1. Introduction
The colonization of non-marine water bodies by animals was one of the major ecological radiations of life. However, there is little known about the early colonization of the non-marine realm by ostracods, which are one of the most abundant animal groups. Ostracods are numerous, widespread and diverse in non-marine and marine waters today. Ostracods occur in the fossil record from the Early Ordovician onwards (Salas, Vannier & Williams, Reference Salas, Vannier and Williams2007; Siveter, Reference Siveter2008) and appear to have been entirely marine during the Early Palaeozoic (for example see Williams et al. Reference Williams, Floyd, Salas, Siveter, Stone and Vannier2003). Their ecological migration from marine to freshwater aquatic environments occurred during the Late Palaeozoic, with the most complete record of this transition in the Carboniferous (Bennett, Reference Bennett2008). Putative brackish water ostracods have been recorded in the Silurian (Siveter, Reference Siveter1984; Clarkson, Harper & Hoey, Reference Clarkson, Harper and Hoey1998; Floyd & Williams, Reference Floyd and Williams2003), with more frequent attempts at colonizing brackish waters being recorded from the Late Devonian onwards (for example Bless, Reference Bless1983; Bless, Streel & Becker, Reference Bless, Streel and Becker1988; Williams et al. Reference Williams, Leng, Stephenson, Andrews, Wilkinson, Siveter, Horne and Vannier2006).
In the Late Silurian to Devonian a non-marine macrofossil lacustrine fauna (including freshwater fish and arthropods) became established worldwide, but it was not until the Carboniferous that most non-marine terrestrial environments were colonized (Buatois et al. Reference Buatois, Mángano, Genise and Taylor1998). The earliest Late Palaeozoic arthropods that are possibly taxonomically ‘close’ to ostracods and which apparently colonized freshwater are leperditicopes, a group which also originated in the Early Ordovician and which are recorded from Early Devonian brackish and putative freshwater environments (Friend & Moody-Stuart, Reference Friend and Moody-Stuart1970; Friedman & Lundin, Reference Friedman and Lundin1998; Knox & Gordon, Reference Knox and Gordon1999). The timing of non-marine colonization in the Carboniferous by invertebrate detritus-feeders can be linked to the timing of land plant evolution (Bateman et al. Reference Bateman, Crane, DiMichele, Kenrick, Rowe and Speck1998; Buatois et al. Reference Buatois, Mángano, Genise and Taylor1998). The ‘passive’ versus ‘active’ (Gray, Reference Gray1988) nature of invertebrate non-marine colonization is key to understanding the mechanism of this ostracod radiation event, that is, whether invertebrates were passively stranded in coastal plain water bodies that freshened over time or if they exploited the food available from the land and actively activity adapted to freshwater conditions.
Living non-marine ostracods belong to the podocope superfamilies Cypridoidea, Darwinuloidea and Cytheroidea, all of which have possible Late Palaeozoic origins. The Palaeozoic Carbonitoidea may be related to the Cypridoidea or Darwinuloidea, and a group of non-marine Cytheroidea (limnocytherids) occur in the Late Carboniferous (Carbonel et al. Reference Carbonel, Colin, Danielopol, Löffler and Neustrueva1988; Horne, Reference Horne, Park and Smith2003). The migration from marine to freshwater by the ostracod Carbonita Strand, Reference Strand1928 first occurred during the Carboniferous; from brackish-influenced environments in the Mississippian (Pollard, Reference Pollard1966; Anderson, Reference Anderson1970; Bless & Pollard; Reference Bless and Pollard1973; Sohn, Reference Sohn1985; Tibert & Scott, Reference Tibert and Scott1999), to subsequently occupy a wide range of freshwater environments by the Pennsylvanian (Schultze, Maples & Cunningham, Reference Schultze, Maples and Cunningham1994; Vannier, Thiery & Racheboeuf, Reference Vannier, Thiery and Racheboeuf2003; Schäfer, Reference Schäfer, Schindler and Heidtke2007). Herein, Carbonita is recorded from the early Mississippian in what are interpreted as freshwater sediments. Along with darwinuloideans, the carbonitoideans radiated to a high diversity of species in the Late Carboniferous and Permian (Carbonel et al. Reference Carbonel, Colin, Danielopol, Löffler and Neustrueva1988; Horne, Reference Horne, Park and Smith2003) and were the most successful Late Palaeozoic ostracods in freshwater environments.
This study draws on a range of palaeontological, sedimentological and palaeoecological data from the Carboniferous of the Midland Valley of Scotland (MVS) to assess: (1) the absolute range of environments that ostracods were colonizing during the Carboniferous; (2) the types of ostracods that were making this transition and whether or not their adaptation was of short-term or long-term success; and (3) the possible mechanisms, both intrinsic (genetic) and extrinsic (environmental), that were driving or facilitating this ecological shift.
2. Geological background
The Strathclyde Group of the MVS was deposited in a range of environments, from shallow marine, restricted marine lagoons and estuaries, to coastal floodplains, fluvial systems and freshwater lakes and swamps (Browne et al. Reference Browne, Dean, Hall, McAdam, Monro and Chisholm1999). The well-preserved and abundant macrofossils and ostracods in the Strathclyde Group make it an ideal sequence to study a marine to non-marine ecological shift during the Mississippian. At this time the MVS was situated on the margins of a restricted marine seaway at an equatorial latitude (Fig. 1). Siliciclastic sediment, sourced mainly from the highlands to the north was deposited in prograding alluvial to lacustrine deltaic systems, with infrequent marine transgressions (Browne et al. Reference Browne, Dean, Hall, McAdam, Monro and Chisholm1999). A study of the older (early Mississippian, Courceyan) Ballagan Formation demonstrated ostracods in hypersaline and brackish water bodies (Williams et al. Reference Williams, Stephenson, Wilkinson, Leng and Miller2005, Reference Williams, Leng, Stephenson, Andrews, Wilkinson, Siveter, Horne and Vannier2006), associated with possible freshwater to brackish algal palynomorphs (Stephenson et al. Reference Stephenson, Williams, Leng and Monaghan2004a, Reference Stephenson, Williams, Monaghan, Arkley, Smith, Dean, Browne and Lengb).
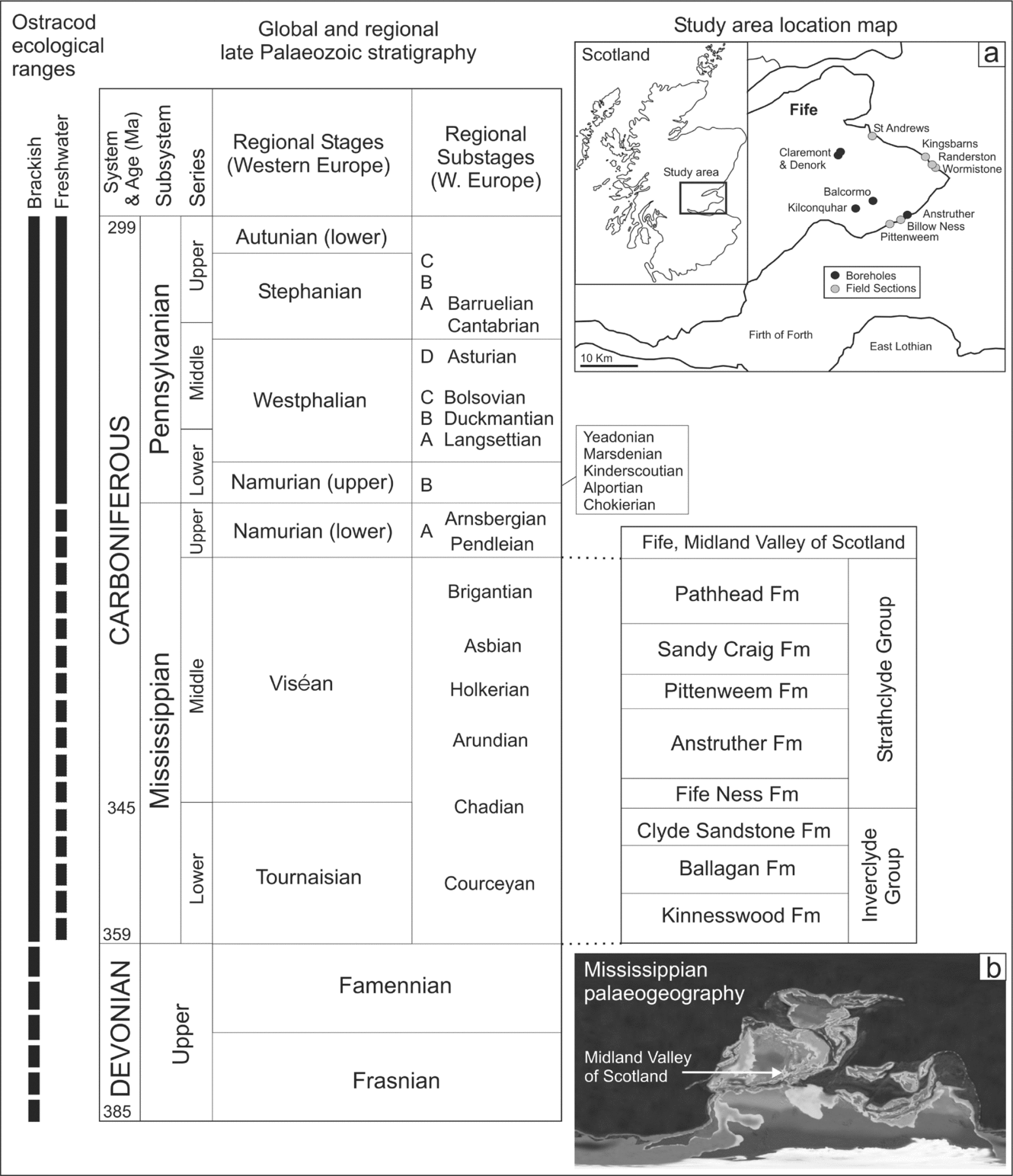
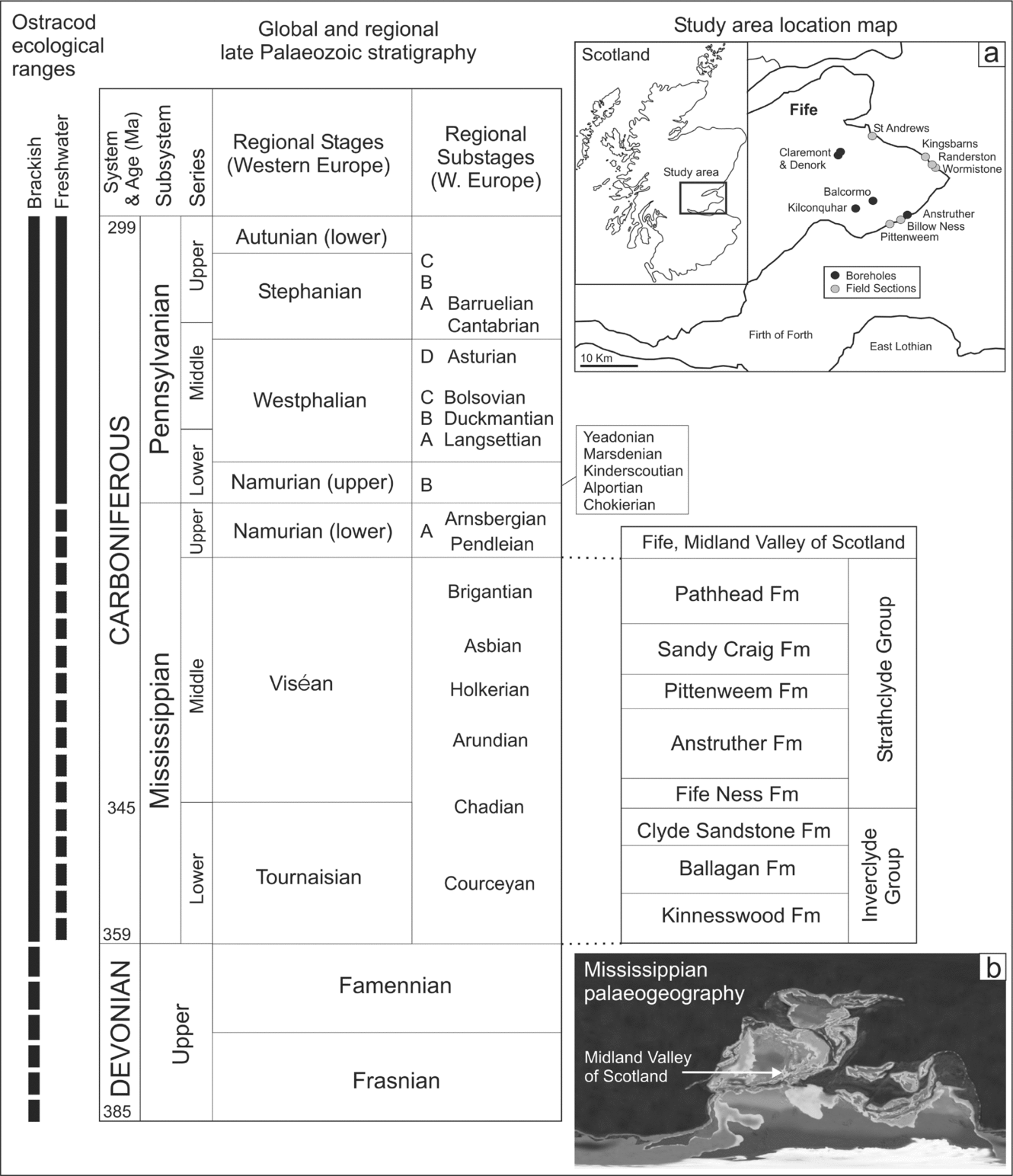
Figure 1. Late Devonian and Carboniferous lithostratigraphy for Fife in the Midland Valley of Scotland. Inset (a) shows the location map of sections studied; inset (b) shows Mississippian palaeogeography. The ecological radiation of ostracods relative to the chronostratigraphy is plotted in terms of their occurrence in brackish and freshwater, from other published records. Solid lines represent a confident age assignment for brackish or freshwater conditions, dashed lines a tentative assignment or only rare occurrence. Carboniferous stratigraphy is adapted from Heckel & Clayton (Reference Heckel and Clayton2006) for Western Europe and Browne et al. (Reference Browne, Dean, Hall, McAdam, Monro and Chisholm1999) for Fife. Dates are from the International Commission of Stratigraphy timescale 2004. The Mississippian palaeogeographic map is for 340 Ma, downloaded from Prof. R. Blakey's website http://jan.ucc.nau.edu/~rcb7.
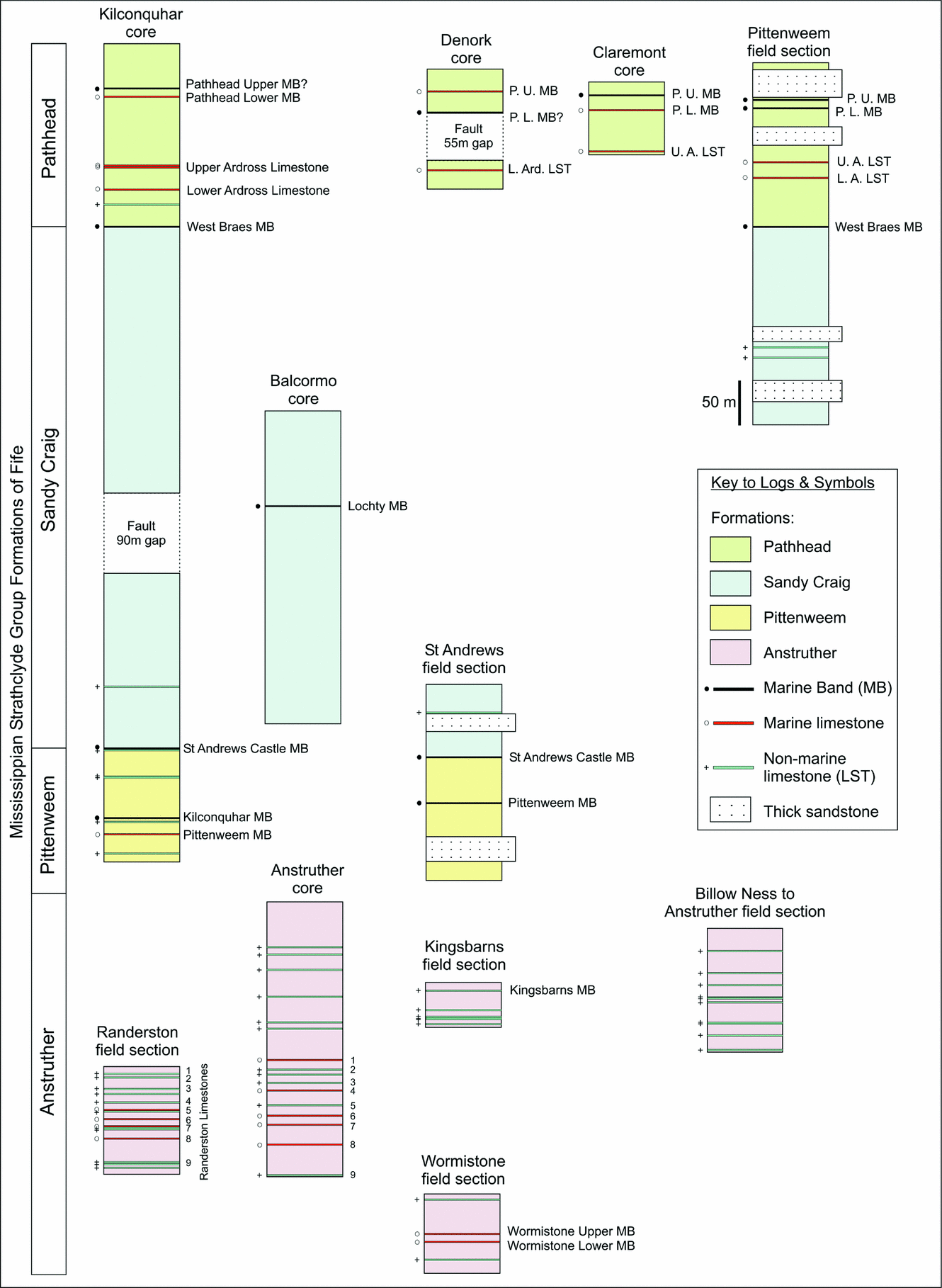
The formations of the Strathclyde Group are correlated across Fife in Scotland using distinctive marine horizons and non-marine limestones (Browne et al. Reference Browne, Dean, Hall, McAdam, Monro and Chisholm1999; Wilson, Reference Wilson1989). The group is well dated by lava flows, lapilli-tuffs and intrusions and by the presence of spores (Monaghan & Parrish, Reference Monaghan and Parrish2005; Owens et al. Reference Owens, McLean, Simpson, Shell and Robinson2005; Stephenson et al. Reference Stephenson, Williams, Monaghan, Arkley, Smith, Dean, Browne and Leng2004b; Fig. 1). The Anstruther Formation has the most non-marine limestones (Fig. 2); the Pittenweem Formation contains fully marine and marginal marine horizons (‘marine bands’) belonging to the Macgregor Marine Bands (Wilson, Reference Wilson1989); the Sandy Craig Formation is essentially non-marine; and the Pathhead Formation contains the thickest fully marine horizons.
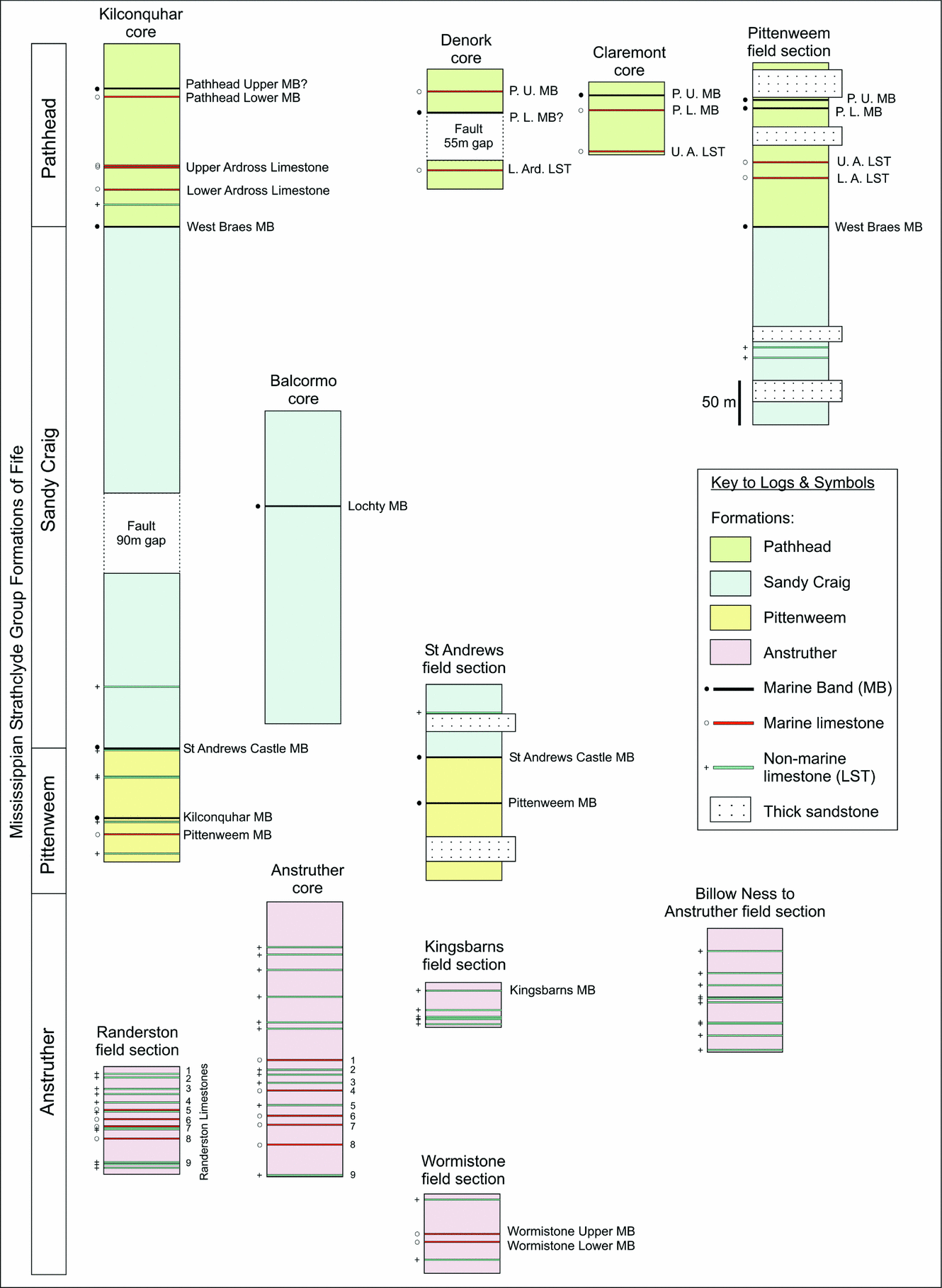
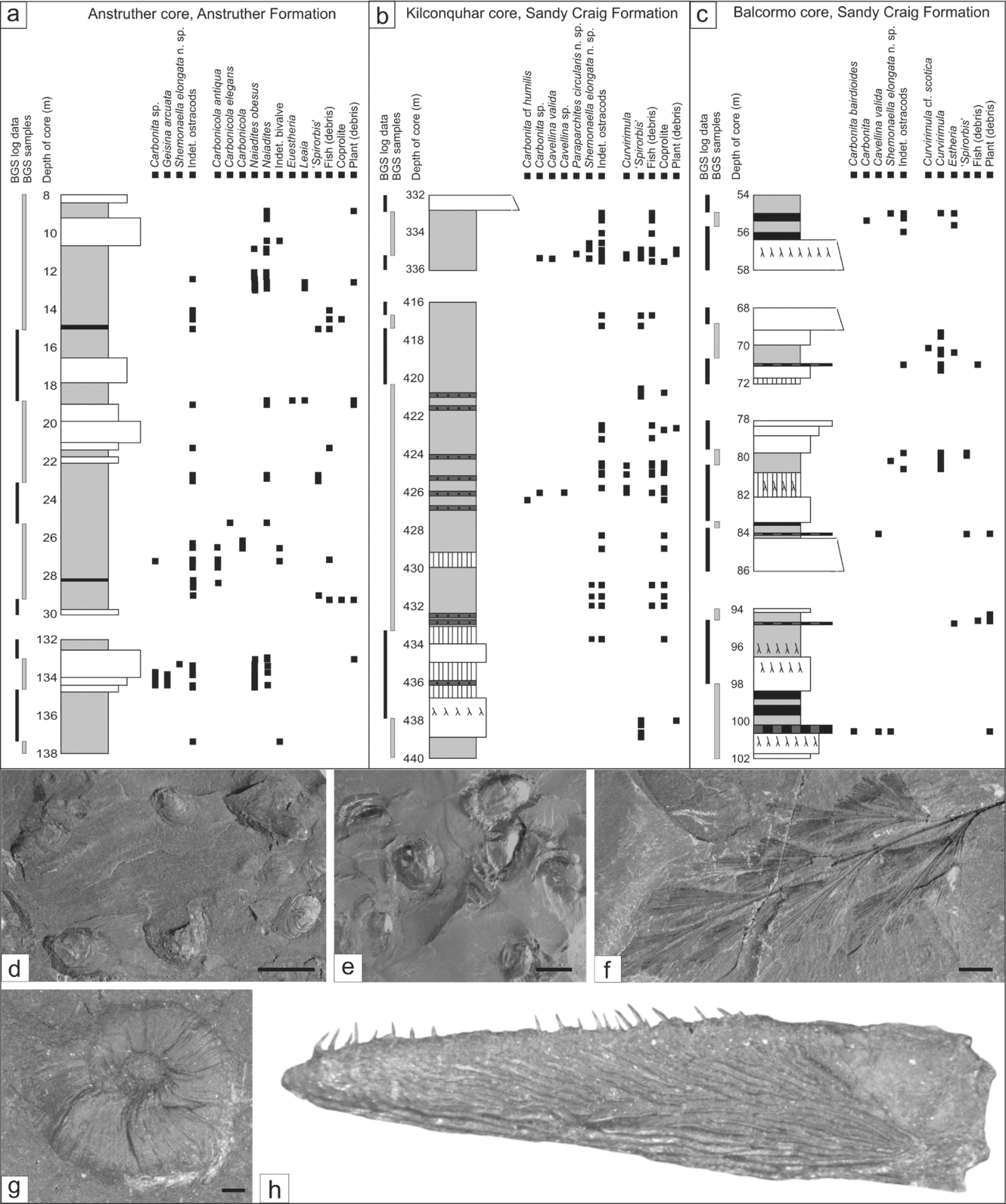
Figure 2. Borehole and field section correlation panel for the Strathclyde Group. Sandstone units (Facies 3a) with a thickness greater than 20 m are shown. British Geological Survey (BGS) boreholes, from 1964–1980, are stored at the BGS in Edinburgh. A sedimentary log and a list of macrofauna were described from these cores by BGS workers (additionally the Balcormo and Kilconquhar boreholes were also described by National Coal Board workers). Field samples were collected as part of this study between 2005 and 2008.
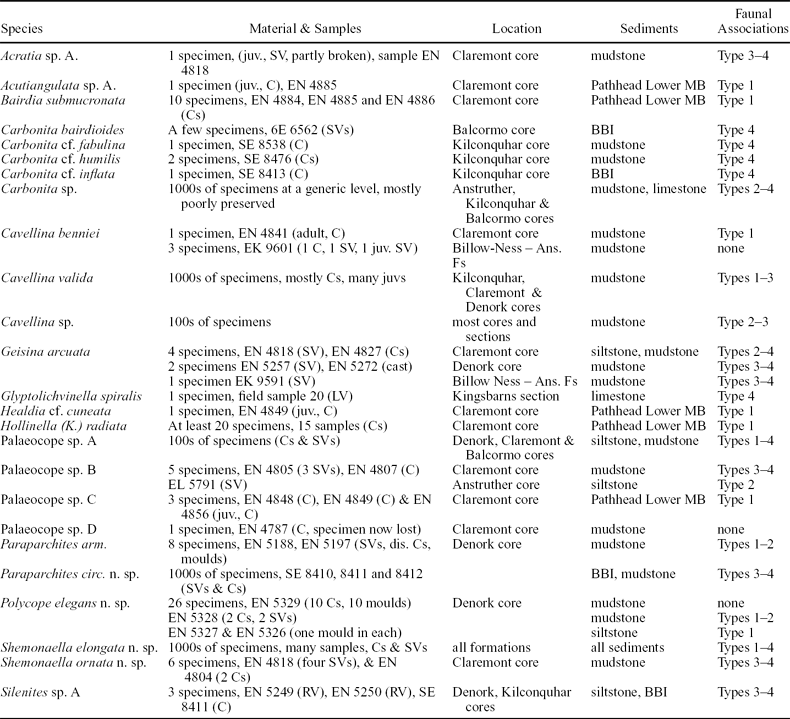
Twenty-five ostracod species are reported from the Strathclyde Group, belonging to the orders Podocopida, Leiocopida, Palaeocopida and Myodocopida (Table 1). Ostracods occur in a range of sediment types and faunal associations (Table 2). The single most diverse ostracod assemblage contains 14 species (Table 3). Ostracods are well preserved, with the exception of those from the Pittenweem Formation, in which most of the carapaces have been dissolved to leave only moulds, and only one species has been identified. The association of particular ostracod species within particular macrofaunal assemblages has been used to determine their ecology.
Table 1. Taxonomic scheme for the ostracods of the Strathclyde Group featured in this study. The higher classification follows Whatley et al. (Reference Whatley, Siveter, Boomer and Benton1993), supplemented by consideration of Sohn (Reference Sohn1985) for the Superfamily Carbonitoidea, and Dewey & Fåhraeus (Reference Dewey and Fåhraeus1987) for the Family Geisinidae

Table 2. Ostracod material, sediments and macrofaunal associations
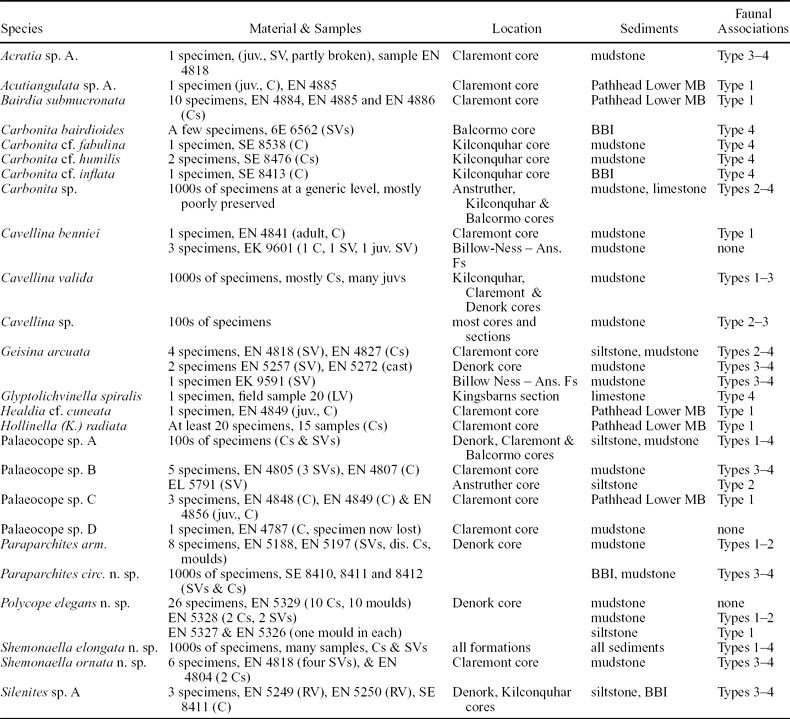
Sample numbers refer to sedimentary rocks containing the ostracods (from BGS boreholes, stored in Edinburgh). Faunal association indicates the ostracod occurrence in particular macrofaunal assemblages.
Abbreviations: C – carapace; SV – single valve; RV – right valve; LV – left valve; juv. – juvenile; dis – disarticulated; Ans. Fs – Anstruther field section; MB – Marine Band; BBI – blackband ironstone.
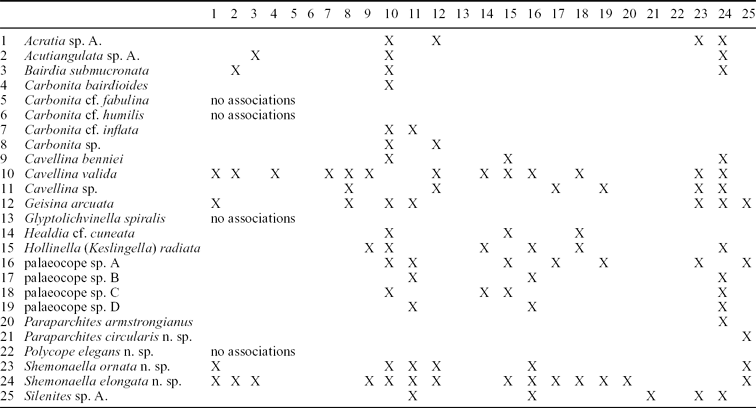
Table 3. Ostracod faunal associations of species from the Strathclyde Group
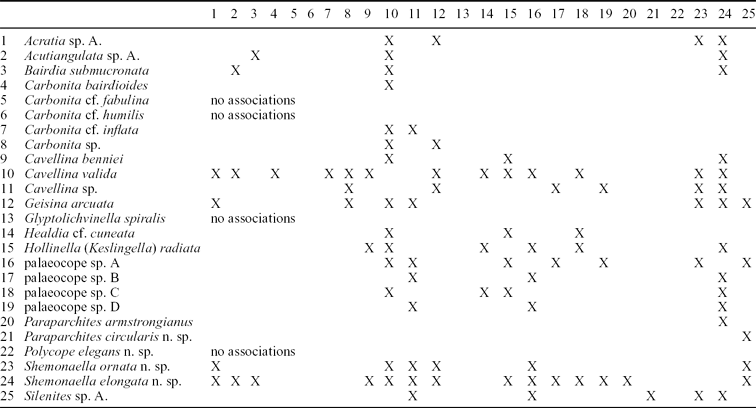
These data do not include the frequency of occurrences; this information is shown for four selected non-marine ostracods in Table 5.
3. Methodology
The sedimentology and fauna were documented from a wide range of rock specimens together with 39 polished thin-sections (cut to a standard thickness of 30 μm) of samples derived from boreholes and field exposures. Ostracods picked from bedding plane surfaces were imaged using a Hitachi S-3600N Scanning Electron Microscope (SEM) at the University of Leicester. Some fossils were photographed on bedding plane surfaces using a Zeiss Axiocam light photography digital imaging system at the Natural History Museum, London.
A taphonomic assessment is important to interpret the ecology of macrofossils and ostracods. An ostracod thanatocoenosis is signalled by the presence of adults and juveniles of different instars, a high proportion of carapaces to single valves and the (rare) occurrence of open (‘butterfly’) carapaces. Mostly single or broken valves, stacked valves and a valve size bias signal a taphocoenosis (sensu Boomer, Horne & Slipper, Reference Boomer, Horne, Slipper, Park and Smith2003). In the sedimentary logs and faunal association tables (Tables 2–5, Figs 3–5), all occurrences of a particular fauna are noted. The problem of apparent ecological ranges being obscured by transportation (Horne, Reference Horne, Park and Smith2003) is addressed by reference to the supposed ecologies of particular fossils, and the taphonomy is carefully considered when assigning an ecological range to any fossil group.
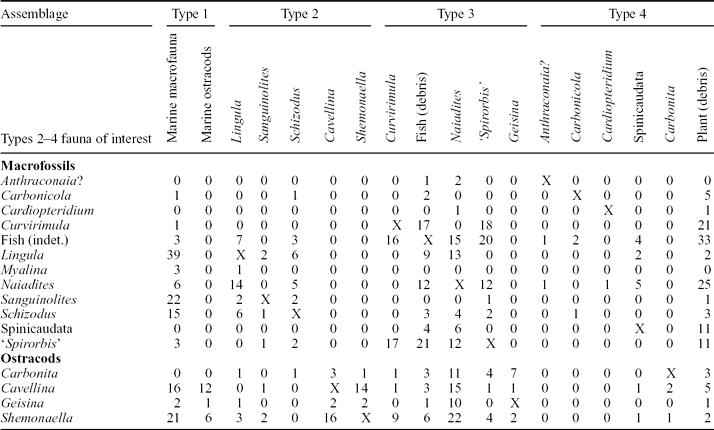
Table 4. The macrofossils and ostracods of the Strathclyde Group, grouped into their respective assemblage types

Three ostracod species do not occur with other fauna and therefore have an uncertain ecology.
Table 5. Faunal associations of common Strathclyde Group non-marine macrofossils and ostracods
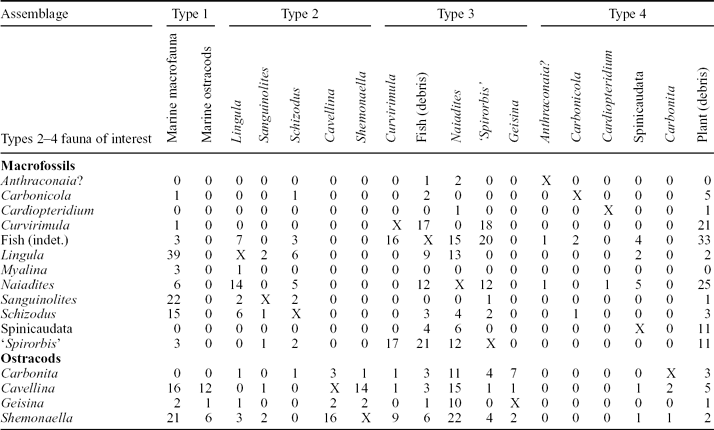
Numbers represent the frequency with which the fauna occur in the same assemblage. An X refers to where the same fauna crosses, for example the association of Cavellina with itself. Marine macrofauna include groups such as brachiopods, bryozoa and gastropods, as defined for the Midland Valley of Scotland by Wilson (Reference Wilson1989), and in this study as a Type 1 Assemblage. For ostracods, the occurrence data are compiled from the single or often multiple species present for that genus.
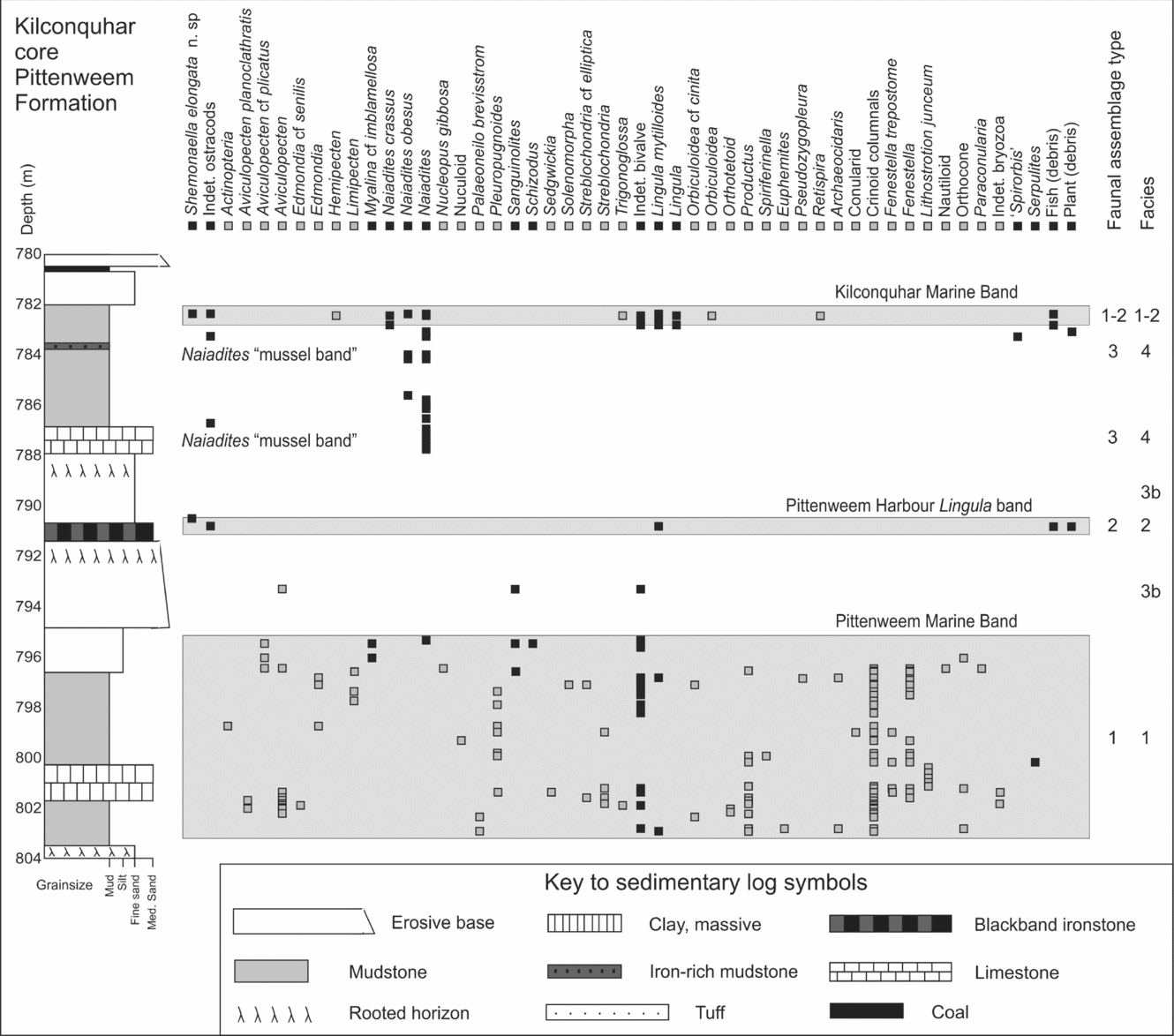
Figure 3. Sedimentary log and fauna of part of the Pittenweem Formation. Type 1 Assemblage elements are represented by a grey colour square and Type 2 to 4 assemblage elements by a black square, at the height in the core at which they occur. The sedimentary log key covers Figures 3–5.
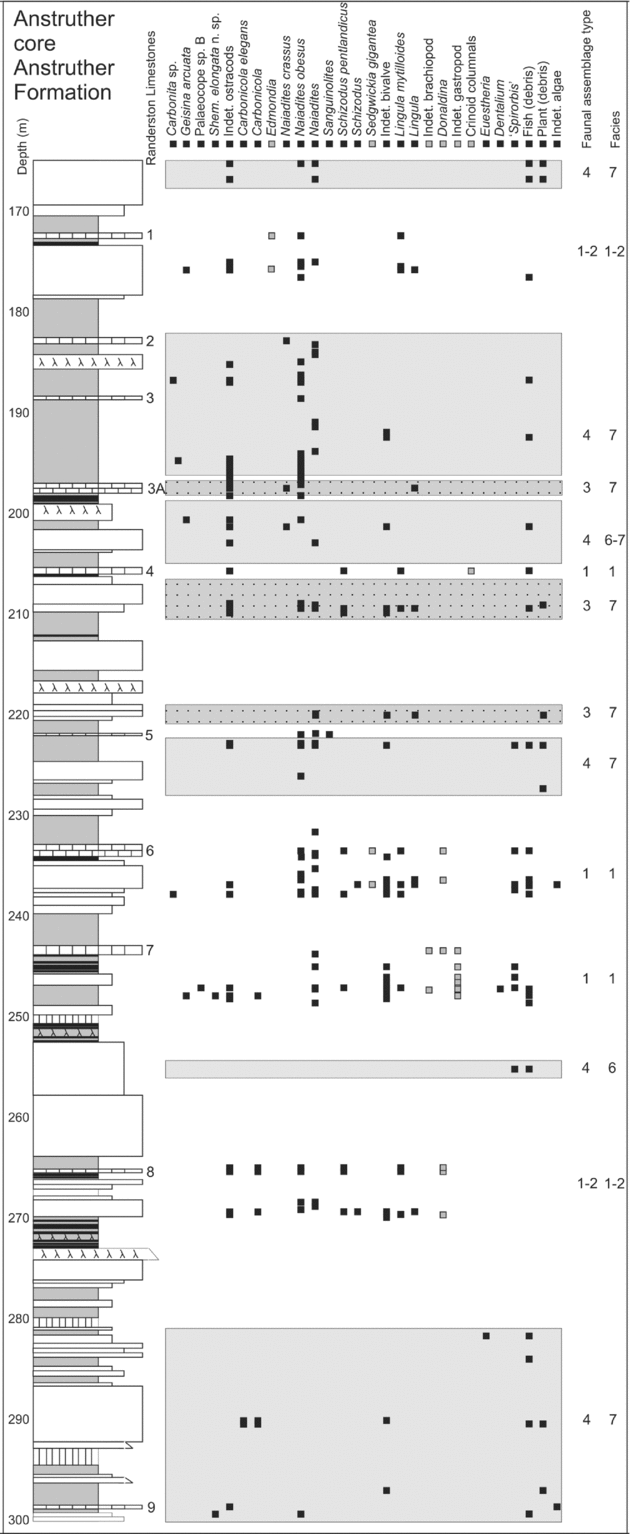
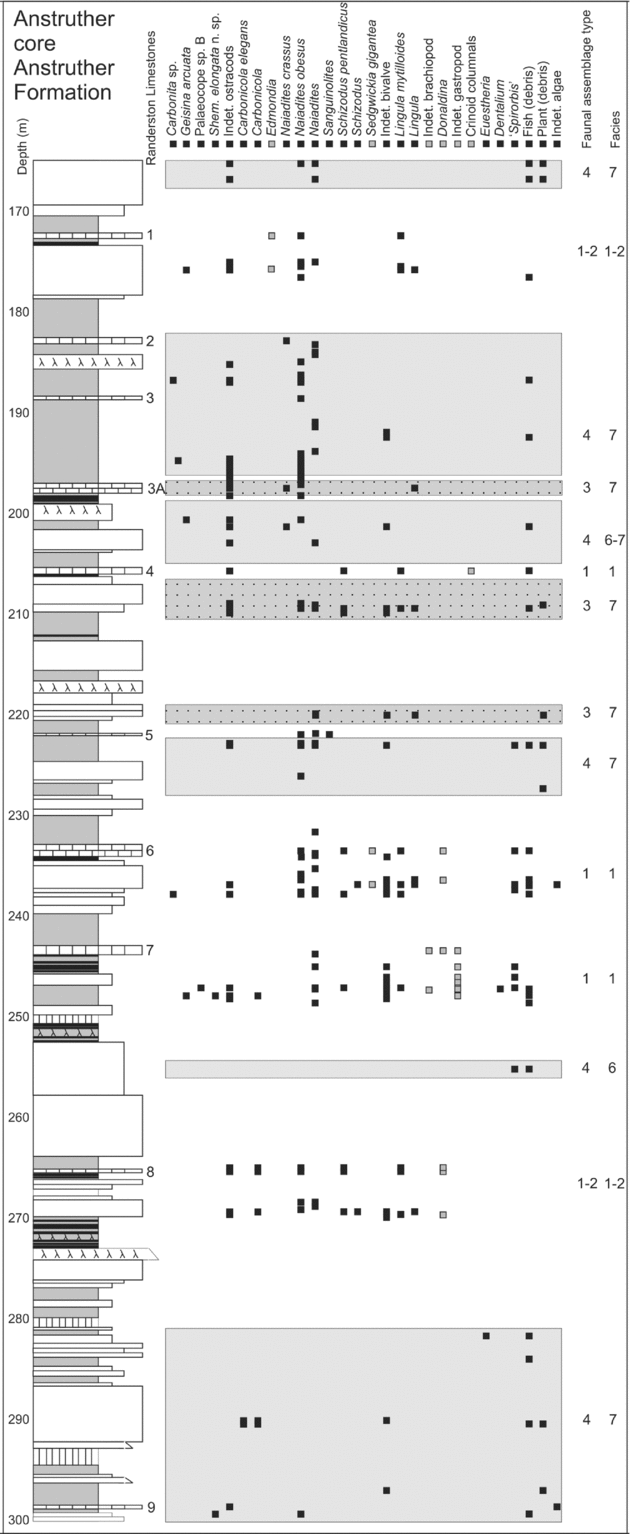
Figure 4. Sedimentary log and fauna of part of the Anstruther core (Randerston Section), Anstruther Formation. Sediments containing Type 3 and 4 assemblages are highlighted with shaded boxes in the background. The Randerston limestones 6 and 7 are typical of those containing a Type 1 Assemblage from this formation.
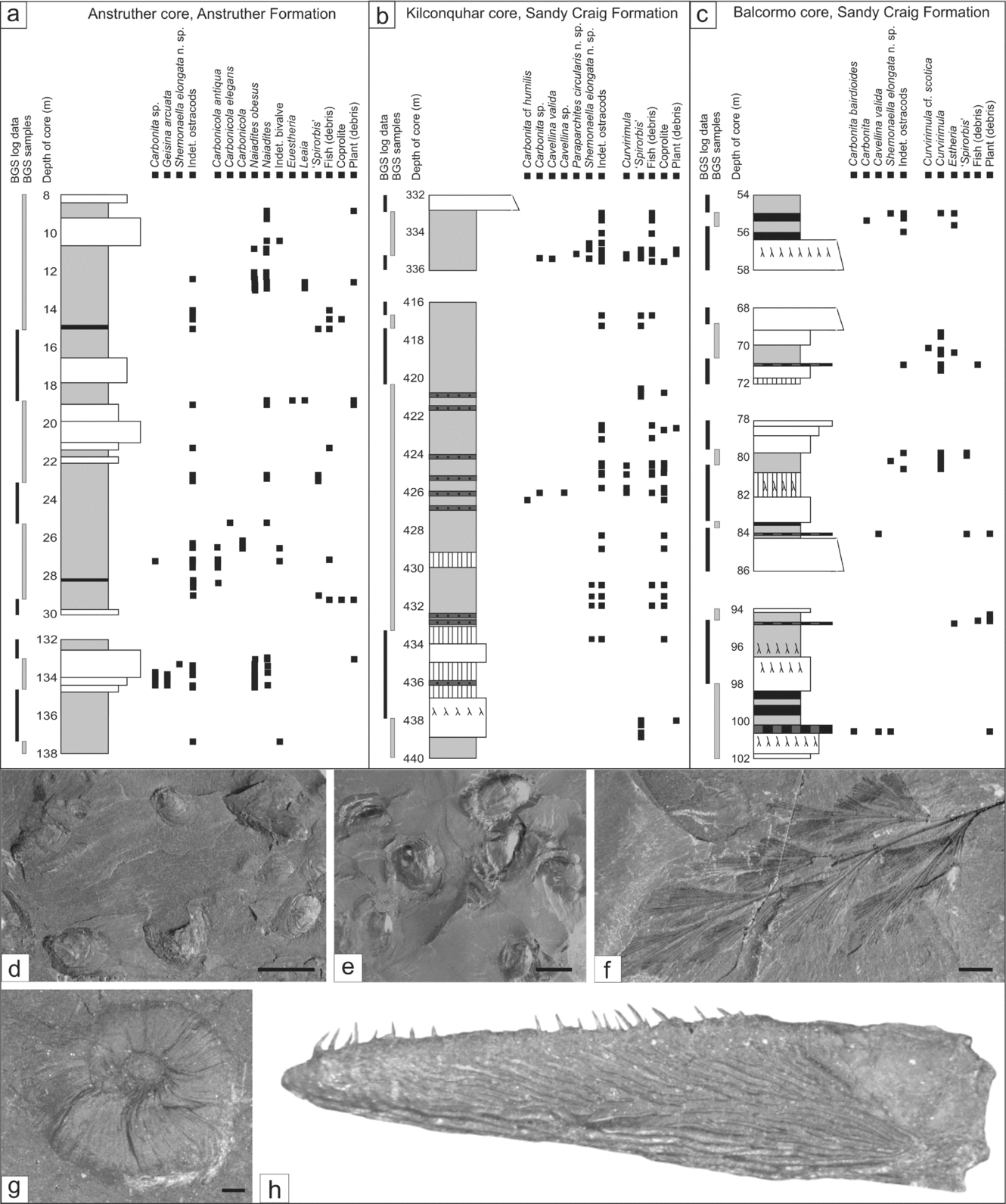
Figure 5. Sedimentary logs of Facies 5 and 6 containing ostracods (a–c) and common Type 3 and 4 assemblage macrofossils (d–h). Mudstones contain the most fossils. (d) Curvirimula cf. scotica, pyritized, GSE 15376. (e) Naiadites obesus, GSE 15378. (f) Telangium affinae, GSE 15380. (g) ‘Spirorbis’, GSE 15379. (h) Fish jaw plate with teeth, 10.5 mm in length, GSE 15382. Light photographs, scale bars 5 mm (d, f) and 0.2 mm (g). Specimens are stored in the palaeontology collection at the BGS, Edinburgh.
4. Faunal assemblages and sedimentary environments
The faunas of the Strathclyde Group can be grouped into recurrent assemblages (Tables 4, 5) and facies (Figs 3–6):

Figure 6. Facies 6 (blackband ironstone; BBI) and Facies 7 (algal limestones) containing ostracods. Table of fauna occurring within BBIs, the most common fauna is Spinicaudata. (a) Polished thin-section (PTS) of a BBI from the Sandy Craig Formation, containing abundant Paraparchites circularis n. sp., MPK 14009. (b) Well-formed stromatolites, Pittenweem Formation, SE 8699. (c–f) Images of the Randerston limestone 9, Anstruther Formation. (c) Vertically stacked hemispheroids and oncoids, field photograph. (d) Size-separated layers of oncoids and pisoliths, field photograph. (e) Abundant ooliths; the concentric laminae are a few microns in thickness, MPK 14004, PTS. (f) Abundant ostracod carapaces, single valves and fish teeth, MPK 14000, PTS, BSEM image. (g, h) Pisolithic plant-rich limestone, Kingsbarns section. (g) Top surface of the limestone bed with numerous log traces (see arrow), field photograph. (h) Cut section through the limestone bed, field sample 19. Scale bars 5 mm (a), 2 cm (b–d), 500 μm (e, f) and 10 mm (h).
Type 1 Assemblage: a high-diversity assemblage of 11 ostracod species and crinoids, orthocones, bryozoans, brachiopods, trilobites, goniatites, gastropods, echinoids and bivalves and the feeding-dwelling trace Chondrites (Table 4). The macrofaunal elements are mainly benthic, with rare nektonic elements such as cephalopods. Hollinella (Keslingella) radiata (Jones & Kirkby, Reference Jones and Kirkby1886) is the most common Type 1 Assemblage ostracod species. This assemblage occurs in Facies 1 and 3a; Facies 1 is fossiliferous limestones within thick (up to 10 m) mudstone intervals or mudstones, with a diverse fauna (Fig. 3). The Pathhead Lower Marine Band has the highest faunal diversity; containing 30 macrofossil and 10 ostracod species. Only very rare Type 1 Assemblage elements are present in Facies 3a; thick, medium–coarse-grained, quartz arenite sandstone successions (from 10–26 m in thickness), present in sections of the formations studied (Fig. 2). Structures include channel forms, parallel laminations, large-scale cross-bedding (1.5 m height planar cross-beds) and extensive convolute-bedding (some convoluted units are 10 m thick) leading to a hummocky weathering pattern. In some cases the tops of large cross-beds are convoluted.
Type 2 Assemblage: includes six ostracod species, the brachiopod Lingula, the bivalves Myalina, Schizodus, Sanguinolites and fish (Table 4). Lingula is most common in the Type 1 Assemblage, but also occurs in Type 2, 3 and 4 assemblages (Table 5), as do Schizodus and Sanguinolites. The assemblage is typified by an association of the ostracods Cavellina valida (Jones, Kirkby & Brady, Reference Jones, Kirkby and Brady1884), Shemonaella elongata n. sp. and palaeocope sp. A. A Type 2 or low-diversity Type 1 Assemblage occurs in Facies 2 mudstones and limestones, most common in the Pathhead and Pittenweem formations (Fig. 3).
Type 3 Assemblage: comprises 11 ostracod species, the most common being Paraparchites circularis n. sp., ‘Spirorbis’, the bivalves Curvirimula scotica and Naiadites obesus and fish (Table 4). Naiadites is the most common and ‘Spirorbis’ is frequently associated with Curvirimula, Naiadites, fish and plant debris (Table 5). Modern Spirorbis is a polychaete worm, but Palaeozoic ‘spirorbids’ are related to lophophorates (Microconchida; Taylor & Vinn, Reference Taylor and Vinn2006), encrust hard substrates (such as stromatolites: Burchette & Riding, Reference Burchette and Riding1977) and were probably suspension feeders. Specimens are planispiral (230–2000 μm diameter) evolute, prostrate discoidal calcareous tubes. Type 3 Assemblage elements occur in Facies 4; mudstones and limestones such as Naiadites ‘mussel bands’, are particularly common in the Pittenweem (Fig. 3) and Anstruther (Fig. 4) formations. These mussel bands represent a taphocoenosis, but Naiadites is also present in thanatocoenosis deposits of low-abundance, monospecific or low-diversity elements of Type 3 and 4 assemblages (Fig. 4).
Type 4 Assemblage: includes 15 ostracod species, most commonly Carbonita sp., the bivalves Anthraconaia, Carbonicola antique, C. elegans, Cardiopteridium and the branchiopod conchostracans Spinicaudata (Table 4). Curvirimula, Naiadites, fish and ‘Spirorbis’ occur in both Type 3 and 4 assemblages. Anthraconaia, Carbonicola and Cardiopteridium commonly occur as low-diversity assemblages associated with Naiadites, plant and fish debris (Bennison, Reference Bennison1960, Reference Bennison1961 and this study). Euestheria, Estheria and Leaia occur in mudstones, associated with plants, fish, Naiadites and ostracods (Table 5). Fish debris (fragments, scales and teeth) are ubiquitous in a range of faunal associations throughout the Strathclyde Group, but occur frequently with Type 3 and 4 assemblages (Table 5). The most complete specimen is a small fish jaw, with affinities to the actinopterygian Rhadinichthys ferrox Traquair (Z. Johanson pers. comm.).
Type 4 Assemblage elements are present in Facies 3b, 5, 6 and 7. Facies 3b is medium-grained, quartz arenite sandstone units (2–10 m in thickness), with Stigmaria roots and bioturbated bases and tops of beds, common in all studied sections. Sedimentary structures include sigmoidal surfaces, trough cross-bedding, planar cross-bedding, current ripples and rare desiccation cracks. Sandstones are commonly interbedded with mudstones on a centimetre to millimetre scale. The thinner beds (commonly 1 m or less), compared to the thicker or convoluted beds of Facies 3a, can be clearly distinguished at exposure. Low-diversity ichnofossil assemblages are most common in this facies and include Monocraterion, Arenicolites, Diplocraterion, Skolithos and Teichichnus and Palaeophycus and Planolites. Rare body fossils include Naiadites, fish, plants, ‘Spirorbis’, Lingula, Spinicaudata, Type 1 and Type 2 Assemblage bivalves and poorly preserved ostracods. Facies 5 sediments are mudstones, carbonaceous shales, siltstones and sandstones with plentiful plant debris; common in the Anstruther and Sandy Craig formations (Fig. 5a–h). The Type 4 Assemblage ostracods are most diverse in Facies 5.
Facies 6 sediments are blackband ironstones composed of alternating iron-rich mudstone (occasionally siltstone) laminae, black carbonaceous mudstone laminae and coal with abundant plant debris; present in sections of the Pathhead, Sandy Craig and Pittenweem formations (Fig. 5). Common fauna in this facies are Spinicaudata, Lingula, Curvirimula, fish, ‘Spirorbis’ and ostracods (Fig. 6a). Ostracods are mostly preserved as carapaces and occur on both ironstone and mudstone laminae, but are more common on the iron-rich laminae. Paraparchites circularis n. sp. occurs within a 20 cm thick blackband ironstone in great abundance, associated with plant debris, rare fish and rare Carbonita cf. inflata and Silenites sp. A. In this thanatocoenosis ostracod carapaces are randomly oriented; adult carapaces are most abundant, with some juveniles (Fig. 6a).
Coals and extensively rooted sandstones or siltstones (‘seat-earths’) are associated with Facies 6. No ostracods have been identified from coals although Carbonita is common in Pennsylvanian coal deposits elsewhere (see, for example, Bless & Pollard, Reference Bless and Pollard1973; Sohn, Reference Sohn1977; Schäfer, Reference Schäfer, Schindler and Heidtke2007). The ‘seat-earths’ commonly underlie shaly coal or coal beds, range in thickness from 0.1–1 m, and do not contain ostracods or macrofauna. The roots are composed of large 1 cm diameter siderite-filled vertical roots and smaller (millimetre thick) organic branching rootlets. Thin units with remnant sedimentary structures are common in all formations and are often overlain by cross-bedded or laminated sandstones. Thicker units (1 m thick) with pervasive root structures are prominent because the roots have destroyed any sedimentary structures. Three of these thick units are identified from field exposures of the Pittenweem and Sandy Craig formations and each is overlain by coals.
Facies 7 dolomitized algal limestones are most numerous in the Anstruther Formation (Fig. 2), and are either stromatolitic or of oncoidal-type concentric form. The fauna includes ‘Spirorbis’, fish and plant debris, coprolites, Naiadites, and the ostracods Carbonita sp., rare Glyptolichvinella spiralis, Shemonaella elongata n. sp. and mostly indeterminate ostracods. Most limestone beds contain elements of both vertically stacked hemispheroids (stromatolites) and concentrically laminated spheroids (oncoids). Oncoids range in size (centimetres to millimetres), while stromatolites vary in form from laminated to well-formed hemispheroids. Vertically stacked hemispheroids have Cryptozoon-like club or columnar shapes in outline (Logan, Rezak & Ginsburg, Reference Logan, Rezak and Ginsburg1964) and some are brecciated at the top (probably owing to desiccation). The basal radius of the laminae increases upwards and domes at the top (Fig. 6b). The spaces between the hemispheroids and within desiccation cracks are filled with oncoids, detrital sediment, fish fragments and abundant ostracods. Freshwater stromatolites are typically composed of a diverse community of algae and microinvertebrates (Freytet & Verrecchia, Reference Freytet and Verrecchia1998). The Strathclyde Group limestones have been extensively dolomitized (Searl, Reference Searl1991) and particular algal species cannot be determined.
In the Anstruther Formation Randerston section (Fig. 6c–f) an algal limestone with both stromatolitic (Fig. 6c) and oncoidal forms (Fig. 6d, f) contains the earliest record of Carbonita from the Midland Valley. Some of the oncoids incorporate pisoliths and ostracods in their centres. The ostracod assemblage is composed of 30% carapaces, whole single valves, and a range of adults and juveniles, representing a thanatocoenosis (Fig. 6f). In other areas there are abundant single valves, densely packed together, representing a taphocoenosis. Overall, the ostracod taphonomy in most algal limestone beds suggests only some limited localized transport.
Pisolithic limestones are the most common in the Anstruther Formation and can contain ‘Spirorbis’, fish (debris), ostracods, rare Lingula, rare Myalina and abundant plant debris. In the Kingsbarns section a layer with irregular-shaped pisoliths (some of which contain ostracods in the centre) is overlain by siltstone packed with wood debris, including logs up to 4 m in length (Fig. 6g, h). Poorly preserved ostracods are present in both layers, with single valves composing 95 % of the assemblage.
5. Ecological interpretation of the Strathclyde Group macrofauna and ostracods
The interpretation of the ecology of the ostracods is based on macrofauna–ostracod or ostracod faunal associations, with reference to known Mississippian ostracod distribution patterns (Tables 2, 4; Fig. 7). Palaeoenvironments are interpreted based on faunal and sedimentological evidence (Fig. 8).
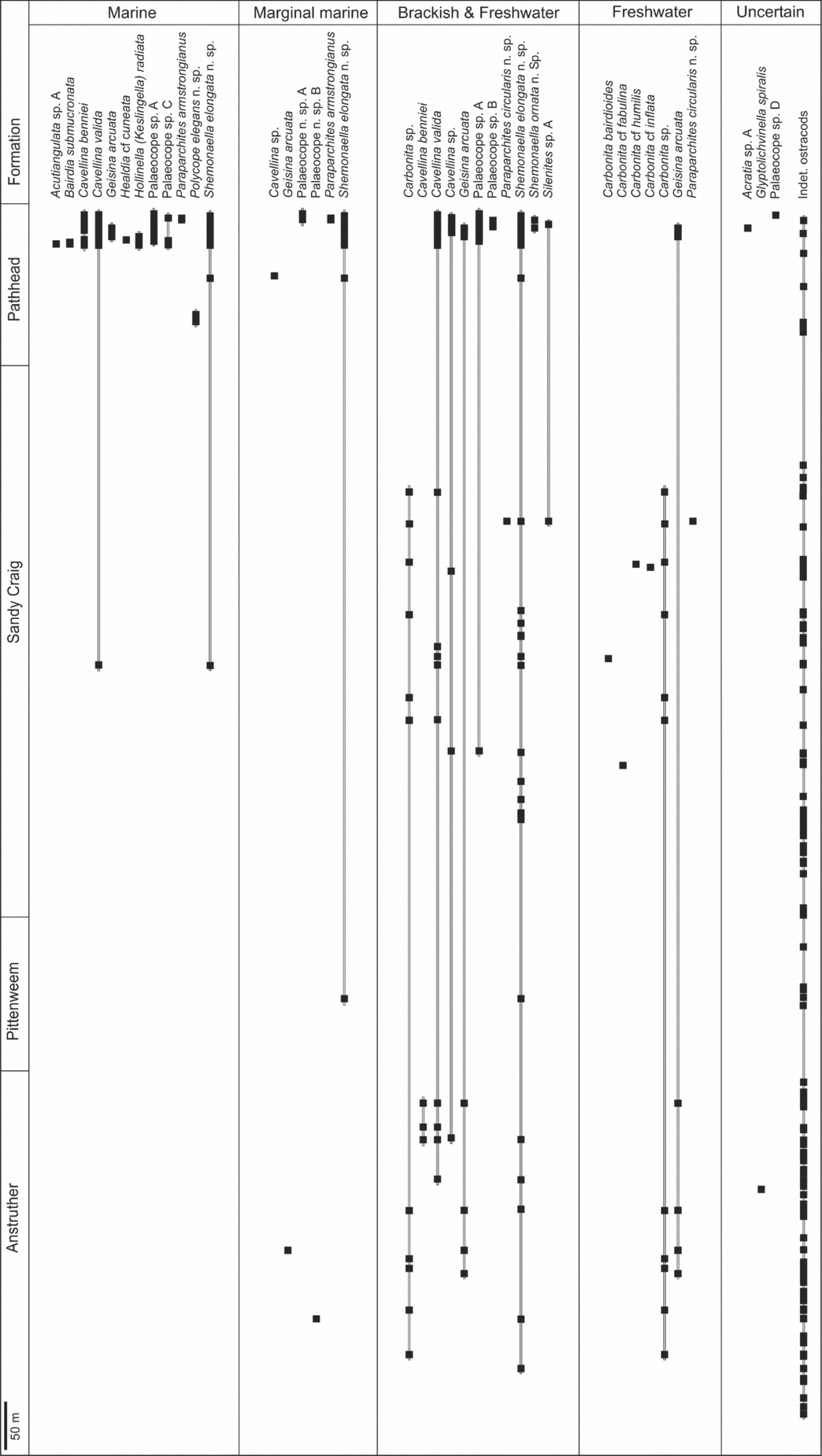
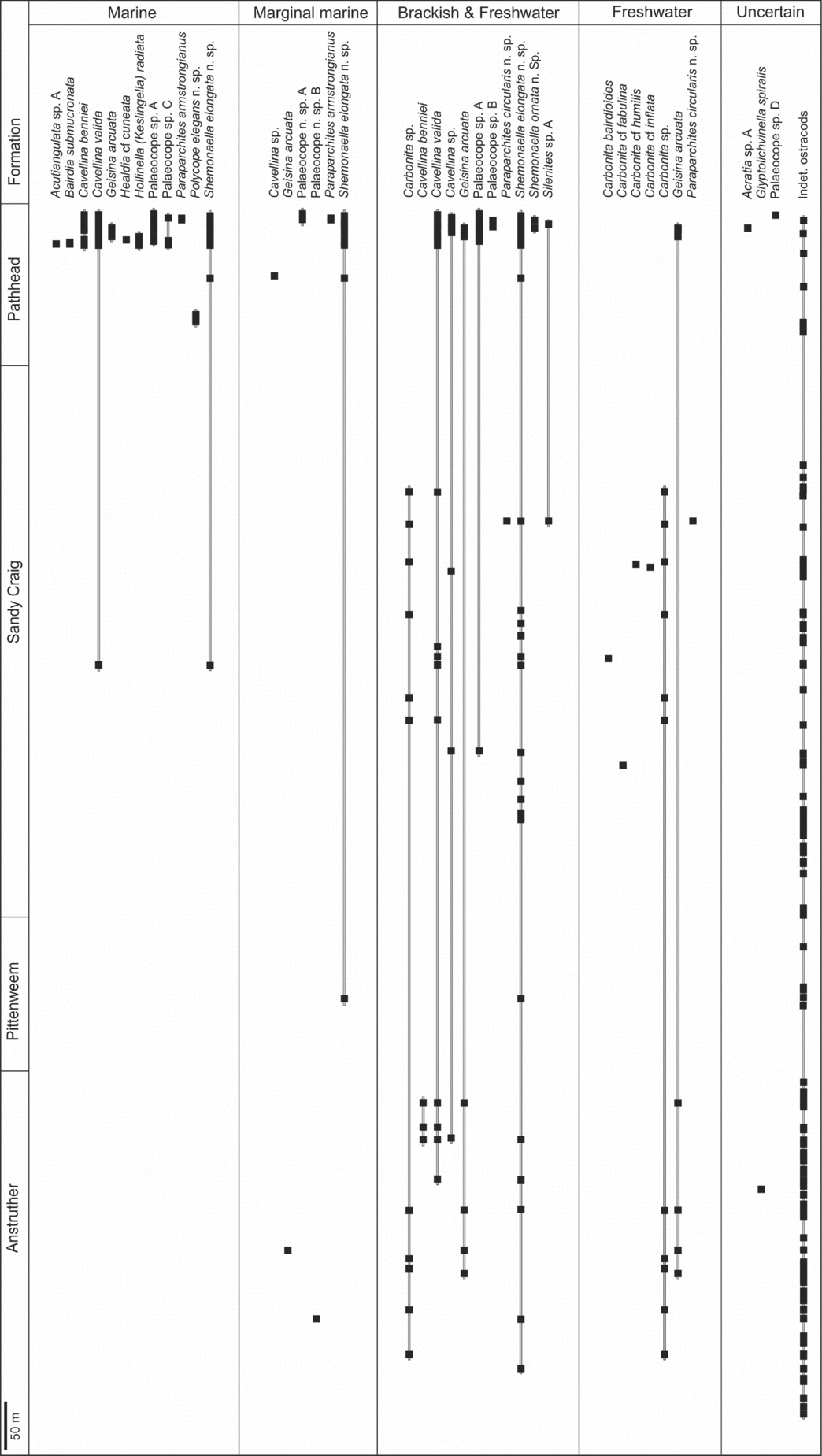
Figure 7. Stratigraphic range and palaeoenvironments of the Strathclyde Group ostracods. Cavellina valida, Geisina arcuata, palaeocope sp. A and Shemonaella elongata n. sp. are the most long ranging.

Figure 8. Palaeoenvironmental model of the ecological range of ostracods for the Anstruther to Pathhead formations. The environmental range of each ostracod species is plotted using the data from Tables 2 and 4, and does not include ostracods that have an indeterminate taxonomy or ecology. Plants are representations of a Carboniferous lycopsid and of Valmeyerodendron. Abbreviations used for ostracod species in the key: H. – Hollinella; Par. – Paraparchites; Shem. – Shemonaella. In the Anstruther Formation blackband ironstones are not present and coals are uncommon, so swampy conditions are minimized in the model. The presence of only Shemonaella elongata n. sp. ostracods in the Pittenweem Formation is due to poor preservation. The Sandy Craig Formation is dominated by non-marine conditions. Stromatolitic limestones are absent in the Pathhead Formation, so a near-shore lake is excluded from the model.
5.a. Type 1 Assemblage: marine
These fossil groups have been determined in the MVS as marine (Wilson, Reference Wilson1989). Chondrites is a Palaeozoic marine ichnogenus (Buatois et al. Reference Buatois, Gingras, Maceachern, Mángano, Zonneveld, Pemberton, Netto and Martin2005). Most pelagic Type 1 Assemblage fossils are fragmented, while well-preserved benthic shelf faunas are the richest, and contain the most abundant fossils.
Twelve ostracod species from this assemblage are considered marine (Fig. 7). Of these, Acutiangulata sp. A, Bairdia submucronata, Healdia cf. cuneata, Hollinella (Keslingella) radiata, palaeocope sp. C and Polycope elegans n. sp. are considered to be stenohaline, as they are only associated with marine macrofossils. Bairdia is abundant in Mississippian marine assemblages (Bless, Reference Bless1983; Bless, Streel & Becker, Reference Bless, Streel and Becker1988), as are Healdia and Hollinella (Přibyl, Reference Přibyl1960; Olempska, Reference Olempska1993; Athersuch et al. Reference Athersuch, Gooday, Pollard, Riley, Whittaker and Hart2009). Acutiangulata is known for its possible brackish-water tolerance, in association with Shemonaella, Cavellina and ‘Spirorbis’ (Athersuch et al. Reference Athersuch, Gooday, Pollard, Riley, Whittaker and Hart2009; Robinson, Reference Robinson, Bate and Robinson1978). Polycope elegans n. sp. is associated with marine macrofossils in a thanatocoenosis. Polycopids are supposed nektobenthic ostracods (Horne, Reference Horne, Park and Smith2003) and are reported as marine in the Mississippian (Dewey & Fåhraeus, Reference Dewey and Fåhraeus1987).
Facies 1 sediments are interpreted as near-shore, shallow marine deposits. Metres-thick successions of mudstones (containing articulated orthocones), representing fairly prolonged marine conditions, occur in the Pathhead Formation (Fig. 8), but are rare. Frequent short-lived marine transgressions are represented by thin marine mudstones overlain by coarsening-upwards successions. The low diversity of the marine ostracod fauna suggests short-lived open marine conditions. Only six species are interpreted as stenohaline and other common Mississippian marine ostracods such as Kirkbya and Amphissites (Coen, Michiels & Parisse, Reference Coen, Michiels and Parisse1988; Olempska, Reference Olempska1993) are absent. The tens of metres thick sandstone units of Facies 3a, with convolute-bedding and channel forms indicate proximal deltaic mouth bar deposition, with a rare marine macrofauna incorporated at the delta front.
5.b. Type 2 Assemblage: marginal marine
The lower-diversity Type 2 Assemblage is interpreted as marginal marine. Lingula squamiformis from the Mississippian of the MVS has been interpreted as a brackish species (Ferguson, Reference Ferguson1963). Here, Schizodus, Sanguinolites and Lingula have similar faunal associations and are interpreted as marginal marine. Myalina is recorded as a common freshwater bivalve by Ferguson (Reference Ferguson1962), but in the present study its associations (Table 5) determine it as marginal marine, albeit based only on low numbers of specimens.
Six species from the Type 2 Assemblage, that occur in a range of marine, marginal marine and brackish to freshwater sediments (Fig. 7), are considered as eurytopic or marginal marine. Post-Palaeozoic ostracods of the Suborder Platycopina are exclusively marine (Horne, Reference Horne, Park and Smith2003), but the Mississippian platycopes Cavellina, Geisina and Glyptolichvinella are euryhaline or of uncertain ecology. At a generic level, ostracod–macrofaunal associations reveal that Cavellina and Shemonaella have a wide salinity tolerance (Table 5). In particular, the species Cavellina valida and Shemonaella elongata n. sp. are abundant (Table 2) and occur in a wide range of environments (Fig. 7) with many other ostracod species (Table 3) and are considered to be eurytopic. Cavellina is known for its marginal marine to brackish-water tolerance in Carboniferous environments (Robinson, Reference Robinson, Bate and Robinson1978; Williams et al. Reference Williams, Stephenson, Wilkinson, Leng and Miller2005, Reference Williams, Leng, Stephenson, Andrews, Wilkinson, Siveter, Horne and Vannier2006). Many Mississippian Shemonaella species are interpreted as marine (for example, Crasquin, Reference Crasquin1985; Dewey, Reference Dewey and Maddocks1983), but some are considered as brackish (Tibert & Scott, Reference Tibert and Scott1999). Mississippian species of Paraparchites with dorsal spines occur in association with typically marine genera such as Amphissites and Bairdia (Sohn, Reference Sohn1969). Here, Paraparchites armstrongianus is interpreted as marginal marine (Fig. 7, Table 5). Marginal marine environments include lagoons, embayments or estuaries associated with lower salinities (Fig. 8). Many ostracods are eurytopic and these environments may have provided a pathway for marine to non-marine transitions.
5.c. Type 3 Assemblage: brackish–freshwater
Palaeozoic spirorbids are reported from freshwater, brackish, marine and hypersaline environments (Taylor & Vinn, Reference Taylor and Vinn2006; Wilson, Reference Wilson1989). Here, the faunal associations of ‘Spirorbis’ suggest that it is brackish-water tolerant. Curvirimula, Naiadites, fish and ‘Spirorbis’ are interpreted to have a brackish to freshwater tolerance.
Four ostracod species are interpreted as brackish to freshwater tolerant. Geisina arcuata is mostly associated with brackish to freshwater (Type 3) macrofossils (Table 5), and this is a common environmental interpretation for this species in the Mississippian (Pollard, Reference Pollard1966, Reference Pollard1969; Anderson, Reference Anderson1970; Bless & Pollard, Reference Bless and Pollard1973; Bless, Streel & Becker, Reference Bless, Streel and Becker1988). Paraparchites circularis n. sp. occurs in great abundance in blackband ironstones, which may represent opportunistic reproduction in quickly colonizing this plant-rich environment. Carboniferous species of Paraparchites have been recorded from environments interpreted as marine (Dewey, Reference Dewey1988), non-marine (Kummerow, Reference Kummerow1953; Williams et al. Reference Williams, Stephenson, Wilkinson, Leng and Miller2005) and hypersaline (Dewey, Reference Dewey and Maddocks1983, Reference Dewey1987, Reference Dewey1988; Williams et al. Reference Williams, Stephenson, Wilkinson, Leng and Miller2005, Reference Williams, Leng, Stephenson, Andrews, Wilkinson, Siveter, Horne and Vannier2006). The associations of Shemonaella ornata n. sp. (ostracods, Naiadites, fish and plant debris) indicate more brackish-water tolerance than S. elongata n. sp. Despite its faunal associations here, Mississippian Silenites has been recorded from marine sediments (Crasquin, Reference Crasquin1985).
Naiadites is commonly associated with brackish macrofauna such as Curvirimula and ‘Spirorbis’ or occurs as a monospecific assemblage in Facies 4 sediments. Its rare occurrence in association with marine faunas may be owing to post-mortem transport. Naiadites mussel bands have undergone post-depositional transport by currents and wave action, as is seen in shallow marine bivalve and oyster coquinas (Wakefield, Reference Wakefield1995). As the exact palaeoenvironmental conditions of Naiadites and other macrofauna are not known, there is uncertainty regarding a brackish or a freshwater interpretation.
5.d. Type 4 Assemblage: freshwater
Anthraconaia, Carbonicola and Cardiopteridium are interpreted as freshwater bivalves (Bennison, Reference Bennison1960, Reference Bennison1961). Anthraconaia and Carbonicola are commonly associated with Naiadites in Pennsylvanian freshwater Coal Measures (for example, Jenkins, Reference Jenkins1960; Hartley, Reference Hartley1993; Brand, Reference Brand1996; Eagar & Belt, Reference Eagar and Belt2003). Anthraconaia, Carbonicola, Curvirimula and Naiadites occur in brackish (Ballèvre & Lardeux, Reference Ballèvre and Lardeux2005) or lacustrine Mississippian sediments (Guirdham et al. Reference Guirdham, Andrews, Browne and Dean2003) and freshwater Pennsylvanian sediments (Brand, Reference Brand1994; Anderson et al. Reference Anderson, Dunlop, Eagar, Horrocks and Wilson1999; Falcon-Lang, Reference Falcon-Lang2005; Falcon-Lang et al. Reference Falcon-Lang, Benton, Braddy and Davies2006).
Extant Spinicaudata typically live in freshwater, but can tolerate salinities up to 6‰ NaCl. Late Palaeozoic Spinicaudata are found in a range of freshwater to brackish coastal plain sediments (Knox & Gordon, Reference Knox and Gordon1999; Jones & Chen, Reference Jones and Chen2000; Park & Gierlowski-Kordesch, Reference Park and Gierlowski-Kordesch2007). Some brackish water records are interpreted as a taphocoenosis (for example Webb, Reference Webb1979), and Late Palaeozoic spinicaudants mostly occur in freshwater environments, including coals and lake sediments (Orr & Briggs, Reference Orr and Briggs1999; Vannier, Thiery & Racheboeuf, Reference Vannier, Thiery and Racheboeuf2003; Hmich et al. Reference Hmich, Schneider, Saber, Voight, El Wartiti, Lucas, Cassinis and Schneider2006).
The association of possible actinopterygian and sarcopterygian fish debris in Type 3 and 4 assemblages is consistent with a brackish to freshwater ecology. Freshwater actinopterygians and sarcopterygians are common in the Late Palaeozoic (for example, Daeschler, Reference Daeschler2000; Trewin & Davidson, Reference Trewin and Davidson1996; Turner, Kemp & Warren, Reference Vannier, Thiery and Racheboeuf1999). In other contemporaneous MVS sedimentary rocks, fish occur in a range of water bodies, from deep-lagoonal (the Wardie Shales) to a semi-permanent lake on a coastal plain (the Foulden Beds; see Dineley & Metcalf, Reference Dineley, Metcalf and Palmer1999).
Species of Carbonita are mostly associated with brackish to freshwater macrofossils (Table 5) and, importantly, only species of Carbonita occur exclusively in sediments interpreted as freshwater (Fig. 7): Carbonita bairdioides and Carbonita cf. inflata occur in blackband ironstones; Carbonita cf. fabulina and Carbonita cf. humilis in siltstones–mudstones associated with plant and fish debris. Carbonita is described from Mississippian brackish water sediments (Pollard, Reference Pollard1985; Sohn, Reference Sohn1985; Tibert & Scott, Reference Tibert and Scott1999). Vannier, Thiery & Racheboeuf (Reference Vannier, Thiery and Racheboeuf2003) first document unambiguous freshwater Carbonita from Pennsylvanian sediments deposited in an intramontane temporary pond.
The cross-bedded sandstones with sigmoidal structures of Facies 3b are interpreted as meandering fluvial channels. The low-diversity ichnofauna typify brackish to estuarine conditions (Buatois et al. Reference Buatois, Gingras, Maceachern, Mángano, Zonneveld, Pemberton, Netto and Martin2005), consistent with a macrofauna of Naiadites, fish and plants. Overbank deposits contain Stigmaria roots and desiccation cracks. The mouth bar and alluvial environments are important components of the environment, but lack ostracods (Fig. 8). Facies 5 mudstones containing Type 3–4 assemblages and abundant plant debris are interpreted as freshwater inland lake or temporary pond deposits. These are present in all except the Pittenweem Formation (Fig. 8). Temporary pools or shallow lakes are a common habitat for Spinicaudata (Vannier, Thiery & Racheboeuf, Reference Vannier, Thiery and Racheboeuf2003) and fish (Dineley & Metcalf, Reference Dineley, Metcalf and Palmer1999) in the Carboniferous. The ostracod-bearing pisolithic plant-rich algal limestone is interpreted to have formed in a shallow carbonate-rich temporary pond or lake where pisoliths formed. The original ostracod ecology is uncertain owing to transport; the ostracods may have originated in a nearby freshwater swamp where wood accumulated, and were later transported to the site of deposition.
The blackband ironstones of Facies 6 represent a key depositional environment for freshwater ostracods. The macrofaunal content of these sediments favours a freshwater interpretation. Blackband ironstones are common in the Pennsylvanian Coal Measures, generally associated with upper delta-plains to alluvial floodplains and coastal plain swamps with small lakes (Boardman, Reference Boardman1989). The Strathclyde Group depositional setting is interpreted as a swampy wetland where plant debris had time to accumulate, creating the black laminae alternating with iron-rich mudstone–siltstone deposited in shallow water stream-fed pools. Fine grain size suggests still-water deposition and the laminae indicate shallow to fluctuating water levels and the periodic accumulation of plant debris.
The ‘seat-earths’ present below coal beds are interpreted as exposure surfaces on a well-drained floodplain, where vegetation had time to develop. Most of these horizons are thin, and are interpreted as relatively brief periods of sub-aerial exposure. The three thicker, extensively rooted horizons are interpreted as palaeosols that developed over a longer time period, and would be classified as a spodosol (sandy forest soil; Retallack, Reference Retallack2001). These units are important for interpreting terrestrial conditions.
The non-marine limestones/dolostones (Facies 7) and associated sedimentary rocks of the Anstruther Formation are a key environment for early non-marine ostracods (Fig. 8). Within these lake-deltaic cycles, occasional marine transgressions occurred, but the predominance of Type 3 and 4 assemblages indicates that brackish to freshwater salinity conditions were the most prevalent. Freshwater conditions were unstable, seen in the variation of stromatolitic to oncoidal algal forms (Logan, Rezak & Ginsburg, Reference Logan, Rezak and Ginsburg1964) and the presence of desiccation cracks, although the periods of exposure were short-lived enough not to result in pedogenesis (cf. MacNeil & Jones, Reference MacNeil and Jones2006). Carboniferous freshwater algal limestones have been reported from the MVS (Guirdham et al. Reference Guirdham, Andrews, Browne and Dean2003), France (Freyet, Broutin & Durand, Reference Freytet, Broutin and Durand2000) and Illinois (Scott, Reference Scott1944). In the Pennsylvanian of Illinois stromatolitic limestones contain abundant indeterminate ostracods and ‘Spirorbis’, interpreted to have lived in a shallow water lake (Scott, Reference Scott1944). Oncoidal-type grains are more problematic, as they have been described from a range of marine to non-marine settings (Davaud & Girardclos, Reference Davaud and Girardclos2001; Peryt, Reference Peryt and Peryt1983). The algal species of the present study cannot be determined, but despite this, we know that ostracods lived in association with these algal forms and that the environment was probably freshwater.
5.e. Uncertain environment
When there is only one ostracod specimen for a particular species, with few or no associated faunas, the palaeoecology is uncertain, as in the case of Acratia sp. A, Glyptolichvinella spiralis and palaeocope sp. D. Acratia is reported as marine (Olempska, Reference Olempska1993), and Glyptolichvinella as marginal marine to brackish (Williams et al. Reference Williams, Stephenson, Wilkinson, Leng and Miller2005, Reference Williams, Leng, Stephenson, Andrews, Wilkinson, Siveter, Horne and Vannier2006).
6. The ostracod radiation into non-marine environments
The success of the initial colonization of non-marine water bodies by ostracods was probably dependent on a number of factors, including intrinsic adaptations of ostracod species to lower salinities; extrinsic mechanisms to drive non-marine colonization, such as changing sea-levels; and a favourable aqueous continental environment to foster ostracods in this new environment.
6.a. Intrinsic adaptations of non-marine ostracods
Of the brackish to freshwater ostracods from this study, their origin and affinity to known living freshwater ostracods is poorly resolved, particularly because all of these Carboniferous genera (Carbonita, Cavellina, Geisina, Paraparchites, Shemonaella and Silenites) became extinct by the end-Permian (Moore, Reference Moore1961). The Carbonitoidea have no extant relatives or preserved soft parts for comparison. The origins of the Carbonitoidea are unknown; hypotheses link them to the marine Healdioidea (Retrum & Kaesler, Reference Retrum and Kaesler2005), Darwinulocopina or marine Sigilloidea (Horne, Reference Horne, Park and Smith2003). Retrum & Kaesler (Reference Retrum and Kaesler2005) noted that the muscle scar pattern of Permian freshwater Carbonita is more like that of the Healdioidea than the Cytheroidea or Cypridoidea. While the Carbonitoidea have been compared to living podocopes such as Cypridopsis (Neale, Reference Neale1984), the evidence for true cypridoideans in the Carboniferous is debated: some argue for a Late Palaeozoic origin (Lethiers & Damotte, Reference Lethiers and Damotte1993; Swain, Reference Swain1976); others place the origin of the Cypridoidea in the Mesozoic (Horne Reference Horne, Park and Smith2003; Tibert et al. Reference Tibert, Colin, Leckie and Babinot2003; Whatley & Ballent, Reference Whatley and Ballent1996).
Despite the problems of relating Carboniferous ostracods to living species, it is clear that important and fundamental physiological changes would be needed for ostracods to adapt to reduced salinities. Of primary importance are osmoregulation and reproductive changes, along with modifications in lifestyle. For the individual animal to survive it would have to regulate the salt intake and output. Osmoregulation takes place by gaseous exchange through the integumental circulatory system in the inner lamella (Aladin & Potts, Reference Aladin and Potts1996; Vannier & Abe, Reference Vannier and Abe1995). Some living ostracods such as Cyprideis torosa are very successful at this and can survive in salinities of 1–40‰ NaCl (Keyser, Reference Keyser2005; Van Harten, Reference Van Harten2000). Other ostracods, for example Cavellina species, which are interpreted as having been eurytopic herein, may have been as adaptable as Cyprideis torosa, although there are no visible signs to indicate how they dealt with osmoregulation, such as variation in carapace ornamentation. For Palaeozoic fossils with no preserved soft parts, inferences have to be made from the carapace. For paraparchitoidean ostracods, the strategy of having a large, thick carapace and an associated integumental circulatory system may have been beneficial in dealing with osmotic pressures caused by changing salinities. This has been suggested for the Leperditicopida (Vannier & Abe, Reference Vannier and Abe1995; Vannier, Wang & Coen, Reference Vannier, Wang and Coen2001), which have a similarly proportioned carapace and are also adapted to non-marine environments, although it is not an exclusively freshwater adaptation.
Reproductive strategy to survive non-marine environments focuses on the ability to reproduce rapidly in often short-lived water bodies (r-strategy) and the development of desiccation-resistant eggs. Evidence for the first of these strategies comes from the hypothesis that Carboniferous paraparchitoideans may have used progenesis and parthenogenetic reproductive strategies to reproduce rapidly in hypersaline conditions (Dewey, Reference Dewey1987). The production of desiccation and transport-resistant resting eggs has been proposed for the Cypridoidea, which had a successful non-marine diversification the Early Cretaceous (Whatley, Reference Whatley1990a, Reference Whatley, Whatley and Mayburyb; Lethiers & Damotte, Reference Lethiers and Damotte1993). This adaptation would enable the survival of ostracods that lived in ephemeral water bodies such as seasonal freshwater lakes, like the resting eggs produced by the Spinicaudata of the Carboniferous Monteceau Lagerstätte, which are associated with Carbonita (Vannier, Thiery & Racheboeuf, Reference Vannier, Thiery and Racheboeuf2003). Although Carbonita is fairly diverse in the Carboniferous (Anderson, Reference Anderson1970 identifies 17 species), no ostracod resting eggs have ever been found.
Possible modifications to lifestyle, such as feeding and locomotion changes, are more speculative. In the Pennsylvanian and Permian, Carbonita was better adapted than Geisina to different sedimentary niches, and it was more taxonomically diverse (Bless & Pollard, Reference Bless and Pollard1973). It has been proposed that Carbonita may have been a deposit feeder like the Recent Cypridopsis, and was perhaps therefore more adaptable than Geisina, which may have been a filter feeder like modern platycopes (Neale, Reference Neale1984; Pollard, Reference Pollard1966). In terms of locomotion, some have argued the adaptation to a swimming mode of life was a key part to the success of the cypridoideans in colonizing non-marine environments in the mid-Mesozoic (along with the adaptation of desiccation-resistant eggs and parthenogenesis: Whatley, Reference Whatley1990a, Reference Whatley, Whatley and Mayburyb, Reference Whatley, Mateer and Chen1992), and it is possible that this was also adopted by Carboniferous Carbonitidae.
6.b. Mechanisms for non-marine radiation
Like many aquatic animals, ostracods appear to have made the transition from marine to non-marine waters in a coastal setting. For example, early Mississippian Carbonita is associated with near-shore marine to low-salinity coastal ponds (Tibert & Scott, Reference Tibert and Scott1999). In the Pennsylvanian, Carbonita is also found in coastal environments, such as brackish waters and poorly drained coastal plain sediments (Falcon-Lang et al. Reference Falcon-Lang, Benton, Braddy and Davies2006; Tibert & Dewey, Reference Tibert and Dewey2006). This ‘estuary effect’ of non-marine colonization (Gray, Reference Gray1988; Park & Gierlowski-Kordesch, Reference Park and Gierlowski-Kordesch2007) is important as it allowed invertebrates to exploit marine transgressions and varying salinity to inhabit otherwise insupportable non-marine environments. In the Carboniferous of the MVS, the stratigraphical pattern of marine bands shows that there were fairly frequent but mostly short-lived marine transgressions and glacio-eustatic cycles from the late Viséan onwards (Kassi et al. Reference Kassi, Weir, McManus and Browne2004).
Following this estuarine or marine transgression pathway, it is possible that ostracods undertook ‘active’ and ‘passive’ (see Gray, Reference Gray1988) non-marine colonization. Active invasion from the sea by euryhaline ostracods may have occurred at times of high sea-level. Marine species may have developed an osmoregulatory adaptation, to allow them to survive in rapidly changing salinities such as in an estuary. The ‘incentive’ for this costly physiological adaptation would have been access to a rich food source and relatively sparsely populated new habitat. Over time such ostracod species would become more tolerant of freshwater conditions and able to survive in freshwater bodies such as lakes. Evidence for this long-term ecological adaptation is suggested in the fossil record, with the first cases of ostracod-like animals close to land at delta-front settings in the early Cambrian (Loughlin & Hillier, Reference Loughlin and Hillier2010; Siveter & Williams, Reference Siveter and Williams1995), putative marginal marine to brackish ostracods from the Silurian (for example Floyd & Williams, Reference Floyd and Williams2003), brackish ostracods in the Devonian (for example Bless, Reference Bless1983) and finally successful freshwater colonization in the Carboniferous (for example Vannier, Thiery & Racheboeuf, Reference Vannier, Thiery and Racheboeuf2003).
Passive invasion may have occurred owing to fluctuating sea-levels and the restriction of ostracods to isolated habitats, for example in subtidal areas that were isolated and freshened when sea-level fell. As mentioned previously, reproductive adaptations such as resting eggs may also have enabled ostracods to exploit temporary coastal plain water bodies. However, the fairly rapid salinity change that would occur in this scenario means that only species that were already pre-adapted to euryhaline conditions would survive, owing to the pressures of osmoregulation. The ‘patchy’ fossil record of non-marine ostracods in the Devonian and earlier may be explained by a series of passive invasion events and ‘failed’ non-marine colonizations. Non-aquatic means of passive invasion have been proposed, such as the transport of ostracods or desiccation-resistant ostracod eggs on the body of tetrapods, or blown by humid winds (Lethiers & Damotte, Reference Lethiers and Damotte1993).
Whether adaptation was active or passive, there may have been evolutionary advantages in moving out of the marine realm. For example, the Late Devonian ocean anoxia (Algeo et al. Reference Algeo, Berner, Maynard and Scheckler1995) may have driven adaptation into new environments. The onset of the Carboniferous glaciation in the Mississippian (Fielding, Frank & Isbell, Reference Fielding, Frank and Isbell2008; Mii, Grossman & Yancey, Reference Mii, Grossman and Yancey1999) would have dramatically affected sea-level and destabilized the shallow marine shelf environment. There is evidence in marine invertebrate populations of low levels of origination, extinction and diversity, from the Mississippian/Pennsylvanian boundary (where there was a second order extinction) for the following 50 Ma (into the Permian; Stanley & Powell, Reference Stanley and Powell2003). Changes in the marine environment and ostracod populations may thus have driven some species to invest in non-marine adaptations.
6.c. Early non-marine environments
The earliest known record of freshwater ostracods is preserved in lacustrine algal limestones in the Anstruther Formation of the MVS, which were colonized by Carbonita sp. Ostracods may have initially colonized this environment owing to passive transport during marine transgression over the lake and remained there as the water freshened. The algae would have provided a suitable food source; a similar adaptation has been proposed for certain leperditicopids associated with stromatolites (see Siveter, Reference Siveter1984; Vannier, Wang & Coen, Reference Mii, Grossman and Yancey2001; Warshauer & Smosna, Reference Warshauer, Smosna, Löffler and Danielopol1977).
Coal seams and blackband ironstones are common in the Strathclyde Group, and are interpreted as wetland swamp deposits. No terrestrial macrofauna were observed (such as the Pennsylvanian land snail Archandon; Hebert & Calder, Reference Hebert and Calder2004), but these wetland freshwater environments are important for ostracods. Carbonita is recorded from blackband ironstones in this study and is even more common in the Pennsylvanian Coal Measures (see, for example, Sohn, Reference Sohn1985). Ostracods may have been fostered in these environments by exploiting the rich plant detritus coming from the land; the increase in the diversity of detritus-feeding epifauna can be linked to the increased abundance of Carboniferous land plants (Buatois et al. Reference Buatois, Mángano, Genise and Taylor1998).
Were the ostracods associated with coal deposits terrestrial? Genetic studies estimate that terrestrial ostracods may have been present as early as the Ordovician (Newman, Reference Newman2005). The ostracods in organic-rich sediments in the present study are assumed to be fully aquatic, but it is possible that the absence of a fossil record of terrestrial ostracods may be a preservational rather than ecological issue (Horne, Reference Horne, Park and Smith2003), as carapaces are unlikely to be preserved in the acid peat-bog environment of coal deposits. Further studies on coal seams are needed to confirm an ostracod presence in coal deposits and a possible semi-terrestrial lifestyle for Mississippian ostracods.
6.d. Timing and duration of non-marine colonization
Of the most successful Carboniferous freshwater ostracods, Geisina became extinct by the mid-Permian, Carbonita at the end-Permian, while Darwinula survived but with a much reduced diversity (Horne, Reference Horne, Park and Smith2003). Only darwinulids and limnocytherids survived the end-Permian extinction and are found in freshwaters today. This study supports the notion of brackish ostracods in the Mississippian (Tibert & Scott, Reference Tibert and Scott1999; Williams et al. Reference Williams, Leng, Stephenson, Andrews, Wilkinson, Siveter, Horne and Vannier2006). Previous studies have recorded freshwater Carbonita from the late Mississippian (Pendleian; Sohn, Reference Sohn1985), while this study records them from the mid-Mississippian (Arundian). The colonization of non-marine environments by ostracods is evident from the Carboniferous until the end-Permian, but it was only one of a series of such ostracod radiations, to be repeated many times. For example, the Cytheroidea are thought to have undertaken seven distinct non-marine invasions from the Late Carboniferous to the present, the most active of which was during the Early Cretaceous (Horne, Reference Horne, Park and Smith2003; Tibert et al. Reference Tibert, Colin, Leckie and Babinot2003).
7. Ostracod taxonomic notes
Four new ostracod species are described in the Systematic Palaeontology Section 8 (authored by Bennett). Here, taxonomic notes are provided for the other species (Table 2, Figs 9–11), noting in some instances their first occurrence in the Mississippian sections studied. The familial and suprafamilial classification of ostracods from this study follows Whatley et al. (Reference Whatley, Siveter, Boomer and Benton1993), supplemented by consideration of Sohn (Reference Sohn1985) for the Superfamily Carbonitoidea, and Dewey & Fåhraeus (Reference Dewey and Fåhraeus1987) for the Family Geisinidae (Table 1).
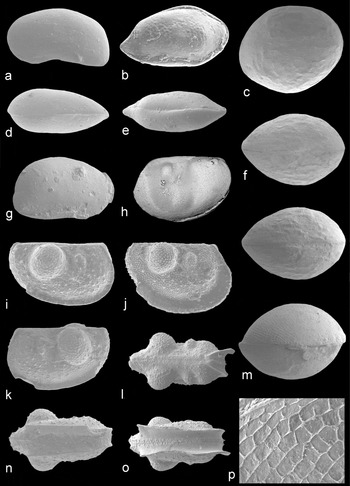
Figure 9. Marine ostracods. Specimen numbers are from the palaeontology collections of the BGS, Keyworth. All images are scanning electron micrographs. Acutiangulata sp. A: (a, d) carapace (MPK 13938; length 440 μm), left lateral and dorsal views, ×100. Bairdia submucronata Jones & Kirkby, Reference Jones and Kirkby1879a: (b, e) carapace (MPK 13939; length 830 μm), right lateral and dorsal views, ×60. Healdia cf. cuneata Robinson, Reference Robinson, Bate and Robinson1978: (g) carapace (MPK 13941; length 420 μm), right lateral view, ×110. Palaeocope sp. C: (h) carapace (juvenile; MPK 13989; length 350 μm), left lateral view, ×129. Polycope elegans n. sp: (c, f) carapace (holotype; MPK 13942; length 1800 μm), right lateral and dorsal view (stereo pair), ×26; (m, p) carapace (MPK 13943; length 1550 μm), subdorsal view (×33), and rectangular reticulation (image 230 μm wide; ×187). Hollinella (Keslingella) radiata (Jones & Kirkby, Reference Jones and Kirkby1886): (i, k, n) carapace, tecnomorph (MPK 13973; length 875 μm), right lateral, left lateral and right ventral views, ×54; (j, l, o) carapace, heteromorph, (MPK 13977; length 1000 μm), right lateral, dorsal and ventral views, ×48.
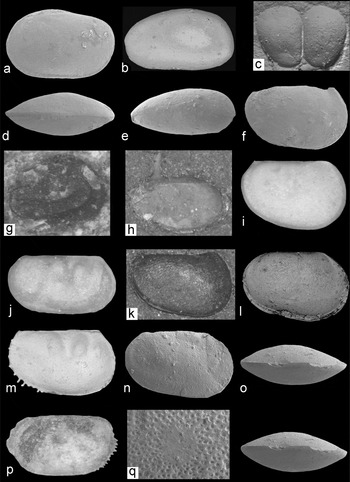
Figure 10. Eurytopic ostracods. Specimen numbers are from the palaeontology collections of the BGS, Keyworth. Images are scanning electron micrographs (a–f, l–o, q) or light photographs (g–k, p). Cavellina benniei (Jones, Kirkby & Brady, Reference Jones, Kirkby and Brady1884): (a, d) carapace (large juvenile; MPK 13944; length 540 μm) left lateral and dorsal views, ×91. Cavellina valida (Jones, Kirkby & Brady, Reference Jones, Kirkby and Brady1884): (b, e) carapace (MPK 13945; length 570 μm), left lateral and dorsal views, ×84. Glyptolichvinella spiralis (Jones & Kirkby, Reference Jones and Kirkby1880) – environment uncertain: (g) left valve (MPK 13949; length 1000 μm), lateral view, specimen partly obscured by dolomite crystals, ×46. Paraparchites armstrongianus (Jones & Kirkby, Reference Jones and Kirkby1886): (h) left valve of a disarticulated carapace (MPK 13950; length 1600 μm), lateral view, ×29. Shemonaella elongata n. sp.: (c) carapace (juvenile; MPK 13968; height of valve 385 μm), silicon rubber cast, ×73; (f) left valve (MPK 13965; length 1220 μm), lateral view, specimen broken at dorsal margin, ×41; (i) left valve (MPK 13964; holotype; CEB7; length 1200 μm), lateral view, ×39; (k) right valve (MPK 13967; length 1000 μm), internal mould, with anastomosing structures, ×48. Shemonaella ornata n. sp.: (l, o) carapace (MPK 13966; holotype; CEB6; length 1600 μm), left lateral and ventral (stereo pair) views, ×30; (n, q) left valve (MPK 13969; length 1400 μm), lateral view (×34), and external view of the adductor muscle scar (120 μm diameter, ×131). Palaeocope sp. A: (j) right valve (MPK 13980; length 850 μm), lateral view, ×58. Palaeocope sp. B: (m) carapace (MPK 13984; length 630 μm), right lateral view, ×75; (p) left valve (MPK 13986; length 700 μm), lateral view, flattened specimen, ×70.
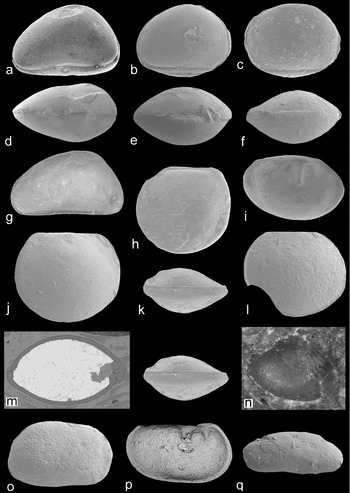
Figure 11. Freshwater (a–g), freshwater-brackish (h–o) and ‘ecology uncertain’ (p, q) ostracods. Specimen numbers are from the palaeontology collections of the BGS, Keyworth. Images are scanning electron micrographs (a–f, h–m, o–q) or light photographs (g, n). Carbonita cf. fabulina (Jones & Kirkby, Reference Jones and Kirkby1879b): (a, d) carapace (MPK 13955; length 940 μm), left lateral and ventral views, ×51. Carbonita cf. inflata (Jones & Kirkby, Reference Jones and Kirkby1879b): (b, e) carapace (MPK 13953; length 875 μm), left lateral and dorsal views, ×48. Carbonita cf. humilis (Jones & Kirkby, Reference Jones and Kirkby1879b): (c, f) carapace (MPK 13956; length 840 μm), left lateral and ventral views, ×50. Carbonita bairdioides (Jones & Kirkby, Reference Jones and Kirkby1879b): (g) carapace (MPK 13957; length 800 μm), left lateral view, ×63. Geisina arcuata (Bean, Reference Bean1836): (i) carapace (MPK 13959; length 610 μm), right lateral view, ×74. Paraparchites circularis n. sp.: (h, k) carapace (juvenile; MPK 13958; length 850 μm), left lateral and ventral (stereo pair) views, ×49; (j) left valve (holotype; MPK 13960; length 800 μm), lateral view, ×58; (l) right valve (MPK 13961; length 720 μm), lateral view, ×64; (m) carapace (juvenile; MPK 13962; thin-section; length 600 μm), the valves are outlined in black, the hinge is to the left, ×87; (n) carapace (MPK 13963; image 800 μm wide), oblique view of an internal skeinkern, central muscle spot and anastomosing structures, ×54. Silenites sp. A: (o) right valve (MPK 13971; length 680 μm), lateral view, ×68. Palaeocope sp. D: (p) carapace (MPK 13991; length 700 μm), right lateral view, ×71. Acratia sp. A: (q) single valve (MPK 13951; length 324 μm), lateral view, ×133.
7.a. Order Podocopida Müller, Reference Müller1894
7.a.1. Suborder Podocopina Sars, Reference Sars1866
Nine species from the families Bairdiidae (genera Acratia, Acutiangulata, Bairdia), Bairdiocyprididae (Silenites) and Carbonitidae (Carbonita) are recognized. Acratia sp. A. is identified by its lateral valve outline (Fig. 11q), which most closely resembles Acratia acuta (Jones & Kirkby, Reference Jones and Kirkby1895). Acutiangulata sp. A has a distinctly sub-quadrate lateral outline of the ventroposterior margin (Fig. 9a, d). Bairdia submucronata Jones & Kirkby (Reference Jones and Kirkby1879a) (topotypes designated herein as NHM specimens In 42133, OS 7457 and OS 7458, the latter two figured by Jones & Kirkby, Reference Jones and Kirkby1879a and Robinson, Reference Robinson, Bate and Robinson1978) possesses a postero-dorsal margin inclined at 10° to the horizontal in lateral view (Fig. 9b). This species is similar to Bairdia altaica Buschmina & Kononova, Reference Buschmina and Kononova1981 and B. beedei Ulrich & Bassler, Reference Ulrich and Bassler1906, but with more central inflation to the carapace. Silenites sp. A is identified by its sub-ovate lateral carapace outline and external surface ornament of 20 μm diameter reticulae (Fig. 11o). The posterior end of the carapace is higher and more inflated than the anterior, which may indicate sexual dimorphism.
Carbonita has a long history of taxonomic revision (see Pollard, Reference Pollard1966; Anderson, Reference Anderson1970; Sohn, Reference Sohn1977; Horne, Reference Horne, Park and Smith2003 and Tibert & Dewey, Reference Tibert and Dewey2006 for a summary). The Scottish specimens lack details of the muscle scar, and their assignment to Carbonita is tentative. Carbonita bairdioides (Jones & Kirkby, Reference Jones and Kirkby1879b) (lectotype designated here as NHM I 2566, fig. d in Athersuch et al. Reference Athersuch, Gooday, Pollard, Riley, Whittaker and Hart2009) is distinguished by its sub-triangular, asymmetrical, lateral carapace outline and arched dorsal margin (Fig. 11g). Carbonita cf. fabulina (Jones & Kirkby, Reference Jones and Kirkby1879b) has a more strongly arched dorsal margin and greater valve height versus length than C. bairdioides (Fig. 11a). Carbonita cf. humilis (Jones & Kirkby, Reference Jones and Kirkby1879b) is identified by its ovate, symmetrical, lateral carapace outline and external surface ornamentation of 20 μm diameter reticulae (Fig. 11c). C. humilis is one of the most common non-marine ostracods in the Pennsylvanian of Britain (Pollard, Reference Pollard1966; Anderson, Reference Anderson1970; Athersuch et al. Reference Athersuch, Gooday, Pollard, Riley, Whittaker and Hart2009) and exhibits sexual dimorphism (Bless & Pollard, Reference Bless and Pollard1975). Carbonita cf. inflata (Jones & Kirkby, Reference Jones and Kirkby1879b) is sub-triangular to sub-rounded in lateral carapace outline (Fig. 11b), but lacks the punctate ornament of Pollard's (Reference Pollard1966) material of C. inflata. Anderson (Reference Anderson1970) regarded Bythocypris tumidus, Cypridopsis fabulina, Gutschickia ovata and Whipplella cuneiformis to be junior synonyms of C. inflata.
Our material extends the range of certain genera in the Mississippian of Britain: Acutiangulata from the Lower Asbian (Robinson, Reference Robinson, Bate and Robinson1978) up to the Brigantian (this study); Bairdia submucronata from the Asbian (Robinson Reference Robinson, Bate and Robinson1978; Turner, Dewey & Fordham, Reference Turner, Dewey and Fordham1997) to the Brigantian, and Carbonita from the Pennsylvanian (Athersuch et al. Reference Athersuch, Gooday, Pollard, Riley, Whittaker and Hart2009) downwards to the Mississippian.
7.a.2. Suborder Metacopina Sylvester-Bradley, Reference Sylvester-Bradley and Moore1961
Healdia cf. cuneata Robinson, Reference Robinson, Bate and Robinson1978 (from the family Healdiidae) is identified by its 85° lateral angle of the posterior margin (Fig. 9g), and the presence of small posterior and postero-ventral spines (between 7–10 μm in length). While H. cuneata lacks spines (Robinson, Reference Robinson, Bate and Robinson1978), other species have much larger spines, such as Healdia cornigera (Jones & Kirkby, Reference Jones and Kirkby1867).
7.a.3. Suborder Platycopina Sars, Reference Sars1866
Four species from the families Cavellinidae (Cavellina), Geisinidae (Geisina) and Glyptolichvinella (family uncertain) are recognized. Owing to a lack of soft-part evidence, the relationship between Recent and Carboniferous platycopes is poorly understood (Horne, Reference Horne, Park and Smith2003). Cavellina benniei (Jones, Kirkby & Brady, Reference Jones, Kirkby and Brady1884) (topotypes designated here as NHM OS 7339, OS 7340 and specimens from slide I. 1725, the former two figured in Jones, Kirkby & Brady, Reference Jones, Kirkby and Brady1884 and Robinson, Reference Robinson, Bate and Robinson1978) has a sub-quadrate lateral carapace outline (Fig. 10a). Cavellina valida (topotypes designated herein as NHM OS 7346 and OS 7347, fig. d in Jones, Kirkby & Brady, Reference Jones, Kirkby and Brady1884 and Robinson, Reference Robinson, Bate and Robinson1978) is defined by the 85° lateral angle of the posterior margin, lack of spines and right-over-left valve overreach (Fig. 10e). Varying posterior inflation in adults indicates possible domiciliar sexual dimorphism, while the lack of inflation in C. benniei may reflect the small number of specimens identified (Table 2). Geisina arcuata Bean (Reference Bean1836) is identified by a shallow adductorial sulcus and a strong right-over-left valve overlap (Fig. 11i). A surface ornament of polygonal pitted reticulation (Pollard, Reference Pollard1966) is absent. Glyptolichvinella spiralis Jones & Kirkby, Reference Jones and Kirkby1880 (lectotype assigned here as NHM OS 7384, fig. d in Jones & Kirkby, Reference Jones and Kirkby1885 and Robinson, Reference Robinson, Bate and Robinson1978) has an external ornamentation of costae in an open spiral pattern (Fig. 10g). The carapace size is larger than other species with similar costal patterns, such as Glyptolichvinella annularis (Kummerow, Reference Kummerow1939).
7.b. Order Leiocopida Schallreuter, Reference Schallreuter1973
Four species from the family Paraparchitidae (Paraparchites armstrongianus (Jones & Kirkby, Reference Jones and Kirkby1886), Paraparchites circularis n. sp., Shemonaella ornata n. sp. and Shemonaella elongata n. sp.) are recognized. The new species are described in the Systematic Palaeontology (Section 8). Paraparchites armstrongianus (Jones & Kirkby, Reference Jones and Kirkby1886) (lectotype assigned here as NHM I. 1756, fig. d in Jones & Kirkby, Reference Jones and Kirkby1886) has a distinct antero-cardinal spine (Fig. 10h), larger than that of other paraparchitids (such as species of Shishaella and Shivaella (Robinson, Reference Robinson, Bate and Robinson1978; Sohn, Reference Sohn1972)).
7.c. Order Palaeocopida Henningsmoen, Reference Henningsmoen1953
Five species from the family Hollinellidae (Hollinella (Keslingella)) and palaeocopes of uncertain affinity are identified. Hollinella (Keslingella) radiata (topotype assigned herein as NHM OS 7331, fig. d in Jones & Kirkby, Reference Jones and Kirkby1886 and Robinson, Reference Robinson, Bate and Robinson1978) is distinguished by a small antero-dorsal node, a large postero-dorsal bulb, and an external ornament of tubercles and spines. The histium is twice as wide and the carapace is wider in some specimens (Fig. 9j), which are identified as heteromorphs (Kellett, Reference Kellett1936). Two adventral spines are present in juveniles, typical of the subgenus (Bless & Jordan, Reference Bless and Jordan1970).
Palaeocopes spp. are classified into four species (A–D), but are left in open nomenclature owing to the small number of specimens identified (Table 2) and their differences to known palaeocope genera. All have a straight dorsal margin, preplete carapace and lobate ornamentation. A large posterior lobe or inflated posterior end in some specimens indicates sexual dimorphism. Species A–D are distinguished by differences in ornamentation. Palaeocope sp. A is quadrilobate (Fig. 10j), palaeocope sp. B trilobate, with spines on the anterior or anterior and posterior free margins (Fig. 10m, p). The spines are 20 μm in length and vary in shape (triangular, clavellate (widens distally) or needle like) and position (closely or distally spaced) between specimens, indicative of intra-species variation. Jonesina fastigiata (Jones & Kirkby, Reference Jones and Kirkby1867) (fig. d in Robinson, Reference Robinson, Bate and Robinson1978, pl. 4, figs 2a–b) resembles palaeocope sp. B, but has more spherical lobes. Palaeocope sp. C is trilobate, distinguished by a postero-cardinal spine (30 μm in length) on the posterior lobe (Fig. 9h). Palaeocope sp. D is trilobate, with flattened valve free margins, reticulate external ornament and no spines (Fig. 11p).
7.d. Order Myodocopida Sars, Reference Sars1866
Polycope elegans n. sp. is the only representative of the family Polycopidae and is described in the following section.
8. Systematic palaeontology (C.Bennett)
Four new species are described from the Strathclyde Group. A differential diagnosis is given owing to the problems of otherwise distinguishing between species that have no or little carapace ornamentation.
Order LEIOCOPIDA Schallreuter, Reference Schallreuter1973
Suborder PARAPARCHITICOPINA Gramm & Ivanov, Reference Gramm and Ivanov1975
Superfamily Paraparchitoidea Scott, Reference Scott1959
Family Paraparchitidae Scott, Reference Scott1959
Genus Paraparchites Ulrich & Bassler, Reference Ulrich and Bassler1906
Type species. By original designation Paraparchites humerosus Ulrich & Bassler, Reference Ulrich and Bassler1906.
Diagnosis. See Dewey & Fåhraeus (Reference Dewey and Fåhraeus1987), p. 108.
Paraparchitescircularis n.sp. Figure 11h, j–n
Holotype. MPK 13960, a left valve; length 800 μm; Fig. 11j.
Derivation of name. Latin circularis, ‘circular’, referring to the lateral valve shape.
Type locality. Sample SE 8411, at 363.2 m core depth, Kilconquhar borehole; the Sandy Craig Formation, Fife [National grid reference NO 4844 0304].
Material. See Table 2. The average (mean) size of adult carapaces is length 840 μm, height 740 μm, width 420 μm.
Differential diagnosis. Species of Paraparchites with a sub-circular carapace outline in lateral view, except for a straight dorsal margin. The carapace has a high height:length ratio of 1:1.1. External surface of the valves have shallow circular punctae, each approximately 12 μm in diameter, and a smooth sub-circular central muscle scar spot approximately 60 μm in diameter. Paraparchites circularis n. sp. has a valve height:length that is greater than other large Mississippian paraparchitoideans which also have a similar outline shape, such as Chamishaella suborbiculata (Münster, Reference Münster1830), Paraparchites carbonaria (Hall), Paraparchites scotoburdigalensis (Hibbert, Reference Hibbert1836) and Shemonaella scotoburdigalensis (Hibbert, Reference Hibbert1836). Paraparchites discus Williams et al. Reference Williams, Stephenson, Wilkinson, Leng and Miller2005, has an incised dorsum and smaller height:length ratio than this species.
Description. Carapace sub-circular in lateral outline, amplete, symmetrical. Dorsal margin straight, two-thirds the total carapace length, ventral, anterior and posterior margins rounded. Height slightly less than length, valves are centrally inflated. Internal moulds have anastomosing structures radiating from a central muscle spot. Right-over-left valve overlap to give a ridge around the valve free margins.
Discussion. The anastomosing structures on the internal surface of valves may reflect part of the circulatory system, as has been proposed for leperditid arthropods (Vannier, Wang & Coen, Reference Vannier, Wang and Coen2001). This species commonly occurs in monospecific assemblages or associated with rare Carbonita cf. inflata and Silenites sp. A. The genus is recorded from the Mississippian of Scotland (Latham, Reference Latham1932; Williams et al. Reference Williams, Stephenson, Wilkinson, Leng and Miller2005), Canada (Dewey, Reference Dewey1988) and the USA (Benson, Reference Benson1955; Sohn, Reference Sohn1971).
Genus Shemonaella Sohn, Reference Sohn1971
Type species. By original designation Shemonaella dutroi Sohn, Reference Sohn1971.
Diagnosis. See Dewey & Fåhraeus (Reference Dewey and Fåhraeus1987), p. 109.
Shemonaellaelongata n.sp. Figure 10c, f, i, k
Holotype. MPK 13964, a left valve; length 1200 μm; Fig. 10i.
Derivation of name. The Latin elongata, ‘long, or elongate’, referring to the unusual length to height carapace proportions.
Type locality. Sample EN 4805, at 11.53 m core depth, Claremont borehole; the Pathhead Formation, Fife [National grid reference NO 4518 1419].
Material. See Table 2. Average dimensions: length 1200 μm, height 760 μm.
Differential diagnosis. Species of Shemonaella with a long dorsal margin (80% of the carapace length), shallow carapace inflation and an unusually high height:length ratio for this genus of 1:1.6. Many paraparchitoideans have the same carapace size, shape and lack of external ornamentation as this species. These include the postplete Shemonaella sp. A of Williams et al. Reference Williams, Stephenson, Wilkinson, Leng and Miller2005, Paraparchites inornatus McCoy, Reference McCoy1844, Paraparchites superbus (Jones & Kirkby, Reference Jones and Kirkby1886) and Shishaella sohnella Crasquin, Reference Crasquin1985, and the preplete Leperditia okeni Münster (Jones & Kirkby, Reference Jones and Kirkby1865), Paraparchites nicklesi (Ulrich, Reference Ulrich1891), Paraparchites okeni Münster, Reference Münster1830 and Shishaella nanaformis Crasquin, Reference Crasquin1985. However, these species are all more centrally inflated, with a shorter carapace height and a shorter dorsal margin to carapace length.
Description. Carapace semicircular in lateral outline, subamplete to postplete. Dorsal margin straight, 80% the total carapace length. Ventral margin curved, anterior and posterior margins rounded. Carapace large, inflated centrally and towards the posterior. Surface smooth, internal moulds have anastomosing structures radiating from a central muscle spot, which may preserve the circulatory system. Left-over-right valve dorsal overreach.
Discussion. This is one of the most common species in the Strathclyde Group, often present in abundance, in all formations and in a range of ecological settings (Tables 2, 3, 5, Figs 7, 8). The genus is recorded from the Mississippian of Britain (Robinson, Reference Robinson, Bate and Robinson1978), Germany (Coen, Reference Coen1990) and Canada (Crasquin, Reference Crasquin1985; Dewey, Reference Dewey, McKenzie and Jones1993; Dewey & Fåhraeus, Reference Dewey and Fåhraeus1987).
Shemonaellaornata n.sp. Figure 10l, n, o, q
Holotype. MPK 13966, a carapace; length 1600 μm; Fig. 10l, o.
Derivation of name. The Latin ornata, ‘ornate’, referring to the distinctive pitted ornament.
Type locality. Sample EN 4804, at 11.53 m core depth, Claremont borehole; the Pathhead Formation, Fife [National grid reference NO 4518 1419].
Material. See Table 2. Average dimensions: length 1450 μm, height 920 μm, width 500 μm.
Differential diagnosis. Species of Shemonaella with an ornament of 10 μm diameter circular pits and a circular, smooth muscle scar spot (120 μm diameter) situated at the midpoint. This species has a larger carapace size and a unique ornament for Shemonaella, compared to other species of the genus. The dorsal margin is comparatively shorter than that of Shemonaella elongata n. sp. Specimens of Leperditia youngiana Jones & Kirkby, Reference Jones and Kirkby1867 from the NHM ‘Jones collection’ has a similar carapace size, but an ornament of 5 μm diameter pits, not the 10 μm diameter pits of this new species.
Description. Carapace sub-ovate in lateral outline, postplete. Dorsal margin straight, two-thirds the total carapace length. Ventral margin straight, sloping towards the anterior; anterior and posterior ends rounded. The posterior end is distinctly higher than the anterior end. Carapace large for the genus, centrally inflated. Right-over-left valve overlap around all free margins excluding the dorsal margin.
Discussion. This species is limited in number of specimens and restricted in distribution compared to Shemonaella elongata n. sp. (Table 2, 3, Figs 7, 8).
Order MYODOCOPIDA Sars, Reference Sars1866
Suborder CLADOCOPINA Sars, Reference Sars1866
Superfamily Polycopoidea Sars, Reference Sars1866
Family Polycopidae Sars, Reference Sars1866
Genus Polycope Sars, Reference Sars1866
Type species. By original designation Polycope orbicularis Sars, Reference Sars1866.
Diagnosis. See Sars (Reference Sars1928), pp. 29–30.
Polycopeelegans n.sp. Figure 9c, f, m, p
Holotype. MPK 13942, a carapace; length 1800 μm; Fig. 9c, f.
Derivation of name. The Latin elegans, ‘beautiful/elegant’, referring to the beautiful, fine reticulate ornamentation.
Type locality. Sample EN 5329, at 74.93 m core depth, Denork borehole; the Pathhead Formation, Fife [National grid reference NO 4540 1409].
Material. See Table 2. Dimensions: length 1800 μm, height 1580 μm, width 1250 μm: MPK 13942.
Differential diagnosis. Polycope with a large carapace size and a unique radial pattern of hexagonal (at the valve centre) to rectangular (at the valve edge) reticulation. The reticulae are 40 μm in diameter. Other Mississippian Polycope mostly have a smaller carapace size, for example Polycope sphaerula (Gründel, Reference Gründel1961) and Polycope spinula Dewey & Fåhraeus, Reference Dewey and Fåhraeus1987. Polycope youngiana Jones, Kirkby & Brady, Reference Jones, Kirkby and Brady1874 resembles the new species in size and shape, but it has an ornament of concentrically ringed grooves rather than reticulae, that is distinctly different.
Description. Carapace sub-circular in lateral outline, sub-spherical in shape, postplete. Dorsal margin straight, one-third the total carapace length, all other margins rounded. Carapace large, thick shelled, tumid. External surface ornament of reticulae arranged in a radial pattern from the midpoint. Reticulae change shape from hexagonal or polygonal in the valve centre, to rectangular or square at the edges of the valve. No valve overlap.
Discussion. This species is only found in the Denork core of the Pathhead Formation. The four samples that contain Polycope elegans n. sp. are each spaced at least a metre apart, suggesting that this species was present for a significant time interval. Other species of Polycope have been described from the Devonian/Carboniferous of Germany (Becker, Claus-Dieter & Klaus, Reference Becker, Claus-Dieter and Klaus1993), the Mississippian of Northumberland (Dewey, Reference Dewey, McKenzie and Jones1993) and the Maritimes Basin of Canada (Dewey, Reference Dewey1988; Dewey & Fåhraeus, Reference Dewey and Fåhraeus1987).
9. Conclusions
(1) The Strathclyde Group of Fife, Scotland, represents a range of different depositional settings and environments, from fully marine conditions to deltaic sediments, marginal marine estuaries and lagoons, and brackish to freshwater lakes, swamps and fluvial systems. Ostracods and other fossils inhabited marine, brackish and freshwater environments. These deposits contain 25 ostracod species, including 4 that are new species.
(2) Macrofossil indicators of freshwater are the bivalves Anthraconaia, Carbonicola and Cardiopteridium, Spinicaudata, fish and plant debris.
(3) The Mississippian of the MVS contains some of the earliest freshwater ostracods globally. Freshwater Carbonita is described from the Arundian, middle Mississippian, in organic-rich mudstones and stromatolitic limestones. This study places the first freshwater ostracods approximately 15 Ma earlier than previously documented at 325 Ma.
(4) Brackish to freshwater macrofauna includes Naiadites, Curvirimula and ‘Spirorbis’. Brackish water ostracods are Geisina arcuata, Paraparchites circularis n. sp., Shemonaella ornata n. sp. and Silenites sp. A.
(5) Typical macrofossil indicators of marginal marine conditions are Schizodus, Sanguinolites and Lingula. Ostracods that are eurytopic, with a wide salinity tolerance, are Cavellina benniei, Cavellina valida, palaeocope species A and B, Paraparchites armstrongianus and Shemonaella elongata n. sp.
(6) Marine ostracods are Acutiangulata sp. A, Bairdia submucronata, Healdia cf. cuneata, Hollinella (Keslingella) radiata, palaeocope sp. C and Polycope elegans n. sp. They occur with a high diversity of marine macrofauna such as brachiopods, bryozoans, echinoderms and gastropods.
(7) The drivers of non-marine colonization by ostracods in the Mississippian can only be speculated upon, but may involve environmental (sea-level) change due to glaciation. The mechanisms to adapt to lower salinity such as osmoregulation and changes in reproduction are not fully known owing to a lack of knowledge of relevant soft parts. For example, there is insufficient evidence to suggest that desiccation-resistant eggs had developed during the Carboniferous, but links can be made to contemporaneous arthropods and living freshwater ostracods.
(8) Despite the success of the non-marine platycopes, podocopes and leiocopes, the majority did not survive the end-Permian extinction. Instead ostracods underwent a further series of terrestrial aquatic colonization events in the Mesozoic.
Acknowledgements
The Natural Environment Research Council (NERC) funded the project, grant number NER/S/A/2005/13368. The British Geological Survey (BGS) provided additional CASE award funding through the British Universities Funding Initiative. The following are thanked from the BGS for their advice and providing access to samples: Drs Maxine Akhurst, Sarah Arkley and Mike Howe, and Mr Mark Dean. All figured specimens are housed in the BGS palaeontology collections, Keyworth. The following are thanked for correspondence and/or discussions about Carboniferous ostracods and fossils: Prof. Alan Lord, and Drs Dave Horne, Chris Dewey, Neil Tibert, John Pollard, Adrian Rundle, Luis Carlos Sánchez de Posada and Zerina Johanson. Two authors (IPW and MB) publish with permission of the Executive Director of the BGS (NERC).