Staphylococcus aureus is an important cause of healthcare-associated infections. S. aureus is transmitted through direct or indirect contact with a colonized or infected person, and healthcare workers (HCWs) often serve as the vector for S. aureus transmission. Because of concerns about worse outcomes and limited treatment options, infection prevention practices are typically more aggressive for methicillin-resistant S. aureus (MRSA) than for methicillin-sensitive S. aureus (MSSA).Reference Calfee, Salgado and Milstone1,Reference Cosgrove, Sakoulas, Perencevich, Schwaber, Karchmer and Carmeli2 As a result, contact precautions are used for patients colonized with MRSA to prevent transmission to other patients, while standard precautions are typically used for patients colonized with MSSA. However, both MRSA and MSSA have similarly severe clinical manifestations. Despite increasing interest, a limited number of studies of S. aureus transmission include MSSA to justify this difference in infection prevention strategies.Reference Price, Golubchik and Cole3
The risk factors for S. aureus transmission are multifactorial and are under active investigation.Reference Roghmann, Johnson and Sorkin4 Although it seems intuitive that the burden of colonization would also confer a greater risk of transmission, only a few studies have quantified MRSA colonization, and even fewer have assessed MSSA colonization or the relationship between colonization burden and transmission.Reference Calfee, Salgado and Milstone1,Reference Datta, Shah and Huang5,Reference Stenehjem and Rimland6 Herein, we examine the risk factors for S. aureus transmission—including both MRSA and MSSA—from community-based nursing home residents to HCW gowns and gloves to assess whether the risk of transmission is higher with a greater burden of colonization.
Methods
Study design
We conducted a multicenter, prospective cohort study to estimate the frequency of and risk factors for S. aureus transmission to gowns and gloves worn by HCWs when providing care to nursing home residents as previously reported.Reference Roghmann, Johnson and Sorkin4 The protocol was approved by the institutional review boards of the University of Maryland Baltimore and University of Michigan.
Population
We screened 2,148 nursing home residents, of whom 695 were ineligible: 425 were not expected to stay >1 week from enrollment, 201 were identified by nursing home staff as being combative or having behavioral problems, and 69 did not speak English. Of the 1,453 eligible residents, 1,050 were not enrolled: 564 did not consent, 176 their legally authorized representative did not respond, and 310 were not approached about the study. The remaining 403 residents were enrolled from 13 community-based nursing homes in Maryland and Michigan. Residents were included in this study if they were colonized with S. aureus upon enrollment, as determined by swab culture from the anterior nares or perianal skin. However, 232 were not colonized and thus excluded from this analysis, and 2 were withdrawn prior to being swabbed, yielding our cohort of 169 S. aureus–colonized residents.
Data collection
We recorded clinical data about the residents obtained from the minimum data set, medical records, and nursing home personnel.7 The HCWs were asked to wear gowns and gloves immediately prior to interacting with residents for up to 28 days after resident enrollment. Each interaction was comprised of 1 or more usual care activities (eg, toileting, then bathing, then dressing). A research coordinator observed and recorded the type of care activities delivered during each interaction. After the interaction, the coordinator swabbed the HCW’s gown and gloves, as described previously.Reference Morgan, Liang and Smith8–Reference Snyder, Thom and Furuno10
Laboratory procedures
Specimens from residents, gowns, and gloves were cultured for S. aureus at a central laboratory as previously described.Reference Roghmann, Johnson and Sorkin4 Staphylococcus aureus DNA was extracted from medium inculcated with resident swabs. Bacterial cells were lysed with lysostaphin (cat. no. L7386-15MG; Sigma-Aldrich, St Louis, MO) at a final concentration of 200 μg/mL at 37°C for 1 hour. The DNA was then purified from bacterial cell lysate with the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). Quantitative polymerase chain reaction (qPCR) targeting the nuc gene was performed on those swabs that grew S. aureus with the iQ5 Real Time Detection System, iQ Supermix (Bio-Rad Laboratories, Hercules, CA). The nuc gene is present as a single copy in almost all S. aureus, but not other bacteria, and thus should correlate with colony-forming units (CFU) of S. aureus. It is a common molecular target for the rapid detection of S. aureus in blood and food samples and can be used to determine S. aureus colonization status.Reference Emswiler-Rose, Johnston, Harris and Lee11–Reference Redel, Gao and Li13 In in vitro validation of our q-PCR methods, the number of nuc gene copies isolated from serial dilutions of standard MSSA (ATCC 43300) and MRSA (ATCC 29213) isolates (American Type Culture Collection, Manassas, VA) correlated well across a range of 103–107 CFU with the quantitative cultures of these isolates (r = 0.997 and 0.999, respectively).
Each S. aureus isolate was typed by DNA sequencing analysis of a single locus, the protein A (spa) gene hypervariable region as previously described.Reference Harmsen, Claus and Witte14 Alleles were identified based on comparison to the sequences in the database at http://spaserver.ridom.de/. The spa types were grouped for each table by multilocus sequence type and were divided into each of 4 main groups: (1) t002 and those related to sequence type 5, (2) t008 and those related to sequence type 8, (3) t1081, and (4) other.
Statistical analysis
We defined the outcome of S. aureus transmission between a resident and HCW as the recovery of S. aureus isolates with matching methicillin susceptibility profiles from the patient (ie, colonization of the anterior nares or perianal skin) and the HCW (ie, the gown or glove worn during an interaction with a resident). The risk of S. aureus transmission for each resident was estimated as a fraction: the number of interactions resulting in S. aureus transmission divided by the total number of interactions. We did not analyze specific care activities because of small sample sizes and bundling within the same interaction (eg, toileting, then bathing, then dressing). We also reasoned that resident characteristics might correlate with specific types of care (eg, residents with wounds receive wound care). Residents were divided into the following 3 groups defined by the percentage of interactions resulting in transmission of S. aureus: (1) no transmission (0% of interactions), (2) low transmission (1%–30% of interactions), and (3) high transmission (>30% of interactions). The mean qPCR values from the perianal skin were divided into 3 categories of S. aureus colonization: (1) no colonization (0 CFU), (2) low burden of colonization (1–100 CFU), and (3) high burden of colonization (>100 CFU). The low- and high-colonization burden groups were compared to the no-colonization group as a reference. The mean qPCR values from the anterior nares were categorized as low burden of colonization (0–1,000 CFU) and high (>1,000 CFU) burden of colonization; 1,000 CFU was the lower limit of persistent nasal colonization described by Nouwen et al.Reference Nouwen, Ott and Kluytmans-Vandenbergh15 A series of the Pearson χReference Cosgrove, Sakoulas, Perencevich, Schwaber, Karchmer and Carmeli2 tests for trend (for categorical data) or Kruskal-Wallis tests (for continuous variables) examined the association of transmission with a single independent variable. Odds ratios for transmission—dichotomized into transmission versus no transmission groups—were determined for those categorical variables with a P < .20.
The final multivariable model included risk factors with the odds ratios that were statistically significant: male sex, the presence of diabetes, pressure ulcer, indwelling urinary catheter, total dependence for transfer, residential care, burden of S. aureus colonization of the perianal skin by qPCR, and MRSA colonization. S. aureus colonization burden of the anterior nares by qPCR, while not statistically significant, was included in the multivariable model because of biological plausibility. The multivariable logistic regression (SAS proc GENMOD, employing a logit link, and binomial distribution) produced adjusted odds ratios for each of the independent variables in the model. The generalized estimating equations method (GEE) of Liang and Zeger, with an exchangeable covariance structure, was used to account for serial auto-correction of repeat measures from the same subjects.Reference Liang and Zeger16 All analyses were performed with and without outliers as identified by Cook’s distance. Statistical tests were 2-tailed, and P values <0.05 were considered statistically significant. Statistical analyses were conducted using Stata version 15 software (StataCorp, College Station, TX) and SAS version 9.3 software (SAS Institute, Cary, NC).
Results
Gown and glove cultures were collected from 1,418 total interactions from the 169 subjects colonized with S. aureus in the anterior nares or perianal skin; 37% of the residents were male and 75% were non-Hispanic white. Table 1 lists resident groups by their risk of transmitting to gowns or gloves (eg, the high transmission group transmitted S. aureus to gowns or gloves in >30% of the care interactions). Residents in the high-transmission group were more likely to be male, to be diabetic, to have a pressure ulcer, to have S. aureus colonization at the perianal skin, to have a relatively high burden of S. aureus colonization at the perianal skin, or to be colonized with MRSA. q-PCR values were significantly greater in the high-transmitter group for both the anterior nares and perianal skin. We did not find an association between high S. aureus transmission and spa type, though a novel spa type t1081 was noted in 16 isolates. The association between S. aureus transmission and antibiotic use at enrollment, total dependence on a HCW for transfer, residential care, and acute-care hospitalization in the past 3 months were likewise not statistically significant.
Table 1. Characteristics of Staphylococcus aureus–Colonized Residents (n=169) Stratified by Level of Transmission to HCW Gowns and Gloves

Note. HCW, healthcare worker; SD, standard deviation; IQR, interquartile range; MRSA, methicillin-resistant S. aureus.
a Units unless otherwise specified.
b Comparing transmission groups.
c Stage 1 or worse.
d 4 isolates were not typable.
The bivariate and multivariable analyses included 168 subjects and 1,417 interactions, with a median cluster size of 7 interactions per subject (range, 1–22); 1 subject and 1 interaction were excluded because of missing data. In the bivariate analysis, the odds of transmission increased >2-fold if the patient was male, had diabetes, had an indwelling urinary catheter, was totally dependent on a HCW for transfer, had perirectal colonization with S. aureus, or had MRSA colonization (Table 2). The odds of transmission increased markedly with increasing burden of perirectal colonization. The bivariate odds were also increased if the subject had a pressure ulcer or was in residential care. Neither antibiotic use at enrollment, nor a high burden of S. aureus in the anterior nares were significant risk factors.
Table 2. Odds Ratios of S. aureus Transmission to HCW Gowns and Gloves Given Resident- and Culture-Based Characteristics Using GEE to Adjusting for Within-Resident Clustering
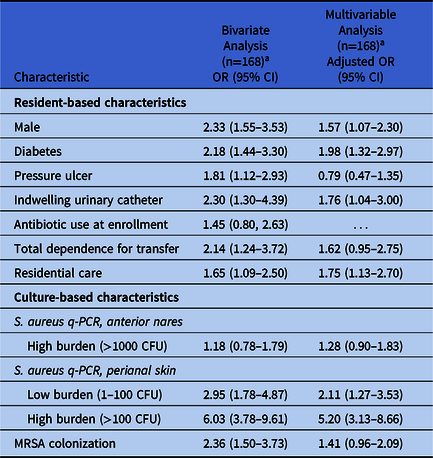
Note. HCW, healthcare worker; GEE, generalized estimating equations; OR, odds ratio; CI, confidence interval; q-PCR, quantitative polymerase chain reaction; CFU, colony-forming units;
a1 resident was excluded because of missing data.
In the multivariable analysis, colonization of the perianal skin had the greatest adjusted odds for S. aureus transmission, with increasing odds as the burden of colonization increased (Table 2). The multivariable odds ratios of transmission associated with being male, having diabetes, and having a urinary catheter were lower than the bivariate odds ratios but still statistically significant; the adjusted odds ratio of being in residential care remained unchanged. Having a pressure ulcer, being totally dependent on an HCW for transfer, and MRSA colonization were no longer statistically significant in the multivariable model. These odds ratios were unchanged when potential outliers were deleted based on Cook’s distance.
Discussion
The transmission of S. aureus between a resident and a HCW is a key step in the spread of S. aureus in healthcare facilities, and it appears to be influenced by resident- and culture-based characteristics. In this study, nursing home residents with a high burden of perianal S. aureus colonization were more likely to transmit S. aureus to HCW gowns and gloves, and it is possible that some of the other risk factors for transmission share this common causal pathway.
The presence of S. aureus on the perianal skin conferred the greatest odds for S. aureus transmission to HCW gowns and gloves in the multivariable analysis. Here, the odds of S. aureus transmission increased with increasing burden of colonization, with the greatest odds ratio corresponding to those residents with >100 CFU from their perianal swab. Nasal carriers of S. aureus who are also perineal carriers have been reported to have higher S. aureus loads and disperse more S. aureus, which is consistent with our findings.Reference Ridley17–Reference Squier, Rihs and Risa19 In contrast, a high burden of nasal colonization (>103 CFU) was not a significant risk factor in the multivariable model, which may reflect that HCWs are more likely to come in contact with other colonization sites (eg, axilla, groin, or perianal region).
Having a pressure ulcer and total dependence for transfer were not associated with S. aureus transmission after adjusting for perianal colonization burden, perhaps because these variables share a common pathway. Previous studies have linked perianal S. aureus colonization to S. aureus-specific lesions (eg, furuncles or carbuncles) of the lower half of the body,Reference Solberg20 and areas of skin breakdown, such as pressure ulcers, are often colonized with S. aureus. Reference de Wert, Rensen, Soons, Poeze, Bouvy and Penders21,Reference Wolcott, Hanson and Rees22 Residents who are totally dependent for transfer are likely at increased risk for developing a pressure ulcer because of additional risk factors such as immobility or incontinence. Conversely, residents with pressure ulcers are more dependent on HCWs.
Diabetes and male sex remained significant risk factors for transmission after adjusting for perianal burden of colonization. Although diabetes has been linked to both nasal S. aureus colonization and perianal colonization burden, it is possible that diabetics could be colonized at additional sites outside of the nares or perineum and lead to greater transmission.Reference Ahluwalia, Sood, Sood, Lakshmy, Kapil and Pandey23,Reference Mermel, Cartony, Covington, Maxey and Morse24 The link between being male and transmission is unclear, though higher levels of colonization have been attributed to hormonal differences.Reference Graham, Lin and Larson25,Reference Nowak, Borkowska and Pawlowski26
Being in residential care—rather than post-acute care—and the presence of a urinary catheter were also significant risk factors for transmission in the multivariable model. Patients in residential care may have greater care needs, which result in a higher cumulative exposure to their healthcare workers and facilities. Although S. aureus is not a typical urinary pathogen, an indwelling catheter provides a surface for colonization and a portal for infection, possibly via colocalization of S. aureus with fibrinogen,Reference Walker, Flores-Mireles and Pinkner27 which may be exacerbated by manipulation of the catheter by the resident or HCW. Moreover, the association between having an indwelling catheter and S. aureus colonization has been reported previously.Reference Harinstein, Schafer and D’Amico28
MRSA colonization was not a significant risk factor after adjusting for confounders, likely because colonization with any S. aureus—and not necessarily just MRSA—is the driver of transmission risk. We did not observe a difference in the risk of transmission among residents colonized with different spa types, suggesting that the strain of S. aureus may not be a determining factor in transmission. We did note an unusual spa type t1081 as a common cause of perianal colonization, which has been reported as a common spa type in nursing homes and hospitals in both The Netherlands and Hong Kong.Reference Cheng, Chan and Lau29–Reference Luk, Ho and Ng34 Interestingly, spa type t1081 has also been associated with increased transmissionReference Cheng, Chan and Lau29,Reference Hetem, Bootsma, Troelstra and Bonten32 and patients in a bedbound state,Reference Luk, Ho and Ng34 possibly due to its predilection for perianal colonization.
The strengths of this study include its prospective, multicenter design with representation from different parts of the nation. This was a community-based study with similar demographics to those of the US nursing home population with respect to gender and ethnicity.Reference Harrington, Carrillo, Dowdell, Tang and Blank35–Reference Harris-Kojetin, Sengupta, Park-Lee and Valverde37 Additionally, surveillance cultures were obtained at enrollment to document colonization, rather than using historical colonization data. Limitations include that gown and glove transmission is a surrogate outcome for the transmission of S. aureus from resident to HCW to another resident, though mechanistically reasonable to study in lieu of resident-to-resident S. aureus acquisition, which is a relatively rare outcome. We could not adjust for repeated measurements of the same nursing home staff member because HCW participation was anonymous. Among 13 nursing homes, however, a variety of staff members were involved, and we used the GEE in our analysis to account for multiple interactions with the same resident. Selection bias toward those residents capable of providing informed consent might have affected our findings, though we engaged the legally authorized representatives of those who were unable to provide informed consent to obtain a representative sample.
In our study, we identified factors that promote S. aureus transmission to HCW gowns and gloves in nursing homes. We have shown that the burden of S. aureus colonization on the perianal skin is a marker for those most likely to transmit to HCW gowns and gloves. To our knowledge, this is the first study to relate the burden of colonization to the risk of transmission. Unlike most other studies, we examined all S. aureus transmission because both MRSA and MSSA are clinically important entities. Our results suggest that targeting MRSA-colonized residents alone may not be the optimal approach to controlling S. aureus transmission. Perianal skin colonization can be used to identify those residents who are most likely to transmit S. aureus to others, and who may warrant infection control interventions such as contact precautions or enhanced barrier precautions.38 Future studies should assess whether decreasing the burden of perianal colonization, perhaps through enhanced hygiene, could decrease S. aureus transmission.
Acknowledgments
We thank the participating nursing home personnel and residents. The opinions expressed in this document are those of the authors and do not reflect the official position of Agency for Healthcare Research and Quality (AHRQ) or the US Department of Health and Human Services. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Financial support
This project was funded by the US Department of Health and Human Services AHRQ (grant no. 1R18HS019979-01A1). E.M.S. is supported by an NIGMS Initiative for Maximizing Student Development (grant no. 2 R25-GM55036). L.M. is supported by the VA Ann Arbor Geriatrics Research, Education, and Clinical Center, National Institute on Aging (grant nos. R01 HS025451, K24 050685AG, and T32 AG062403) and by the University of Michigan Claude D. Pepper Older Americans Independence Center (grant no. P30 AG024824). J.D.S. and M.R. are supported by the VA Maryland Healthcare System Geriatrics Research, Education, and Clinical Center, and University of Maryland Claude D. Pepper Older Americans Independence Center (grant no. P30AG028747).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





