1. Introduction
Fortey & Chatterton (Reference Fortey and Chatterton1988) and Fortey (Reference Fortey1990) regarded the ventral median suture as a key synapomorphy of the Order Asaphida, and included all the groups that had a ventral median suture in their cladistic analysis, providing a cladogram depicting the relationships within the Order Asaphida. They (Fortey & Chatterton, Reference Fortey and Chatterton1988, p. 200) noted that the earlier (late Furongian–Tremadocian) genera of the Remopleuridioidea have been described to have a ventral median suture, and considered the loss of the ventral median suture in some ‘kainellids’ as secondary. Accordingly, the Superfamily Remopleuridioidea was included in their cladistic analysis of the Order Asaphida as a constituent group of the order, and this superfamily came out as the sistergroup of the Superfamily Dikelocephaloidea in the analysis (Fortey & Chatterton, Reference Fortey and Chatterton1988). In addition, the globular protaspis, termed asaphoid protaspis by Fortey & Chatterton (Reference Fortey and Chatterton1988), of the Ordovician remopleuridioid trilobites was treated as another key Asaphida-related characteristic of the superfamily (Fortey & Chatterton, Reference Fortey and Chatterton1988, p. 186). However, as stated by Fortey & Chatterton (Reference Fortey and Chatterton1988, p. 200), the morphology and ontogeny of the earlier representatives of the Remopleuridoidea were not well known.
Park & Choi (Reference Park and Choi2009) reported that the tsinaniid trilobite Tsinania canens (Walcott, Reference Walcott1905) developed a ventral median suture during ontogeny, proving that a ventral median suture could independently have evolved within the order Corynexochida. Park & Choi (Reference Park and Choi2010a) also demonstrated that the mode of formation of the ventral median suture in the dikelocephaloid trilobite Asioptychaspis subglobosa Sun, Reference Sun1924 can be distinguished from those of Tsinania canens and the suggested model by Chatterton et al. (Reference Chatterton, Speyer, Hunt and Fortey1994). They showed that there are three different modes by which the ventral median suture of trilobites evolved, namely: (1) by the disappearance of an inverted-triangular rostellum in front of a connective suture as suggested by Chatterton et al. (Reference Chatterton, Speyer, Hunt and Fortey1994); (2) by the reduction of a triangular rostral plate behind a connective suture as in Tsinania canens (see Park & Choi, Reference Park and Choi2009); and (3) by splitting of a yoked free cheek as in Asioptychaspis subglobosa.
Recently, Adrain, Peters & Westrop (Reference Adrain, Peters and Westrop2009) reported a Marjuman cedariid trilobite, Cedarina schachti Adrain, Peters & Westrop, Reference Adrain, Peters and Westrop2009, which is morphologically similar to plesiomorphic members of the Remopleuridioidea. They suggested that remopleuridioids may have arisen from a stock of cedariids, rendering the Cedariidae paraphyletic, and that the Remopleuridioidea should not be included in the Order Asaphida.
This study reports the ontogeny of the middle Furongian remopleuridioid trilobite Haniwa quadrata Kobayashi, Reference Kobayashi1933 from the Hwajeol Formation of Korea. The ontogeny of this, one of the oldest remopleuridioid trilobites, will provide information to test whether the Remopleuridioidea is truly related to the members of the Order Asaphida as suggested by Fortey & Chatterton (Reference Fortey and Chatterton1988), or is not related as suggested by Adrain, Peters & Westrop (Reference Adrain, Peters and Westrop2009).
2. Fossil locality, material and note on taxonomy
All the material considered in this study was collected from the lowermost part of the Hwajeol Formation at the Sagundari section (129°01′03.4″ E, 37°04′57.0″ N) in the Taebaeksan Basin, Korea (Fig. 1). The Hwajeol Formation is an alternating succession of limestone and shale beds with occasional intercalation of limestone conglomerate beds (Choi et al. Reference Choi, Chough, Kwon, Lee, Woo, Kang, Lee, Lee, Sohn, Shinn and Lee2004). The depositional setting of the formation is interpreted as inner to outer ramp environments (Kwon et al. Reference Kwon, Chough, Choi and Lee2006). Three trilobite faunas have been recognized within the formation: the Asioptychaspis Zone, the Quadraticephalus Zone, and the saukiid-dominated fauna, in ascending order (Sohn & Choi, Reference Sohn and Choi2007). The material for this study was obtained from the Asioptychaspis Zone, which is of middle Furongian age. Sohn & Choi (Reference Sohn and Choi2007) reported Pseudagnostus planulatus (Raymond, Reference Raymond1924), Asioptychaspis subglobosa (Sun, Reference Sun1924), Haniwa sosanensis Kobayashi, Reference Kobayashi1933, and Tsinania canens (Walcott, Reference Walcott1905) from the Asioptychaspis Zone of the Sagundari section.

Figure 1. Location maps. (a) Tectonic map of Korean peninsula and surrounding area showing the location of the Taebaeksan Basin. Q-D – Qinling-Dabie belt, S – Sulu Belt, N – Nangnim Massif, P – Pyeongnam Basin, I – Imjingang Belt, G – Gyeonggi Massif, O – Okcheon Belt, T – Taebaeksan Basin, Y – Yeongnam Massif. (b) Geological map of the Taebaeksan Basin which shows the distribution of the lower Palaeozoic Joseon Supergroup in the Taebaeksan Basin. The asterisk indicates the location of the Sagundari section from which the material for this study was collected.
Limestone–shale couplets from the horizon 2.5 m above the base of the formation were digested by hydrochloric acid, and the silicified specimens of trilobites were collected from the residues, which included Pseudagnostus planulatus, Asioptychaspis subglobosa, Tsinania canens, Haniwa quadrata, Koldinioidia sp., Guanxiaspis? sp., a missisquoid gen. et sp. indeterminate and a dikelocephaloid gen. et sp. indeterminate. Among these, Park & Choi (Reference Park and Choi2009) and Park & Choi (Reference Park and Choi2010a) studied the ontogeny of Tsinania canens and Asioptychaspis subglobosa, respectively. For the ontogenetic study of Haniwa quadrata, 24 protaspides, 133 cranidia, 41 free cheeks, 27 thoracic segments and 103 post-protaspid pygidia were collected. All of the specimens illustrated in this study are deposited in the palaeontological collections of Seoul National University with registered SNUP numbers.
Sohn & Choi (Reference Sohn and Choi2007) obtained specimens of Haniwa from both the Asioptychaspis Zone and Quadraticephalus Zone, and assigned all the specimens to Haniwa sosanensis Kobayashi, Reference Kobayashi1933. The holotype of H. sosanensis has a parallel-sided anterior branch of the facial suture (Kobayashi, Reference Kobayashi1933, pl. 15, fig. 2; re-illustrated in Sohn & Choi, Reference Sohn and Choi2007, fig. 6p), while that of H. quadrata has a divergent forward anterior branch of the facial suture (Kobayashi, Reference Kobayashi1933, pl. 15, figs 7, 8; re-illustrated herein in Fig. 6z). The mature specimens of Haniwa collected from the Asioptychaspis Zone in this study have a divergent forward anterior branch of the facial suture, and thus are assigned to H. quadrata Kobayashi, Reference Kobayashi1933. The specimens of Haniwa figured in Sohn & Choi (Reference Sohn and Choi2007) do not have a parallel-sided anterior branch of the facial suture, hence requiring a taxonomic revision.
3. Ontogeny
Terminology for ontogenetic description mainly follows Chatterton & Speyer (Reference Chatterton, Speyer and Kaesler1997) and Hughes, Minelli & Fusco (Reference Hughes, Minelli and Fusco2006). Length (sag.) and width (tr.) measurements were taken for all protaspides, post-protaspid cranidia shorter than 3 mm, and post-protaspid pygidia shorter than 1.2 mm.
Protaspid period. All the collected protaspides seem to form a single instar-corresponding stage (Fig. 2), but the smallest protaspis has a rather distinctive morphology, implying that it may represent an earlier protaspid stage.

Figure 2. Scatter plot of length versus width for protaspid exoskeletons of Haniwa quadrata Kobayashi, Reference Kobayashi1933.
The protaspides of Haniwa quadrata are circular to oval in outline, 0.50–0.70 mm long and 0.46–0.57 mm wide, moderately convex in lateral view, with moderately effaced surface. Five pairs of short marginal spines are present (Fig. 3); the anteriormost pair is located just behind the palpebral lobes, and projects slightly posterolaterally from the cranidium; the second pair projects posterolaterally; the third pair is directed postero-dorsally; and the two posteriormost pairs project posteriorly. The cranidium takes up 62–66 % of the total length in dorsal view. The glabella is oblong in outline, widest at glabellar midlength, poorly defined by shallow axial furrows. The preglabellar field is very short (Fig. 3f), and is clear in the slightly posteroventral view (Fig. 3q). The palpebral lobes are relatively large and prominent, occupying the anterior part of the cranidum. The occipital ring is defined by a shallow occipital furrow and the posterior marginal cranidial furrow which is more deeply incised than the occipital furrow. The inverted-trapezoidal or inverted-triangular trunk is convex and slopes down rearward. The axial furrows are not visible, but the axial part is higher than the pleural field. The pygidial doublure is short and slightly in-turned (Fig. 3q, s, t).

Figure 3. Protaspides of Haniwa quadrata Kobayashi, Reference Kobayashi1933. (a–c) SNUP6001, dorsal, lateral and posterior views; (d–g) SNUP6002, dorsal, slightly anterodorsal, anterolateral and lateral views; (h, i) SNUP6003, dorsal and lateral views; (j, k) SNUP6004, lateral and dorsal views; (l–n) SNUP6005, dorsal, posterior and lateral views; (o–u) SNUP6006, dorsal, ventral, slightly posteroventral, lateral, slightly ventrolateral, ventrolateral and posterior views; (v–aa) the smallest protaspis, possibly belonging to an earlier protaspid stage; SNUP6007, dorsal, anterior, anterolateral, left lateral, posterior and right lateral views.
The short preglabellar field may be reminiscent of the protaspides of the Order Proetida (see Chatterton & Speyer, Reference Chatterton, Speyer and Kaesler1997), but they cannot be regarded homologous to each other, as the protaspides of the Order Proetida are flattened and have completely adult-like benthic morphology, while those of Haniwa quadrata are slightly bulbous, and hence distinguished from the conventional adult-like morphology.
The smallest protaspis (Fig. 3v–aa) is 0.50 mm long and 0.47 mm wide, and is distinctive in that it has a more bulbous morphology with only two pairs of marginal spines. The glabella is wider and is poorly defined by very shallow axial furrows.
Post-protaspid cranidial development. The posterolateral projections of Haniwa quadrata are narrow and usually not preserved well, and hence the posterior cranidial width, or ‘J1’ (Shaw, Reference Shaw1957), cannot be measured with confidence. Accordingly, the palpebral cranidial width, or ‘J’ (Shaw, Reference Shaw1957), is measured and plotted against the cranidial length (Fig. 4). The development of the post-protaspid cranidia is divided into five stages according to the size and morphology. The first developmental stage may represent the earliest meraspid instar-corresponding cluster (Fig. 4), but such clustering is not recognizable for the subsequent development. To visualize the allometric growth of cranidial development, 14 landmarks were selected, and the Partial Procrustes distance of each cranidium by the reference of the consensus of the three smallest cranidia was plotted against the centroid size (Fig. 5). Ninety-four specimens in which the fourteen landmarks were available were measured. This method has been recently used for trilobites by Webster (Reference Webster2007) and Hopkins & Webster (Reference Hopkins and Webster2009) to study allometric growth. The software TpsDig 2, developed by F. James Rohlf (freely available at http://life.bio.sunysb.edu/morph/), was used to digitize landmark coordinates, while the Procrustes coordinates and the centroid size were obtained by CoordGen 6.0, which was created by David Sheets (freely available at http://www.canisius.edu/~sheets/morphsoft.html).

Figure 4. Scatter plot of cranidial length versus palpebral cranidial width for post-protaspid cranidia of Haniwa quadrata Kobayashi, Reference Kobayashi1933.

Figure 5. Partial Procrustes distance of cranidia from a reference form of the consensus of the three smallest cranidia against centroid size, and a diagram showing the selected landmarks on cranidium. The slope of the Procrustes distance becomes almost horizontal during the developmental stage 4. The slope becoming horizontal indicates the cessation of allometric growth, thus representing the attainment of morphological maturity. Symbols are the same as in Figure 4.
The developmental stage 1 cranidia (Fig. 6a–e) are 0.43–0.59 mm long with the maximal cranidial width across the palpebral lobes (palpebral cranidial width heareafter) of 0.43–0.59 mm. They have a sub-trapezoidal outline with rounded anterior margin in dorsal view. The axial furrows are clearly incised. The glabella is parallel-sided with a rounded anterior margin. The occipital furrow is shallow and straight. The posterior occipital margin is rounded rearward. The preglabellar field is short, about 0.1 of the cranidial length. The palpebral lobes are defined by shallow palpebral furrows, and about 0.35 of the cranidial length. The anterior branch of the facial suture is convergent anteriorly and convex abaxially, and the posterior branch of the facial suture runs transversely outward, and then abruptly runs backward, forming a right angle. The posterior border furrows are transverse and shallow, and the posterior cranidial margin is abaxially deflected posteriorly. A pair of protaspid marginal spines, located just behind the palpebral lobes of the protaspis, is absent, implying that there was an abrupt degeneration of the marginal spine during protaspid/meraspid transition. Such a phenomenon is also recognized during the protaspid/meraspid transition of the Ordovician remopleuridioid trilobite Remopleurides caelatus Whittington, Reference Whittington1959 (see Whittington, Reference Whittington1959, pl. 3, fig. 1–9, and also Fortey & Chatterton, Reference Fortey and Chatterton1988, text-fig. 11.4, 5 for the reconstruction). Specimens studied: n = 12.

Figure 6. Post-protaspid cranidia of Haniwa quadrata Kobayashi, Reference Kobayashi1933. (a–e) Developmental stage 1 cranidia; (a) SNUP6008; (b–d) SNUP6009, dorsal, lateral and ventral views; (e) SNUP6010. (f–i) Developmental stage 2 cranidia; (f) SNUP6011; (g, h) SNUP6012, dorsal and lateral views; (i) SNUP6013. (j–n) Developmental stage 3 cranidia; (j) SNUP6014; (k, l) SNUP6015, dorsal and lateral views; (m) SNUP6016, ventral view; (n) SNUP6017. (o–s) Developmental stage 4 cranidia; (o, p) SNUP6018, dorsal and lateral views; (q) SNUP6019, ventral view; (r) SNUP6020; (s) SNUP6021. (t–y) Developmental stage 5 cranidia; (t) SNUP6022; (u) SNUP6023; (v) SNUP6024, ventral view; (w–y) SNUP6025, lateral, dorsal and ventral views. (z) Holotype of Haniwa quadrata from the Tsinania canens Zone of Liaotung, North China, stored in the University Museum, University of Tokyo, Japan, PA0424.
The developmental stage 2 cranidia (Fig. 6f–i) are 0.58–0.90 mm long with the palpebral cranidial width of 0.64–0.97 mm, and are distinguished from the developmental stage 1 cranidia in having a straight to slightly curved anterior border furrow and longer posterior fixigenal projection. The anterior margin of the glabella is less rounded than that of the previous stage cranidia. The preglabellar area is 0.14–0.19 of the cranidial length. The preglabellar field is weakly convex dorsally. The occipital furrow is moderately incised. The anterior border is short (sag.) and the cranidial anterior margin is broadly rounded. The palpebral ridge is moderately thick and short. The length of palpebral lobes is 0.41–0.43 of the cranidial length. The anterior branch of the facial suture is slightly convergent to slightly divergent forward. The posterior fixigenal projection is exsagittally short and transversely long. The posterior border furrow is shallow and straight. The most severe allometric growth can be recognized during this developmental stage (Fig. 5). n = 25.
The developmental stage 3 cranidia (Fig. 6j–n) are 0.87–1.36 mm long with a palpebral cranidial width of 0.90–1.62 mm, and have a more rounded anterior margin. The preglabellar area occupies 0.21–0.23 of the cranidial length. The occipital furrow is straight, clearly incised and laterally continues to the straight posterior border furrow. Three pairs of glabellar furrows are wide and shallow. The palpebral ridge is obsolete. The length of the palpebral lobes is 0.41–0.47 of the cranidial length. The anterior branches of the facial suture diverge forward. The palpebral area of fixigena is slightly narrower than that of the developmental stage 2 cranidia. The posterior cranidial border slightly gets wider abaxially with a rounded posterolateral margin. n = 31.
The developmental stage 4 cranidia (Fig. 6o–s) are 1.49–2.21 mm long with the palpebral cranidial width of 1.59–2.70 mm. The furrows are moderately effaced, compared to the cranidia of the previous stage, but some specimens retain clearly incised palpebral, axial, occipital and glabellar furrows (Fig. 6s). The frontal margin of the glabella is weakly truncated in large specimens (Fig. 6r, s). The palpebral area of the fixigena is reduced, so that the anterior and posterior tips of the palpebral lobes almost abut the glabella. The preglabellar area is 0.23–0.27 of the cranidial length. The palpebral lobe is large and semi-circular in outline, and is 0.46–0.50 of the cranidial length. The anterior branches of the facial suture are forwardly divergent. The slope of the Procrustes distance against the centroid size becomes less steep during this developmental stage (Fig. 5), indicating that the large specimens of the developmental stage 4 may have reached the ‘geometrically’ mature morphology. n = 17.
The developmental stage 5 cranidia (Fig. 6t–y) are longer than 2.60 mm with the palpebral cranidial width more than 2.65 mm. The cranidia at this stage are morphologically mature (Fig. 5). The surface is moderately effaced. Notably, the anterior border furrow is completely effaced, so that the frontal area is weakly convex dorsally. The S1, S2, and the occipital furrow are shallowly impressed, while S3 glabellar furrows are not recognizable. The glabella is proportionally wider than those of the previous developmental stages. The preglabellar area is 0.26–0.30 of the sagittal cranidial length. The relative length of the palpebral lobes is generally shorter than that of the previous stage with 0.38–0.48 of the cranidial length. The anterior branches of the facial suture diverge forward. The posterior branches of the facial suture weakly curve forward, making the posterior fixigenal projection look like a ‘cedariform’ (Fig. 6w–y). The posterolateral part of the posterior fixigenal projection is slightly faceted (Fig. 6w, x). n = 48.
Free cheek development. The smallest free cheek at hand (Fig. 7a) could be fitted into a developmental stage 3 cranidium, and is anteriorly yoked. The free cheek remained anteriorly yoked throughout the subsequent development. The protaspid free cheeks and the free cheeks of the developmental 1 and 2 cranidia should also have been yoked anteriorly, judging from the fact that the protaspis of the Ordovician remopleuridioid, Remopleurides aff. R. eximius Whittington, Reference Whittington1959, had anteriorly yoked free cheeks (Fortey & Chatterton, Reference Fortey and Chatterton1988, pl. 17, figs 1–5). The smallest free cheek has a very narrow genal field with a narrow lateral border defined by a weak lateral border furrow. The eye socle is narrow. The lateral border gets slightly narrower anteriorly, but does not disappear at the anteriormost part.

Figure 7. Free cheeks of Haniwa quadrata Kobayashi, Reference Kobayashi1933. (a) SNUP6026; (b) SNUP6027; (c) SNUP6028; (d) SNUP6029; (e) SNUP6030; (f–h) SNUP6031, dorsal, lateral, and ventral views; (i, j) SNUP6032, dorsal, and ventral views; (k) SNUP6033; (l, m) SNUP6034, dorsal and ventral views.
Subsequently, the genal field gets wider and so does the lateral border (Fig. 7b). The genal spine is not well preserved, but relatively short. The free cheeks which would have fitted into the developmental stage 4 cranidia (Fig. 7c–e) have a slightly effaced dorsal surface; the lateral border furrow is shallow. The length of the genal spine is 0.6 of the cranidial length in the smaller specimen (Fig. 7c), but in the larger one the genal spine is as long as the cranidial length and slightly curved adaxially (Fig. 7d).
Large specimens which would have fitted into the developmental stage 5 cranidia have a highly effaced surface, so that there is no distinction between lateral border and genal field (Fig. 7f–m). The eye socle is relatively high in the largest specimen (Fig. 7i). The small specimen (Fig. 7f–h) has a wide doublure. The lateral border-equivalent genal field gets narrower anteriorly, and almost disappears at the anteriormost part. The length of the genal spine is about 1.2 of the cranidial length in the small specimen (Fig. 7f–h), while it is 0.8 of the cranidial length in the largest specimen (Fig. 7i, j). The slightly abaxially curved genal spine of the smallest specimen (Fig. 7f–h) seems to represent an intraspecific variation, given the fact that the others (Fig. 7i–m) are generally slightly curved adaxially. The genal spine and the ventral side of the doublure show terrace lines which run parallel to the margin.
Thoracic segment development. The morphology of thoracic segments within the complete specimens of Haniwa longa Zhu & Zhou in Yao & Wang, Reference Yao and Wang1978, reported by Zhu & Wei (Reference Zhu and Wei1991), helps to determine the relative position of disarticulated thoracic segments within the thorax in this study. Thoracic segments can be divided into three groups: thoracic segments from the anterior part of thorax have a wide axis with relatively straight pleurae (Fig. 8a–g); those from the middle part are transverse to slightly curved rearward with a narrow axis (Fig. 8h–o); and those from the posterior part have abaxially curved rearward pleurae (Fig. 8p–w).

Figure 8. Thoracic segments of Haniwa quadrata Kobayashi, Reference Kobayashi1933. (a–g) Thoracic segments from the anterior part of thorax; (a, b) small thoracic segments assigned to H. quadrata with doubt, SNUP6035, dorsal and posterior views; (c) SNUP6036; (d–g) SNUP6037, dorsal, ventral, posterior and lateral views. (h–o) Thoracic segments from the middle part of thorax; (h) small thoracic segment retaining the macropleural spines, SNUP6038; (i–k) SNUP6039, dorsal, posterior and ventral views; (l, m) SNUP6040, dorsal and posterior views; (n, o) SNUP6041, dorsal and lateral views. (p–w) Thoracic segments from the posterior part of the thorax; (p) SNUP6042; (q) SNUP6043; (r, s) SNUP6044, dorsal and lateral views; (t) SNUP6045; (u–w) SNUP6046, dorsal, posterior and ventral views.
Thoracic segments from the anterior part of the thorax (Fig. 8a–g) have a wide axis; the maximum width of the axis is about 0.4 of the width of the thoracic segment. The small thoracic segment (Fig. 8a, b) is assigned to Haniwa quadrata with reservation, as it has narrow, clearly incised, abaxially curved rearward pleural furrows, and an articulating half-ring much longer than the axial ring. The posterior view of this small thoracic segment displays a relatively high convexity (Fig. 8b), compared to other thoracic segments. The larger ones (Fig. 8c–g) have a somewhat effaced dorsal surface, and a rounded forward-articulating half-ring, separated from the axial ring by a shallow articulating furrow. The articulating half-ring is as long as the axial ring. The posterior margin of the axial ring is transverse, or weakly indented. The pleura runs horizontally only to the fulcrum, distal to which it dips ventrally. The axial furrow is shallow in the large specimen (Fig. 8d). The anterolateral margin has a prominent articulating facet. The doublure is narrow and bounds from the lateral margin to the posterolateral margin (Fig. 8e).
Thoracic segments from the middle part of the thorax (Fig. 8h–o) have an articulating half-ring which is shorter than the length of the axial ring. The articulating furrow is clearly incised. The posterior margin of the axial ring is transverse. The width of the axis is slightly less than one-third of the width of the thoracic segment. The pleura is weakly curved rearward abaxially. Pleural furrows are wide and moderately incised. The articulating facet is relatively narrow, compared to the thoracic segments from the anterior part of the thorax. A broad doublure bounds the lateral margin and continues to a narrow doublure bounding the abaxial part of the posterior margin (Fig. 8k). Terrace lines on the doublure run along the lateral margin of the thoracic segment (Fig. 8k). A panderian notch and a panderian protuberance are recognizable in the ventral view (Fig. 8k) and the posterior view (Fig. 8j), respectively. The smallest specimen (Fig. 8h) has a pair of long macropleural spines. The macropleural spines of this thoracic segment must be homologous to the macropleural spines of immature pygidia. Zhu & Wei (Reference Zhu and Wei1991) reported an early holaspis of Haniwa longa, which has prominent macropleural spines in the third thoracic segment, which was, however, degenerated later in development. The macropleural spines of the small thoracic segment of Haniwa quadrata also may have been degenerated with growth, since no large thoracic segments with macropleural spines have been discovered in association with Haniwa quadrata in this study.
Thoracic segments from the posterior part of the thorax (Fig. 8p–w) have abaxially curved rearward pleurae. The articulating half-ring is shorter than the axial ring. The posterior margin of the axial ring is transverse or slightly arched forward. The width of the axis is 0.4 of the width of the thoracic segment. The pleura runs horizontally to the fulcrum, distal to which it abruptly curves rearward. The pleural furrow is wide and moderately incised. The articulating facet is relatively narrower than that of the thoracic segments from the middle part of the thorax, and extends from the anterolateral margin to the lateral margin of the thoracic segment. A panderian protuberance and a panderian notch are prominent in the posterior view (Fig. 8v) and the ventral view (Fig. 8w), respectively.
The overall morphology of thoracic segments of Haniwa quadrata is slightly different from that of H. longa, illustrated by Zhu & Wei (Reference Zhu and Wei1991); thoracic segments of H. quadrata do not have distinctive pleural spines, whereas those of H. longa do. However, in H. longa, there is a trend that posteriorly located thoracic segments within the thorax are more spinose than the anteriorly located ones. This is also true for H. quadrata; thoracic segments from the anterior part of the thorax have a rounded posterolateral margin, whereas those from the middle and posterior part have a weakly pointed posterolateral margin (see Fig. 8). In addition, the morphologically mature pygidium of H. quadrata, which will be described below, has a smooth posterior margin, while the pygidium of H. longa is spinose. In short, the trunk of H. quadrata has less spinose pleurae than that of H. longa.
Post-protaspid pygidial development. Sohn & Choi (Reference Sohn and Choi2007) mistakenly assigned the pygidia of Haniwa to Quadraticephalus elongatus Kobayashi, Reference Kobayashi1935 (Sohn & Choi, Reference Sohn and Choi2007; fig. 5p, t, u), and did not assign any pygidium to Haniwa. The pygidia of Haniwa illustrated by Sohn & Choi (Reference Sohn and Choi2007) under the name of Q. elongatus are, however, different from those of Haniwa quadrata illustrated in this study in having a more transverse outline, thus requiring a taxonomic revision.
In this study, the development of pygidia is divided into nine stages (Fig. 9), according to morphology. The last three stages are considered to represent the holaspid period, and thus the first six should belong to the meraspid period. The complete specimens of Haniwa longa (Zhu & Wei, Reference Zhu and Wei1991) have eleven thoracic segments, and all the so-far discovered remopleuridioid trilobites whose thorax is articulated are known to have at least eleven thoracic segments (see Adrain, Peters & Westrop, Reference Adrain, Peters and Westrop2009, p. 39). The possible primitive sister taxon of remopleuridioid trilobites, Cedarina schachti Adrain, Peters & Westrop, Reference Adrain, Peters and Westrop2009, had ten thoracic segments (Adrain, Peters & Westrop, Reference Adrain, Peters and Westrop2009). Accordingly, Haniwa quadrata may have at least ten thoracic segments, hence a minimum of ten meraspid degrees. The six meraspid stages (stage A–F) of Haniwa quadrata in this study, therefore, do not strictly correspond to the meraspid degrees; some stages must include more than one meraspid degree within them. However, due to the gradational morphological change, further division of meraspid stages is currently impossible. In addition, poorly preserved surface structure hampers further differentiation of pygidial development.

Figure 9. Scatter plot of pygidial length versus width for post-protaspid pygidia of Haniwa quadrata Kobayashi, Reference Kobayashi1933.
The Stage A pygidia (Fig. 10a–j) are 0.32–0.43 mm long and 0.47–0.64 mm wide, and characterized by having two pairs of macropleural spines which must be homologous to the two posteriormost pairs of marginal spines of the protaspides (Fig. 3). The anterior second and third pairs of marginal spines of the protaspides are not recognized in these earliest pygidia, implying a possible sudden degeneration during protaspid/meraspid transition. However, it cannot be ruled out that the possible earliest meraspid pygidia which would have had the second and third pairs of marginal spines-bearing segments were not recovered. The pygidia are convex dorsally in lateral view (Fig. 10b, e, h). The relatively high convexity of the pygidium is similar to the convex protaspid morphology, indicating that no significant metamorphosis was involved during the protaspid/meraspid transition. The poor preservation hinders further detailed observation, but very shallow pleural and interpleural furrows are visible in a well-preserved specimen (Fig. 10g). The anterior pair of macropleural spines is longer than the posterior one. It is not clear how many segments are present in front of the first macropleural-bearing segment. Nevertheless, the relatively wide variation in size for this earliest stage (Fig. 9) suggests that this stage may include more than one meraspid degree. n = 19.

Figure 10. Post-protaspid pygidia of Haniwa quadrata Kobayashi, Reference Kobayashi1933. (a–j) Stage A pygidia; (a–c) SNUP6047, dorsal, lateral and posterior views; (d–f) SNUP6048, dorsal, lateral and posterior views. (g–i) SNUP6049, dorsal, lateral, posterior views; (j) SNUP6050, ventral view. (k–p) Stage B pygidia; (k–m) SNUP6051, dorsal, posterior and lateral views; (n–p) SNUP6052, dorsal, posterior and lateral views. (q–v) Stage C pygidia; (q–s) SNUP6053, dorsal, lateral and posterior views; (t, u) SNUP6054, dorsal and lateral views; (v) SNUP6055, ventral view. (w–cc) Stage D pygidia; (w, x) SNUP6056, dorsal and posterior views; (y–aa) SNUP6057, dorsal, lateral and posterior views; (bb, cc) SNUP6058, dorsal and oblique posterior views. (dd–kk) Stage E pygidia; (dd–hh) SNUP6059, dorsal, posterior, oblique posterior, ventral and lateral views; (ii–jj) SNUP6060, dorsal and oblique posterior views; (kk) SNUP6061. (ll–ss) Stage F pygidia; (ll–nn) SNUP6062, dorsal, lateral and oblique posterior views; (oo–qq) SNUP6063, dorsal, lateral and oblique posterior views; (rr, ss) SNUP6064, dorsal and oblique posterior views.
The Stage B pygidia (Fig. 10k–p) are 0.35–0.45 mm long and 0.49–0.59 mm wide. They can be distinguished from Stage A by the anteriorly located macropleural spine-bearing segment. The overall convexity is lower than that of the Stage A pygidia (Fig. 10m, p). In one specimen there is nothing in front of the macropleural spine-bearing segment (Fig. 10k–m), but in other specimens there is a pair of small pleural spines in front of the first pair of macropleural spines (Fig. 10n–p). However, it is not clear whether the pair of small spines represents the presence of a segment in front of the macropleural spine-bearing segment. The anterior two macropleural spines are as long as the sagittal length of the pygidium, and the posterior two macropleural spines are about 1.4 of the sagittal pygidial length. The overall size of the Stage B pygidia is not much different from that of the Stage A pygidia (Fig. 9). Such stability in pygidial size during development was shown in the pygidial development of Hintzeia plicamarginis Simpson, Hughes, Kopaska-Merkel & Ludvigsen, Reference Simpson, Hughes, Kopaska-Merkel and Ludvigsen2005 (Simpson et al. Reference Simpson, Hughes, Kopaska-Merkel and Ludvigsen2005) and Cyclolorenzella convexa (Resser & Endo in Endo & Resser, Reference Endo and Resser1937) (Park & Choi, Reference Park and Choi2010b). Simpson et al. (Reference Simpson, Hughes, Kopaska-Merkel and Ludvigsen2005) demonstrated that the stability in size during pygidial development of H. plicamarginis represents a depletion phase in the late meraspid period, during which segment release from the anterior part of the pygidium into the thorax continued after the generation of new segments in the rear part of the pygidium had stopped. The size stability during pygidial development in C. convexa occurred in a relatively later phase of the meraspid period (Park & Choi, Reference Park and Choi2010b). It is noteworthy that the stability in size during pygidial development is seen in the early meraspid phase in Haniwa quadrata. However, due to poor preservation, it is uncertain whether the pygidial size stability during the early phase of the meraspid period in H. quadrata is ascribable to the presence of a depletion phase. n = 6.
The Stage C pygidia (Fig. 10q–v) are 0.30–0.38 mm long and 0.61–0.82 mm wide, and have one pair of macropleural spines. They are less convex than the Stage B pygidia (Fig. 10r, u). The length of the macropleural spines is about twice the pygidial length. The macropleural spines project abaxially rearward. The maximum width of the axis is about 0.3 of the maximum pygidial width. There are at least four axial rings and four pairs of small marginal spines behind the macropleural spines. It is interesting to note that the Stage C pygidia are generally wider, but shorter than those of the previous stages (Fig. 9). n = 13.
The Stage D pygidia (Fig. 10w–cc) are 0.33–0.45 mm long and 0.68–0.80 mm wide. The macropleural spine-bearing segment was released to the thorax and thus is not present at this stage. Pleural furrows are weakly incised. The pygidial length is about 0.45–0.58 of the pygidial width. The maximum width of the axis is 0.25–0.28 of the pygidial width. There are at least four axial rings. Five pairs of marginal spines are present, but some specimens (e.g. Fig. 10bb, cc) have one extra pair of small spines at the posterior end of the pygidium. n = 9.
The Stage E pygidia (Fig. 10dd–kk) are 0.42–0.55 mm long and 0.81–0.98 mm wide. The pygidial length is about 0.48–0.66 of the pygidial width. The maximum width of the axis is 0.26–0.28 of the pygidial width. They have at least five axial rings and six pairs of marginal spines. The pleural furrows are wide and moderately deep. Interpleural furrows are not recognizable. n = 8.
The Stage F pygidia (Fig. 10ll–ss) are 0.39–0.46 mm long and 0.78–1.03 mm wide. The pygidial length is about 0.45–0.50 of the pygidial width. The maximum width of the axis is 0.28–0.31 of the pygidial width, thus relatively wider than that of the Stage E pygidia. There are at least five axial rings and six pairs of marginal spines. The smallest specimen (Fig. 10qq, rr) has somewhat distinctive morphology in which the marginal spines, except the anteriormost pair, are slightly conjoined at the base, implying that more than one meraspid degree is included within this stage. This single specimen has five pairs of marginal spines and may represent the last degree of the meraspid period, as the marginal spines conjoined at the base are characteristics of the early holaspid pygidia (Fig. 11). n = 9.
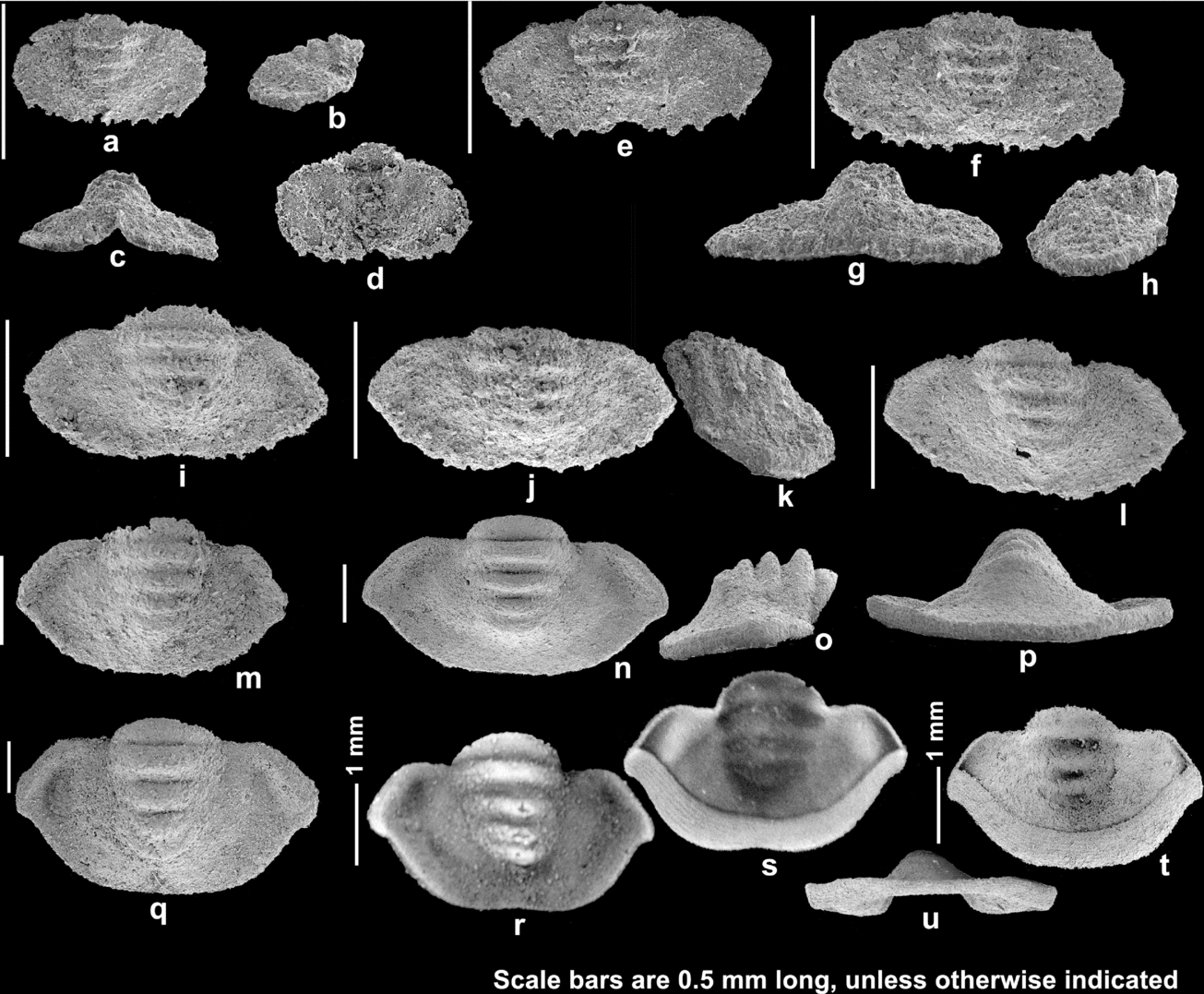
Figure 11. Holaspid pygidia of Haniwa quadrata Kobayashi, Reference Kobayashi1933. (a–h) Holaspid stage A pygidia; (a–d) SNUP6065, dorsal, lateral, posterior and ventral views; (e) SNUP6066; (f–h) SNUP6067, dorsal, posterior and lateral views. (i–l) Holaspid stage B pygidia; (i) SNUP6068; (j, k) SNUP6069, dorsal and lateral views; (l) SNUP6070. (m–u) Holaspid stage C pygidia; (m) SNUP6071; (n–p) SNUP6072, dorsal, lateral and posterior views; (q) SNUP6073; (r, s) SNUP6074, dorsal and ventral views; (t, u) SNUP6075, ventral and posterior views.
The Holaspid stage A pygidia (Fig. 11a–h) are 0.33–0.42 mm long and 0.64–0.97 mm wide, and are characterized by a smooth pleural field, and four pairs of short marginal spines which are conjoined at the base. The smooth pleural field is a characteristic of the holaspid pygidia of Haniwa quadrata, and thus this stage can be confidently considered to be the earliest stage of holaspid period. The maximum width of the axis is 0.32–0.36 of the pygidial width. The smallest pygidium of this stage (Fig. 11a–d) is shorter and narrower than the Stage D pygidia (Fig. 9). The posterolateral part of the pleural field slopes strongly downward distally, and continues to the flat marginal spines. The length of the smallest pygidium is about 0.52 of the pygidial width, while those of the other two pygidia are 0.40 and 0.43, respectively. There are four axial rings. n = 3.
The Holaspid stage B pygidia (Fig. 11i–l) are 0.50–0.67 mm long and 0.99–1.27 mm wide and are defined by having an uneven posterolateral margin, which indicates that the marginal spines are being degenerated or completely conjoined with growth. The maximum width of the axis is 0.38–0.40 of the pygidial width. The inter-ring furrows are sagittally wider than those of the previous stage. The anterior margin runs horizontally to the fulcrum and then dips ventrally distally, probably in parallel with the posterior margin of the posteriormost thoracic segment (see Fig. 8p–w). There is a gentle depression in the anterolateral part of the pleural field. n = 6.
The Holaspid stage C pygidia (Fig. 11m–u) have a smooth posterolateral margin. The axial rings are highly convex and prominent (Fig. 11o). The most abaxial part of the pygidium is slightly pointed, compared to the smooth posterolateral margin. The doublure is broad, and sculptured with terrace lines that are sub-parallel to the margin (Fig. 11t). There is no change in the number of axial rings since the onset of the holaspid period. n = 40.
To see if there was an allometric growth within the holaspid period, seven landmarks were chosen for 49 holaspid pygidia in which the landmarks were available to be digitized, and the Partial Procrustes distance of each specimen by the reference of the smallest holaspid pygidium was plotted against the centroid size (Fig. 12). Although the ontogenetic morphological variation of the holaspid pygidia enables the tripartite division of the holaspid period in this study, allometric growth was not identified during the holaspid period apart from the transition from the morphology of the smallest pygidium into those of the other holaspid pygidia (Fig. 12). However, it cannot be ruled out that the allometric growth during the early holaspid period, if any, was masked by the significant morphological variation shown by the morphologically mature holaspid pygidia (Fig. 12), or the number of landmarks was not enough to reveal the pygidial morphology in the first place.

Figure 12. Partial Procrustes distance of holaspid pygidia from a reference form of the smallest holaspid pygidium (Fig. 11a) against centroid size, and a diagram showing the selected seven landmarks on holaspid pygidium. As there is a significant morphological variation even among the smallest three holaspid pygidia (Fig. 11a–h), only the smallest one was selected for the reference form. Note that no significant allometric growth can be detected except for the obviously immature morphology of the smallest pygidium (see text). Symbols are the same as in Figure 9.
The number of axial rings and marginal spines decreases when entering into the holaspid period, and does not change throughout the holaspid period. As the epimorphic phase during which the number of trunk segments did not change any more preceded the onset of holaspid period during development, the developmental mode of H. quadrata can be considered protomeric (sensu Hughes, Minelli & Fusco, Reference Hughes, Minelli and Fusco2006).
4. Discussion
4.a. Phylogenetic position of Haniwa
The phylogenetic position of Haniwa has been a source of debate. Kobayashi (Reference Kobayashi1935) assigned the genus to the family Ptychopariidae, but Shergold (Reference Shergold1975) considered Haniwa as a family Incertae Sedis under the Superfamily Remopleuridioidea. Later, Guo & Duan (Reference Guo and Duan1978), Qiu et al. (Reference Qiu, Lu, Zhu, Bi, Lin, Zhang, Qian, Ju, Han and Wei1983) and Chen et al. (Reference Chen, Qian, Lin, Zhang, Wang, Yin and Erdtmann1985) regarded this genus as a member of the family Anomocaridae. Zhu & Wei (Reference Zhu and Wei1991) placed Haniwa in the remopleuridioid family Richardsonellidae, pointing out the resemblance of the well-preserved specimens of Haniwa with the members of the Richardsonellidae. Jell & Adrain (Reference Jell and Adrain2003) did not regard the Richardsonellidae as a valid family, and assigned Haniwa to the Remopleurididae of the expanded familial concept which included all the previous members of the Richardsonellidae (see also Adrain, Peters & Westrop, Reference Adrain, Peters and Westrop2009).
The ontogenetic information of Haniwa quadrata corroborates the remopleuridioid affinity of this trilobite. Although the protaspis of Haniwa quadrata is not highly bulbous, it is still similar to that of the Ordovician remopleuridioid Remopleurides caelatus Whittington, Reference Whittington1959 (see Whittington, Reference Whittington1959, pl. 3, fig. 1–5, and also Fortey & Chatterton, Reference Fortey and Chatterton1988, text-fig. 11.4 for reconstruction) in having relatively large palpebral lobes, smooth surface and marginal spines just behind the palpebral lobes projecting posterolaterally. This suggests the close phylogenetic relationship of Haniwa quadrata with the Ordovician remopleuridioids, and thus H. quadrata is considered to represent the primitive form of the Remopleuridioidea. If the ‘cedariform’-like appearance of the small posterior fixigenal projection in the cranidia of H. quadrata is phylogenetically homologous to the cedariform of the Marjuman trilobite family Cedariidae, the claim of Adrain, Peters & Westrop (Reference Adrain, Peters and Westrop2009) that the Remopleuridioidea may have arisen from a stock of the Cedariidae would be accepted. On the other hand, Haniwa did not possess the long axial spine on the eighth thoracic segment and pits in the anterior border furrow which were noted as synapomorphies grouping the Marjuman cedariid Cedarina, and the Ordovician remopleuridioid trilobites (Adrain, Peters & Westrop, Reference Adrain, Peters and Westrop2009); the complete specimens of Haniwa longa do not have such a long axial spine (Zhu & Wei, Reference Zhu and Wei1991), no thoracic segment with a long axial spine was associated with Haniwa quadrata in this study, and the cranidia of Haniwa lack the pits in the anterior border furrow. If Cedarina is a true plesiomorphic sister taxon of the younger remopleuridioid trilobites, the lack of the prominent axial spine and the pits in the anterior border furrow may have been an autapomorphic feature of Haniwa.
Fortey & Chatterton (Reference Fortey and Chatterton1988, p. 200) gave a diagnosis of the Remopleuridioidea, in which the spinose pygidium with flattened spines united at the base was considered as one of the main features of the superfamily. Haniwa longa has a pygidium with such morphology (see Zhu & Wei, Reference Zhu and Wei1991). The morphologically mature pygidium of Haniwa quadrata, however, does not have a spinose pygidial margin, but a smooth posterolateral margin. Interestingly, the Holaspid stage A pygidia, representing the earliest holaspid period of H. quadrata, are spinose with flattened spines united at the base. This fact suggests that the typical spinose pygidium of the Remopleuridioidea may have been a result of paedomorphic evolution of the primitive remopleuridioid trilobites with a smooth pygidial margin such as H. quadrata. The possible Marjuman primitive sister taxon of remopleuridioid trilobites, Cedarina, also has a pygidium with smooth margin (see Adrain, Peters & Westrop, Reference Adrain, Peters and Westrop2009).
4.b. Phylogenetic position of Remopleuridioidea
Fortey & Chatterton (Reference Fortey and Chatterton1988, p. 200) noted that the relatively primitive members of the Remopleuridioidea, such as Pseudokainella, Menoparia and Elkanaspis, had ventral median sutures, and accordingly included the Remopleuridioidea in their cladistic analysis of the Order Asaphida. However, the middle Furongian remopleuridioid trilobite, Haniwa quadrata, possessed a yoked free cheek, lacking a ventral median suture, as did H. longa (see Zhu & Wei, Reference Zhu and Wei1991). The yoked free cheek may not have been an autapomorphic condition of Haniwa, but may have been a plesiomorphic condition of the Remopleuridioidea, because Taishania Sun, Reference Sun1935, which is an older remopleuridioid trilobite than Haniwa, also had a yoked free cheek (see Qian, Reference Qian1994, pl. 15, fig. 8). If this is the case, the ventral median sutures of the younger remopleuridioid trilobites must have been derived from a yoked free cheek as were those of Asioptychaspis (Park & Choi, Reference Park and Choi2010a), and they should be distinguished from the ventral median sutures of other members of the Order Asaphida, which were considered to be attained by the loss of an inverted-triangular rostellum in front of a connective suture (Chatterton et al. Reference Chatterton, Speyer, Hunt and Fortey1994).
The protaspid morphology of H. quadrata also supports the exclusion of the Remopleuridioidea from the Order Asaphida. The Ordovician remopleuridioid trilobites are known to possess a highly globular protaspis with enrolled doublure, termed asaphoid protaspis by Fortey & Chatterton (Reference Fortey and Chatterton1988) (see Ross, Reference Ross1951; Whittington, Reference Whittington1959; Chatterton, Reference Chatterton1980). Although the protaspis of the primitive Furongian remopleuridioid Haniwa quadrata is more convex and globular than the typical benthic protaspides, it is not as globular as those of the Ordovician relatives and lacks an enrolled doublure (Fig. 13). Moreover, no significant radical metamorphosis occurred during the development of H. quadrata, except the possible sudden disappearance of the anterior three pairs of the protaspid marginal spines during the protaspid/meraspid transition. This demonstrates that the plesiomorphic remopleuridioid trilobites did not possess a typical asaphoid protaspis. Adrain, Peters & Westrop (Reference Adrain, Peters and Westrop2009) also mentioned that, based on unpublished material, the plesiomorphic remopleuridid Elkanaspis did not have a highly globular protaspis. This corroborates the idea that the asaphoid protaspis was not the plesiomorphic condition of the Remopleuridioidea. The highly globular protaspides of the Ordovician remopleuridioid trilobites with an enrolled doublure, therefore, must have evolved from a less globular protaspis without an enrolled doublure. Accordingly, the highly globular protaspid morphology of the Ordovician remopleuridioid trilobites is not homologous to the ‘asaphoid protaspis’ of the Asaphidae, making the exclusion of the Remopleuridioidea from the Order Asaphida tempting. In addition, the protaspid morphology of H. quadrata differs from that of the dikelocephaloid trilobite, Asioptychaspis subglobosa (Sun, Reference Sun1924), reported by Park & Choi (Reference Park and Choi2010a), proving that the Remopleuridioidea is not phylogenetically close to the Dikelocephaloidea.

Figure 13. Sagittal sections of five protaspides for the comparison of convexity (anterior is to the left). (a) Typical benthic protaspis of the middle Cambrian ptychoparioid, Spencella sp. (reconstructed on the basis of Fortey & Chatterton, Reference Fortey and Chatterton1988, text-fig. 11.12c); (b) slightly bulbous protaspis of Haniwa quadrata (reconstructed on the basis of Fig. 3r, this study); (c, d) highly bulbous Ordovician remopleuridioid protaspides with enrolled doublure; (c) Remopleurides sp. aff. R. eximius Whittington, Reference Whittington1959 (modified from Fortey & Chatterton, Reference Fortey and Chatterton1988, text-fig. 9.1a); (d) Remopleurides caelatus Whittington, Reference Whittington1959 (reconstructed on the basis of Fortey & Chatterton, Reference Fortey and Chatterton1988, text-fig. 11.4a); (e) typical asaphoid protaspis of Isotelus sp. which belongs to the Asaphidae (reconstructed on the basis of Fortey & Chatterton, Reference Fortey and Chatterton1988, text-fig. 11.9b).
In short, the yoked free cheek and the protaspid morphology of Haniwa quadrata corroborate the claim of Adrain, Peters & Westrop (Reference Adrain, Peters and Westrop2009) that Remopleuridioidea is not a member of the Order Asaphida.
4.c. Multiple evolutions of a highly globular protaspis and its implication for the current Order Asaphida
It is significant in defining the concept of the Order Asaphida that the highly globular protaspides with enrolled doublure of the Ordovician remopleuridioid trilobites evolved independently from the asaphoid protaspis of the Asaphidae. While reporting the first convincing case of the polyphyletic evolution of ventral median suture, Park & Choi (Reference Park and Choi2009) suggested the Order Asaphida be defined exclusively by the presence of the asaphoid protaspis. However, as shown above, a highly globular protaspis with enrolled doublure could also have evolved polyphyletically, and thus the possession of a highly globular protaspis alone cannot guarantee the membership of the Order Asaphida.
The trilobite families and superfamilies that have a highly globular protaspis, summarized in Fortey & Chatterton (Reference Fortey and Chatterton1988), include the Asaphidae, Nileidae, Remopleuridioidea and Trinucleoidea. Later, Berard, Clarkson & Taylor (Reference Berard, Clarkson and Taylor2000) assumed that Taihungshania miqueli (Bergeron, Reference Bergeron1893), which belongs to the Taihungshaniidae, had an asaphoid protaspis. The inclusion of these trilobite groups within one order, therefore, should be reconsidered. In particular, the Superfamily Trinucleoidea has been placed in the Order Asaphida, based on the presence of a ventral median suture in the possible middle Cambrian sister taxon, Liostracina (Fortey & Chatterton, Reference Fortey and Chatterton1988, p. 211), and globular protaspis in the Ordovician representatives (Fortey & Chatterton, Reference Fortey and Chatterton1988; Chatterton et al. Reference Chatterton, Speyer, Hunt and Fortey1994). As the possession of both a ventral median suture and a highly globular protaspis does not guarantee the Asaphida affinity, the inclusion of the Superfamily Trinucleoidea within the Order Asaphida would require further examination.
Taken together, only the five derived families, the Ceratopygidae, Asaphidae, Taihungshaniidae, Nileidae and Cyclopygidae, can confidently remain within the Order Asaphida of Fortey & Chatterton (Reference Fortey and Chatterton1988) and Fortey (Reference Fortey1990). However, as the key characters which defined the Order Asaphida, a ventral median suture and a highly globular protaspis, turn out to be subject to polyphyletic evolution, the concept of the Order Asaphida should first be emended. More comprehensive cladistic analysis including all pertinent groups and characters is required in order to represent the ingroups and redefine the concept of the order.
5. Conclusions
(1) The ontogeny of the middle Furongian remopleuridioid, Haniwa quadrata, reveals that H. quadrata had a slightly globular protaspis without enrolled doublure, and the free cheek remained yoked during the whole of the development.
(2) As the primitive member of the Remopleuridioidea, Haniwa quadrata, possessed neither the ventral median suture nor a highly globular protaspis, the Superfamily Remopleuridioidea should be excluded from the Order Asaphida, as suggested by Adrain, Peters & Westrop (Reference Adrain, Peters and Westrop2009).
(3) The evolution of the highly globular protaspis with enrolled doublure of the Ordovician remopleuridioid trilobites from the less bulbous protaspis without enrolled doublure demonstrates the multiple evolution of a highly globular protaspis. Therefore, the concept of the Order Asaphida is in need of revision.
Acknowledgements
We are grateful to Mark Webster and Jonathan M. Adrain for their constructive reviews. Thanks are extended to S. M. Lee and J. W. Sohn for their help in the field. Rudy Lerosey-Aubril, Thomas A. Hegna and Paul S. Hong provided some literature for this study. Jikhan Jung gave advice on the morphometric analyses. This work was supported by a grant from the National Research Foundation of Korea (Grant No.–2010–0000310). This paper is a contribution of the BK 21 Project of the School of Earth and Environmental Sciences, Seoul National University.















