1. Introduction
Mud-mounds or stromatactis mounds are widespread features in the Phanerozoic rock record and are spatially distributed around the globe (e.g. Pratt, Reference Pratt1982; Krause et al. Reference Krause, Scotese, Nieto, Sayegh, Hopkins and Meyer2004). They are generally believed to be formed in slightly deeper, more offshore environments than typical reefs with a more rigid framework and lesser amounts of finely disintegrated shell debris and mud (Pratt, Reference Pratt1982). After their first appearance in the Cambrian Period they became increasingly common through the Early Palaeozoic and reached their acme during Carboniferous times (Krause et al. Reference Krause, Scotese, Nieto, Sayegh, Hopkins and Meyer2004).
Upper Ordovician carbonate mud-mounds of subsurface Gotland, Sweden, were recently studied in detail by Sivhed et al. (Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004). These mounds consist of relatively pure limestones with minor amounts of siliciclastic material and have a diameter of up to 800 m and an amplitude of up to 50 m. Similar and even larger mounds (up to 3 km in diameter) have also been found in adjacent offshore areas (Flodén et al. Reference Flodén, Puura, Söderberg, Tuuling and Suuroja1994; Tuuling & Flodén, Reference Tuuling and Flodén2000). Comparable structures are also known from other regions in Baltoscandia, such as Dalarna, on the Swedish mainland (Jaanusson, Reference Jaanusson, Bruton and Williams1982), and Estonia (Nestor, Reference Nestor1995; Harris et al. Reference Harris, Sheehan, Ainsaar, Hints, Männik, Nõlvak and Rubel2004). The Swedish mud-mounds were primarily investigated from the mid-1970s and through the early 1990s for their hydrocarbon potential and they contained enough oil to support small-scale oil production during those years (Sivhed et al. Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004).
Sivhed et al. (Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004) analysed a number of drill cores that penetrate into and around the Gotland mud-mounds (Fig. 1). From that investigation, which included petrological, sedimentological, geochemical, palaeontological and palaeoecological aspects, it became clear that the mounds and surrounding strata contained a relatively diverse fossil assemblage. Subsequently, Bergström, Löfgren & Grahn (Reference Bergström, Löfgren and Grahn2004) studied the recovered conodonts and chitinozoans in closer detail in order to achieve more precise biostratigraphical age determinations of the mud-mounds (see below). This sparked our interest in developing a more in-depth study including the scolecodonts, or the jaws of polychaete annelid worms, particularly because these fossils turned out to be the most diverse and one of the most abundant faunal elements associated with these submarine structures. Evidently, jawed polychaetes played an important role in the mud-mound communities and can thus add to our understanding of their palaeoecology. Moreover, except for a few scolecodonts illustrated by Eisenack (Reference Eisenack1976) from the island of Öland, the assemblage described here forms the first record of Ordovician scolecodonts from Sweden. As such it can be compared to coeval assemblages known from other regions and increase our knowledge of the palaeobiogeographical distribution of these metazoans.

Figure 1. (a) Map showing the general configuration of the Baltoscandian palaeobasin and the location of Gotland and Estonian drill core sites used for comparison. The confacies boundary is modified from Nõlvak (Reference Nõlvak, Raukas and Teedumäe1997). The North Estonian Confacies is characterized by relatively shallow water environments, whereas the Central Baltoscandian Confacies is characterized by deeper water environments. (b) Detail map of northern Gotland showing the location of the six scolecodont-yielding drill cores (see also Sivhed et al. Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004; Bergström, Löfgren & Grahn, Reference Bergström, Löfgren and Grahn2004).
2. Stratigraphy and facies
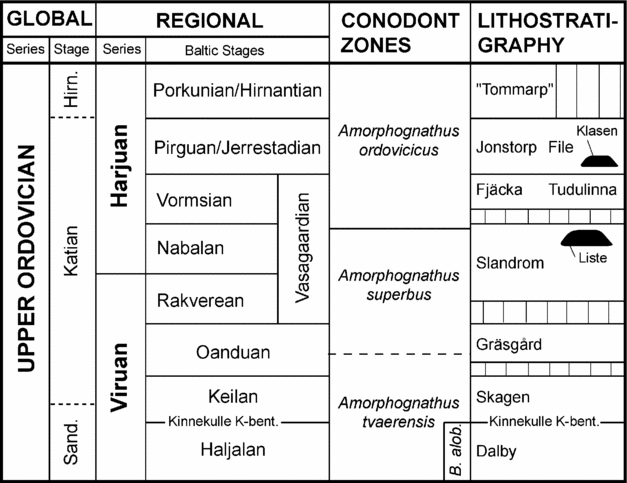
In the Gotland subsurface wells, Bergström, Löfgren & Grahn (Reference Bergström, Löfgren and Grahn2004) recognized two generations of mounds: the Rakveran–Nabalan Liste mounds and the Pirguan Klasen mounds, of which the latter are most common. In terms of conodont biostratigraphy, the Liste mounds belong to the Amorphognathus superbus Zone, whereas the Klasen mounds were inferred to belong to the A. ordovicicus Zone (Fig. 2). No conodonts were recovered that could confirm the latter age, but because the Klasen mounds occur stratigraphically above the Tudulinna Formation, they are believed to be correlated with the A. ordovicicus Zone (Bergström, Löfgren & Grahn, Reference Bergström, Löfgren and Grahn2004). Neither the Liste mounds nor the surrounding strata yielded any scolecodonts, so the entire collection described herein belongs to the Klasen Member, using the terminology of Bergström, Löfgren & Grahn (Reference Bergström, Löfgren and Grahn2004). In terms of global Ordovician stratigraphy, this member belongs to the Katian Stage of the middle–upper part of the Upper Ordovician Series (e.g. Webby et al. Reference Webby, Cooper, Bergström, Paris, Webby, Paris, Droser and Percival2004; Nõlvak, Hints & Männik, Reference Nõlvak, Hints and Männik2006).

Figure 2. Correlation between lithostratigraphical units in the Gotland subsurface, international and regional series and stages, conodont zonation, and the proposed chronostratigraphical position of the Klasen and Liste mounds (after Bergström, Löfgren & Grahn, Reference Bergström, Löfgren and Grahn2004). B. alob. – Baltoniodus alobatus conodont Subzone; Hirn. – Hirnantian; Sand. – Sandbian.
The mounds and their surrounding strata were described in petrographical detail by Sivhed et al. (Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004). Briefly, those authors distinguished four different types of lithofacies (Fig. 3). (1) The intra-mound (or mound core) facies is characterized by dense algal packstones/boundstone interbedded by wackestones with a higher content of micritic mud. This facies frequently contains stromatolites and algae providing an organic framework. Stromatactis and stylolites are common in places and the mound facies is primarily autochthonous with only local redeposition and reworking. (2) The mound cap and flank facies is dominated by bioclastic packstones and wackestones with a mottled texture inferred to be the result of bioturbation. The transition from the intra-mound facies to this facies is gradational. (3) The supra-mound facies mostly consists of dense variably argillaceous wackestones interbedded with greyish green muddy limestone. (4) The sub-mound facies is similar to the supra-mound facies except that the former also includes coarser fragments and abundant crinoidal debris.

Figure 3. Different facies types, dominating lithology and fossil elements identified above, within and below the Upper Ordovician mud-mounds of subsurface Gotland, Sweden. For detailed description of lithological characteristics, see Sivhed et al. (Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004).
3. Materials and Methods
In their original survey, Sivhed et al. (Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004) collected 36 samples (of 150–330 g each), from 12 drill cores (32 mm diameter) for studies of the microfossil content and biostratigraphy. Of these samples, ten yielded scolecodonts. For the present study we were able to obtain three additional samples (04E1-1 to 3, of approximately 350 g each) from the Stormyr-2 drill core to be processed for scolecodonts. All samples were digested in accordance with the acetic acid technique of Jeppsson, Fredholm & Mattiasson (Reference Jeppsson, Fredholm and Mattiasson1985) and Jeppsson, Anehus & Fredholm (Reference Jeppsson, Anehus and Fredholm1999), and the dried and sieved residues were picked down to 0.063 mm. With the exception of two samples from the sub-mound facies, all scolecodonts were derived from the supra-mound facies (Table 1). A total number of more than 300 scolecodonts and five semi-articulated jaw apparatuses were recovered.
Table 1. Scolecodont-yielding samples and distribution of taxa

Counts are based on the first maxillae (MI) only, except for Tetraprion sp. A and Lunoprionella? sp., in which the MII were used. An apparatus is counted as one specimen.
Except for the complementary samples (04E1-1 to 3), less than 15 scolecodonts were recovered from each one of the scolecodont-yielding samples. The former samples yielded considerably more, up to over 100 specimens (Table 1). This discrepancy could partly be attributed to an actual variability in scolecodont yield for certain intervals and/or facies, but probably more important, by different picking methods and sample size; the additional samples were, as indicated above, slightly larger and picked primarily for scolecodonts. The overall rather limited number of specimens hampers detailed analyses and discussions of relative frequencies of various taxa; however, some estimations at the supra-specific level could be made for the richer samples.
All figured specimens are deposited at the Department of Geology, Lund University, Lund, Sweden (depository acronym LO, for Lund Original). The Swedish Geological Survey are in possession of the drill cores.
4. Mud-mound biota and palaeoecology
Submarine organic build-ups, such as mounds and reefs, usually provide refugia for a variety of organisms, and therefore such environments can produce diverse fossil faunas. The carbonate mud-mounds of subsurface Gotland and the surrounding strata contained a variable biota as is evident by the recorded fossils that include remains of sessile and vagrant benthos, as well as nektonic and planktonic organisms. Several faunal elements characteristic of Late Ordovician shelf communities, such as trilobites, brachiopods and graptolites, are rare or lacking, however.
There is a significant variability in terms of taxonomic composition, diversity and disparity between the different facies represented in and around the mounds (Fig. 3). The sub-mound facies is dominated by echinoderm fragments, particularly crinoids, but it also yielded some conodonts, brachiopods, ostracodes, and rare chitinozoans and scolecodonts. The intra-mound facies has the lowest fossil diversity and is composed primarily of algae, stromatolites and some bryozoans. Some conodonts, as well as fragments of echinoderms, were also reported (Sivhed et al. Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004; Bergström, Löfgren & Grahn, Reference Bergström, Löfgren and Grahn2004). Thus, firm-bottom, epifaunal, suspension-feeding organisms dominate the mound core biota, whereas infaunal organisms are scarce. The cap and flank facies is faunally relatively diverse and yielded echinoderms, algae, bryozoan fragments, gastropods, bivalves, tabulates and micritized pellets of probable faecal origin, as well as probable bioturbation generated by infaunal activity (Sivhed et al. Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004, p. 123). The most diverse assemblages were recorded from the supra-mound facies, which yielded a wide variety of fossil elements, for example, condonts, brachiopods, scolecodonts, spicules of hexactinellid sponges, organic-walled hydrozoan(?) tubes, bryozoans, graptolites (recovered from the new samples of the Stormyr-2 core; Fig. 4a, b) and chitinozoans, and it is particularly rich in echinoderm fragments (Fig. 3).

Figure 4. SEM micrographs of selected graptolites (a, b) and representative scolecodonts and polychaete jaw apparatuses (c–aa) from the drill cores, subsurface Gotland, Sweden. All scale bars are 100 μm. All scolecodonts are in dorsal view unless stated otherwise. (a, b) Proximal parts of graptolite rhabdosomes. (a) From sample 04E1-2, LO 10440; (b) from sample 04E1-3, LO 10441. (c) Oenonites sp. A, from sample Gt01-7, right MI, LO 10442. (d) Oenonites sp. C, from sample Gt01-29, left MI, LO 10443. Note that some glue covers parts of the jaw surface, which also may have slightly affected the outline of the inner wing. (e–k) Oenonites sp. D; note that the left MI in (e) possibly does not belong to this species and also that the jaw apparatuses (h, i) were tentatively assigned to Oenonites sp. D. (e) From sample 04E1-3, left MI, LO 10444; (f) from sample 04E1-3, right MI, LO 10445; (g) from sample 04E1-3, right MI, LO 10446; (h) apparatus of consisting of fused MI, from sample 04E1-3, LO 10447, seen from two different views (h1, h2); (i) apparatus of consisting of fused MI and a broken ventral mandible, from sample 04E1-3, LO 10448, seen in two different views (h1, h2); (i) apparatus of consisting of fused MI and a broken ventral mandible, from sample 04E1-3, LO 10448, seen in two different views (i1, i2); (j) Oenonites sp. D, from sample 04E1-3, left MI, LO 10449; (k) Oenonites sp. D, from sample Gt01-29, left MI, LO 10450. (l, m) Oenonites sp. F, from sample 04E1-3. (l) Left MI, LO 10451; (m) right MI, LO 10452. (n) Oenonites sp. E, from sample 04E1-2, right MI, LO 10453. (o) Gen. et sp. indet. A, from sample 04E1-3, right MI, LO 10454. Specimen appears intermediate between Kalloprion and Oenonites. (p) Gen. et sp. indet. B, from sample Gt01-29, right MI, LO 10455. (q–s) Pistoprion transitans, from sample 04E1-3. (q) Right MII, LO 10456; (r) left MI, LO 10457; (s) right MI, LO 10458. (t–v) Mochtyella aff. cristata, from sample 04E1-3. (t) Left MI, LO 10459; (u) left MI, LO 10460 (u1 close-up of the posterior part of the entire jaw, as seen in u2); (v) left MI, LO 10461. Note that specimens u and v differ slightly from each other in the position of the anterior, additional denticulated ridge situated on the inner face, sub-parallel to the main dentary. (x) Mochtyella ex gr. fragilis, from sample 04E1-2, left MI, LO 10462. (y) Mochtyella aff. duplicidentata, from sample 04E1-1, left MI, LO 10463. (z) Indeterminate placognath from sample 04E1-1, left MI, LO 10464. (aa) Semi-articulated jaw apparatus of Mochtyella, from sample 04E1-2, LO 10465.
Bergström, Löfgren & Grahn (Reference Bergström, Löfgren and Grahn2004) reported a moderately diverse conodont fauna, dominated by members of Panderodus and Amophognathus, and a few chitinozoans primarily belonging to the Belonechitina wesenbergensis complex. Conodonts were recorded in all facies types and the most prolific faunas were recovered from the mound facies, whereas the supra-mound facies is least productive. In terms of conodont biofacies, Bergström, Löfgren & Grahn (Reference Bergström, Löfgren and Grahn2004) placed their fauna in the Amorphognathus–Plectodina Biofacies, possibly mixed with the Hamarodus–Dapsilodus–Scabbardella Biofacies, the latter of which is also present in the mounds of the Boda Limestone of Sweden (see Sweet & Bergström, Reference Sweet and Bergström1984). Chitinozoans were only reported from three (out of 36) samples, two of which are from the supra-mound facies and one from the sub-mound facies. The new samples from Stormyr-2 also yielded a few chitinozoans, at least one of which belongs to Cyathochitina. The low chitinozoan yield probably reflects a relatively coarse sieve size (> 63 μm) rather than an unproductive lithology.
Although a preservational bias cannot be entirely ruled out, it seems that except for the mound-building organisms (stromatolites, algae and bryozoans), the mounds themselves did not have an otherwise diverse fauna and apparently did not provide a hospitable environment for vagrant benthos. Remains of the latter are virtually absent, and it is basically only the presumably nektonic conodonts that seem to have been able to live in direct connection with the growing mounds. In the supra-mound facies, scolecodont-bearing polychaetes were not only one of the most abundant faunal elements but also the most diverse one.
The precise palaeobathymetry of the subsurface mud-mounds of Gotland is difficult to estimate. Because algae seem to have been the main constructors, Sivhed et al. (Reference Sivhed, Erlström, Bojsen-Koefoed and Löfgren2004) argued that they must have occurred within the photic zone. Because the conodont fauna recorded is more diverse than what is usually extracted from shallow water reef and mound cores, Bergström, Löfgren & Grahn (Reference Bergström, Löfgren and Grahn2004) noted that the mounds seem to be of a more off-shore type. As discussed below, the scolecodont data provide some additional information as to the palaeoenvironmental setting.
5. The polychaete fauna
The recorded polychaete fauna is diverse, particularly considering the relatively small sample size and limited amount of specimens recovered. The entire collection is provisionally regarded as representing a taxonomically single type fauna, and the only sample that deviated from the others to some extent is 04E1-3. It yielded a number of characteristic taxa not present in the other samples (Table 1). The supra-mound facies seems to be slightly richer in scolecodonts than the sub-mound and yielded some tens up to a couple of hundreds of specimens per kilogram of rock.
The specimens recovered belong to at least 12 genera and 27 species (Table 1; Figs 4, 5). The fauna is dominated by polychaetaspids and mochtyellids, just like many coeval Baltoscandian assemblages (e.g. Hints & Eriksson, Reference Hints and Eriksson2007a,Reference Hints and Erikssonb), and the most common genera include Oenonites Hinde, Reference Hinde1879; Mochtyella Kielan-Jaworowska, Reference Kielan-Jaworowska1961; and Pistoprion Kielan-Jaworowska, Reference Kielan-Jaworowska1966.

Figure 5. SEM micrographs of selected representative scolecodonts and polychaete jaw apparatuses from the drill cores, subsurface Gotland, Sweden. All scale bars are 100 μm. All specimens are in dorsal view, except (p) lateral view. (a) A minute semi-articulated jaw apparatus of an undescribed placognath taxon, from sample 04E1-2, LO 10466. (b–e) Atraktoprion cf. contractus, from sample 04E1-1. (b) Left MI, LO 10467; (c) left MI, LO 10468; (d) right MI, LO 10469; (e) basal plate, LO 10470. (f) Atraktoprion sp., from sample 04E1-2, right MI, LO 10471. (g) Leptoprion sp., from sample 04E1-3, left MI, LO 10472. (h, i) Kalloprion? sp., from sample Gt01-32. (h) Left MI, LO 10473; (i) right MI fused with basal plate, LO 10474. (j) Protarabellites rectangularis, from sample 04E1-3, left MI, LO 10475. (k, l) Pteropelta gladiata, from sample 04E1-3. (k) Left MI, LO 10476; (l) right MI, (dorsal view slightly tilted right), LO 10477. (m–o) Pteropelta sp., from sample 04E1-3. (m) Left MI, LO 10478; (n) probable right MI of this species, LO 10479; (o) basal plate, LO 10480. (p) Lunoprionella? sp., from sample 04E1-2, MII, LO 10481. (q) Tretoprion astae, from sample 04E1-3, left MI, LO 10482. (r) Xanioprion cf. borealis, from sample Gt01-7, right MII, LO 10483. (s) Xanioprion sp. B sensu Hints (Reference Hints2000), from sample Gt01-7, left MI, LO 10484.
The polychaetaspids are most abundant and diverse, generally forming approximately 50% of the scolecodonts (Table 1). Representatives of this family form the bulk of many faunas of Ordovician and Silurian age (e.g. Kielan-Jaworowska, Reference Kielan-Jaworowska1966; Eriksson & Bergman, Reference Eriksson and Bergman2003; Eriksson, Bergman & Jeppsson, Reference Eriksson, Bergman and Jeppsson2004; Hints & Eriksson, Reference Hints and Eriksson2007a). Most of the polychaetaspids belong to Oenonites and at least six species were distinguished and assigned to Oenonites sp. A to F (Fig. 4c–n; Table 1). Counting of these specimens is complicated because of their sensitivity to deformation, and therefore some were lumped together as Oenonites spp. in Table 1. One left first maxilla (MI) of Oenonites sp. C appears superficially similar in its ramus and inner wing to Dubichaetaspis bergmani Eriksson, Reference Eriksson1998 from the Silurian of Gotland, and the species identified here might be ancestral to the latter (Fig. 4d). The species referred to as Oenonites sp. D is most abundant (Table 1; Fig. 4e–k). It shows some similarities to O. gadomskae (Kielan-Jaworowska, Reference Kielan-Jaworowska1966) and O. wyszogrodensis (Kozłowski, Reference Kozłowski1956). Two small partial jaw apparatuses probably also belonging to Oenonites sp. D were recorded (4h, i). Oenonites sp. F (Fig. 4l, m) has an overall morphology, and particularly the ramus of the right MI, that resembles O. olavi Eriksson, Reference Eriksson1997. It is also intermediate between Polychaetaspis sp. B and Polychaetaspis sp. A of Hints (Reference Hints1998) (Polychaetaspis Kozłowski, Reference Kozłowski1956 is a junior synonym of Oenonites). In addition to these Oenonites species, some intermediate forms were recovered: Gen. et sp. indet. A (Fig. 4o), showing characteristics transitional between Oenonites and Kalloprion, and the Oenonites-reminiscent Gen. et sp. indet. B (Fig. 4p). The less species-rich polychaetaspid genus Kozlowskiprion Kielan-Jaworowska, Reference Kielan-Jaworowska1966 is merely represented by one broken right MI, too poorly preserved to warrant an unequivocal generic assignment.
The mochtyellids are represented by Pistoprion transitans Kielan-Jaworowska, Reference Kielan-Jaworowska1966 and at least three species of Mochtyella. In addition, most of the specimens assigned to the ‘Placognatha indet.’ category (Table 1) probably belong to Mochtyella. Isolated placognath-type jaws can be difficult to identify because their number of diagnostic characters is often limited, and taxa with structurally different apparatuses can possess similar, or even homeomorphic, elements. Thus, in the case of small collections yielding more than one species, apparatus reconstructions can be problematic. Specimens were none the less counted for statistical purposes.
Pistoprion transitans (Fig. 4q–s) is one of the most common placognaths in the collection, accounting for up to 15% of the assemblage. In eastern Baltica this species ranges from the Haljala Stage and, most probably, extends well into the Silurian. In the Upper Ordovician of Estonia, it is also one of the most common species, frequently comprising 10–30% of the assemblages (Hints, Reference Hints1998, Reference Hints2000; Hints et al. Reference Hints, Hints, Meidla and Sohar2003). Some Pistoprion species are regarded as environmentally sensitive, showing preferences to shallow shelf rather than basinal settings (e.g. Hints, Reference Hints2000). Hence the common occurrence of P. transitans in Gotland could infer environmental conditions similar to those in northern and central Estonia (see also below). Of the three named Mochtyella species, M. aff. cristata is most common (Fig. 4t–v). Compared to M. cristata Kielan-Jaworowska, Reference Kielan-Jaworowska1961, it has a shorter and more posteriorly located second ridge. M. aff. cristata differs from M. polonica Kielan-Jaworowska, Reference Kielan-Jaworowska1966, another closely related species, in having more robust maxillae and a longer second ridge with more prominent denticles. A lesser number of specimens were assigned to M. ex gr. fragilis (Fig. 4x; see also Szaniawski, Reference Szaniawski1970) and M. aff. duplicidentata (Fig. 4y). Of the unidentified placognaths, the left MI shown in Figure 4z shows some Mochtyella characteristics, but the prominent basal ridge and, to some extent, the main dentary, deviate from those of the common members of the genus. Also recovered from the collection at hand were one semi-articulated jaw apparatus of Mochtyella (Fig. 4a) and a minute one of probable placognath type (Fig. 5a).
Prionognath taxa and their allies are rare, forming just a few per cent of the assemblage, when present (Table 1). Atraktoprion Kielan-Jaworowska, Reference Kielan-Jaworowska1962 is represented by two species, both of which have closely similar analogues in the Silurian of Gotland. The larger of the two (Fig. 5b–e) was tentatively assigned to Atraktoprion contractus (Hinde) sensu Bergman (Reference Bergman, Jaanusson, Laufeld and Skoglund1979). A minute right MI with a small hook was assigned to Atraktoprion sp. (Fig. 5f). Similar small atraktoprionids also occur in eastern Baltic sections, but their systematic position and relationships with other species remain uncertain. One incomplete kalloprionid jaw apparatus was recovered. Eriksson (Reference Eriksson2006) noted the problems with distinguishing between Kalloprion Kielan-Jaworowska, Reference Kielan-Jaworowska1962 and Leptoprion Kielan-Jaworowska, Reference Kielan-Jaworowska1966, and Kalloprion? sp. (Fig. 5h, i; Table 1) is indeed intermediate between these genera. Its left MI resembles those of typical Leptoprion species, whereas the right MI and basal plate are similar to those of Kalloprion. One recovered left MI was assigned to Leptoprion with confidence (Fig. 5g).
Ramphoprionids were recorded in only one sample: 04E1-3. Most specimens seem to belong to Protarabellites rectangularis Eriksson, Reference Eriksson2001 (Fig. 5j). That species has a patchy distribution in the Silurian of Gotland (Eriksson, Reference Eriksson2001) and the present occurrence extends its range down into the Upper Ordovician. So far, P. rectangularis has not been recovered in the eastern Baltic sections, where the closely related P. staufferi Eriksson, Reference Eriksson2001 commonly occurs in coeval strata (Hints, Reference Hints1998, Reference Hints2000).
Polychaeturids were recorded in sample 04E1-3, where they form approximately 8% of the assemblage (Table 1). Pteropelta gladiata (Eisenack, Reference Eisenack1939) (= Polychaetura gracilis Kozłowski, Reference Kozłowski1956) (Fig. 5k, l) is one of the most widespread and common polychaete species in the Baltic Ordovician, already appearing in the lowermost Darriwilian (e.g. Hints, Reference Hints1998, Reference Hints2000; Hints et al. Reference Hints, Hints, Nemliher and Nõlvak2007). It is particularly common in the Rakvere Stage, where its relative frequency may reach 40% (Hints, Reference Hints2000). Pteropelta gladiata preferred relatively shallow-water environments, like those that were present in northern and central Estonia during that time, whereas in the coeval strata of basinal settings in southern Estonia it was absent or very rare. In addition to P. gladiata, a few specimens of another species, Pteropelta sp., were recovered (Table 1; Fig. 5m–o). Its left MI resembles that of P. kielanae (Hints, Reference Hints1998), but differs in having a wider posterior margin and longer and more rectangular inner wing. Another polychaeturid species that occurs abundantly in the Nabala–Vormsi interval of Estonia and Poland (Hints, Reference Hints2000, pl. II: 1–2; Hints et al. Reference Hints, Hints, Nemliher and Nõlvak2007) has a much narrower posterior margin of the left MI and distinct, transversally stretched, anteriormost denticles. The basal plate of Pteropelta sp. (Fig. 5o) is intermediate between those of the aforementioned species. A single left MI closely similar to the Gotland Pteropelta sp. specimens has been found in the shoal limestone of the Vasalemma Formation (Keila–Oandu stages) of northwestern Estonia.
One second maxilla (MII) of Tetraprion sp. A sensu Hints (Reference Hints2000), that is, belonging to the ctenognath family Tetraprionidae (see Kielan-Jaworowska, Reference Kielan-Jaworowska1966), was recorded (Table 1). Tetraprion sp. A is long-ranging, but the most abundant occurrences have been recorded in the Porkuni Stage, where it can make up more than 20% of the assemblages (Hints, Reference Hints and Põldvere2001).
Although only one left MI of the tretoprionid Tretoprion astae Hints, Reference Hints1999 was recorded, its characteristic denticles and hollowed outer face allowed confident identification (Fig. 5q). This species has a rather long stratigraphical range, beginning in the Haljala Stage, becoming more abundant in the Vormsi Stage, and extending at least into the lower Wenlock (Hints, Reference Hints1999; Hints et al. Reference Hints, Killing, Männik and Nestor2006). Tretoprionids have not, however, been recorded from the exposed Silurian strata of Gotland (Eriksson, Bergman & Jeppsson, Reference Eriksson, Bergman and Jeppsson2004).
Xanioprionids are represented by two species. One is closely similar to Xanioprion borealis Kielan-Jaworowska, Reference Kielan-Jaworowska1962, but additional elements are needed to confirm this affinity (Fig. 5r). The species referred to as Xanioprion sp. B sensu Hints (Reference Hints2000) has distinctive MI and MII, the horseshoe-like MII having a bent posterior margin to fit the elongated MI (cf. Hints, Reference Hints2000, pl. I: 11; Hints & Eriksson, Reference Hints and Eriksson2007a, fig. 3J). The MI recovered (Fig. 5s) is most likely conspecific with the forms found in coeval strata of Estonia. The stratigraphical range of this taxon (or group of taxa) extends from the Darriwilian into the Wenlock.
The specimen superficially similar to Lunoprionella Eisenack, Reference Eisenack1975 (Fig. 5p) has many fewer denticles than those typical of the genus. Probably the same species occurs in Upper Ordovician and Silurian strata of Estonia.
The Gotland polychaete assemblage includes some species that are believed to be environmentally sensitive, particularly P. transitans and P. gladiata (see Hints, Reference Hints2000; Hints et al. Reference Hints, Hints, Nemliher and Nõlvak2007). These species occur abundantly in shallow-shelf (North Estonian Confacies) or ramp settings of northern and central Estonia, but are missing in the basin environments of southern Estonia (the Livonian Tongue area, Central Baltoscandian Confacies). Moreover, forms typical of deeper-water environments such as Rakvereprion balticus (Eisenack, Reference Eisenack1975), which dominate approximately coeval strata of the Valga core (Hints, Reference Hints and Põldvere2001), were not recovered from the present collection. This fits well with the general palaeogeographical models of the Baltic Palaeobasin (cf. Männil, Reference Männil1966; Jaanusson, Reference Jaanusson1995), where Gotland is positioned in a transitional area between the North Estonian and Central Baltoscandian Confacies (Fig. 1).
6. Coeval polychaete faunas from other regions
Not surprisingly, the recovered polychaete fauna is similar in taxonomic composition to coeval ones known from other areas of the Baltic palaeobasin (e.g. Kielan-Jaworowska, Reference Kielan-Jaworowska1966; Hints, Reference Hints1998, Reference Hints2000) and thereby also differs quite substantially from those of Laurentia (e.g. Eriksson & Bergman, Reference Eriksson and Bergman2003; Eriksson, Leslie & Bergman, Reference Eriksson, Leslie and Bergman2005; Hints & Eriksson, Reference Hints and Eriksson2007a,Reference Hints and Erikssonb). While the recovered assemblage is generally similar to those in Estonia, some differences can be noted. First of all, the present collection is taxonomically less diverse, and many taxa common in coeval strata of Estonia were not recorded in the Gotland material, such as Vistulella kozlowskii Kielan-Jaworowska, Reference Kielan-Jaworowska1961, Mochtyella cristata, Oenonites varsoviensis (Kielan-Jaworowska, Reference Kielan-Jaworowska1966), Protarabellites staufferi, Lunoprionella, Tetraprion pozaryskae Kielan-Jaworowska, Reference Kielan-Jaworowska1966, and some species of Kalloprion. This also applies to the species that are considered to have some biostratigraphical value, among them distinctive species of Oenonites, Kozlowskiprion, Xanioprion and Kalloprion (Hints, Reference Hints2000, fig. 2). To a large extent, the lack of these taxa is attributed to the relatively small size of the samples at hand, although effects of unfavourable facies and restricted geographical distribution cannot be excluded. Paulinitids are rare in Late Ordovician polychaete assemblages from Baltica and no representatives were recorded from the Gotland subsurface samples. The oldest record in Baltoscandia of Kettnerites Žebera, Reference Žebera1935, the most common paulinitid genus, is in the Nabala Stage. However, as noted by Hints (Reference Hints2000), they are very rare here, so they are not likely to be represented in small samples, such as the present ones. By contrast, paulinitids are abundant and taxonomically diverse in Silurian strata from this palaeocontinent (Bergman, Reference Bergman1989; Eriksson, Bergman & Jeppsson, Reference Eriksson, Bergman and Jeppsson2004) and can be quite common also in Upper Ordovician strata of Laurentia (Eriksson & Bergman, Reference Eriksson and Bergman2003; Eriksson, Leslie & Bergman, Reference Eriksson, Leslie and Bergman2005). Although recorded in one sample only (04E1-3), polychaeturids are a characteristic faunal element. They are known to be common in many Middle through Upper Ordovician successions of Baltica, while being virtually absent in coeval strata of Laurentia (Hints, Reference Hints1998, Reference Hints2000; Eriksson & Bergman, Reference Eriksson and Bergman2003; Hints & Eriksson, Reference Hints and Eriksson2007a). In addition to P. gladiata, the yet undescribed Pteropelta Eisenack, Reference Eisenack1939 species strengthen the idea that polychaeturids were diverse in the Upper Ordovician of Baltica and that their distribution was environmentally controlled. More detailed comparisons and discussions of Late Ordovician polychaete assemblages of Baltica and Laurentia are provided by Eriksson & Bergman (Reference Eriksson and Bergman2003), Eriksson, Leslie & Bergman (Reference Eriksson, Leslie and Bergman2005), and Hints & Eriksson (Reference Hints and Eriksson2007a,Reference Hints and Erikssonb).
In order to compare the Gotland assemblage with approximately coeval ones (Pirgu Stage) from other regions of Baltoscandia, the relative frequency of the dominant genera and other higher groups of taxa was calculated. The Gotland assemblage (Fig. 6a) was subsequently compared to assemblages recorded from three drill cores, Orjaku, Laeva and Valga, from Estonia (Figs 1, 6b–d; Hints, Reference Hints2000), representing different environmental settings and positions on the palaeoplatform. The results reveal an obvious correspondence, at least at the super-specific rank, particularly between the Gotland assemblage and those of the shallow-water Orjaku drill core but also to the transitional Laeva assemblage. These assemblages are all characterized by abundant occurrences of Oenonites, Pistoprion, Mochtyella and other placognaths (Fig. 6a–c). The assemblage recorded from the Valga core deviates substantially from the other three (Fig. 6d). The latter is from a deeper shelf setting and is characterized by a great abundance of taxa here referred to as ‘other placognaths’, that is, excluding Mochtyella, Pistoprion and including, among others, Rakvereprion Mierzejewski, Reference Mierzejewski1978.

Figure 6. Pie charts showing the average relative abundance (%) of common genera and higher groups of taxa from the present study (a) compared with coeval, Pirgu Stage, assemblages from Estonia (b–d) from different environmental settings (see also Fig. 1). Note that ‘Other placognatha’ also includes representatives of Tetraprionidae and ‘prionognaths’ includes Atraktoprion, Leptoprion, and Kalloprion. The Gotland chart (a) is composed of the average from the three most productive samples (04E1-1 to 3). The Estonian charts are based on: (b) the average from six samples from the Orjaku drill core, representing a shallow shelf setting; (c) the average from 14 samples from the Laeva drill core, representing a transitional area; and (d) the average from eight samples from the Valga drill core, representing a deeper shelf setting (for further information, see Hints, Reference Hints2000; Hints & Eriksson, Reference Hints and Eriksson2007a).
The same dataset used for calculating the relative frequency was processed by PAST software (Hammer, Harper & Ryan, Reference Hammer, Harper and Ryan2001), and a principal component analysis was performed and is expressed as a scatter plot (Fig. 7). From this analysis it is also apparent that the Gotland samples group particularly well with the Orjaku assemblage. The reason that the Gotland samples do not plot even more tightly together is probably because of the relatively limited number of specimens obtained from each sample.

Figure 7. Scatter plot of principal component analyses showing differentiation of the jawed polychaete assemblages of the Pirgu Stage from samples from Gotland and Estonia. The plot was drawn using the PAST software (Hammer, Harper & Ryan, Reference Hammer, Harper and Ryan2001) utilizing a correlation matrix of the same variables as shown in Figure 6. Dominant taxa for factor 1 are Oenonites and ‘Other placognaths’ and for factor 2 Mochtyella, Pistoprion and prionognaths.
7. Discussion and conclusions
Scolecodont-bearing polychaetes formed an important and considerable part of the faunal communities associated with the Late Ordovician mud-mounds of subsurface Gotland, Sweden. Their diversity greatly exceeds that of any other faunal group investigated from the sampled drill cores, with at least 27 species belonging to 12 genera identified. A number of taxa recovered from the collection at hand have previously not been known from Sweden, or were known from younger (Silurian) strata. For example, this study includes the first record of polychaeturids from Sweden, a common family from the Ordovician of Baltoscandia.
The overall lower diversity in fossil elements observed in the intra-mound facies compared to the surrounding facies probably reflects a combination of a lower degree of biotic colonization and increased rate of deposition. The scarcity of scolecodonts from the sub-mound facies is puzzling since it is closely similar in lithological aspect to the productive supra-mound facies. Additional samples from the sub-mound facies would probably diminish this difference. The reddish and greenish colour in some intra-mound intervals and sub-mound intervals may signal oxidized sediments which would help explain the lack of scolecodonts (as well as chitinozoans and other organic-walled microfossils) in these strata. It is also possible that the intra-mound stratum was inhospitable for benthic and mainly burrowing organisms. Most extant eunicidans (the order to which the fossil members here identified belong) are infaunal burrowers, epifaunal crawlers or tube builders (Paxton, Reference Paxton, Beesley, Ross and Glasby2000). However, based on the knowledge of extant polychaetes, we know that many jaw-bearing eunicidan polychaete species successfully inhabit reefs and reef-like structures. Some of these use their jaw apparatuses to excavate and bore into the coral frameworks and hence are efficient bioeroders (Paxton, Reference Paxton, Beesley, Ross and Glasby2000 and references therein). The colonization of reefs may have occurred later during their evolutionary history.
Diverse polychaete assemblages are known from the Rakverean–Nabalan of Baltoscandia (e.g. Hints, Reference Hints2000), suggesting that the Liste mounds should also yield scolecodonts. The lack of scolecodonts from those mounds can be explained by the fact that the two samples processed for microfossils derive from the intra-mound facies (Bergström, Löfgren & Grahn, Reference Bergström, Löfgren and Grahn2004, table 1), which, as shown here, is unproductive for scolecodonts.
This study reinforces the fact that the diversity and abundance of scolecodonts often are almost the inverse of that of conodonts (e.g. Eriksson, Leslie & Bergman, Reference Eriksson, Leslie and Bergman2005; Hints et al. Reference Hints, Killing, Männik and Nestor2006). This suggests that these faunal elements occupied different ecological niches in vivo but also that they respond differently to taphonomic processes. In terms of applicability, this indicates that scolecodonts could serve as an important complement to conodonts, for example in biostratigraphy, in rocks where the latter are rare or lacking. Bergström, Löfgren & Grahn (Reference Bergström, Löfgren and Grahn2004) noted that only one of the 16 samples representing the intra-mound facies (mound core) lack conodonts and other microfossils. They moreover argued that the apparent uniformity of the conodont faunas from the mounds and their vicinity may indicate that the environmental differences between the mounds and their immediate surroundings were not substantial enough to affect the conodont faunas markedly. This is in stark contrast to the scolecodont-yield, as no samples from the intra-mound facies yielded scolecodonts. This could be explained by the fact that the presumably nektonic or nekto-benthic conodont animals (cf. Barnes & Fåhræus, Reference Barnes and Fåræus1975) had a better ability of living within, or immediately above, the mound than the largely benthonic polychaetes, many of which probably burrowed down into the substrate. Hence, the polychaetes seemingly did not favour these bottom conditions.
The multivariate analyses closely relate the Gotland polychaete assemblage to those occurring in shallow water to transitional shelf environments in Estonia. Thus, during Late Ordovician times, the environments of northern Gotland were most similar to those in North Estonia (North Estonian Confacies, Fig. 1), indicating that the mud-mounds could have been formed in such environments and not in a deeper shelf to basinal setting.
Acknowledgements
We wish to thank A. Löfgren (Department of Geology, Lund) for providing some of the scolecodonts studied here, and U. Sivhed and M. Erlström (both of the Swedish Geological Survey, Lund) for allowing MEE to re-sample the Stormyr-2 core. The Swedish Research Council funds the research of MEE, and OH was supported by the Estonian Science Foundation Grants 7640 and 7674. This study is a contribution to IGCP Project 503 ‘Ordovician Palaeogeography and Palaeoclimate’. Two anonymous referees critically improved the manuscript.