1. Introduction
While the damselfly fossil record has greatly increased during recent years, thanks to the study of the mid-Cretaceous Burmese amber fauna, these are mainly Caloptera Belyshev & Haritonov, Reference Belyshev and Haritonov1983 and Coenagrionomorpha Bechly, Reference Bechly1996. The recorded Mesozoic Lestomorpha Bechly, Reference Bechly1996 (= Hemiphlebiidae Tillyard, Reference Tillyard1926 + Lestiformia Bechly, Reference Bechly1996) (= Lestoidea Calvert, Reference Calvert, Godman and Salvin1901, sensu Dijkstra et al. Reference Dijkstra, Kalkman, Dow, Stokvis and Van Tol2014) are mainly Hemiphlebiidae, and three or four Lestiformia in the families Cretacoenagrionidae Bechly, Reference Bechly1996, Perilestidae Tillyard & Fraser, Reference Tillyard and Fraser1938 and Synlestidae Tillyard, Reference Tillyard1917. The oldest current records of the highly diverse extant Lestidae are from the Latest Eocene (Nel & Paicheler, Reference Nel and Paicheler1994; Nel & Fleck, Reference Nel and Fleck2014). This family is currently subdivided into Lestinae and Sympecmatinae, the former being diverse and frequently found in the Oligocene and the Miocene lacustrine sediments of Europe, while the latter is known by a Sympecma sp. from the Late Miocene of Italy, and Sympecma incerta (Piton, Reference Piton1934) from the Pliocene of France (Cavallo & Galetti, Reference Cavallo and Galletti1987; Nel & Paicheler, Reference Nel and Paicheler1994). No fossil Lestidae is currently recorded outside of Europe, while the family is cosmopolitan (except Antarctica).
Here we report a new fossil site and describe the oldest-known Lestidae from the late Eocene of Tibet (China), attributing it to the extant Western Palaearctic lestine genus Chalcolestes Kennedy, Reference Kennedy1920. It demonstrates the very broad distribution of Lestidae during the Eocene and supports its potential greater antiquity, in accordance with its extant distribution. We also discuss the palaeoclimatic implications of the new discovery.
2. Materials and methods
The fossils including the well-preserved Lestidae were collected in a new late Eocene deposit in northern Kanggale Hill (NK, 31° 58′ 21.38″ N, 88° 26′ 10.19′ E, 4662 m a.s.l.) of the south Nima Basin, which is a large Cenozoic continental basin in central Tibet, SW China (Fig. 1). It is an east–west-trending elongated rift basin resting on a Jurassic–Cretaceous marine succession, situated in the Bangong–Nujiang suture zone formed by the Mesozoic collision of the Lhasa and Qiangtang blocks (Kapp et al. Reference Kapp, Yin, Harrison and Ding2005, Reference Kapp, DeCelles, Gehrels, Heizler and Ding2007; DeCelles et al. Reference DeCelles, Kapp, Ding and Gehrels2007a) The fossiliferous strata comprise greyish-green mudstones and calcareous shales, interbedded with mudstones, sandstones and limestones (Fig. 2). Abundant fossils including Odonata, Orthoptera, Hemiptera, Hymenoptera and cyprinid fish occur in the greyish-white laminated limestones, dominated by the aquatic hemipteran Aquarius lunpolaensis and cyprinid fishes (Fig. 3), previously also recorded in the Dayu area of Lunpola Basin. The latest palaeomagnetic study of the Dayu Section provided a late Eocene (c. 39.5–37 Ma) age for the fossil layers (middle Niubao Formation) (Fang et al. Reference Fang, Dupont-Nivet, Wang, Song, Meng, Zhang, Nie, Zhang, Mao and Chen2020), thus indicating a late Eocene age for the new fossiliferous layers in the northern Kanggale Hill.

Fig. 1. Geological map showing fossil site in northern Kanggale Hill of Nima Basin, central Tibet, SW China.

Fig. 2. Photograph showing fossiliferous outcrop in Kanggale Hill.

Fig. 3. Representative fossils from fossiliferous outcrop in Kanggale Hill. (a) Chalcolestes tibetensis sp. nov., holotype (NIGP174550). (b) Aquarius lunpolaensis (Hemiptera). (c) Aquarius lunpolaensis and a cyprin fish. (d) Cyprinid fish. (e) Orthoptera: Grylloidea. Scale bars = 10 mm.
The specimens were examined dry using a Nikon SMZ1000 stereomicroscope. Observation was augmented by temporary wetting with laboratory alcohol, which improved the contrast between the fossil and the matrix, eliminating the surface irregularity of the latter. Photographs were taken using a Canon 5D digital camera, and line drawings were prepared from these photographs using image-editing software (CorelDraw X8 and Adobe Photoshop CS6). The specimens NIGP 174550 are housed in the Nanjing Institute of Geology and Palaeontology (NIGP), Chinese Academy of Sciences, China. We follow the wing venation nomenclature of Riek & Kukalová-Peck (Reference Riek and Kukalová-Peck1984), modified by Nel et al. (Reference Nel, Martínez-Delclòs, Paicheler and Henrotay1993), Bechly (Reference Bechly1996, Reference Bechly2016) and Jacquelin et al. (Reference Jacquelin, Desutter-Grandcolas, Chintauan-Marquier, Boistel, Zheng, Prokop and Nel2018). Abbreviations. AA, Analis anterior; AP, Analis posterior; Arc, arculus; Ax, primary antenodal cross-vein; C, Costa; CuA, Cubitus anterior; CuP Cubitus posterior; dc, discoidal cell; IR, Interradius; MA, Media anterior; MP, Media posterior; N, nodus; Pt, pterostigma; RA, Radius anterior; RP, Radius posterior; Rspl, radial supplementary longitudinal vein; sdc, subdiscoidal cell; sn, subnodus.
3. Systematic palaeontology
Order Odonata Fabricius, Reference Fabricius1793
Family Lestidae Calvert, Reference Calvert, Godman and Salvin1901
Genus Chalcolestes Kennedy, Reference Kennedy1920
Chalcolestes tibetensis sp. nov.
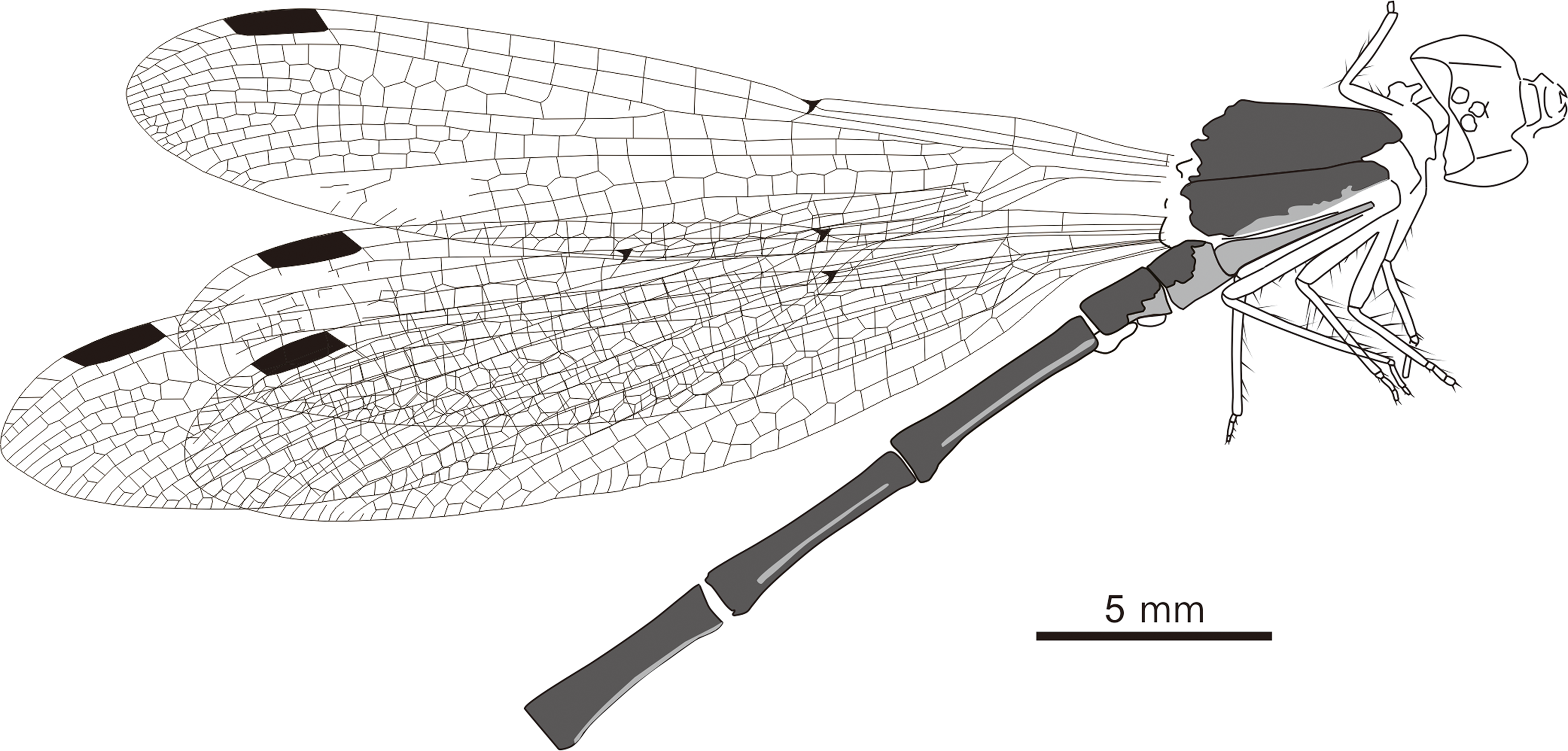
Fig. 4. Line drawing of Chalcolestes tibetensis sp. nov., holotype (NIGP174550).
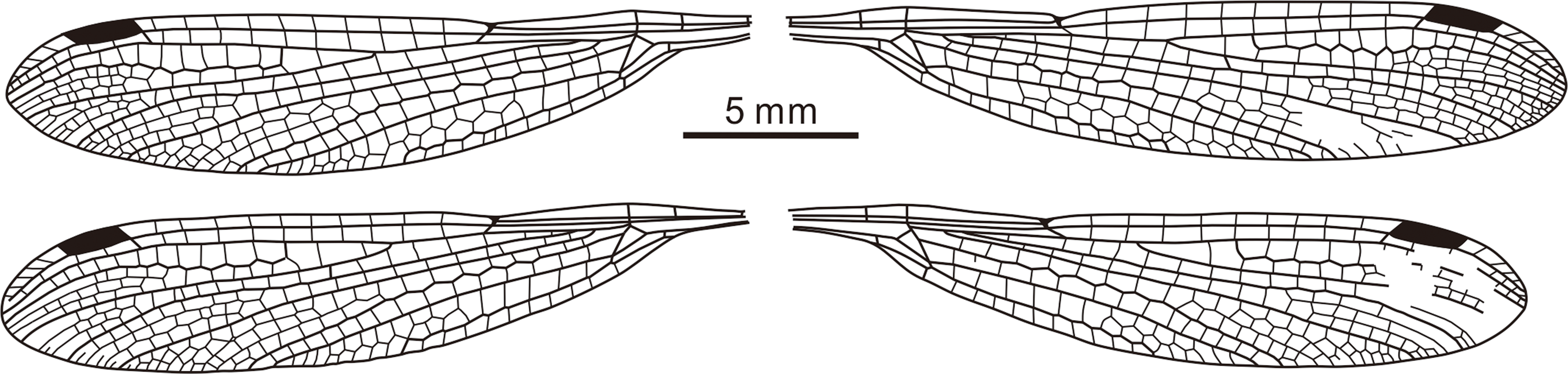
Fig. 5. Line drawing of four wings of Chalcolestes tibetensis sp. nov., holotype (NIGP174550).
Material. Holotype NIGP174550; imprint of a body with attached legs and wings, only five abdominal segments preserved; head deformed by the compression, so that the eyes seem to be closer than in an extant Lestidae, as occurs frequently for compression fossil damselflies; deposited in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, China.
Etymology. Named after the Tibet region where the type was found.
Age and outcrop. Late Eocene; Kanggale Hill, south Nima Basin, Central Tibet, China.
Diagnosis. Wings c. 21 mm long; only ten postnodal cross-veins; pterostigma covering two cells; base of AA clearly basal to CuP; five to six cells in supplementary row between MP and CuA in forewing and three rows in hind wings.
Description. Body length c. 17 mm, head length c. 1 mm, thorax length c. 3 mm and abdominal length c. 13 mm; head dark, broader than long. Eyes not well preserved, separated by gap of 0.8 mm. One foreleg, two mid-legs and two hind legs preserved (Fig. 3). Left forewing complete (Fig. 4). Wing length 21.2 mm; length from wing base to Arc 3.3 mm, from Arc to N 4.1 mm, from N to Pt 9.9 mm, from Pt to wing apex 3.8 mm. Primary antenodal cross-veins preserved, Ax0 close to wing base, Ax1 1.9 mm distal of Ax0, Ax2 1.3 mm distal of Ax1; no secondary antenodal and antesubnodal cross-veins present. Ten postnodal cross-veins nearly aligned with ten postsubnodal cross-veins present before Pt. Four postnodal and six postsubnodal cross-veins present distal of Pt, non-aligned. Arc aligned with Ax2. dc basally closed, free with strong distal angle; length of basal side 0.6 mm, of anterior side 0.3 mm, of distal side 0.8 mm, of posterior side 1.2 mm. sdc free and elongate, 1.7 mm long and maximum 0.3 mm wide. CuP 0.3 mm distal of level of Ax1. Nodal structures well preserved, Sn aligned with Cr. Midfork (base of RP3/4) lying closer to dc than to Arc, and 1.0 mm distal of Arc. Base of IR2 one cell and 0.6 mm distal of midfork. RP2 originating three cells distal of Sn, lying 3.0 mm distally, nearer to N than to Pt. IR1 long, originating two cells distal of base of RP2 and six cells basal of Pt, lying 2.7 mm distal of base of RP2; one row of cells present between IR1 and RP1, and three cells between IR1 and RP2. Rspl well present, with one row of cells between it and IR2, MA distally zigzagged and long. MP long and slightly curved, with one or two rows of cells between it and CuA. CuA long, with one row of cells between it and posterior wing margin. Pt long, hyaline, covering two cells and well braced, 2.0 mm long and 0.5 mm wide; posterior side of Pt thickened and fused with strongly thickened pterostigmal part of RA to form U-shaped structure; Pt brace in same oblique with Pt base.
4. Discussion
4.a. Relationship between Chalcolestes tibetensis sp. nov. and Lestidae
The wing venation of Chalcolestes tibetensis sp. nov. is characteristic of the Lestidae and more precisely of the subfamily Lestinae, because of the elongate pterostigmata; bases of RP3/4 and IR2 midway between arculus and nodus; an oblique cross-vein ‘O’; distal discoidal vein MAb very oblique, with distal angle of discoidal cell very acute; MA strongly zigzagged; area between IR2 and RP3/4 distally strongly widened with three rows of cells between these two veins; fore- and hind-wing discoidal cells of the same width and shape (excluding the Sympecmatinae Fraser, Reference Fraser1951 that have discoidal cell in forewings narrower and shorter than in hind wings); arculus mainly formed by the basal discoidal cross-vein, so that the discoidal cell is nearly touching the RA.
The Eocene Eolestes syntheticus Cockerell, Reference Cockerell1940 (type species of the Eolestidae Greenwalt & Bechly, Reference Greenwalt and Bechly2014) and Chalcolestes tibetensis sp. nov. have similar wing venations especially in the base of AA quite basal to CuP and the presence of secondary rows of cells between MP and CuA, but with the following major differences: area between RP3/4 and IR2 distally strongly widened with three rows of cells between these veins in C. tibetensis sp. nov. (as in other Lestidae); MA more strongly zigzagged in C. tibetensis sp. nov. as in other Lestidae; and pterostigma clearly shorter in C. tibetensis sp. nov. than in Eolestes. The synlestid genus Megalestes has a venation similar to those of the Lestidae and of C. tibetensis sp. nov., but differing in the absence of the oblique vein ‘O’ (although a rudimentary one can be present in some specimens) and clearly less acute distal angle of the discoidal cell with a longer anterior side of the same cell (Münz, Reference Münz1919: pl. 7, fig. 39; Payra et al. Reference Payra, Dawn, Subramanian, Deepak, Chandra and Tripathy2021). The enigmatic Eocene taxon Lutetialestes uniformis Greenwalt & Bechly, Reference Greenwalt and Bechly2014 (in Lestinoidea Calvert sensu Bechly, Reference Bechly1996, but of uncertain family) shares with C. tibetensis sp. nov. the base of AA quite basal to CuP and the presence of secondary rows of cells between MP and CuA, but differs from it in the MA very weakly zigzagged and ScP less oblique.
Within the Lestidae, the three sympecmatine genera Sympecma Burmeister, Reference Burmeister1839 , Indolestes Fraser, Reference Fraser1922 and Austrolestes Tillyard, Reference Tillyard1913 (incl. Ceylonolestes Kennedy, Reference Kennedy1920) have the discoidal cell of the hind wing of different shape than that of the forewing, more than l.15 times, usually 1.3–1.5 times, longer than that of the forewing (Fraser, Reference Fraser and Stephenson1933; Watson & Moulds, Reference Watson and Moulds1979; Seehausen, Reference Seehausen2017). Austrolestes also has the sectors of arculus not appressed to RA (Tillyard, Reference Tillyard1913: 422, fig. 2).
The classification of the Lestinae has been the subject of numerous changes in the past. Numerous genera, considered as valid by Fraser (Reference Fraser1951), were put as subgenera of Lestes by Pinhey (Reference Pinhey1980), viz. Chalcolestes, Paralestes Schmidt, Reference Schmidt1951, Xerolestes Fraser, Reference Fraser1951, plus the three subgenera Pseudochalcolestes Pinhey, Reference Pinhey1980, Icterolestes Pinhey, Reference Pinhey1980 and Malgassolestes Pinhey, Reference Pinhey1980. Pinhey (Reference Pinhey1980) synonymized the subgenus Africalestes Kennedy, Reference Kennedy1920 with the subgenus Lestes. Vajda et al. (Reference Vajda, Szabó, Cserháti and Dévaia2018) re-separated Chalcolestes from Lestes on the basis of body characters of adult and nymph. After Fraser (Reference Fraser1951), the vein AA separating from AP distinctly basal to vein CuP is a character proper to the genus Chalcolestes, vs AA emerging at the level of CuP or even slightly more basal in the other Lestinae (see also Tillyard, Reference Tillyard1913: fig. 1). This character is present in Chalcolestes tibetensis sp. nov. and even more pronounced than in the two extant species Chalcolestes parvidens (Artobolevsky, Reference Artobolevsky1929) and C. viridis (Van der Linden, Reference Van der Linden1825). Nevertheless, Jödicke (Reference Jödicke1997) indicated that not all specimens of C. viridis have this character, rendering it weaker than supposed by Fraser (Reference Fraser1951). C. tibetensis sp. nov. further differs from these two species in the presence of two rows of —five to six cells in the forewings, and three cells in the hind wings, between MP and CuA.
We investigated the potential affinities of Chalcolestes tibetensis sp. nov. with the other lestine genera. Archilestes Selys-Longchamps, Reference Selys-Longchamps1862 (including Superlestes Williamson, Reference Williamson1921) has a very broad pterostigma, and the base of RP2 only one cell distal to subnodus, unlike C. tibetensis sp. nov. (Münz, Reference Münz1919: pl. 7, fig. 40; Williamson, Reference Williamson1921; Fraser, Reference Fraser1951; Pérez-Gutiérrez, Reference Pérez-Gutiérrez2012). Cyptolestes Williamson, Reference Williamson1921, synonymized with Archilestes by Gloyd (Reference Gloyd1980), but considered by Donnelly (Reference Donnelly1981) as a subgenus of Archilestes, has a shorter pterostigma than the other species of Archilestes. Nevertheless, both also differ from C. tibetensis sp. nov. in the more numerous postnodal cross-veins (more than 12 instead of 9–10) (Williamson, Reference Williamson1921). Superlestes also has the apex of IR2 opposite the base of the pterostigma.
Platylestes Selys-Longchamps, Reference Selys-Longchamps1862 has a pterostigma quadrate or subquadrate (Münz, Reference Münz1919). Icterolestes has a short pterostigmata (l.5 mm or less, vs 2.0 mm in Chalcolestes tibetensis sp. nov.) and wing apices rounded instead of being acute in C. tibetensis sp. nov. (Pinhey, Reference Pinhey1980). Sinhalestes Fraser, Reference Fraser1951 has 18–20 postnodal cross-veins, unlike C. tibetensis sp. nov. (Fraser, Reference Fraser and Stephenson1933). Orolestes McLachlan, Reference McLachlan1895 has a longer pterostigma, covering more than four cells, and the wings of males broadly banded or spotted with brown colour (McLachlan, Reference McLachlan1895; Martin, Reference Martin1903; Fraser, Reference Fraser1951). The Lestes subgenera Paralestes and Malgassolestes have only long intercalary veins in the area between MA and RP3/4, unlike C. tibetensis sp. nov. (Fraser, Reference Fraser1951; Schmidt, Reference Schmidt1951; Pinhey, Reference Pinhey1980). Pseudochalcolestes differs from C. tibetensis sp. nov. in the base of AA opposite CuP in hind wing and the presence of only one supplementary longitudinal vein between MA and RP3/4 (Schmidt, Reference Schmidt1951). Xerolestes differs from C. tibetensis sp. nov. in the longest intercalary vein between RP3/4 and MA beginning well basal to base of RP2 (Pinhey, Reference Pinhey1980). The subgenus Lestes differs from C. tibetensis sp. nov. in the base of AA opposite CuP in fore- and hind wing.
Several fossils currently attributed to Lestes with a supplementary row of cells in the area between MP and CuA are known from the latest Eocene and Oligocene in Europe. We need to compare Chalcolestes tibetensis sp. nov. to these taxa. C. tibetensis sp. nov. has a wing venation very close to that of the latest Eocene Lestes regina Théobald, Reference Théobald1937 (Gard, France), even in the wings’ sizes (20–22 mm long in L. regina and 22 mm in C. tibetensis sp. nov.), but the latter has 11–12 postnodals, four cells between subnodus and base of RP2 and 5–6 cells in the supplementary row between MP and CuA in all wings (Théobald, Reference Théobald1937; Jattiot et al. Reference Jattiot, Latutrie and Nel2020). Nel & Fleck (Reference Nel and Fleck2014) described a latest Eocene fossil as Lestes aff. regina (Isle of Wight, UK), very similar to the French fossils. The early Oligocene Lestes forsterii Hess, Reference Hess1895 (Haut-Rhin, France) and L. vicina Hagen, Reference Hagen1858 (Germany) have five cells in the supplementary row between MP and CuA but 11–12 postnodal cross-veins (Hagen, Reference Hagen1858; Théobald, Reference Théobald1937). The Oligocene Lestes ceresti Nel, Reference Nel1985 (Vaucluse, France) also has a venation very close to that of C. tibetensis sp. nov., but many more cells in the supplementary row between MP and CuA (8–12 cells), more postnodal cross-veins and longer wings (25.2–28.8 mm long) than C. tibetensis sp. nov. It seems that the base of AA and CuP are closer to each other in L. ceresti than in C. tibetensis sp. nov. (Nel & Papazian, Reference Nel and Papazian1985; Nel & Paicheler, Reference Nel and Paicheler1994;). The Oligocene Lestes brisaci Nel et al. Reference Nel, Martínez-Delclòs, Papier and Oudard1997 (Vaucluse, France) has only two to four cells in the supplementary row between MP and CuA, but four cells between subnodus and base of RP2 (Nel et al. Reference Nel, Martínez-Delclòs, Papier and Oudard1997). The Oligocene Lestes statzi Schmidt, Reference Schmidt1958 (Germany) has many more postnodals than C. tibetensis sp. nov., base of RP2 three to four cells distal of subnodus, and only one to three cells in the supplementary row between MP and CuA (Schmidt, Reference Schmidt1958). The latest Oligocene Lestes aquisextana Nel, Reference Nel1985 has only one cell in the same area but two rows of cells between CuA and posterior wing margin (Nel, Reference Nel1985; Nel & Paicheler, Reference Nel and Paicheler1994).
In the sister clade (Synlestidae + Perilestidae) of the Lestidae, the base of AA is well basal to CuP in Megalestes (see Gyeltshen et al. Reference Gyeltshen, Kalkman and Orr2017; Payra et al. Reference Payra, Dawn, Subramanian, Deepak, Chandra and Tripathy2021) while AA separates from AP quite distal to CuP in Synlestes and Nubiolestes and there is no distinct AA in Perilestes (Münz, Reference Münz1919; Schmidt, Reference Schmidt1942). But as Megalestes is the sister group of all the others (Synlestidae + Perilestidae), it is likely that the situation in this genus is plesiomorphic, while the other representatives of this clade have a strong tendency to pronounced petiolation with a distal ‘migration’ of AA. The presence of the same situation in Hemiphlebia mirabilis (Hemiphlebiidae, sister group of all other Lestomorpha) supports this hypothesis. Also, the Early Cretaceous Cretalestes martinae Jarzembowski et al. Reference Jarzembowski, Martínez-Delclòs, Bechly, Nel, Coram and Escullié1998 and two Eocene taxa Eolestes syntheticus and Lutetialestes uniformis have the base of AA well basal to CuP, suggesting that it is the plesiomorphic state of character for the Lestoidea. Chalcolestes would have kept this state of character. Thus, the attribution of C. tibetensis sp. nov. to this genus is based on a plesiomorphy, rendering it only tentative. Nevertheless, without other evidence to the contrary and any autapomorphic character that would allow the creation of a new lestid genus, we prefer to keep it in this genus. Also a phylogenetic analysis of the whole Lestidae is necessary to clarify the relationships in this family.
The synlestid genus Megalestes has a venation very similar to those of the Lestidae and Chalcolestes tibetensis sp. nov., but differing in the absence of oblique veins ‘O’ and clearly less acute distal angle of the discoidal cell with a longer anterior side of the same cell (Münz, Reference Münz1919: pl. 7, fig. 39).
4.b. Remarks
Jattiot et al. (Reference Jattiot, Latutrie and Nel2020: 435) indicated that a ‘supplementary row of cells between MP and CuA can be found also in some specimens but not in others, of extant Lestes species (L. dryas Kirby, Reference Kirby1890)’, but it is remarkable that a majority of Lestinae from the Eocene and Oligocene share this character, while those from the Miocene have only one row of cells in this area. This character is also rare among the extant Lestinae. This phenomenon needs further material to be confirmed, but the present discovery of Chalcolestes tibetensis sp. nov. is one more case supporting it. The presence of the same character in the two Eocene Eolestes syntheticus and Lutetialestes uniformis would suggest that it could also be a plesiomorphy in the Lestiformia, but this hypothesis needs to be confirmed because the Hemiphlebiidae do not share it.
5. Conclusion
5.a. Fossil calibration
Chalcolestes tibetensis sp. nov. is the oldest record of the crown group of Lestidae, as the previous ones were from the Latest Eocene of France and the UK (33.9–28.4 Ma). The widespread distribution of this family during the Eocene suggests a greater antiquity. Unfortunately, the putative older record of Lestes zalesskyi Piton, Reference Piton1940 from the Palaeocene of Menat (France) is very uncertain because its original description is very imprecise and the holotype is lost (Nel & Paicheler, Reference Nel and Paicheler1994: 7).
5.b. Palaeoclimatic implications
The modern-day hinterland of the Tibetan Plateau has a relatively low-relief topography, in stark contrast to the high-relief Himalaya and surrounding mountain ranges (Fielding et al. Reference Fielding, Isacks, Barazangi and Duncan1994); such a unique low-relief landscape in central Tibet has been formed since at least the late Eocene (Han et al. Reference Han, Sinclair, Li, Wang, Tao, Qian, Ning, Zhang, Wen, Lin, Zhang, Xu, Dai, Zhou, Liang and Cao2019). The Palaeogene Lunpola–Nima sediment depo-centre is located between the palaeo-Gangdese and the palaeo-Qiangtang (Tangulha) mountain ranges to the south and north of the basin (DeCelles et al. Reference DeCelles, Quade, Kapp, Fan, Dettman and Ding2007b), respectively. It is generally accepted that Qiangtang Block in the north entered into collision by the Cretaceous and has since been in an intracontinental setting (Yin & Harrison, Reference Yin and Harrison2000). The Gangdese Mountains in the south have probably also maintained high elevations since at least the Palaeocene (Ding et al. Reference Ding, Xu, Yue, Wang, Cai and Li2014). However, the Palaeogene topographic characteristics and palaeoclimatic history of the Lunpola–Nima sediment depo-centre between the palaeo-Gangdese and the palaeo-Qiangtang (Tangulha) mountains are very poorly known.
Some isotope studies suggest a high Lunpola Basin floor (4–5 km) (Rowley & Currie, Reference Rowley and Currie2006; Decelles et al. Reference DeCelles, Quade, Kapp, Fan, Dettman and Ding2007b; Polissar et al. Reference Polissar, Freeman, Rowley, McInerney and Currie2009; Quade et al. Reference Quade, Breecker, Daëron and Eiler2011), a high palaeo-Gangdese (∼4.5 km; Ding et al. Reference Ding, Xu, Yue, Wang, Cai and Li2014) and a high palaeo-Qiangtang/Tangulha mountain range (>5.0 km; Xu et al. Reference Xu, Ding, Zhang, Cai, Lai, Yang and Zeng2013; Lin et al. Reference Lin, Dai, Zhuang, Jia, Zhang, Ning and Wang2020). However, evidences from palaeobotany (Wang et al. Reference Wang, Dutta, Kelly, Rudra, Li, Zhang, Zhang, Wu, Cao, Wang, Li and Zhang2018) and palaeontology (Deng et al. Reference Deng, Wang, Xie, Li, Hou and Sun2011; Sun et al. Reference Sun, Xu, Liu, Zhang, Xue and Zhao2014; Ma et al. Reference Ma, Wang, Meng, Ma, Zhao, Li and Wang2017; Wu et al. Reference Wu, Miao, Chang, Shi and Wang2017; Su et al. Reference Su, Farnsworth, Spicer, Huang, Wu, Liu, Li, Xing, Huang, Deng, Tang, Xu, Zhao, Srivastava, Valdes, Deng and Zhou2019) suggest much lower elevations (1–3 km). Moreover, emerging numerical simulations of palaeoelevations based on temperature constraints and thermal lapse rates are also widely divergent (e.g. Botsyun et al. Reference Botsyun, Sepulchre, Donnadieu, Risi, Licht and Caves2019; Valdes et al. Reference Valdes, Lin, Farnsworth, Spicer, Li and Tao2019). In view of this situation, the fundamental reason for the contradiction of the above-mentioned palaeoelevation reconstruction results is that the stratigraphic age of the Lunpola basin has not been well constrained (Fang et al. Reference Fang, Dupont-Nivet, Wang, Song, Meng, Zhang, Nie, Zhang, Mao and Chen2020). In other words, the high altitude of the Lunpola Basin proposed by Rowley & Currie (Reference Rowley and Currie2006) mainly refers to the late Oligocene period of 26.5–23.3 Ma, while palaeontological teams proposed that the low elevation was mainly for the Eocene period of 39.7–37 Ma (Wu et al. Reference Wu, Miao, Chang, Shi and Wang2017; Su et al. Reference Su, Farnsworth, Spicer, Huang, Wu, Liu, Li, Xing, Huang, Deng, Tang, Xu, Zhao, Srivastava, Valdes, Deng and Zhou2019). Most importantly, after the comprehensive study of a large number of plant fossils by Su et al. (Reference Su, Spicer, Wu, Farnsworth, Huang, Del Rio, Deng, Ding, Deng, Huang, Hughes, Jia, Jin, Li, Liang, Liu, Liu, Sherlock, Spicer, Srivastava, Tang, Valdes, Wang, Widdowson, Wu, Xing, Xu, Yang, Zhang, Zhang, Zhang, Zhao and Zhou2020), a subtropical forest called the ‘Shangri La’ ecosystem was also proposed for the Middle Eocene (∼47 Ma). Its palaeogeomorphological features are low valleys distributed in an east–west direction, at ∼1500 m elevation within an east–west-trending valley under a monsoonal climate with a mean annual temperature of ∼19 °C and a cold-month mean temperature of 7 ± 2.9 °C with rare frosts.
Our finding provides evidence for a regional climate system and its possible response to Tibetan landscape evolution. The steady specific ecosystem directly affects insect population dynamics and geographic distributions. As ectotherms, insects are highly sensitive to ambient temperatures and may respond very quickly to temperature fluctuations (Robinet & Roques, Reference Robinet and Roques2010). The recent Chalcolestes parvidens and Chalcolestes viridis have Western Palaearctic distributions (Southern, Eastern and Central Europe, Syria, Jordan, Israel, Turkey, and Maghreb in North Africa), all under low altitudes (Wildermuth & Martens, Reference Wildermuth and Martens2019). Chalcolestes viridis has a rather narrow temperature tolerance between 14 °C and 24 °C (Romero, Reference Romero1988). They occur in still or slow-flowing water in ditches, ponds, lakes and canals. The discovery of a Chalcolestes in the late Eocene of Tibet indicates a low altitude for the Nima basin during this period and further suggests that the genus is quite old and was more widespread during the Palaeogene.
This conclusion enables us to have a clearer understanding of the palaeogeomorphological constraints of the Nima and the surrounding Lunpola basins during the Palaeogene: the Tibetan landscape comprised a high (>4 km) Gangdese mountain range along the southern margin of the Lhasa terrane and a Tanghula upland on the more northerly Qiangtang terrane; while the Nima, Lunpola and other basins in the middle of the Bangong Nujiang suture zone were almost in the valley area, with relatively low altitudes, c. 1500– m a.s.l.
This discovery provides a warm and humid climate with low latitude for the Niima and Lunpola basins during the late Eocene.
Acknowledgements
We offer our sincere gratitude to the editors and anonymous reviewers for their very useful comments on an earlier version of the manuscript. This research was supported by the National Natural Science Foundation of China (41972115), the Second Tibetan Plateau Scientific Expedition and Research (2019QZKK0706), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000).
Conflict of interest
We declare that we have no conflict of interest.







