1. Introduction
Testudinoidea is the most diverse group of living turtles, which represents about half of the diversity of the order Testudines today (Claude & Tong, Reference Claude and Tong2004; Lourenço et al. Reference Lourenço, Claude, Galtier and Chiari2012). Known since the Mid Cretaceous, the early stages of the radiation in this group remain largely unknown, and the pre-Eocene fossil record is exclusively found in Asia, suggesting that they originated in this region during the Cretaceous. For a long time, all pre-Eocene stem testudinoid turtles have been assigned to the paraphyletic Lindholmemydidae (or more certainly polyphyletic; see Tong et al. Reference Tong, Li, Li, Chen, Li, Yu, Yu, Cheng, Di and Claude2016). This composite assemblage is characterized by a set of primitive characters and no apomorphy. Among the plesiomorphies of Lindholmemydidae, the presence of well-developed inframarginal rows makes them unique by comparison with the modern families. On the other hand, the modern families (Emydidae, Geoemydidae, Testudinidae and Platysternidae) almost all apparently start their fossil record in the Eocene in Asia, Europe and North America, while so-called Lindholmemydidae (testudinoids with inframarginals) disappeared from the fossil record before the Eocene. To date, none of these stem testudinoids has been reported after the Palaeocene/Eocene boundary, suggesting that the stem testudinoids became extinct before that time. Here we report the first post-Palaeocene stem testudinoid turtles, discovered from the early Eocene of Wutu, in Shandong Province, China.
The first turtle remains from the early Eocene Wutu Formation were reported by Ye (Reference Ye1995). Based on the presence of a complete row of inframarginals and absence of the mesoplastron, Ye (Reference Ye1995) referred this juvenile turtle shell (IVPP RV 95001) to the family Dermatemydidae, but refrained from erecting a new taxon because of the absence of available characters of the carapace. Since that time, additional material has been discovered, including several incomplete shells and shell fragments collected by the Sino-Belgian expeditions in the Wutu coal mine in 2006 and 2008. In this paper we provide a re-examination of the shell described by Ye in 1995 and a systematic study of the new material collected by the Sino-Belgian team. In order to better understand the early stages of the testudinoid radiation, we conducted the first comprehensive phylogenetic analysis of the group, including most basal taxa. Since our analysis indicates that the members of the family Lindholmemydidae do not form a monophyletic group, we use the term ‘stem testudinoids’ instead of that family name throughout our paper.
Institutional abbreviations: IVPP – Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing; PEPZ (IBCAS) – Peking National Herbarium Paleozoological collection (Institute of Botany, Chinese Academy of Sciences), Beijing.
2. Geological setting
The new material was collected from the surface tailings of the Wutu coal mine near the town of Wutu, Linqu County, Shandong Province, China (Fig. 1), in 2006 and 2008. The Wutu Formation, which reaches c. 1000m in thickness, was established by Geological Team No 121, Shandong Coal and Geology Exploration Bureau, in 1960 (Zhao, Reference Zhao1981). It consists of several members, namely from bottom to top: the lower coal-bearing Member, the oil shale Member, the middle coal-bearing Member (containing 12 coal beds) and the upper coal-bearing Member (Fig. 2).

Figure 1. Map showing the location of the Wutu coal mine (modified from Zhang et al. Reference Zhang, Smith, Yang and Li2016).

Figure 2. General stratigraphic column of the Wutu Formation, indicating fossil layers B5 and B7 where the fossil turtles come from (modified from Li et al. Reference Li, Smith, Liu, Awasthi, Yang, Wang and Li2011).
The new specimens are fragmentary carapaces, some associated with plastron. The fossils were embedded in black shale at the contact of coal beds 5 and 7 from the middle coal-bearing Member at c. 250 m below the ground (Li et al. Reference Li, Smith, Liu, Awasthi, Yang, Wang and Li2011; Fig. 2). The specimens collected are compressed, some of them being deformed.
The Wutu coal mine in the Wutu Basin of the Shandong Province is an important fossil locality. Mainly known for its mammal fauna that allows the Wutu Formation to be assigned to an early Eocene age (Tong & Wang, Reference Tong and Wang1998), the site has also yielded the oldest Asian records of Nuphar (Nymphaeaceae) and Prunus (Rosaceae) based on well-preserved seeds (Chen, Manchester & Chen, Reference Chen, Manchester and Chen2004; Li et al. Reference Li, Smith, Liu, Awasthi, Yang, Wang and Li2011). Although Beard & Dawson (Reference Beard and Dawson1999) even proposed a late Palaeocene age for the fossil site, based on the presence of some primitive mammals with North American affinities such as the neoplagiaulacid multituberculate Mesodmops dawsonae and the carpolestid plesiadapiform Carpocristes oriens, it is now widely accepted to be of early Eocene age. The presence of a diversified mammal association (51 species) mainly including derived taxa belonging to modern orders such as the hyaenodontan Preonictis youngi, the miacid carnivoran Zodiocyon zetesios, the perissodactyls Pappomoropus taishanensis, Chowliia laoshanensis and Homogalax wutuensis, and the artiodactyl Wutuhyus primiveris (Tong & Wang, Reference Tong and Wang2006) clearly pertains to an Eocene age. Finally, the palynological assemblage from the Wutu Formation suggests a late Early Eocene to early Middle Eocene age with a warm temperate vegetation succession comprising mixed needle- and broad-leaved forests (Wang, Wang & Zhang, Reference Wang, Wang and Zhang2005; Zhang et al. Reference Zhang, Smith, Yang and Li2016).
3. Material
The material studied herein consists of three partial shells and other shell fragments. This material is housed in the Institute of Botany, Chinese Academy of Sciences, Beijing, and Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing (see holotype and referred specimens).
The comparative material of pre-Eocene testudinoids includes all the taxa that are sufficiently well known to allow comparisons and phylogenetic analyses: Anhuichelys spp. from the Palaeocene of China (Tong et al. Reference Tong, Li, Li, Chen, Li, Yu, Yu, Cheng, Di and Claude2016); Amuremys planicoctata (Riabinin, Reference Riabinin1930) from the Latest Cretaceous of Russia and China (Danilov et al. Reference Danilov, Bolotsky, Averianov and Donchenko2002); Elkemys australis Ye, Reference Ye1974 from the Early Palaeocene of Guangdong, southern China (first-hand observation of H.T.; Ye, Reference Ye1974; Danilov, Claude & Sukhanov, Reference Danilov, Claude and Sukhanov2012); Gravemys (including G. barsboldi Sukhanov & Narmandakh, Reference Sukhanov and Narmandakh1976 from the Late Cretaceous of Mongolia (Danilov, Reference Danilov2003) and G. hutchisoni Danilov, Reference Danilov2003 from Inner Mongolia, China (first-hand observation of H.T.; Danilov, Reference Danilov2003)); Hokouchelys chenshuensis Ye, Reference Ye1974 from the Middle Palaeocene of Guangdong, southern China (first-hand observation of H.T.; Ye, Reference Ye1974); Hongilemys kurzanovi Sukhanov & Narmandakh, Reference Sukhanov, Narmandakh, Danilov and Parham2006 (Sukhanov, Reference Sukhanov, Benton, Shishkin, Unwin and Kurochkin2000; Sukhanov & Narmandakh, Reference Sukhanov, Narmandakh, Danilov and Parham2006); Lindholmemys elegans Riabinin, Reference Riabinin1935 and L. martinsoni from the Late Cretaceous of Uzbekistan and L. occidentalis from the Late Cretaceous of Mongolia (Riabinin, Reference Riabinin1935; Nessov & Krassovskaya, Reference Nessov and Krassovskaya1984; Danilov, Reference Danilov1999; Sukhanov, Reference Sukhanov, Benton, Shishkin, Unwin and Kurochkin2000; Danilov & Sukhanov, Reference Danilov and Sukhanov2001); Paramongolemys khosatzkyi Danilov & Sukhanov, Reference Danilov and Sukhanov2013 from the Late Palaeocene of Mongolia (Danilov & Sukhanov, Reference Danilov and Sukhanov2013); Pseudochrysemys gobiensis Sukhanov & Narmandakh, Reference Sukhanov and Narmandakh1976 (Sukhanov & Narmandakh, Reference Sukhanov and Narmandakh1976; Danilov, Claude & Sukhanov, Reference Danilov, Claude and Sukhanov2012); Shandongemys dongwuica Li et al. Reference Li, Tong, Wang, Chen and Xu2013 (first-hand observation of H.T.; Li et al. Reference Li, Tong, Wang, Chen and Xu2013); Tsaotanemys rugosus Bohlin, Reference Bohlin1953 from the late Early Cretaceous of Gansu, China (Bohlin, Reference Bohlin1953); and the Palaeocene stem testudinoids which were previously assigned to the genus Mongolemys, but the generic assignment is doubtful: ‘M.” tatarinovi Sukhanov & Narmandakh, Reference Sukhanov and Narmandakh1976; ‘M.’ reshetovi Sukhanov & Narmandakh, Reference Sukhanov and Narmandakh1976 and ‘M.’ trufanensis Ye, Reference Ye1974 (Sukhanov & Narmandakh, Reference Sukhanov and Narmandakh1976; Danilov, Reference Danilov1999; Sukhanov, Danilov & Narmandakh, Reference Sukhanov, Danilov and Narmandakh1999; Danilov, Reference Danilov2003).
4. Systematic palaeontology
Testudines Linnaeus, 1758
Cryptodira Cope, 1868
Testudinoidea Batsch, 1788
Wutuchelys eocenica n. gen. n. sp.
(Figs 3–6)
Etymology: The genus name is from Wutu, the locality where the turtle specimens have been collected; the species name derives from the Eocene age of the specimen.

Figure 3. Wutuchelys eocenica n. gen. n. sp. PEPZ WT012 (holotype), shell in dorsal (a, b) and ventral (c, d) views and detail of the ornamentation on the carapace (e) and plastron (f). Scale bar = 5 cm (a–d) and 2 cm (e, f). Abbreviations: if, inframarginal; n, neural; py, pygal; spy, suprapygal.
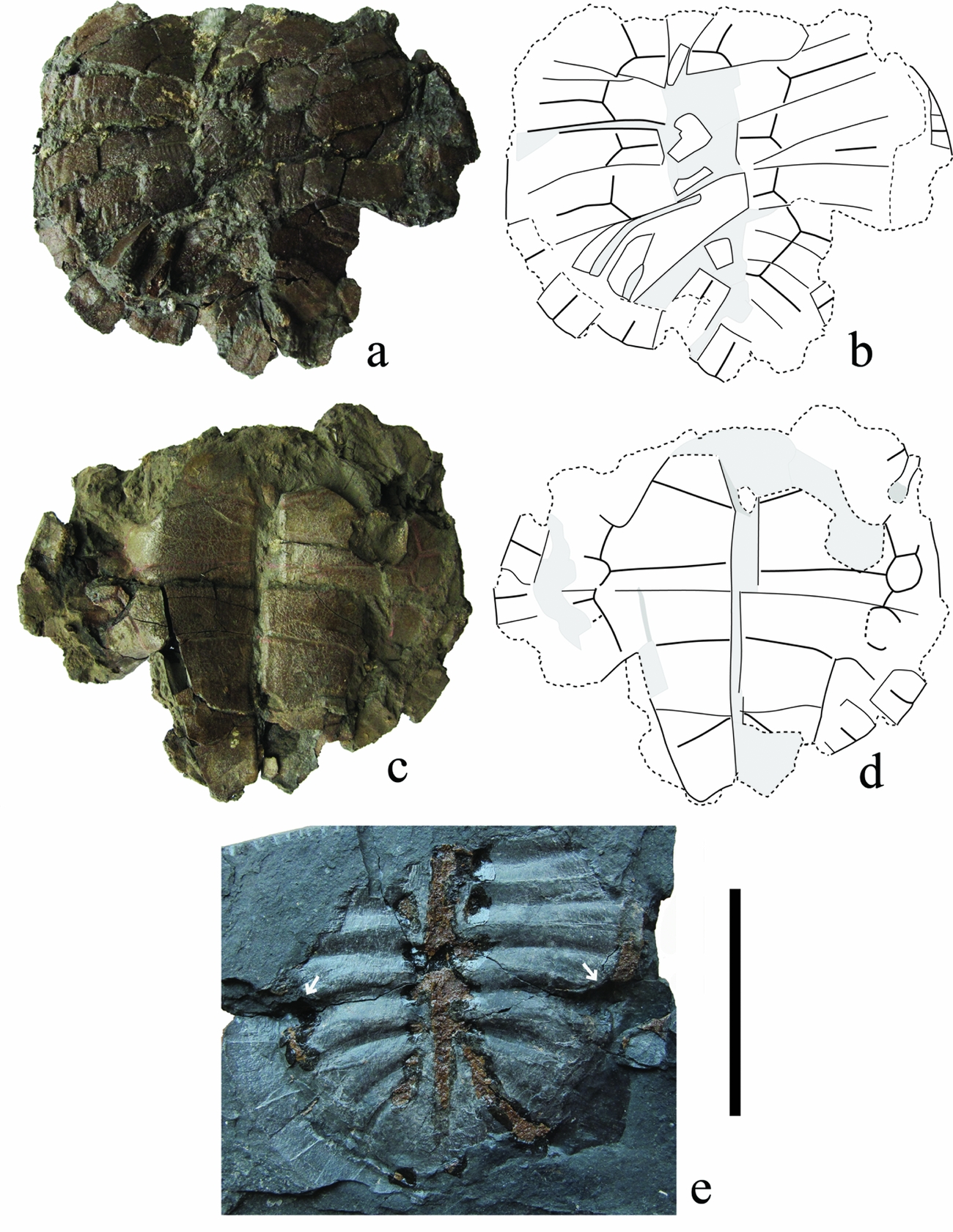
Figure 4. Wutuchelys eocenica n. gen. n. sp. a–d: IVPP RV 95001, a juvenile shell in dorsal (a, b) and ventral (c, d) views. (e) PEPZ WT013, internal mould of carapace. Arrows indicate the insertion site of the inguinal buttresses on the inner surface of costal 5. Scale bar = 5 cm.

Figure 5. Wutuchelys eocenica n. gen. n. sp. PEPZ WT003b, a shell in dorsal (a, b) and ventral (c, d) views. Arrow indicates the insertion scar of the axillary buttress on the inner surface of costal 1. Scale bar = 5 cm.

Figure 6. Reconstruction of carapace (a) and plastron (b) of Wutuchelys eocenica n. gen. n. sp.
Holotype: PEPZ WT012, a partial shell with articulated carapace and plastron; the right one-third of both carapace and plastron and the anterior portion of the carapace are missing; the anterior end of the plastron is damaged and the xiphiplastra are disarticulated.
Referred specimens: PEPZ WT003, including three individuals (WT003a: a smaller carapace preserved mostly as imprint exposed in dorsal view; WT003b: a partial shell with very damaged carapace articulated with incomplete plastron, the plastron lacking both epiplastra and xiphiplastra; WT003f: a partial plastron); PEPZ WT006a–g: a fragment of plastron, exposed in dorsal view; PEPZ WT009: an isolated right peripheral 1; PEPZ WT010: a fragment of carapace; PEPZ WT013: a small shell preserved mostly as internal mould, only part of the carapace preserved and exposed in inner view; all from the type locality. IVPP RV 95001, an almost complete juvenile shell with articulated carapace and plastron. The shell is severely crushed dorsoventrally, so some plates are overlapping on one another and crushed and the neural series is not visible; the carapace lacking notably the nuchal and some peripherals. On the plastron, both epiplastra and the left xiphiplastron are missing; the entoplastron is damaged. The specimen has been further damaged since the description of Ye (Reference Ye1995); we are unable to completely restore the left xiphiplastron and the left bridge region.
Type locality and horizon: Wutu coal mine, Linqu County, Shandong Province, China; middle coal-bearing Member of Wutu Formation, Early Eocene.
Diagnosis: A stem testudinoid differing from all post-Eocene testudinoids by the presence of a full row of well-developed inframarginal scutes and differing from all other pre-Eocene stem testudinoids by the following combination of characters: carapace oval in outline that is slightly expanded posteriorly, with a relatively large cervical notch; shell surface covered with a pronounced ornamentation, consisting of anteroposteriorly directed thin ridges and furrows on the carapace and fine vermiculate furrows on the plastron; vertebrals wider than long; trapezoidal vertebral 1 which is expanded anteriorly and reaching the second marginal scute; short bridge by comparison with most pre-Eocene testudinoids; large anal notch; round entoplastron; humeropectoral sulcus located far anterior to the base of the anterior lobe of the plastron and posterior to the entoplastron, and four relatively wide inframarginals which are mainly located on the bridge and slightly extend onto the peripherals.
Measurements: see Table 1.
Table 1. Measurements of Wutuchelys eocenica n. gen. n. sp. (in mm. Estimated complete value in parentheses)

4.a. Description
Carapace: As preserved in PEPZ WT003b and PEPZ WT012, the carapace has a relatively wide oval outline which is slightly expanded posteriorly. As all shells have undergone a dorsoventral crushing, the original height of the carapace is undeterminable. The cervical notch, partly preserved in PEPZ WT003b, is shallow and wide. There are no midline or lateral keels on the carapace, and the carapace margin is smooth without serration. The surface of the carapace is covered with clear ornamentation consisting of thin ridges and furrows, directed anteroposteriorly and slightly anteromedially (Fig. 2E). The damaged carapace of PEPZ WT003a, PEPZ WT003b and PEPZ WT013 exposes the internal structure. On the inner surface of the costal 1, the second thoracic rib forms a sharp edge that extends laterally to end at an oval scar on the lateral part of the plate for the axillary buttress insertion (Fig. 5C, D). The inguinal buttresses insert on the lateral one-fourth of the costal 5 (Fig. 4E). The rib heads are not reduced.
The nuchal is not preserved. The incomplete neural series (neurals 3–8) is preserved in PEPZ WT012. Neural 3 is detached and turned over, so its dorsal surface is exposed on the ventral side of the shell. The preserved neurals are relatively narrow, hexagonal with short anterolateral sides. Neurals 3–5 are longer than wide. Neural 6 is not completely exposed, so its shape is unclear. Neurals 7 and 8 are crushed; it appears that neural 7 is roughly square and neural 8 is slightly longer than wide. Suprapygal 1 is almost complete, and is trapezoidal. Suprapygal 2 is damaged dorsally, but its ventral side is well preserved. It is large and appears wider than suprapygal 1. The pygal has the dorsal surface damaged, but its ventral side is almost intact; it is roughly square. The complete costal series are preserved in IVPP RV 95001IVPP RV 95001; and the left costals 3–8 and the right costal 7 are preserved in PEPZ WT012. Costal 1 is longer than costal 2. Peripheral 1 (PEPZ WT009) is trapezoidal with a long contact with costal 1. The right peripherals 4–11 are preserved in PEPZ WT012. Peripherals 4–6 are relatively narrow; peripherals 7 and those postward are wider.
The vertebrals are preserved in PEPZ WT012 and IVPP RV 95001. Vertebral 1, incomplete in IVPP RV 95001, is wide, with the lateral margins divergent anteriorly. PEPZ WT009 (an isolated peripheral 1) shows that vertebral 1 contacts marginal 2. Vertebrals 2 and 3 appear to be wider than long. The intervertebral sulci cross the first, third, fifth and eighth neurals respectively. The pleural scutes are as wide as the vertebrals. In PEPZ WT012, the interpleural sulci are located closer to the posterior margin of the costal, not at the mid-length as in IVPP RV 95001. We interpret this difference as intraspecific variation. The marginals are restricted in the peripherals, with the pleuromarginal sulci well distant from the costoperipheral suture.
Plastron: The plastron is loosely connected to the carapace. The plastron is broad with a wide and short bridge. The anterior lobe is relatively long and clearly wider than the posterior lobe. The posterior lobe is longer than the bridge, with straight lateral borders which are convergent posteriorly. The surface of the plastron is covered with clear ornamentation consisting of fine vermiculate furrows (Fig. 2F).
The epiplastron is not preserved. The entoplastron, incompletely preserved in PEPZ WT003b and PEPZ WT003f, appears to be almost circular. The hypoplastron has a similar bridge length to the hyoplastron but is longer than the latter at the midline. The xiphiplastron is longer than wide. The anal notch is large and deep.
The gulars are not preserved. The humeropectoral sulcus is located posterior to the entoplastron and far anterior to the line connecting the bottom of the axillary notches. The pectoral is shorter than the abdominal. The femoroanal sulcus forms a wide angle which is located posterior to the hypoxpiphiplastral suture in PEPZ WT012, but reaches the hypoxiphiplastral suture in IVPP RV 95001. A complete row of four inframarginals is preserved on both sides of IVPP RV 95001 and the left side of PEPZ WT012. The inframarginals are relatively wide and located mainly on the bridge, with the lateral border slightly extending onto the peripherals. They separate the plastral scutes completely from the marginal scutes. In PEPZ WT012, the inframarginals 1 and 3 are small while inframarginals 2 and 4 are much larger.
4.b. Comparisons
Based on the general morphology and ornamentation on the shell surface, all specimens from Wutu are referred to a single species. It is assigned to Testudinoidea because the axillary and inguinal buttresses contact the costal plates. It is outside of the clades Geoemydidae, Testudinidae, Platysternidae and Emydidae sensu stricto, all characterized by a reduction or a disappearance of inframarginal scutes. The general shell morphology of Wutuchelys is well comparable to the testudinoids from the Palaeocene and the Cretaceous of Asia.
Wutuchelys has a complete series of four relatively wide inframarginals which are mostly located on the bridge and slightly overlap the peripherals. The right inframarginals of PEPZ WT003b appear to be narrower, but this is due to the lateral shift of the plastron caused by crushing. This inframarginal morphology is different from the strong overlapping of the inframarginals scutes on the peripheral plates of Elkemys, Gravemys and Hokouchelys (Danilov, Claude & Sukhanov, Reference Danilov, Claude and Sukhanov2012). Although Mongolemys and Shandongemys also have wide inframarginals which are restricted in the bridge, they are distinct from Wutuchelys in having only three inframarginals. The inframarginals of Lindholmemys, Amouremys, Tsaotangemys, Hongilemys and Paramongolemys are narrow. Pseudocrysemys has reduced inframarginal series, allowing, in some cases, the contact between the abdominal and the marginal scutes.
IVPP RV 95001 has wide vertebral scutes which may partly be due to the juvenile nature of the specimen. The vertebrals of holotype of Wutuchelys (PEPZ WT012) are incomplete. When reconstructed, vertebrals 2 and 3 would be slightly wider than long. This is different from the longer than wide vertebrals 2 and 3 in Elkemys, Gravemys, Lindholmemys, Paramongolemys and Hokouchelys. In other lindholmemydids, these vertebrals are as long as wide.
Vertebral 1 in Wutuchelys is wider than the nuchal plate with the anteriorly divergent lateral borders, resulting in the contact between vertebral 1 and marginal 2 as in some Mongolemys and Paramongolemys (Danilov & Sukhanov, Reference Danilov and Sukhanov2013). In Elkemys, Gravemys, Hokouchelys, Lindholmemys and Pseudocrhysemys, vertebral 1 is narrower than the nuchal, contacting marginal 1.
The shell surface of Wutuchelys is covered with clear ornamentation, consisting of fine anteroposteriorly directed ridges and furrows on the carapace and vermiculate furrows on the plastron. Elkemys, Hokouchelys and Paramongolemys have a smooth shell surface. The ornamentation of Shandongemys consists of larger ridges, while that of Amuremys is more irregular and stronger.
Wutuchelys is a small turtle; the largest specimen has a carapace length of c. 16 cm. IVPP RV 95001 is a juvenile, with wider shell and open sutures. The largest specimen (PEPZ WT012) is likely an adult or sub-adult individual, with a more elongate shell. All specimens have a loose connection between the plastron and the carapace; the whole plastron is often pushed inward and sometimes laterally by crushing. This loose connection is reminiscent of living Platysternon. One additional important related feature of Wutuchelys is its short bridge. The bridge length / plastron width ratio in this taxon is c. 45%. This ratio is even smaller in IVPP RV 95001 (38%), likely due to the wider shell of the juvenile. Although Shandongemys, Mongolemys and Tsaotanemys also have a short bridge, with a bridge length / plastron width ratio of 50– 58%, it is apparent that Wutuchelys has the shortest bridge among pre-Eocene testudinoids. Elkemys, Gravemys and Hokouchelys have the greatest ratio, ranging from 60% to 73%, which is also comparable to most geoemydids and testudinids.
The detailed comparisons between Wutuchelys and other genera of pre-Eocene stem testudinoids are summarized in Table 2. (Some poorly known taxa are not included: Lindholmemys occidentalis, ‘Mongolemys’ tatarinovi, ‘M.’ reshetovi and ‘M.’ trufanensis.)
Table 2. Comparisons between Wutuchelys eocenica n. gen. n. sp. and other stem testudinoid taxa

5. Phylogenetic analysis
The phylogenetic relationships of the basal testudinoids with modern families of the group (Emydidae, Geoemydidae, Testudinidae, Platysternidae) are not well understood. Molecular dating indicates that Emydidae and Testudinidae+Geoemydidae may have split during the Cretaceous (Lourenço et al. Reference Lourenço, Claude, Galtier and Chiari2012), but this lacks support from the fossil record. To date, no comprehensive analysis has been run; only a few stem testudinoids have been included in the phylogenetic analyses (Claude & Tong, Reference Claude and Tong2004; Cadena, Ksepka & Norell, Reference Cadena, Ksepka and Norell2013; Tong et al. Reference Tong, Li, Li, Chen, Li, Yu, Yu, Cheng, Di and Claude2016). In order to determine the phylogenetic relationships of Wutuchelys, a data matrix of 36 informative characters for 28 taxa, including 12 stem testudinoids, extends the work of Tong et al. (Reference Tong, Li, Li, Chen, Li, Yu, Yu, Cheng, Di and Claude2016). Chelydra, Ordosemys, Dermatemys and Claudius are included for rooting the tree and testing the monophyly of the in-group.
Two analyses were run, one using only morphological characters and one constraining the relationship of living taxa using a molecular scaffold based on the phylogenies obtained by Crawford et al. (Reference Crawford, Parham, Bellas, C., Glenn, Papenfuss, Henderson, Hansen and Brian2015), Lourenço et al. (Reference Lourenço, Claude, Galtier and Chiari2012) and Guillon et al. (Reference Guillon, Guéry, Hulin and Girondot2012). Character description and distribution are given in Appendices 1 and 2. All characters except one were ordered, and all ordered multi-state characters were scaled so that those characters would not have a disproportionate effect above binary characters on phylogeny estimation. Parsimony analyses were performed under PAUP 4.0 b10 (Swofford, Reference Swofford1998) using random addition sequence, and the tree bisection–reconnection branch-swapping algorithm across 10000 replicates.
The unconstrained analysis resulted in two equally parsimonious trees of 104.45 steps (Fig. 7). In this analysis, Wutuchelys is found in a clade with Tsaotanemys, which is the sister group of Shandongemys. Platysternon is at the base of the Testudinoidea radiation, but Dermatemys is found within the in-group with Paramongolemys, suggesting an important convergent pattern between Dermatemydidae and Testudinoids. Together with Paramongelemys, Hongilemys, Lindholmemys and Hokouchelys, Elkemys and Gravemys form a clade with Testudinidae and Geoemydidae. Emydidae and Pseudochrysemys form a monophyletic group, which is the sister group of this clade. Amuremys, Mongolemys and Anhuichelys form a clade which has an intermediate position between Platysternon and Wutuchelys+Tsaotanemys+Shandongemys clade. If we exclude the position of Dermatemys, the analysis is in agreement with the molecular scaffold with the exception of Platysternon, which is more basal. This first result suggests that Lindholmemydidae are polyphyletic, that Anhuichelys is in a more basal position than in Tong et al. (Reference Tong, Li, Li, Chen, Li, Yu, Yu, Cheng, Di and Claude2016) and that based on the position of Lindholmemys, Emydidae split from Testudinidae+Geoemydidae before Turonian.

Figure 7. Strict consensus on the left and the two trees obtained for the unconstrained phylogenetic analysis.
The analysis constrained by a molecular scaffold resulted in four equally parsimonious trees of 112.12 steps (Fig. 8). As in the unconstrained analysis, Wutuchelys and Tsaotanemys are found in a basal clade. Platysternon being constrained with Emydidae, some relationships change but several remain. Hongilemys, Lindholmemys, Gravemys, Hokouchelys and Elkemys are a sister group of Geoemydidae+Testudinidae. The position of Paramongelemys and Shandongemys is variable. Finally, Pseudochrysemys and Emydidae are a sister group of Platysternon, Amuremys and Anhuichelys in all instances. General conclusions are the same as in the unconstrained analysis, with a split between Emydidae+Platysternidae and Testudinidae+Geoemydidae before the Turonian, a basal position for Wutuchelys, and also a basal position for Anhuichelys, which become a convergent form with Testudinidae rather than rooting them.

Figure 8. Strict consensus on the left and the four trees obtained for the constrained phylogenetic analysis.
Our phylogenetic analyses confirm some previous hypotheses. First of all, Lindholmemydidae are not monophyletic and this family should not stand for pre-Eocene Testudinoidea. Furthermore, two notable relationships are found in both constrained and unconstrained analyses. First, Elkemys, Gravemys, Hokouchelys, Geoemydidae and Testudinidae form a monophyletic clade. This relationship is supported by one exclusive synapomorphy (character 35: the contribution of the hyoplastron to the bridge length is greater than that of the hypoplastron) and three non-exclusive synapomorphies (character 33: a long bridge. This character is also present in Anhuichelys by convergence and some geoemydids by reversion. Character 18: a large anal notch and character 31: the presence of four inframarginals. These two characters also occur in the Wutuchelys/Tsaotanemys clade by convergence). It is noteworthy that the number of inframarginals and the contribution of the hyoplastron to the bridge relative to the hypoplastron are partially correlated to the bridge length, even though there is no complete match in the distribution of the different states. Within this group, Elkemys, Gravemys and Hokouchelys form a clade in the constrained analysis as recognized by Danilov et al. (Reference Danilov, Claude and Sukhanov2012), although in the unconstrained analysis Gravemys is closer to Geoemydidae+Testudinidae than the Elkemys+Hokouchelys clade. Second, in all our phylogenetic hypotheses, Wutuchelys and Tsaotanemys form a basal monophyletic clade. This clade is not supported by any exclusive synapomorphy but shares two characters that evolved independently in the clade formed by Elkemys, Gravemys, Hokouchelys, Geoemydidae and Testudinidae: the presence of four inframarginals and a well-developed anal notch. But unlike the Elkemys, Gravemys, Hokouchelys, Geoemydidae and Testudinidae clade, the bridge of Wutuchelys and Tsaotanemys is short. The larger number of inframarginals in this clade seems not to have evolved, as a consequence of bridge lengthening. In addition, in Wutuchelys and Tsaotanemys, the inframarginals do not overlap the peripherals to a great extent as is the case in Elkemys, Hokouchelys and Gravemys. This further suggests that the evolution of the number of inframarginals is convergent in these two groups. The Wutuchelys/Tsaotanemys clade demonstrates that there is an important gap in the fossil record for this clade, spanning from the Cretaceous to the Palaeocene. For both taxa, one-quarter to one-third of the characters are missing in the matrix because of the incompleteness of the material. On the other hand, because fossil testudinoids are poorly documented at present in terms of skull morphology, most conclusions regarding skull evolution within the superfamily have not really been challenged since the work of McDowell (Reference McDowell1964). Further material, notably skull remains, would allow us to test whether this relationship is robust.
6. Conclusion
Wutuchelys eocenica n. gen n. sp. is part of an ancient lineage of Testudinoidea that split from other groups before the Turonian and is characterized by a short bridge. It represents a relict taxon of stem testudinoids which survived after the Palaeocene/Eocene boundary, as some mammals from the same locality. The apparent close relationships between Wutuchelys and Tsaotanemys support the hypothesis that ghost lineages are present in testudinoids and that the fossil record in the late Cretaceous should be investigated to better understand the early radiation of testudinoids.
Acknowledgements
We thank our colleagues who participated in the Wutu paleontological expeditions of 2006, 2008 and 2009 including Annelise Folie, Sandrine Ladevèze, Ya Li, Pieter Missiaen, Bin Sun, and Qian-Qian Zhang. We also thank Fabrice Vanderlinden for preparing the specimens, and Eric De Bast and Hélène Legendre for assisting with photographs of the specimens. This paper is a contribution to a bilateral research project financially supported by the Chinese Ministry of Science and Technology (2009DFA32210) and the Belgian Federal Science Policy Office (BL/36/C54). This research was supported by the Synthesys projects funded by the European Commission (http://www.synthesys.info/) to access the Wutu turtle material deposited in the Royal Belgian Institute of Natural Sciences (BE-TAF-4504) and the comparative living turtle collection housed in the Natural History Museum of Vienna (AT-TAF-2046) to H.T.
Appendix 1. Character states
1. Alternating costal plates: 0 no, 1 polymorphic or intermediate, 2 yes.
2. Inguinal and axillary buttresses contacting costals: 0 no, 1 yes.
3. Second suprapygal larger than first: 0 no, 1 polymorphic or equally larger, 2 yes.
4. Longer than wide pygal plate: 0 no, 1 polymorphic or as long as wide, 2 yes.
5. Marginal 12 relative to pygal: 0 marginals 12 higher than pygal, 1 marginals 12 = pygal, 2 marginals 12 lower than pygal.
6. Neurals: all hexagonal with shortest sides facing anterolaterally: 0 yes, 1 no.
7. Costal 1 reaching peripheral 4: 0 yes, 1 polymorphic, 2 no.
8. Lateral epiplastral lip present: 0 yes, 1 no.
9. Epiplastral lip present in the middle: 0 yes, forming an elevated bulge or a pocket, 1 yes but flat, 2 no.
10. Gular reaching entoplastron: 0 no, 1 polymorhic, 2 yes.
11. Humeropectoral sulcus behind entoplastron: 0 yes, 1 polymorphic, 2 no.
12. Presence of a central carina at least in juvenile, and sometimes persistent in adults: 0 no, 1 yes.
13. Cervical scute: 0 as long as wide as or wider than long, 1 longer than wide, 2 absent.
14. Coalescent trochanters of the femur: 0 no, 1 yes.
15. Bony bridge: 0 no, 1 yes.
16. Anal midline length relative to that of femoral: 0 anal longer than femoral, 1 anal equal to femoral, 2 anal shorter than femoral.
17. Midline length of xiphiplastron greater than the one of the hypoplastron: 0 no, 1 yes.
18. Anal notch: 0 absent, 1 small, 2 clearly present.
19. Inframarginal row: 0 complete and wide, 1 complete and narrow, 2 polymorphic, 3 incomplete.
20. Wide entoplastron: 0 yes, 1 polymorphic or intermediate, 2 no.
21. Three carinae in juveniles, sometimes persistent in adults: 0 no, 1 yes.
22. Pairs of anterior and posterior musk ducts: 0 no, 1 yes.
23. Nuchal emargination: 0 absent, 1 small or variable, 2 well developed.
24. Vertebrals 2–3: 0 wider than long, 1 longer than wide.
25. Vertebral 1: 0 wide, 1 narrow (anterior end included in nuchal plate).
26. Contact between nuchal plate and first peripheral: 0 nearly parallel to body axis, 1 convergent forward.
27. Gular notch: 0 absent, 1 present.
28. Inguinal buttresses: 0 does not reach costal, 1 reaches costal 5 only, 2 reaches costals 5 and 6.
29. Suture between epiplastron and hyoplastron: 0 nearly perpendicular to body axis or backward laterally, 1 forward laterally.
30. Pectoral scutes: 0 present, 1 absent.
31. Number of inframarginal scutes when the row is complete: 0 three, 1 four.
32. Inframarginal scutes on plastron: only on plastron 0, extending slightly on peripheral 1, extending strongly on peripheral 2.
33. Bridge length / plastron width ratio: 0 less than 55%, 1 above 55%.
34. Pleural 3 reaching marginal 6: 0 no, 1 yes.
35. Contribution of hyoplastron and hypoplastron to minimal bridge length: 0 almost equal, 1 greater in hyoplastron.
36. Flange of the prearticular longer than anterior extension of angular in lingual view: 0 no, 1 yes.
Appendix 2. Taxon/character matrix
Achilemys 21000 10010 0?011 10230 ?0011 10{12}00 ??101 ?
Amuremys 01012 01??2 0?0?1 ???11 ?0010 1?100 ?0?0? ?
Anhuichelys 1111{12} 00021 100?1 01{01}31 00111 11100 ??100 ?
Chelydra 00002 {01}2120 01000 01002 10200 00000 00000 0
Chrysemys 01022 01012 21101 00130 00000 10110 ?1000 0
Claudius 00001 02122 01000 11030 10000 10001 ??000 0
Clemmys 01022 02012 21101 00130 00000 10110 ?1000 0
Dermatemys 00022 02122 01001 10200 00110 10011 11100 0
Elkemys 01002 01012 210?1 10200 ?0111 11{12}10 12101 ?
Geochelone 21221 12001 00{12}11 20230 00000 10210 ? 1111 1
Gopherus 21121 12002 00011 20230 00000 10110 ?1111 1
Gravemys 01000 00022 0?0?1 20200 ?0111 10110 12101 ?
Heosemys 01000 10012 21001 20230 11101 10110 ?1011 1
Hokouchelys 01??? 01022 010?1 20200 ?0111 11110 12101 ?
Hongilemys 01??? 02??1 0?0?1 20?11 ?0111 10?10 01100 ?
Lindholmemys 01002 01022 0?0?1 20110 ?0011 10210 01100 ?
Malayemys 01000 00012 21001 20230 11101 10210 ?1101 1
Manouria 2102{01} 12011 00011 20230 00100 10110 ?1101 1
Mongolemys 01022 01021 0?001 10101 00010 00{12}00 00000 0
Ordosemys 00000 1212? 0?000 10002 ?0200 10000 10000 ?
Palaeoemys 01000 00012 01001 20230 11111 10210 ?1101 ?
Paramongolemys 01002 02122 0?1?1 10111 ?0010 10110 00100 ?
Platysternon 00002 {01}2021 11000 11210 0020{01} 10000 00001 0
Pseudochrysemys 01002 02012 0?1?1 10120 ?0111 10110 01001 ?
Rhinoclemmys 01000 11012 21001 20230 0100{01} 10110 ?1101 1
Shandongemys 01??? 02??2 0???1 20?00 ?001? ?1?10 00000 ?
Tsaotanemys 01?02 0???2 0?0?1 20211 ?0100 11110 100?0 ?
Wutuchelys 0101? 0???? 0???1 10201 ?0?0? ??110 11000 ?












