Hospital-acquired infections play a major role in healthcare-associated morbidity and mortality, affecting 5% of all hospitalized patients in the United States each year. Reference Matheos and Heard1 More specifically, central-line–associated bloodstream infections (CLABSIs) are laboratory-confirmed bloodstream infections not related to infection at another site and which occurred >48 hours after the placement of a central line.
In May 2017, our hospital transitioned from Arrow (Teleflex, Gurnee, IL) central venous catheters (CVCs) to Centurion (Centurion Medical Products, Dallas, TX) CVCs. In the following months, the infection control division noted an increase in the rate of CLABSI. Importantly, during this time there were no other changes in hospital policy or procedure protocols. Given these increased rate of CLABSI, the hospital transitioned back to Arrow catheters the following year. Consequently, this gave us the unique opportunity to perform a before–after–before comparison between Arrow catheters (ie, lined by chlorhexidine and silver sulfadiazine [CHSS]) and Centurion catheters (ie, lined by silver-iontophoretic [SI]).
Methods
Subjects and design
This study was approved as a retrospective, nonhuman subject research study by the Institutional Review Board of Eastern Virginia Medical School (EVMS; IRB no. 19-05-NH-0139). The primary objective was to compare the overall rate of CLABSI between the CHSS and SI CVCs. Secondary objectives included comparison of identified organisms and sites of infection. Temporary CVCs that were placed in intensive care units (ICUs) at Norfolk General Hospital between July 1, 2016, and April 30, 2019, were included. Exclusion criteria included any mediport catheters, peripherally inserted CVCs, or hemodialysis catheters or any CVCs placed at an outside hospital. Any cases reported as mucosal barrier injury laboratory-confirmed bloodstream infections were also excluded. Sentara Norfolk General Hospital, located in Norfolk, Virginia, is a 563-bed academic hospital with 6 ICUs, which serves as the primary teaching institution for EVMS.
The infection control division tracks all hospital-acquired infections including CLABIs using the standardized National Healthcare Safety Network (NHSN) definitions. The infection control division confirmed and reported our deidentified data. The NHSN is a widely used healthcare-acquired infection tracking system, and the infections documented include CLABSIs. 2 The following data were also collected: catheter and patient days, catheter type, insertion site, month of CLABSI, and organisms identified from blood cultures. All information was collected in accordance with hospital and institutional review board policies.
Study timeline
Data were collected in three 10-month windows, July 1, 2016, to April 30, 2017, and July 1, 2018, to April 30, 2019, when the CHSS catheter data were collected; and July 1, 2017, to April 30, 2018, when the SI catheter data were collected. We included a 2-month washout period between the collection windows when data were not collected to ensure the use of only 1 catheter.
Data analysis
The total number of CLABIs during each period was recorded as infections per 1,000 central-line days (CLD). The comparison of the rates of CLABSI between the CHSS and SI catheters was conducted using the proportion χ 2 test. Reference Fan, Wang and Wei3
Central venous catheters
Our study included 2 types of catheters: CHSS and SI catheters. Although both provide antiseptic characteristics, the CHSS catheters differ in the addition of chlorhexidine and the distribution of the coating throughout the catheter including the lines, hubs, and indwelling piece, whereas the SI catheters only coat the indwelling part.
Results
In total, 20 CLABSIs were identified during the study period. Also, 5 CLABSIs occurred during the washout period and were excluded. Overall, 5 CLABSIs occurred with the CHSS CVC (overall CLABSI rate, 0.24 per 1,000 CLD) in comparison to 10 with the SI CVC (overall CLABSI rate, 1.03 per 1,000 CLD). Specific to the CHSS-associated CLABSIs, 4 occurred during the initial study period (CLABSI rate, of 0.43 per 1,000 CLD, but not statistically significant), and only 1 occurred during the follow-up period (CLABSI rate 0.08 per 1,000 CLD). The incidence rate ratio of the CHSS versus SI catheters was 0.2318 (95% CI, 0.06216–0.7443; P = .004) (Table 1).
Table 1. Results

Note. CHSS, chlorhexidine and silver sulfadiazine lined catheters; SI, silver ionotrophe-lined catheters.
a Per 1,000 central line.
b The incidence rate ratio of the CHSS versus SI catheters was 0.2318 (95% CI, 0.06216–0.7443; P = .004).
Notably, our hospital policy dictates CVC insertion through the subclavian or internal jugular veins before a femoral vein if possible. The organisms and sites of infection are summarized in Table 2.
Table 2. Organisms Identified and Sites of Central Line
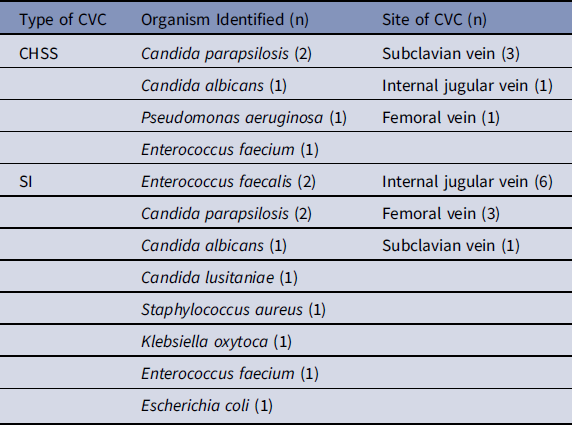
Note. CHSS, chlorhexidine and silver sulfadiazine lined catheters; SI, silver ionotrophe–lined catheters.
Discussion
Over the years, advances in medical technology and infection control techniques have contributed to reducing the risk of CLABSIs. However, multisociety guidelines from the Society of Healthcare Epidemiology of America and Infectious Disease Society of America are predominantly focused on the use of proper antiseptic technique, along with the care and maintenance of the line. Little is mentioned about catheter selection apart from the following: “Use antiseptic or antimicrobial-impregnated central venous catheters in adult patients” (quality of evidence I). Reference Arvaniti4 Literature reviews of the clinical outcomes of various studies demonstrate variable data with significant heterogeneity and, most importantly, a lack of direct comparison between catheter types. Reference Corral5–Reference Niel-Weise, Stijnen and van den Broek7 As such, a hospital’s choice of catheter is determined largely by cost or physician preference rather than comparable outcomes data.
Our study demonstrated that the rate of CLABSIs may be higher among patients receiving SI CVCs (1.03 per 1,000 CLD) than those with CHSS CVCs (0.24 per 1,000 CLD). Unique to our study was a before–after–before design, with catheter selection being the only significant change in infection control during the study period. These results show that the SI catheter’s CLABSI rate reflected a 2.39 times and 12.87 times higher risk for CLABSI than the CHSS catheter in the before and after periods, respectively, and an overall higher risk of 4.29 times throughout the study. The organisms associated with the CHSS catheter were 60% fungal species, with gram-positive and gram-negative organisms accounting for 20% each. The SI isolates were ~40% each for gram-positive and fungal species.
Our study has several limitations. Given the retrospective design, the data were collected in regard to compliance and maintenance policies of the CVC. However, the increased rate of CLABSI following the switch to SI CVCs, without changing any other infection control parameters or hospital protocols, which included antiseptic technique, performing staff members, no additional training during the study period and the same patient population, all lead toward the catheters characteristics and differences being the main differentiating factor. Moreover, there was no identifiable cluster in time or ICU site that could be identified as a potential confounder. Comparing both catheters, the addition of chlorhexidine and extended coating of the CHSS catheters stand out. Reference Lai, Chaiyakunapruk and Lai8 The extension lines and hubs are frequently accessed, and they can act as a gateway for organism entry and possible colonization. The combination of chlorhexidine and extended coating provides a thorough barrier that decreases the chance of colonization, which may be reflective of the decreased rate of CLABSIs in our study.
Although the study is limited by the retrospective design and lack of randomization, the data reflect real-world clinical practice rather than a controlled clinical environment. Overall, our findings suggest that the microbiological preclinical data may not translate into the clinical setting and that the use of CHSS CVCs may be associated with a lower number of CLABSIs than SI CVCs. Our findings highlight a potential difference between the two catheters and the need for further assessment in randomized control trials.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





