III.Reference Rutala, Kanamori and Gergen8 For these reasons, we chose to continue our work with AR 0385.
During this study, 3 products became the first to acquire formal EPA-registered C. auris label claims, including Micro-kill Bleach Germicidal Bleach Wipes (EPA no. 37549-1), Oxivir 1 disinfectant spray (EPA no. 70627-74), and Oxivir 1 disinfectant wipes (EPA no. 70627-77), expanding the range of disinfects recommended against C. auris. However, additional registered products with a broader range of chemistries and delivery mechanisms are still needed to accommodate the context-specific needs of healthcare facilities. To help meet this need, the data generated in this study were used to support an EPA-approved section 18 emergency exemption action under the Federal Insecticide, Fungicide, and Rodenticide Act. This action temporarily permitted off-label use of Oxivir TB spray (EPA reg no. 70627-56), Oxivir TB wipes (EPA reg no. 70627-60), hydrogen peroxide disinfectant spray (EPA reg no. 67619-24), hydrogen peroxide disinfectant wipes (EPA reg no. 67619-25), PDI Sani Prime Spray (EPA reg no. 9480-10), PDI Sani-Cloth Prime (EPA reg no. 9480-12), and PDI Super Sani-Cloth (EPA reg no. 9480-4) to control C. auris in the healthcare setting. Following the emergence exemption approval, the manufacturers of these products applied for and received formal EPA-registered C. auris claims, thus extending the utility of these products for C. auris into the future. Registration of more disinfectant products for use against C. auris remains of public health value to further increase options available for healthcare facilities working to control C. auris.
Acknowledgments
Special thanks to Lisa Smith, Rebecca Pines, Stephan Tomasino and Susan Lawrence in the Microbiology Laboratory Branch at US Environmental Protection Agency for training in OECD test methods MB 35-00 and MB-37-00. The use of product names in this paper does not imply their endorsement by the U.S. Department of Health and Human Services. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
Financial support
D.J.S. was funded by the Oak Ridge Institute for Science and Education (ORISE).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





