1. Introduction
Microfacies analysis commonly aims to determine the origin and distribution patterns of carbonate grains and the dominant biological control on carbonate sedimentation (Flügel, Reference Flügel2004). In addition to cathodoluminescence microscopy, fluid inclusion microscopy, scanning electron microscopy as well as stable isotope chemistry and X-ray techniques, facies analysis has become one of the common tools in sedimentological studies applied in marine settings (e.g. Flügel, Reference Flügel2004). In laminated lake sediments, it is increasingly used for the interpretation of seasonal palaeoclimate signals – including the rate, magnitude and direction of natural changes – as well as human impact on past environments by combining microscopical investigations with high-resolution X-ray fluorescence scanning analyses (e.g. Brauer et al. Reference Brauer, Allen, Mingram, Dulski, Wulf and Huntley2007; Sorrel et al. Reference Sorrel, Oberhänsli, Boroffka, Nourgaliev, Dulski and Röhl2007; Neugebauer et al. Reference Neugebauer, Brauer, Dräger, Dulski, Wulf, Plessen, Mingram, Herzschuh and Brande2012; Francus et al. Reference Francus, Suchodoletz, Dietze, Donner, Bouchard, Roy, Fagot, Verschuren and Kröpelin2013; Swierczynski et al. Reference Swierczynski, Lauterbach, Dulski, Delgado, Merz and Brauer2013). However, the characterization of microfacies (e.g. the fabric of sediments in laminated archives), including the biological contribution using electron and confocal microscopy, remains particularly poorly documented in lacustrine (palaeo)ecosystems. Past biological activity in lake sediments can be recorded as organic matter (OM) content, whose preservation is favoured under dysoxic to anoxic conditions, and fossil biosignatures. The OM content may reflect the water supply to the lake (Turcq et al. Reference Turcq, Albuquerque, Cordeiro, Sifeddine, Simoes Filho, Souza, Abrão, Oliveira, Silva and Capitâneo2002) but is basically derived from both terrigenous inputs and autochthonous production in the lake. It is therefore of crucial importance to characterize the OM origin in order to decipher runoff and productivity related environmental changes (Prasad et al. Reference Prasad, Garg, Singh and Thakur2007). This is usually achieved through comprehensive palynofacies analyses, although studying only OM does not allow microbe–mineral interactions to be deciphered.
Interest in the geomicrobiological approach has grown during the last decade as it has demonstrated the importance of linking geological and microbiological processes in palaeoenvironmental reconstructions. Organo-mineral interactions can be recorded in the geological record as microbial biosignatures. They include: (1) morphologically preserved microfossils; (2) stromatolite-like structures; (3) biologically induced or influenced minerals (e.g. Dupraz et al. Reference Dupraz, Reid, Braissant, Decho, Norman and Visscher2009); and (4) organic chemical biomarkers (i.e. isotopic ratios, lipid biomarkers). In the absence of clearly identifiable remains of organisms, a variety of mineralogical biosignatures may infer past biological activity in an ecosystem (Banfield et al. Reference Banfield, Moreau, Chan, Welch and Little2001). Although authigenic minerals were previously used as palaeosalinity and palaeoproductivity proxies reflecting trophic state and/or early diagenetic conditions, they have also been considered as potential biosignatures of past microbial activity (Vuillemin et al. Reference Vuillemin, Ariztegui, De Coninck, Lücke, Mayr and Schubert2013).
This study investigates organic and mineral laminated sediments of alpine Lake Son Kul (Kyrgyzstan, Central Asia) using an innovative sedimentological and geomicrobiological approach which allows the recognition of morphological biosignatures. This includes a comprehensive and detailed characterization of the microfacies using: (1) microbial communities; (2) microbe–mineral interactions; and (3) mineral morphotypes. For that purpose we used high-resolution microscopy, which includes optical, scanning and transmission electron microscopy, in addition to confocal laser scanning microscopy, X-ray diffraction and X-ray fluorescence analyses of bulk sediments and extracted OM from palynological residues. This study will shed new light on the palaeoenvironmental conditions documented at Lake Son Kul during Holocene time (Huang et al. Reference Huang, Oberhänsli, von Suchodoletz, Prasad, Sorrel, Plessen, Mathis and Usubaliev2014; Lauterbach et al. Reference Lauterbach, Witt, Plessen, Dulski, Prasad, Mingram, Gleixner, Hettler-Riedel, Stebich, Schnetger, Schwalb and Schwarz2014; Mathis et al. Reference Mathis, Sorrel, Klotz, Huang and Oberhänsli2014), and provide new insights to further explore this complex alpine environmental system in central Tien Shan.
2. Geological and climate setting
Lake Son ( = Son Kul or Kol) (41° 50ʹ 52ʹʹ N, 75° 07ʹ 45ʹʹ E, 3016 m asl) is a Kyrgyz alpine lake embedded in the central Tien Shan ranges (Fig. 1). It is the second-largest lake in Kyrgyzstan after Lake Issyk-Kul and also one of largest high-mountain lakes in the world. Today however, most inflow is contributed by groundwater (Beloglasova & Smirnova, Reference Beloglasova and Smirnova1987). The water column is well mixed and oxygenated down to the lake bottom in summer, as indicated by a site survey in July 2007. An oxygen minimum was found at a water depth of 1.5 m, reflecting ongoing respiration in the photic zone. Furthermore, according to earlier observations, H2 and methane occurred near shores during the cold season. The high wind frequency controls the water mixing in the shallow lake. According to previous surveys, the weather is calm only during 11 % of the ice-free season (Beloglasova & Smirnova, Reference Beloglasova and Smirnova1987). This provides for a likely steady mixing of the water column and makes Lake Son Kul mostly a polymictic lake.

Figure 1. Location of Lake Son Kul: (a) global map with focus on western central Asia; (b) central Tien Shan including south Kazakhstan and Lake Balkash (image courtesy of NASA GSFC); and (c) bathymetric map of Son Kul basin with coring site SK07 in the southern part of the lake.
The annual mean air temperature is c. −3.5 °C, with summer temperature c. 11 °C and winter temperatures as low as −20 °C. Unlike Issyk-Kul, the Son Kul Lake water is fresh and is frozen between October and May.
3. Material and methods
3.a. Core sampling and chronology
In this study, we analysed a core retrieved during a field campaign in summer 2007. Piston core SK07 (1.52 m) was collected using a Usinger piston corer (http://www.uwitec.at) at Lake Son Kul (41° 46.196ʹ N, 75° 08.204ʹ E; water depth 12.5 m) (Fig. 2). Due to the coring procedure, the topmost 0.6 m is missing. The chronology of core SK07 is based on 11 AMS 14C dates obtained on core SK07 and another core, SK04 (Mathis et al. Reference Mathis, Sorrel, Klotz, Huang and Oberhänsli2014). Because the age model was already extensively discussed elsewhere (Huang et al. Reference Huang, Oberhänsli, von Suchodoletz, Prasad, Sorrel, Plessen, Mathis and Usubaliev2014; Mathis et al. Reference Mathis, Sorrel, Klotz, Huang and Oberhänsli2014), we refer to those studies for a more comprehensive overview. The age model for core SK07 is depicted in Figure 2.
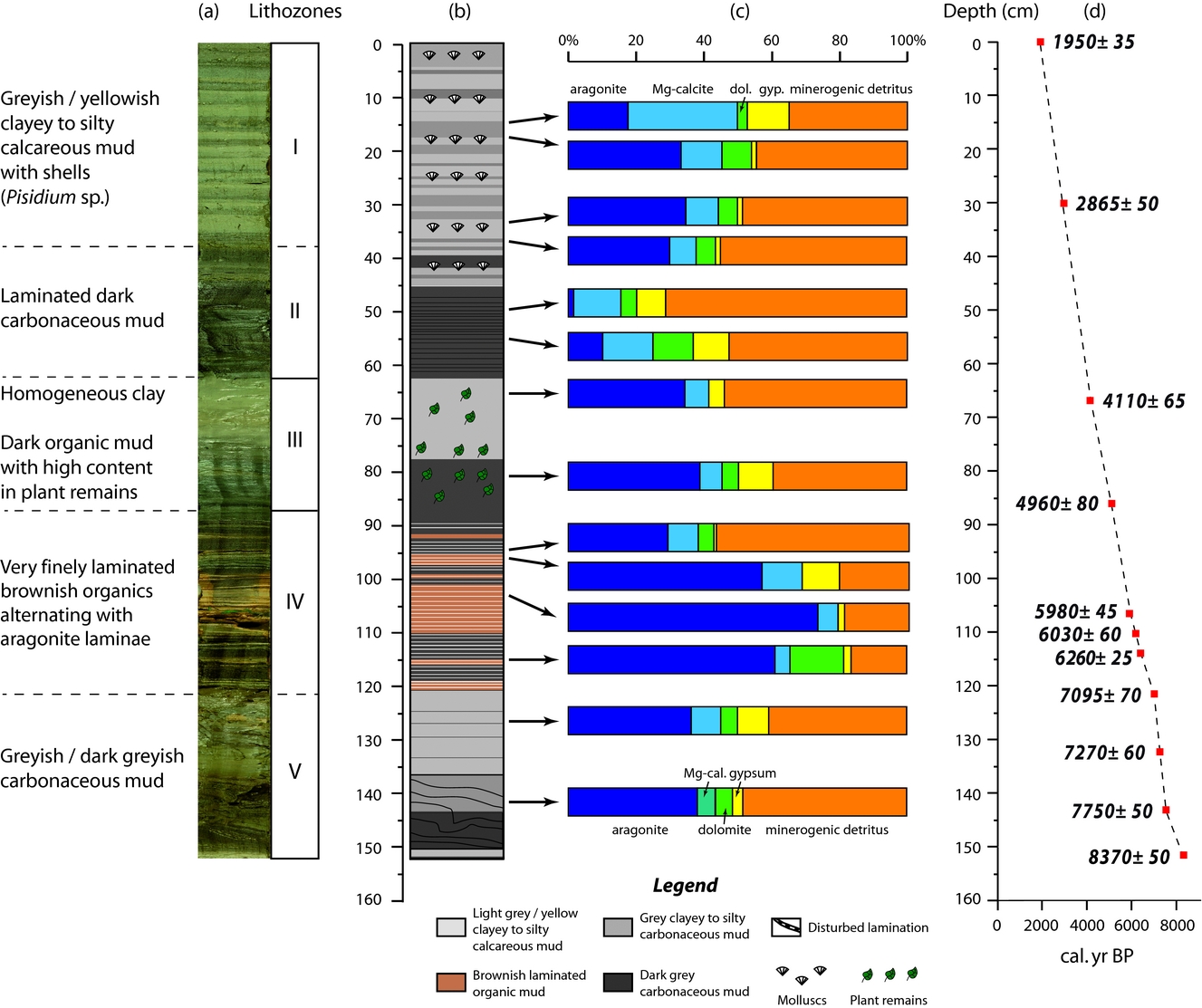
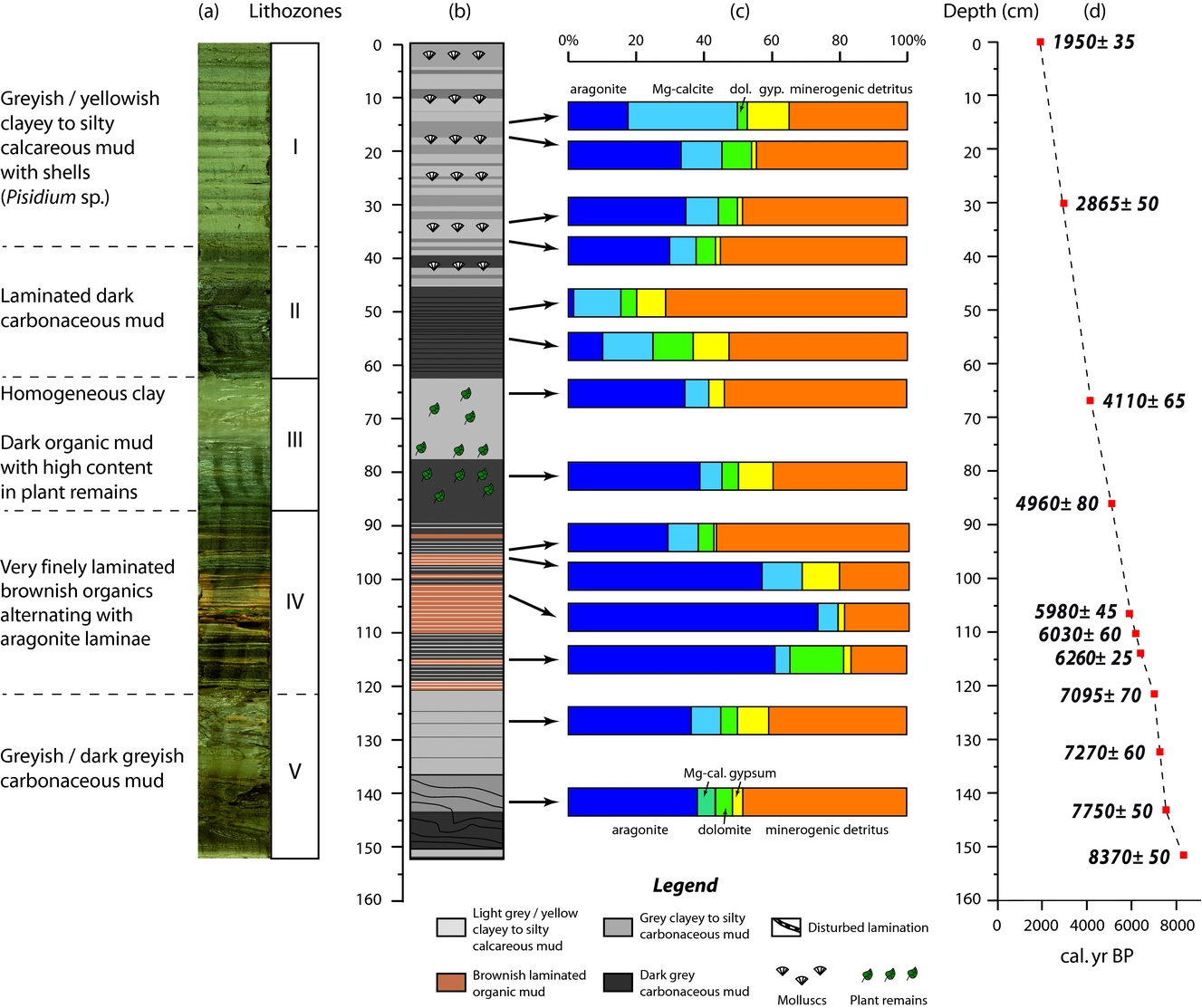
Figure 2. (a) Lithozones, (b) simplified lithology, (c) mineralogical data based on XRD analyses and (d) age model for Son Kul core SK07.
3.b. Mineralogy
For X-ray diffraction analyses (XRD) samples were ground to an ultrafine powder in an agate mortar. Organic-rich samples were treated by bleaching with a sodium hypochlorite solution. Samples were deposited on a plastic wafer in a plastic sample holder. We employed a Bruker AXS D8 Advance instrument, equipped with a linkeye counter and automatic sampler rotating the sample (ETH Zürich).
3.c. Facies analyses
For microfacies analyses and micro-XRF scanning, we prepared a series of 2.5-cm-long sediment samples from the interval 87–95 cm in core SK07 (Fig. 3). The samples were freeze-dried, soaked in a transparent epoxy resin (Araldite® 2020; Vantico, Basel, Switzerland) and subsequently polished at the University of Geneva (Switzerland). Thin-sections were analysed under parallel and polarized light with a microscope Leica DM750P (Germany). Magnifications used were 25× (overview), 40× and 100× (measurement of lamination thickness and microfacies description; error: 20 μm). Thin-section photographs were obtained using a digital camera Leica EC3 equipped with the Leica Acquire 1.0 software.

Figure 3. Thin sections (facies) images of the laminated interval in Unit III (cross polarized right). (A) Macroscopic view of the studied interval (87–95 cm) in core SK07. (B) Petrographic microscope view of (a–h) the fabric of laminated sediments with (i–l) diatom occurrences. (a) Alternating laminae of dense micrite showing fluffy patches of aragonite and organic matter including diatoms; (b–d) dense micrite lamina including plant cuticles (b) and diatoms (c); (e) sharp transition between micrite and organic matter laminae (note the enrichment in ostracods at the top of the micrite lamina); (f) close-up of dense micrite lamina showing fluffy patches of aragonite; (g) transition between micrite and organic matter laminae (note the enrichment in plant cuticles and sheet-like vegetal fragments at the top of the organic lamina and the presence of stomatocysts); and (h) close-up of transition between micrite and organic matter laminae (note the presence in minerogenic detritus in the organic lamina). (i) Anomoneis cf. sphaerophora (100× magnification); (j) Caloneis spp. (40× magnification); (k) Gomphonema spp. (100× magnification); and (l) diatom bloom including Campylodiscus spp. and Caloneis sp. (40× magnification), (C) μ-XRF measurements of selected chemical elements (Ca, Fe) on impregnated block used to make thin-sections. Upper: μ-XRF data show the enrichment of Ca (Fe) in the micrite (organic) laminae; lower panel: same pattern but as elemental map.
3.d. Electron microscopy
Bulk sediments and palynological residues (i.e. isolated OM) were studied using scanning electron microscopy (SEM) prior to fixation with glutaraldehyde and were subsequently platinum- or gold/palladium-coated, using a FEI Quanta 250 FEG at the Centre Technologique des Microstructures at the University Lyon 1 (France). Semi-quantitative elemental analyses of micron-sized spots were obtained using an EDAX energy-dispersive X-ray spectrometer (EDS Centre Technologique des Microstructures) during SEM observations. The OM ultrastructure from two selected samples (3.2–4 cm in Unit I and 88–90 cm in Unit IV) was subsequently investigated using transmission electron microscopy (TEM). For TEM observations, fossil OM was fixed in 1 % glutaraldehyde in 50 mM cacodylate buffer at pH 7.4 (CB) and at room temperature for one hour, washed three times in CB and post-fixed in 1 % osmium tetroxyde in CB for one hour at room temperature. After three washes in CB, the material was embedded in 2.5 % agarose and dehydrated before inclusion in Epon. Ultrathin sections (70 nm) were obtained using a Leica ultra-microtome and stained with lead citrate uranyl acetate for 15 min. TEM observations were performed with a Phillips CM100 transmission electron microscope (Centre Technologique des Microstructures, University Lyon 1) and digital image processing was applied.
3.e. Fluorescence in situ hybridization
The morphology and distribution of microbial species in core SK07 (Fig. 4) was examined by fluorescence in situ hybridization (FISH; e.g. Amann, Ludwig & Schleifer, Reference Amann, Ludwig and Schleifer1995). This approach is based on the combination of oligonucleotide probes, which are specifically designed for identifying precise bacterial species or phyla. When these probes are labelled with fluorescent dyes, they can be used to identify single microbial cells directly by FISH. Twelve samples (1–4 cm long) were stained both with the 16S rRNA probe SRB385 (CGGCGTCGCTGCGTCAGG) labelled with cyanine 3 to image sulphate-reducing bacteria (SRB) and with the 16S rRNA probe ARCH915 (GTG CTC CCC CGC CAA TTC CT) labelled with FTIC to image archaea. The SRB385 oligoprobe targets SRB of the y-proteobacteria (Amann et al. Reference Amann, Krumholz and Stahl1990). Sediment samples were first fixed with an aqueous solution containing 2 % (v/v) paraformaldehyde, air-dried on a glass slide and then dehydrated sequentially by ethanol solutions of 50, 80 and 96 % for 3 min each. The dehydrated sample was air-dried again, and hybridized with 1 μL each of the oligonucleotide probe solutions (100 ng mL−1) and 10 mL of a formamide-containing hybridization buffer (0.9 M NaCl, 0.01 % SDS, 20 mM Tris-HCl, pH 7.2). Hybridization took place at 46 °C in a sealed moisture chamber for 90 min, followed by incubation in washing buffer (0.07 M NaCl, 0.01 % SDS, 20 mM Tris/HCl, 5 mM EDTA, pH 7.2) at 48 °C for 30 min. The glass slide was then rinsed with distilled water and air-dried before examination under a confocal laser scanning microscope (CLSM, Zeiss LSM 510 META).

Figure 4. FISH analysis to localize specific SRB (artificial colour red) and archaea (artificial colour green) populations in sediments from Son Kul core SK07. Scale bars: 20 μm. All panels show confocal laser scanning micrographs. (a) Abundant SRB in dark layers of Unit I in surface sediments (1.5–2 cm depth). (b) Monospecific aggregates of archaea in the yellowish layers of Unit I (at 32 cm). Some aggregates exhibit a yellow fluorescence (arrow) where the SRB and archaea are completely mixed. (c) Syntrophic colony with bacteria intertwined with filamentous archaea in Unit II sediments (49.5–50 cm). (d) Isolated SRB aggregates in Unit III (72 cm). (e) Archaea/SRB consortia resulting in large aggregates in which archaea are tightly associated in monospecific clusters (Unit IV, 112.5–113.5 cm, dark brown organic lamina). (f) Large abundance of SRB over archaea (Unit IV, 112.5–113.5 cm interface between organic and micrite laminae). (g) Archaea and SRB are almost absent from the micrite laminae of Unit V (128–129 cm). (h) SRB aggregates observed in randomized patches from the dark greyish layers in Unit V (131.5–132 cm).
3.f. Micro X-ray fluorescence
Major and trace element mapping were analysed by Micro X-ray fluorescence using an EAGLE III μ-probe spectrometer (μ-XRF; EDAX Inc., Mahwah, NJ, USA; faisceau 50 μm) at the University of Geneva (Switzerland).
4. Results
4.a. Sedimentary facies
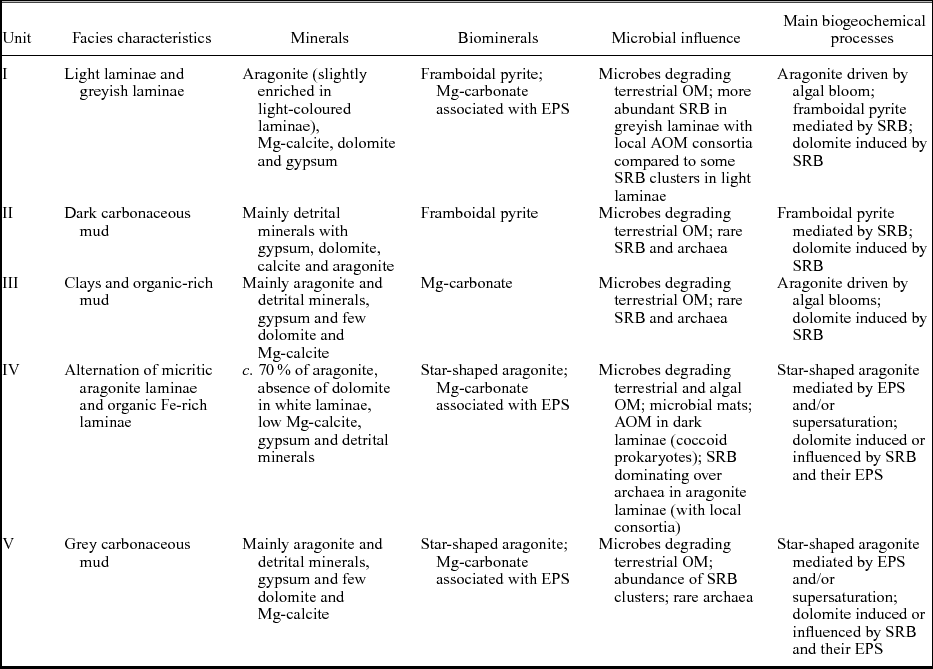
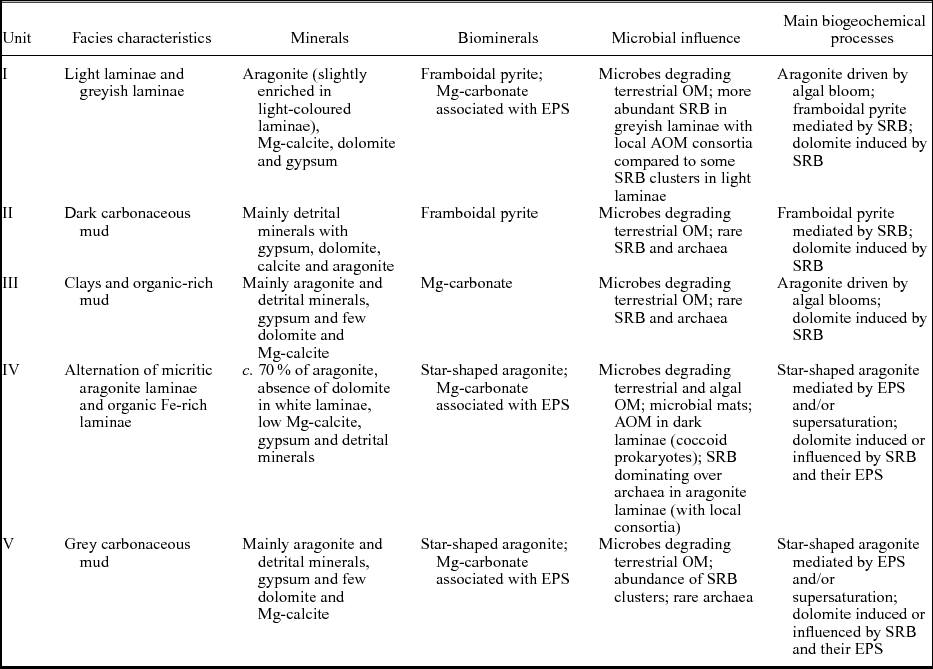
Based on macro- and microfacies properties, texture, macrofossils, mineralogy (e.g. XRD analyses) and morphological biosignatures, we have subdivided core SK07 into five lithological units (I–V; Fig. 2) described in the following. Sediments from core SK07 contain authigenic minerals (aragonite, Mg-calcite, gypsum and dolomite) and detrital silicates (quartz, feldspars such as albite and clay minerals).
Unit I (0–38 cm; c. 3100–1950 cal. BP) is characterized by alternating greyish and yellowish clayey to silty calcareous muds forming 0.5–5-cm-thick beds. Ostracoda and diatoms are rather common in this unit, but mollusc shells remain confined to the greyish thick layers. Sediments from this unit are composed of about 40 % of detrital minerals (mainly albite, quartz and micas) and 50 % of carbonates as based on XRD analyses (Fig. 2). Light-coloured laminae are enriched in aragonite in contrast to the greyish laminae, while dolomite averages c. 9 % of the mineral matrix.
Unit II (38–63 cm; c. 4000–3100 cal. BP) is characterized by a pronounced lithology and colour change. Its laminations consist of interspersed dark and dark-grey calcareous mud layers. Ostracoda and diatoms are abundant in the greyish layers while mollusc shells are absent. Mineralogical analyses show that Unit II exhibits the lowest aragonite content (less than 5 %) for the entire core SK07, while the minerogenic detritus dominates this unit. Content of gypsum (up to 20 %) and Mg-calcite are relatively high, whereas dolomite abundance is about 5 %.
Unit III (63–87 cm; c. 5000–4000 cal. BP) consists of thick greyish calcareous clay layers. This unit displays aragonite and Mg-calcite contents similar to the light-coloured laminae from Unit I, while gypsum content is c. 4 %. It is worth noting that no dolomite was found in this unit.
Macroscopically, the very well-laminated Unit IV (87–122 cm; c. 7100–5000 cal. BP) consists of finely laminated yellowish calcareous mud interbedded with white aragonite and dark brown organic layers. Accordingly, observations under the petrographic microscope reveal two kinds of laminae (Fig. 3a). The first type of lamina is a dense micrite (e.g. aragonitic) lamina with traces of organic matter and very rare detrital grains (Fig. 3b–f). The laminae are enriched in ostracods (Fig. 3e) and diatoms, the latter occurring in the form of blooms in the matrix (Fig. 3c, l). Dominant and subdominant diatom taxa are Anomoneis cf. sphaerophora, Gomphonema spp., Caloneis spp., Campylodiscus spp., Scoliotropis spp., Stauroneis spp. and Pinnularia spp. (Fig. 3i–l). A few chrysophyte stomatocysts are also encountered (Fig. 3g). Under the μ-XRF core scanner, these laminae are Ca rich (Fig. 3C). The second type of lamina, forming the dark brown macroscopic layers, mainly comprises organic matter. The lamina is composed of an organic matrix including angular very fine to medium (e.g. mineral silt and clay) detrital material (mainly quartz and micas), plant cuticles and undifferentiated vegetal fragments. Diatoms are less common in the thin-sections. Some laminae can be as thin as 70 μm in thickness. μ-XRF data show that these laminae are enriched in Fe but poor in Ca, revealing a clear opposite pattern (anti-correlation) between the two types of laminae. XRD analyses reveal that sediments from Unit IV display the highest content in aragonite in core SK07 (Fig. 2). Although the micrite laminae are dominated by aragonite (up to 74 %), they do not show a clear pattern in terms of mineral distribution for dolomite and gypsum when compared with the dark brown laminae. Gypsum seems to be more abundant in the upper part of Unit IV (between 87 and 100 cm), while dolomite exhibits the highest content at the base of Unit IV in micrite laminae (11 %). Note that no dolomite was found in the micrite laminae from the upper part of Unit IV. Mg-calcite is relatively low in this unit, regardless of the dark brown/micrite lamina distribution.
Unit V (122–152 cm; c. 8350–7100 cal. BP), with partly disturbed layering in the lowermost part of the core, consists again of greyish calcareous mud layers interbedded with dark greyish calcareous mud layers. Aragonite and detrital minerals predominantly characterize sediments from Unit V. Mg-calcite, dolomite and gypsum average 6 %, 5 % and 7 %, respectively. It is worth noting that sediments from the interval 120–135 cm are significantly enriched in gypsum (9 %). The disturbed layers at the bottom of the core may have suffered cryoturbation shortly after deposition.
4.b. Electron microscopy (SEM, TEM)
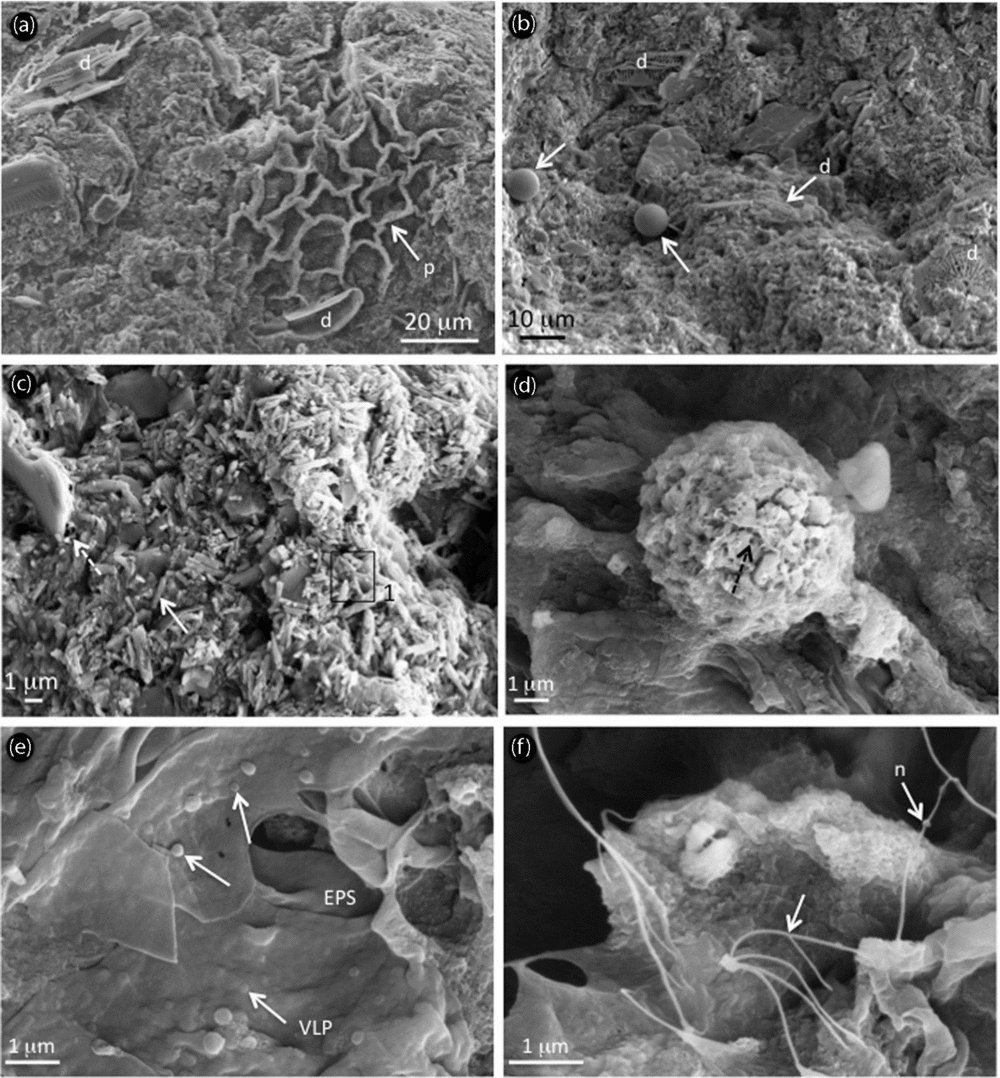
Under SEM, facies analyses in Unit I indicate the presence of slightly dissolved diatoms, biogenic siliceous microspheres named chrysophyte stomatocysts, framboidal pyrites and the chlorococcalean algae Botryococcus braunii and Pediastrum sp. trapped in the carbonaceous matrix (Fig. 5; supplementary Figs S1, 2, available at http://journals.cambridge.org/geo). The main discrepancies between the greyish and light-coloured layers are the presence of distinct aragonite rods in the yellowish layers and the predominance of gypsum in the greyish layers. Both laminae however contain extracellular polymeric substances (EPS) characterized by an alveolar network, which are closely associated with Mg-carbonates and the surrounding Mg–Al silicate matrix (supplementary Fig. S1). SEM investigations of OM reveal B. braunii, Pediastrum sp., plant cuticles and framboidal pyrites in both types of laminae (Fig. 5; supplementary Figs S1, 2). Another difference between yellowish and greyish laminae resides in the preservation state of organic components, enhanced in greyish laminae (supplementary Fig. S2d), along with more abundant framboidal pyrites compared to the light-coloured laminae. Very rare euhedral pyrite was found in the yellowish laminae, showing dissolution features (supplementary Fig. S1f). In both types of laminae microbial signatures are abundant: tracheids are largely colonized by microbes (supplementary Fig. S2e) in the greyish layers, while B. braunii are slightly degraded by microbes as shown by abundant filamentous EPS leading to a fluffy aspect on algae surface. In the yellowish laminae, nanometre-scale spheroids in the size range 60–200 nm are frequently found embedded within EPS (Fig. 5e) and are referred to as virus-like particles (VLPs). Nanofilaments (less than 150 nm in diameter but extending several microns in length) are also encountered and resemble nanowires, which are closely associated with bacteria (Fig. 5f). Ultrastructure of OM in the yellowish laminae shows ultralaminae and fluffy EPS (Fig. 6a) along with plant cuticles, prokaryotic cells with intracellular inclusions (Fig. 6b) and abundant VLPs showing a typical hexagonal shape (Fig. 6c).
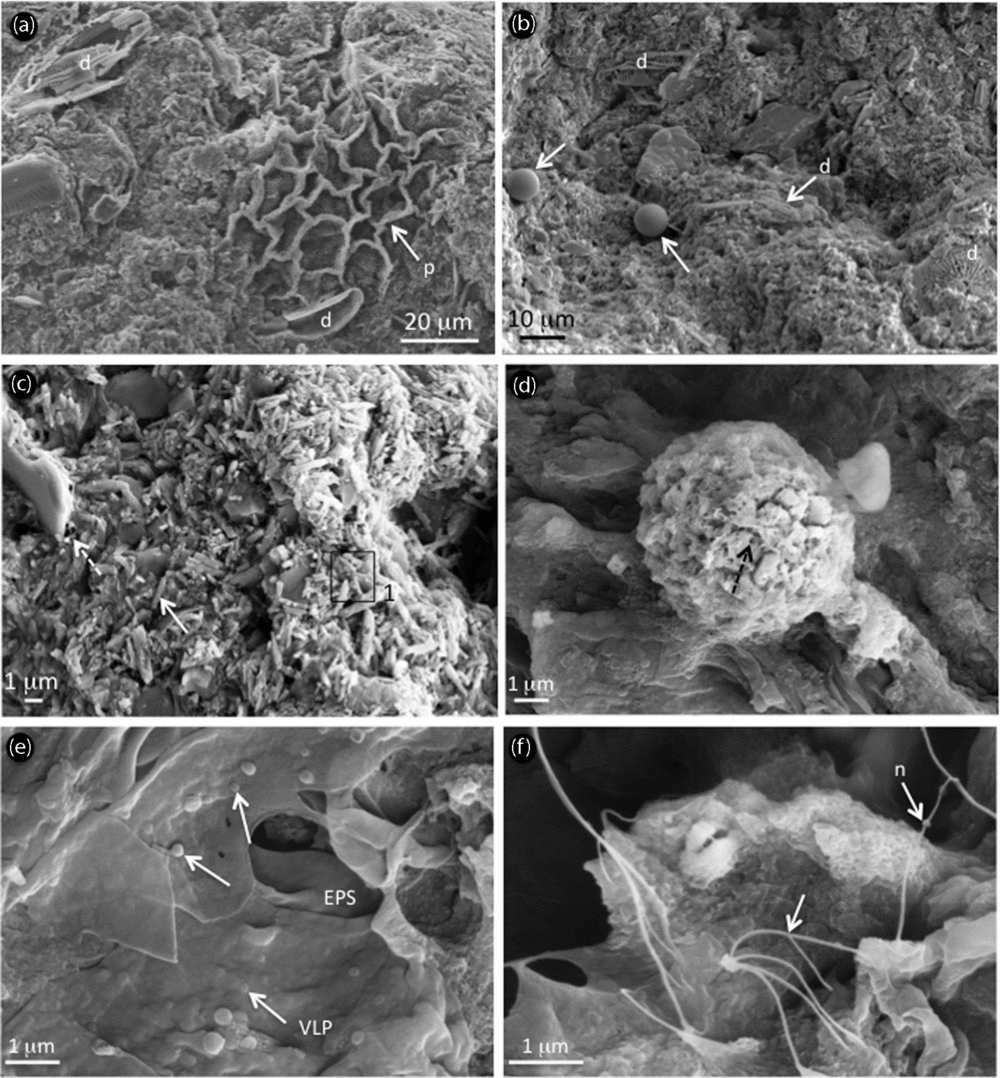
Figure 5. SEM photomicrographs and associated elemental analyses showing (a–d) microfossils and microstructure of precipitates and (d–f) organic matter after acid etching from Unit I in the light-coloured layer. (a) Intact to slightly dissolved diatoms (‘d’) and Pediastrum sp. (‘p’) trapped in the carbonate mud; (b) different diatom populations (‘d’) and chrysophytes (white arrows); (c) aragonite as rods (white arrow), embedded in a matrix containing EPS alveolar structure (dashed arrow); (d) partially dissolved framboidal pyrite as shown by numerous holes (dashed arrow); (e) virus-like particles (‘VLP’, arrows) embedded within EPS; and (f) nanowires (‘n’, arrows).

Figure 6. TEM sections of organic matter after acid etching from (a–c) Unit I in the greyish layer (3.2–4 cm) and (d–f) Unit IV in the dark greyish layer (88–90 cm) showing: (a) ultralaminae as bacterial cell walls (arrows) and EPS (dashed arrows); (b) bacterial cell (black arrow) containing different intracellular inclusions (white arrows) and bacterial cell walls (dashed arrow); (c) virus-like particles (VLP; arrows); (d) small cyanobacterium (black arrow) containing thylakoid membranes (white arrows); (e) EPS characterized by an alveolar network; and (f) VLP characterized by an icosahedral shape (black arrows) and sometimes a tail and fluffy EPS (dashed arrow).
Unit II is made up of thick dark-greyish calcareous mud layers and contains abundant and well-preserved different diatom communities, chrysophyte stomatocysts, B. braunii, microbial filaments and framboidal pyrites (Fig. 7). Most stomatocysts are small, smooth and spherical (less than 5 μm in diameter) with a cylindrical collar (1.2 μm in diameter and 0.3 μm in height). They resemble Stomatocyst 52 as defined by Duff, Zeeb & Smol (Reference Duff, Zeeb and Smol1995). Microfossils are exceptionally well preserved as shown by intact diatom frustules and the presence of a cap still attached to the stomatocysts (Fig. 7e).

Figure 7. SEM photomicrographs showing microfossils and microstructure of precipitates from Unit II: (a) slightly dissolved diatoms (‘d’), Botryococcus braunii (‘b’) and chrysophytes (‘ch’); (b, c) well-preserved diatom frustules (‘d’); (d) filamentous microbes (‘f’); (e) framboidal pyrite (white arrow) and chrysophyte (‘ch’) with the cap still preserved and attached in the pore; and (f) elemental analysis of the chrysophyte confirming the Si cyst.
Unit III consists of homogeneous light-coloured greyish calcareous muds and reflects the dominance of aragonite rods and detrital particles such as clays (Fig. 8). This unit is characterized by the absence of diatoms, while B. braunii is rare. Microbial features are abundant and characterized as filamentous microorganisms, VLPs and EPS (Fig. 8b–e). The latter are closely associated with Mg-carbonate, showing a rhomboedral shape (Fig. 8e–f).

Figure 8. SEM photomicrographs showing microfossils and microstructure of precipitates from Unit III: (a) B. braunii (‘b’) and abundant detrital particles (‘de’); (b) detrital particles (‘de’), aragonite rods (dashed arrows) and EPS (white arrow); (c) EPS as nanofilaments (arrows); (d) filamentous microbes (‘f’), abundant detrital particles (‘de’) and aragonite (‘ar’); (e) nanometre-sized virus-like particles (VLP, arrows) and rhomboedra of Mg-carbonates; and (f) elemental analysis of the Mg-carbonate rhomboedra showing small amount of Si.
Unit IV mostly displays aragonite under SEM. Aragonite is principally in the form of rods (Fig. 9a), but stars are also present in both dark and white laminae (supplementary Fig. S3a, available at http://journals.cambridge.org/geo). Aggregates of carbonates, likely low Mg-calcite, contain small amounts of Si (supplementary Fig. S3b). These aggregates are surrounded by EPS showing an aluminosilicate composition plus Mg. At a higher magnification, EPS embed detrital minerals, aragonite and other Mg-carbonates and seem to create a matrix leading to the preservation of VLPs (Fig. 9b). Examinations of OM under SEM show distinct aspects depending on the types of laminae. Dark brown organic laminae show abundant figured OM such as B. braunii and plant cuticles, which are extensively colonized and degraded by microbes as shown by the wide abundance of EPS (Fig. 9c). Isolated coccoid prokaryotes were found within EPS, but only one pyrite crystal was found in this unit within the organic laminae (supplementary Fig. S3c). Conversely, as well as the colonization of figured OM by microbes (supplementary Fig. S3d, e), the aragonite laminae contain abundant colonies of coccoid prokaryotes closely associated with EPS which seem to be independent of figured OM (Fig. 9d, e; supplementary S3e–f). Abundant VLPs have been found on prokaryote surfaces (Fig. 9d, f). Ultrastructure of OM from both aragonite and organic laminae (88–90 cm depth) reveals ultralaminae (Fig. 6d) and different prokaryotic cells, such as microbial cells with intracellular cytoplasmic membrane resembling thylakoids (Fig. 6d). EPS were distributed as either a fluffy network (Fig. 6f) or condensed alveolar network with holes of different orders of magnitude (Fig. 6e). VLPs are characterized by a hexagonal shape (capsid-like), sometimes with a tail attached to it (Fig. 6f).

Figure 9. SEM photomicrographs showing (a, b) microfossils and microstructure of precipitates and (c) organic matter after acid etching in the dark greyish layer and in the light-coloured layer from Unit IV. (a) EPS (arrows) covering aragonite crystals (‘ar’) and detrital particles (‘de’); (b) needle-like aragonite crystals (‘ar’), nanometre-sized virus-like particles (VLP, black arrow) trapped within EPS (dashed arrow); (c) partially degraded B. braunii (‘b’) covered by EPS (dark arrow); (d) enlargement of coccoid prokaryotes (‘prok’, see square in supplementary Fig. S3d) closely associated with EPS and nanometre-sized virus-like particles (VLP); (e, f) abundant coccoid prokaryotes closely associated with degraded cuticles and alveolar EPS. Note the presence of rutile in (e) and VLP attached to prokaryotic cells in (f).
Unit V is divided in two intervals: (1) greyish calcareous mud layers, interbedded with (2) dark greyish calcareous mud layers. The uppermost interval is dominated by aragonite as rods and stars (Fig. 10a). Other mineral phases have been found such as low Mg-calcite, which occurs as rhomboedra containing minor Si (Fig. 10b; supplementary Fig. S4a, available at http://journals.cambridge.org/geo) and other Mg-carbonate aggregates (Fig. 10c). Aragonite crystals are embedded within an aluminosilicate matrix resembling EPS, which contains mainly Mg and Si (Fig. 10b; supplementary Fig. S4a, b). EPS are widely distributed and can be clearly recognized as filaments (Fig. 10c; supplementary Fig. S4c). The lowermost interval displays well-crystallized aragonite stars (Fig. 10d) and rods (Fig. 10e; supplementary Fig. S4d). Mg-carbonates containing Si were found as nanometre-size rhombs, which are closely associated with EPS (Fig. 10e). Some diatoms were also identified (Fig. 10d). Microbial features are well preserved and widely distributed as EPS in the form of filaments (Fig. 10e; supplementary Fig. S4f) and of stretched-out veils (Fig. 10f; supplementary Fig. S4f). The latter are surrounding Mg-carbonate crystals (Fig. 10f).

Figure 10. SEM photomicrographs showing microfossils and microstructure of precipitates from Unit V. (a) Spherulite-like aragonite (‘ar’, arrow) and aragonite rods (dashed arrow); (b) microbial carbonate (1) and mineralized EPS (2); (c) filaments of EPS (black arrows) and associated Mg-carbonate aggregate; (d) aragonite star (circle) and remains of diatoms (‘d’); (e) fibrous aragonite (‘ar’, dark arrow) and tiny carbonate rhombs (white arrow) associated with EPS (dashed arrow) showing sometimes filamentous structures (small arrow); and (f) tiny crystals of microbial Mg-carbonates (dark arrows) embedded within EPS (white arrow).
4.c. Fluorescence in situ hybridization
The SRB and archaea populations in the sediments were determined based on FISH images using specific probe combinations. Figure 4 illustrates the FISH image using the Archaea-specific probe ARCH915 and the SRB-specific probe SRB385.
FISH surveys of microbial cells in 12 samples revealed a qualitative variable abundance and distribution of SRB and archaea (Fig. 4). Unit I is characterized by an abundance of SRB over archaea in the yellowish laminae (Fig. 4a), while SRB and archaea coexist together in aggregates in the greyish laminae (Fig. 4b). Few microbes were found in Units II and III, but both SRB and archaea occur together in aggregates (Fig. 4c, d) and were sometimes even mixed (Fig. 4c). Unit IV showed distinct patterns of microbial distribution. In the dark brown organic layers archaea/SRB consortia were abundant and resulted in either large aggregates in which archaea are closely associated in monospecific clusters (Fig. 4e), or in SRB prevailing over archaea at the interface between organic and micrite laminae (Fig. 4f). Few microbes were found in the aragonite layers and archaea and SRB occurred as a consortium in aggregates (Fig. 4g) in Unit V. Dark greyish layers are characterized by an abundance of SRB over archaea (Fig. 4h).
5. Interpretation and discussion
5.a. Microbial biosignatures
5.a.1. Microbial processes
Palynological analysis of Son Kul sediments reveals an important contribution of amorphous OM (Mathis et al. Reference Mathis, Sorrel, Klotz, Huang and Oberhänsli2014). This amorphous OM is of granular aspect, suggesting a microbial origin (Pacton, Gorin & Vasconcelos, Reference Pacton, Gorin and Vasconcelos2011). This is in agreement with the wide abundance of filamentous and coccoid microbes along with EPS found in the different units (e.g. Figs 5–10). EPS are usually found as isolated nanofilaments associated with Mg-carbonates or as fluffy particles in palynological residues (with evidences of degradation of figured particles) from all units except Unit IV. In these units, the local occurrence of nanofilaments of EPS along with the numerous bacterial cell walls observed in Unit I, referred to as ultralaminae (see Pacton, Fiet & Gorin, Reference Pacton, Fiet and Gorin2008 for a comprehensive review), support the hypothesis that OM is mainly derived from microbes and the degradation of figured elements by microbial activity. However, EPS exhibit a different aspect in sediments from Unit IV. They are abundant and characterized by a typical alveolar network (with different orders of magnitude), which can only be preserved when associated with a biofilm or a microbial mat structure (Pacton, Fiet & Gorin, Reference Pacton, Fiet and Gorin2007). In that case they are believed to enhance OM preservation, and to provide an efficient chemical and physical barrier against degradation (Pacton, Fiet & Gorin, Reference Pacton, Fiet and Gorin2007). This is confirmed by the higher abundance of EPS in Unit IV compared to other units (e.g. Fig. 9), along with the unusual preservation of numerous colonies of coccoid prokaryotes (Fig. 9) and the preserved intracellular content of some cyanobacteria (Fig. 6). EPS are disconnected in sediments from Unit V, while they are more closely associated with Mg-carbonate crystals in the lowermost interval of Unit V.
Microscopical investigations conducted in the different units would suggest a wide abundance of SRB over depth, while archaea distribution may suggest specific ecological niches. Indeed, the cell aggregates observed in the organic layers of Unit III are similar to syntrophic (i.e. metabolic cross-feeding) colonies of bacteria intertwined with filamentous archaea (Stams & Plugge, Reference Stams and Plugge2009), which points to anaerobic oxidation of methane (AOM). AOM metabolic process is assumed to be a reversal of methanogenesis, coupled with the reduction of sulphate to sulphide involving methanotrophic archaea (ANME) and sulphate-reducing bacteria (SRB) as syntrophic partners (e.g. Hansen et al. Reference Hansen, Finster, Fossing and Iversen1998; Boetius et al. Reference Boetius, Ravenschlag, Schubert, Rickert, Widdel, Gieseke, Amann, Jørgensen, Witte and Pfannkuche2000; Valentine & Reeburgh, Reference Valentine and Reeburgh2000; Schubert et al. Reference Schubert, Vazquez, Lösekann-Behrens, Knittel, Tonolla and Boetius2011). Recent studies have established that at least two phylogenetically different groups of anaerobic methanotrophic Archaea (ANME-I and ANME-II) can mediate this process but seem more sensitive to temperature rather than to sulphate concentration, pH and salinity, especially in cold conditions for ANME-II (Nauhaus et al. Reference Nauhaus, Treude, Boetius and Krüger2005). This consortium is likely highly active in Unit IV. The typical EPS distribution, in addition to the well-preserved microbial bodies, would indicate that these communities were living in biofilms or in microbial mat-like structures (e.g. Pacton, Fiet & Gorin, Reference Pacton, Fiet and Gorin2006). The higher microbial activity recorded in Unit IV might be explained by microbial community structures and potentially anaerobic methanotrophs-associated phylogeny (Vigneron et al. Reference Vigneron, Cruaud, Pignet, Caprais, Gayet, Cambon-Bonavita, Godfroy and Toffin2013). The presence of intact cyanobacterial cells showing intracellular thylakoid membranes implies that they were fossilized in situ, as photosynthetic organisms are rapidly degraded after death in the water column by chemical and physical processes. Such exceptional preservation conditions occur in places where degradation reactions are reduced, as is the case in EPS in microbial mats or biofilms (Pacton, Fiet & Gorin, Reference Pacton, Fiet and Gorin2007, Reference Pacton, Fiet and Gorin2008). The presence of different aerobic and anaerobic prokaryotic metabolims (i.e. cyanobacteria, SRB and archaea) points to a vertical microbial distribution profile, such as in microbial mats. This microbial community structure can be compared with microbial mat systems such as those reported from Arctic saline coldwater springs (Vincent et al. Reference Vincent, Whyte, Lovejoy, Greer, Laurion, Suttle, Corbeil and Mueller2009). In such microbial mats, evidence of AOM was found below the photosynthetic layer of the mat community due to the coexistence of methanogens and SRB (Buckley, Baumgartner & Visscher, Reference Buckley, Baumgartner and Visscher2008).
The composition and structure of microbial communities in Son Kul sediments is quite variable, as demonstrated by the restricted occurrence of microbial mats in Unit IV. In other units, microbes were presumably distributed either in the water column or at the water–sediment interface. In some specific conditions, bacteria can establish connections to terminal electron acceptors through so-called ‘nanowires’, extensions of the outer membrane and periplasm (Pirbadian et al. Reference Pirbadian, Barchinger, Leung, Byun, Jangir, Bouhenni, Reed, Romine, Saffarini, Shi, Gorby, Golbeck and El-Naggar2014). Initially described as connectors to other cells in anoxic conditions, nanowires facilitate the transport of electron acceptors between bacteria (Reguera et al. Reference Reguera, McCarthy, Metha, Nicoll, Tuominen and Lovley2005). Electrically conductive nanowires are not restricted to metal-reducing bacteria, such as Shewanella sp. and Geobacter sp., but are also produced by an oxygenic photosynthetic cyanobacterium and a thermophilic fermentative bacterium (Gorby et al. Reference Gorby, Yanina, McLean, Rosso, Moyles, Dohnalkova, Beveridge, Chang, Kim, Kim, Culley, Reed, Romine, Saffarini, Hill, Shi, Elias, Kennedy, Pinchuk, Watanabe, Ishii, Logan, Nealson and Fredrickson2006). Recent studies suggested that interspecies electron transfer in AOM coupled to bacterial sulphate reduction might be mediated by direct cell–cell contact through nanowires (Thauer & Shima, Reference Thauer and Shima2008; Meulepas et al. Reference Meulepas, Jagersma, Khadem, Stams and Lens2010). Further studies are required to assess the precise role of nanowires in these sediments, for example if they constitute a microbial strategy to the limitation of sulphates.
5.a.2. Virus-like particles
Numerous virus-like particles were found in sediments from Lake Son Kul not only in upper sediments but also in deeper layers (e.g. 88–90 cm; c. 5000 bp). Although viruses are ubiquitous components of aquatic systems (e.g. Suttle, Reference Suttle2007; Yau et al. Reference Yau, Lauro, DeMaere, Brown, Thomas, Raftery, Andrews-Pfannkoch, Lewis, Hoffman, Gibson and Cavicchioli2011), little is known about their preservation potential in the geological record. The lack of viruses in the geological record is attributed to their small size and lack of a unique chemical or isotopic signature (Kyle, Pedersen & Ferris, Reference Kyle, Pedersen and Ferris2008). Previous studies have shown that viral abundance is strong in oxygenated layers from the upper 40 cm of freshwater sediments (Fischer et al. Reference Fischer, Wieltschnig, Kirschner and Velimirov2003). Viruses were also found in larger abundances in top sediments than in the water column of a temperate lake (Duhamel & Jacquet, Reference Duhamel and Jacquet2006), but no data have been yet reported from deeper layers. Viral abundance and distribution was indeed reported from deep-marine sub-seafloor settings, in which genetic material was still present in the capsid (Bird et al. Reference Bird, Juniper, Ricciardi-Rigault, Martineu, Prairie and Calvert2001; Middelboe, Glud & Filippini, Reference Middelboe, Glud and Filippini2011). Fossilized viruses are scarcely reported in the literature, and are found either (1) in acidic waters of the Rio Tinto in Spain (Kyle, Pedersen & Ferris, Reference Kyle, Pedersen and Ferris2008); (2) being silicified either in laboratory experiments (e.g. Orange et al. Reference Orange, Chabin, Gorlas, Lucas-Staat, Geslin, Le Romancer, Prangishvili, Forterre and Westall2011) or in geothermal systems (Peng et al. Reference Peng, Xu, Jones, Chen and Zhou2013); or (3) being mineralized as Mg-carbonates in photosynthetic microbial mats (Pacton et al. Reference Pacton, Wacey, Corinaldesi, Tangherlini, Kilburn, Gorin, Danovaro and Vasconcelos2014). The identification of fossilized viruses in the geological record therefore remains a challenge. Several lines of evidence must support their origin, including size and morphology, while ruling out potential inorganic precipitates. VLPs described in Lake Son Kul sediments match the criteria for identifying fossilized viruses (Pacton et al. Reference Pacton, Wacey, Corinaldesi, Tangherlini, Kilburn, Gorin, Danovaro and Vasconcelos2014), that is, organic nanospheres of size range 20–200 nm, bearing a hexagonal outline that resembles a polyhedral (termed icosahedral) capsid sometimes with a tail attached to it, thus strongly supporting a viral origin. VLPs in surface sediments (e.g. Unit I) are characterized by a capsid full of organic content which is likely genetic material, whereas those from Unit IV look empty suggesting that they probably lost their nucleic acids and that only capsids can be preserved in sediments under anoxic conditions. Viruses are considered to play a key role in the food web as they liberate OM from host cells releasing essential nutrients into the ecosystem (Fuhrman, Reference Fuhrman1999; Wilhelm & Suttle, Reference Wilhelm and Suttle1999; Middelboe & Jørgensen, Reference Middelboe and Jørgensen2006). This is in agreement with a higher primary productivity in Units I and IV, as shown by high total organic content (TOC) and elevated content in amorphous OM (Huang et al. Reference Huang, Oberhänsli, von Suchodoletz, Prasad, Sorrel, Plessen, Mathis and Usubaliev2014; Mathis et al. Reference Mathis, Sorrel, Klotz, Huang and Oberhänsli2014). High content in amorphous OM is commonly used as a proxy for low oxygen content at the sediment–water interface. It also provides evidence for high primary productivity in the photic zone (Prasad et al. Reference Prasad, Garg, Singh and Thakur2007; Pacton, Gorin & Vasconcelos, Reference Pacton, Gorin and Vasconcelos2011). High viral abundance but low virus-to-bacterium ratios were also reported in freshwater lake sediments (e.g. Maranger & Bird, Reference Maranger and Bird1996; Lemke et al. Reference Lemke, Wickstrom and Leff1997). Although sediments favour the preservation of viruses but not their proliferation in tropical environments (Bettarel et al. Reference Bettarel, Bouvy, Dumont and Sime-Ngando2006), our data would point to a similar process in cold, high-altitude environments.
5.b. Mineral biosignatures
5.b.1. Carbonates
In lacustrine systems authigenic carbonate phases reveal key environmental information (e.g. sub-annual precipitation signals, lake productivity, low lake level stages, etc.); however, their primary and possible diagenetic origin may blur the interpretation (Talbot & Kelts, Reference Talbot and Kelts1986). Calcium carbonates in Lake Son Kul sediments are mainly dominated by aragonite (except for Unit II), with lower contributions of Mg-calcite and dolomite.
5.b.1.a. Aragonite
Whether the origin of aragonite in sediments is inorganic or biological is still under debate (DeGroot, Reference DeGroot1965; Shinn et al. Reference Shinn, Steinen, Lidz and Swart1989; Boss & Neumann, Reference Boss and Neumann1993; Milliman et al. Reference Milliman, Freile, Steinen and Wilber1993; Morse, Gledhill & Millero, Reference Morse, Gledhill and Millero2003), but most of these studies are related to warm- and shallow-marine environments. Aragonite formed in cold, oligotrophic, high-latitude settings (e.g. Antarctica; see Hendy et al. Reference Hendy, Healy, Rayner, Shaw and Wilson1979; Lawrence & Hendy, Reference Lawrence and Hendy1985; Bird et al. Reference Bird, Chivas, Radnell and Burton1991; Hendy, Reference Hendy2000) or in montane lakes is highly unusual, and has only been reported in Bear Lake (USA) associated with an increase in salinity (Dean et al. Reference Dean, Rosenbaun, Skipp, Colman, Forester, Liu, Simmons and Bischoff2006). The key variable for aragonite formation appears to be a high Mg:Ca ratio (Müller, Irion & Förstner, Reference Müller, Irion and Förstner1972), and an increase in Mg:Ca ratio is usually accompanied by an increase in salinity (Kelts & Hsü, Reference Kelts, Hsü and Lerman1978). In lake environments, the occurrence of algal blooms in the epilimnion promotes carbonate precipitation by reducing CO2 concentrations, which leads to increasing pH values (e.g. Kelts & Hsü, Reference Kelts, Hsü and Lerman1978; Koschel et al. Reference Koschel, Benndorf, Proft and Recknagel1983; Koschel, Reference Koschel1997). This mechanism is therefore considered as a biologically induced precipitation pathway. Micrometre-sized needle-like particles in Son Kul sediments are similar to those reported from algal blooms (e.g. Hodell et al., Reference Hodell, Schelske, Fahnenstiel and Robbins1998; Sondi & Juracic, Reference Sondi and Juracic2010), suggesting a similar process linked to biological productivity in the lake.
However, whether primary productivity was primarily driven by photosynthetic organisms or by chemotrophic prokaryotes (such as methanogens) remains unknown. The highest content in aragonite is recorded in Unit IV, and corresponds to the clear occurrence of SRB and methanotrophic archaea as consortia. Stable carbon isotopes values on bulk carbonates from Units IV and V are slightly depleted (δ13C of −2 to 4 ‰ compared to the upper units of δ13C of 4–8 ‰; Huang et al. Reference Huang, Oberhänsli, von Suchodoletz, Prasad, Sorrel, Plessen, Mathis and Usubaliev2014), suggesting that AOM (inducing light δ13C) contributed to carbon mineralization prior to (Mg-)calcite precipitation; this is in contrary to methanogenesis producing CO2 enriched in 13C (Waldron, Hall & Fallick, Reference Waldron, Hall and Fallick1999; Jahren, LePage & Werts, Reference Jahren, LePage and Werts2004; Chanton et al. Reference Chanton, Chaser, Glasser, Siegel, Flanagan, Ehleringer and Pataki2005). Such δ13Ccarb values in Unit IV and V are similar to those from Lake Cadagno where AOM is an important process in the carbon cycle (Schubert et al. Reference Schubert, Vazquez, Lösekann-Behrens, Knittel, Tonolla and Boetius2011). Additionally, a new morphotype of aragonite appears in Units IV and V, consisting of micrometre-sized elongated rod- to radial-star-shaped crystal aggregates. This morphotype is similar to the star-shaped type 1 spherulite illustrated in Andreassen, Beck & Nergaard (Reference Andreassen, Beck and Nergaard2012), which evolves to spherulites by increasing the supersaturation. The star-shaped morphotype further resembles spherulite precursors similar to those found in microbial mats and stromatolites (e.g. Spadafora et al. Reference Spadafora, Perri, McKenzie and Vasconcelos2010). Aragonite spherulites have also been reported associated with bacteria as a tracer of increased salinity (Sanchez-Navas et al. Reference Sanchez-Navas, Martìn-Algarra, Rivadeneyra, Melchor and Martìn-Ramos2009) and are frequently found in (hyper)saline environments (Spadafora et al. Reference Spadafora, Perri, McKenzie and Vasconcelos2010; Arp et al. Reference Arp, Helms, Karlinska, Schumann, Reimer, Reitner and Trichet2012). Whether these precipitates are of organic or inorganic origin remain unknown. However, as EPS are of relative importance in Unit IV (a major constituent of microbial mats) and in Unit V, they might have influenced the formation of star-shaped minerals in both units. At Lake Issyk-Kul (Kyrgyzstan) a similar microbial process was proposed for the possible origin of vaterite, a polymorph of CaCO3 (Giralt, Julia & Klerkx, Reference Giralt, Julia and Klerkx2001).
5.b.1.b. Dolomite
Dolomite in sediments is one of the most controversial topics in sedimentary geology (e.g. Warren, Reference Warren2000; McKenzie & Vasconcelos, Reference McKenzie and Vasconcelos2009). This is mostly because no precipitation of dolomite has been rigorously demonstrated in laboratory experiments at low temperatures and in the absence of microbial activity (Lippmann, Reference Lippmann1973; Land, Reference Land1998). More specifically, the metabolic activity of SRB has so far been considered to overcome the kinetic barrier to dolomite formation by increasing pH and carbonate alkalinity (Vasconcelos et al. Reference Vasconcelos, McKenzie, Bernasconi, Grujic and Tiens1995; Warthmann et al. Reference Warthmann, Lith, Vasconcelos, McKenzie and Karpoff2000), although recent studies showed that sulphate reduction is not sufficient to induce carbonate precipitation in microbial mats (Meister, Reference Meister2013). Microbial dolomite is widely encountered in modern saline environments (e.g. Vasconcelos & McKenzie, Reference Vasconcelos and McKenzie1997; Wright, Reference Wright1999; Wright & Oren, Reference Wright and Oren2005; Wright & Wacey, Reference Wright and Wacey2005), while biogenic dolomite precipitation was observed in non-hypersaline inland lakes (Deng et al. Reference Deng, Dong, Lv, Jiang, Yu and Bishop2010) and in freshwater experiments, more specifically associated with methanogenic waters (Roberts et al. Reference Roberts, Bennett, Gonzalez, Macpherson and Milliken2004; Kenward et al. Reference Kenward, Goldstein, Gonzalez and Roberts2009). Dolomite precipitation can occur around cells (Warthmann et al. Reference Warthmann, Lith, Vasconcelos, McKenzie and Karpoff2000) or associated with EPS (Sanchez-Roman et al. Reference Sanchez-Roman, Vasconcelos, Schmid, Dittrich, McKenzie, Zenobi and Rivadeneyra2008; Deng et al. Reference Deng, Dong, Lv, Jiang, Yu and Bishop2010) produced by SRB. Recently, the precipitation of dolomite was shown closely associated with cyanobacterial EPS and organic carbon, while SRB influence was not excluded (Paulo & Dittrich, Reference Paulo and Dittrich2013). However, a variety of microbes other than SRB can trigger dolomite precipitation such as methanogens (Roberts et al. Reference Roberts, Bennett, Gonzalez, Macpherson and Milliken2004; Kenward et al. Reference Kenward, Goldstein, Gonzalez and Roberts2009), sulphide-oxidizing bacteria (Moreira et al. Reference Moreira, Walter, Vasconcelos, McKenzie and McCall2004) and methane-oxidizing archaea in gas hydrate deposits (Sassen et al. Reference Sassen, Roberts, Carney, Milkov, DeFreitas, Lanoil and Zhang2004). Dolomite in Son Kul sediments is more abundant in the light-coloured layers from Units I, II (up to 9 %) and V, while this pattern is more complex in the finely laminated sediments of Unit IV. In the latter, FISH data combined with dolomite occurrences (based on XRD analyses) indicate that the highest dolomite content was recorded close to clear occurrences of SRB/archaea consortia in sediments. However, the wide presence of EPS in these samples precludes the discrimination of methanotrophic archaea coupled with SRB from passive mineralization of EPS (cf. Bontognali et al. Reference Bontognali, McKenzie, Warthmann and Vasconcelos2014) as the main process triggering dolomite precipitation. We propose that dolomite most probably formed in the early diagenetic phase, similar to the other Mg-calcite crystals, when pore water was highly mineralized. It must also be considered that dolomite might be a byproduct related to methanogenesis occurring in soils around the lake (as it has been reported from other lake systems; e.g. Talbot & Kelts, Reference Talbot and Kelts1986), although we cannot rule out the possibility that methanogenesis operated in the lake itself. Monitoring surveys in the 1960s and 1970s indeed reported nearshore emanations of H2 and methane during the cold season (Beloglasova & Smirnova, Reference Beloglasova and Smirnova1987). Considering that core SK07 is a proximal site in Lake Son Kul (therefore with a proximal habitus), the influence of both surface and groundwater inputs of soil-derived methanogenesis-related products on the formation of authigenic carbonates in lake sediments should be considered.
5.b.2. Iron sulphides
Framboidal pyrites have been widely encountered in Son Kul sediments but in Units I and II only, and are almost absent from the underlying units. Numerous studies have demonstrated that the growth of framboidal pyrite at temperatures above 60 °C can occur in abiotic laboratory systems (e.g. Ohfuji & Rickard, Reference Ohfuji and Rickard2005). They are widely found in natural sedimentary environments, although the formation processes are still unclear (Ohfuji & Rickard, Reference Ohfuji and Rickard2005), supporting a biological origin (e.g. Sawlowicz, Reference Sawlowicz2000; Popa, Badescu & Kinkle, Reference Popa, Badescu and Kinkle2004; Vuillemin et al. Reference Vuillemin, Ariztegui, De Coninck, Lücke, Mayr and Schubert2013). More specifically, the presence of framboidal pyrites reflect precipitation from highly supersaturated fluids developed due to metabolic activity of sulphate-reducing bacteria (Habicht & Canfield, Reference Habicht and Canfield1997; Wilkin & Barnes, Reference Wilkin and Barnes1997; Maclean et al. Reference MacLean, Tyliszczak, Gilbert, Zhou, Pray, Onstott and Southam2008). Pyrite sulphur is provided by microbial sulphate reduction (Berner, Reference Berner1984) increasing the alkalinity of the pore water according to the reaction
H2S produced by sulphate reduction may react with Fe2+ leading to precipitation of aggregates of pyrite:
Precipitation of pyrite was also shown to be a common byproduct of sulphate reduction and AOM (Schink, Reference Schink2002). However, FISH surveys in Son Kul sediments showed that AOM occurred mainly in both Units IV and V, suggesting that framboidal pyrites may have been mediated through sulphate reduction only. The lack of framboidal pyrite in Unit IV may be linked to either oxidation of initially formed pyrite, inhibition of crystallization caused by sulphate limitation or a lack of reactive iron availability. Considering the first hypothesis, oxidation of pyrite would have generated an acidic sulphur-rich solution (e.g. Blowes & Jambor, Reference Blowes and Jambor1990; Edwards et al. Reference Edwards, Bond, Druschel, McGuire, Hamers and Banfield2000) that would have been buffered by carbonates. In such case, we would expect to find sulphates and iron oxi-hydroxides (e.g. Charpentier et al. Reference Charpentier, Mosser-Ruck, Cathelineau and Guillaume2004) or even smectite (Gaudin et al. Reference Gaudin, Buatier, Beaufort, Petit, Grauby and Decareau2005). However, none of these minerals was found to be significantly higher in Units IV and V compared to Units I and II. Inhibition of pyrite crystallization is therefore the most likely hypothesis. Unit IV is characterized by the highest abundance of syntrophic consortia of sulphate-reducing bacteria and methanotrophic archaea (Fig. 6; Section 5.a.1). They catalyse the reaction:
(Hinrichs et al. Reference Hinrichs, Hayes, Sylva, Brewer and DeLong1999; Boetius et al. Reference Boetius, Ravenschlag, Schubert, Rickert, Widdel, Gieseke, Amann, Jørgensen, Witte and Pfannkuche2000; Orphan et al. Reference Orphan, House, Hinrichs, McKeegan and DeLong2001; Knittel et al. Reference Knittel, Lösekann, Boetius, Kort and Amann2005; Niemann et al. Reference Niemann, Lösekann, de Beer, Elvert, Nadalig, Knittel, Amann, Sauter, Schlüter, Klages, Foucher and Boetius2006) and do not favour a sulphate limitation as the main factor preventing pyrite formation. Consequently, the absence of reactive iron availability seems to be the most likely explanation for the absence of framboidal pyrite in Units III, IV and V in core SK07.
5.b.3. Stomatocysts
Biogenic siliceous microspheres, referred to as chrysophyte stomatocysts, have been found in the different units from core SK07. Although they display various morphologies, stomatocyst 52 (produced by more than one species; Duff, Zeeb & Smol, Reference Duff, Zeeb and Smol1995) seems to dominate microfossil assemblages. These stomatocysts were found elsewhere in arctic and alpine habitats such as arctic tundra lakes, arctic peats, arctic ponds, alpine ponds and montane lakes, suggesting that stomatocysts are produced by a cold-tolerant form. Chrysophyte stomatocysts (Smol, Reference Smol1988), and silica-scaled chrysophytes (Wilken, Kristiansen & Jürgensen, Reference Wilken, Kristiansen and Jürgensen1995) accumulate in sediments and are used to reconstruct past climate trends, especially in cold regions (Charvet, Vincent & Lovejoy, Reference Charvet, Vincent and Lovejoy2012). Previous studies have shown that chrysophytes respond to a variety of environmental changes, including shifts in pH (Duff & Smol Reference Duff and Smol1991; Facher & Schmidt Reference Facher and Schmidt1996; Wilkinson, Hall & Smol, Reference Wilkinson, Hall and Smol1999), trophic status (Zeeb, Duff & Smol, Reference Zeeb, Duff and Smol1990; Zeeb et al. Reference Zeeb, Christie, Smol, Findlay, Kling and Birks1994; Wilkinson, Hall & Smol, Reference Wilkinson, Hall and Smol1999; Cabala & Piatek, Reference Cabala and Piatek2004; Charvet, Vincent & Lovejoy, Reference Charvet, Vincent and Lovejoy2012), salinity (Pienitz et al. Reference Pienitz, Walker, Zeeb, Smol and Leavitt1992; Zeeb & Smol, Reference Zeeb and Smol1995), pronounced seasonal changes in light availability (Charvet, Vincent & Lovejoy, Reference Charvet, Vincent and Lovejoy2012) and climate (Zeeb & Smol, Reference Zeeb, Smol, Bradbury and Dean1993). Their success is most likely due to their diverse nutritional strategies and ability to produce resistant siliceous resting stages, termed stomatocysts. Unlike most other algal groups that are predominantly autotrophic, many chrysophytes are able to switch between autotrophy, heterotrophy and even phagotrophy. Moreover, their ability to create a resilient resting stage allows them to survive unfavourable conditions (Betts-Piper, Zeeb & Smol, Reference Betts-Piper, Zeeb and Smol2004). According to Holmgren (Reference Holmgren1984), Lake Son Kul falls into the Chrysophyceae-diatoms assemblage classification. Although unornamented cysts are widely distributed across a variety of geographical and ecological gradients (Betts-Piper, Zeeb & Smol, Reference Betts-Piper, Zeeb and Smol2004), the abundance of these morphotypes in Holocene sediments at Son Kul suggests that ecological conditions were continuously favourable for their growth (at least in Units I and II) in this cold, alpine lake during Holocene time. Further investigations are nevertheless required to study the variations in stomatocyst assemblages over depth, in order to more precisely evaluate palaeoenvironmental conditions in Lake Son Kul during the Holocene.
6. Summary and conclusions
This work demonstrates the high potential of coupling a geomicrobiological approach to a classical facies analysis for the study of laminated lacustrine sediments. Microbe–mineral interactions investigated in sediments from Lake Son Kul reveal different environmental processes in which microbes played a fundamental role (Table 1). Microbial communities are dominated by SRB at the water–sediment interface, degrading OM. They promoted dolomite and framboidal pyrite precipitation during early diagenesis. Moreover, SRB lived in consortia with methanotrophic archaea and performed AOM, especially between 7000 and 5000 bp when microbial mat structures producing large quantities of EPS enhanced the preservation of microbial structures such as virus-like particles and coccoid prokaryotes. Aragonite is of primary origin and driven by biological activity similar to algal bloom events (especially in Unit IV). Moreover, another morphotype of aragonite (a spherulite-like precursor) occurs in Unit IV, possibly mediated by EPS in microbial mats. This study further highlights that the preservation of Holocene viral relics at Lake Son Kul can provide promising and new perspectives in the search for viral biosignatures in ancient sediments.
Table 1. The main biogeochemical processes taking place in Core SK07 from Lake Son Kul (with associated specific minerals and biominerals) for lithological Units I–V.
Acknowledgements
The authors thank two anonymous reviewers for their constructive comments. The authors acknowledge Daniel Ariztegui for conducting micro-XRF elemental mappings (University of Geneva) and the Centre Technologique des Microstructures (University Claude Bernard-Lyon 1) for electron microscopy facilities. We thank Lydia Zehnder (ETH Zurich) for her support with X-ray diffraction analysis. We are also grateful to Samuel Mailliot (University Lyon1) for his contribution to Figure 1 and Tharsis-Energy for financial support.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0016756814000831