1. Introduction
Among more than 50 species currently known from the Emu Bay Shale Konservat-Lagerstätte on Kangaroo Island, South Australia, euarthropods represent more than half that diversity and are numerically dominant at the level of individuals (Paterson et al. Reference Paterson, García-Bellido, Jago, Gehling, Lee and Edgecombe2015), a pattern shared with other Cambrian Konservat-Lagerstätten (Caron et al. Reference Caron, Gaines, Aria, Mángano and Streng2014). Fifteen field seasons at Buck Quarry since 2007 (Gehling et al. Reference Gehling, Jago, Paterson, García-Bellido and Edgecombe2011) have yielded more than 5000 registered specimens in the South Australian Museum. A new non-biomineralized euarthropod described herein is known from two specimens that provide a fairly complete picture of the dorsal exoskeletal morphology, as well as information on the antenna, hypostome and doublure. This rare taxon constitutes the oldest record of aglaspidid-like euarthropods in Australia, with Aglaspidida Walcott, Reference Walcott1912 (sensu stricto) known from just one occurrence, in the upper Cambrian of Tasmania (Ortega-Hernández et al. Reference Ortega-Hernández, Braddy, Jago and Baillie2010).
2. Material and methods
Two specimens (SAM P48369 and P46332) form the basis for the description of a new Emu Bay Shale euarthropod, and are housed in the palaeontological collections at the South Australian Museum, Adelaide (prefix SAM P). Photography used a Canon EOS 5D digital SLR camera with a Canon MP-E 65 mm 1–5× macro lens and low-angle NW light to enhance relief. Camera lucida drawings were made under a Leica MZ6 stereomicroscope. Images were edited and plates assembled with Adobe Photoshop Version C5.
Eozetetes gemmelli gen. et sp. nov. was coded using the character matrices of Ortega-Hernández, Legg & Braddy (Reference Ortega-Hernández, Legg and Braddy2013) and Stein et al. (Reference Stein, Budd, Peel and Harper2013), both of which scored a broad range of early euarthropods for 82 and 74 characters, respectively, the former employing a denser taxonomic sampling for Aglaspidida and aglaspidid-like euarthropods in particular. Parsimony analysis with TNT (Goloboff, Farris & Nixon, Reference Goloboff, Farris and Nixon2008) used equal character weights as well as implied weights with varied concavity constants. Heuristic searches used 10000 random stepwise addition sequences saving 50 trees per replicate with TBR branch swapping. Node support was quantified using parsimony jackknifing for equal weights and symmetric resampling for implied weights. Jackknife frequencies and GC values, respectively, above 50% are reported based on 1000 replicates of jackknife resampling with 36% removal probability or 1000 replicates of symmetric resampling with 33% change probability.
3. Systematic palaeontology
EUARTHROPODA Lankester, Reference Lankester1904 VICISSICAUDATA Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013
Discussion. Vicissicaudata was named (Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013) for a putative clade composed of Aglaspidida, Cheloniellida Broili, Reference Broili1932 and a group referred to as Xenopoda Raymond, Reference Raymond1935. The last group conventionally includes Sidneyia Walcott, Reference Walcott1911 and Emeraldella Walcott, Reference Walcott1912, and has sometimes been expanded in scope to also include Cheloniellida (e.g. Hou & Bergström, Reference Hou and Bergström1997). Vicissicaudata was diagnosed by a postabdomen that lacks walking legs. Monophyly of Vicissicaudata has been defended in cladistic analyses using implied character weights for a broad range of artiopodan euarthropods by Ortega-Hernández, Legg & Braddy (Reference Ortega-Hernández, Legg and Braddy2013), Legg, Sutton & Edgecombe (Reference Legg, Sutton and Edgecombe2013) and Legg (Reference Legg2014). A nearly identical clade had been recognized as ‘Clade 5’ of Cotton & Braddy (Reference Cotton and Braddy2004, fig. 8), united by a postabdomen lacking appendages, strongly curved posterior tergites compared to anterior ones and a pre-telson segment with paired, unsegmented appendicular derivatives, in the form of caudal flaps (Xenopoda), furci (Cheloniellida) or postventral plates (Aglaspidida). Members of Vicissicaudata (sensu Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013) were, however, resolved as non-monophyletic in a phylogenetic analysis by Stein et al. (Reference Stein, Budd, Peel and Harper2013), uniting with some additional, mostly Cambrian taxa (Squamacula Hou & Bergström, Reference Hou and Bergström1997; Retifacies Hou, Chen & Lu, Reference Hou, Chen and Lu1989; Molaria Walcott, Reference Walcott1912; Burgessia Walcott, Reference Walcott1912; and Marrellomorpha Beurlen, Reference Beurlen1934).
Genus Eozetetes gen. nov.
Type species. Eozetetes gemmelli sp. nov., by monotypy.
Etymology. Gr. Eo-, early; zetetes, searcher; for the fossil hunter after whom the type species is named.
Diagnosis. Euarthropod with relatively short (sag.), wide (tr.) cephalic shield; genal angles acute, lacking spines; dorsal eyes absent; moderately wide cephalic doublure; paradoublural line curving posteriorly adjacent to attachment of hypostome; 18 trunk tergites with tergopleurae and broad overlap of adjacent tergites; axial part of trunk tergites 1–18 arched anteriorly, bearing paired longitudinally ovate nodes that grade into carinae on posterior segments; caudal tergite (tergite 19) short, ring-like; styliform tailspine longer than remaining part of trunk.
Eozetetes gemmelli sp. nov. Figures 1–4
Material. Holotype, SAM P48369a, b (Figs 1, 2); 10.9 m above the base of the Emu Bay Shale; paratype SAM P46332a, b (Fig. 3), 10.8 m above the base of the Emu Bay Shale. Both from Buck Quarry (35°34'25''S 137°34'36''E), Big Gully, north coast of Kangaroo Island, South Australia; Cambrian Series 2, Stage 4, Pararaia janeae Zone.

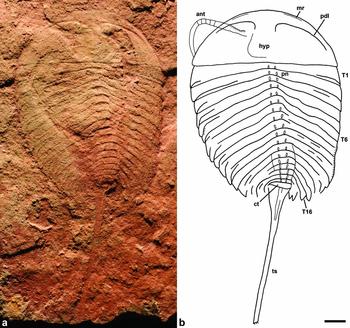
Figure 1. Eozetetes gemmelli gen. et sp. nov., from the Emu Bay Shale, Kangaroo Island, South Australia. Holotype SAM P48369a. (a) Dorsal view of articulated specimen. (b) Interpretive drawing. Abbreviations: ant – antenna; ct – caudal tergite; hyp – hypostome; mr – marginal rim on cephalic shield; pdl – paradoublural line; pn – paired longitudinally ovate nodes on axis; T – tergite; Tp – tergopleura; ts – tailspine. Scale bar 2 mm (same scale for both images).

Figure 2. Eozetetes gemmelli gen. et sp. nov., from the Emu Bay Shale, Kangaroo Island, South Australia. Holotype SAM P48369a, all dorsal views (see Fig. 1 for overview). (a) Cephalic shield and left antenna; arrowheads indicate margins of hypostome. (b) Anterior part of trunk (left side) showing overlapping of tergopleurae; black arrowheads indicate anterior margins of tergopleurae; white arrowheads, posterior margins. (c) Posterior part of trunk, showing proximal part of tailspine. Abbreviations as in Figure 1. Scale bars 2 mm.

Figure 3. Eozetetes gemmelli gen. et sp. nov., from the Emu Bay Shale, Kangaroo Island, South Australia. Paratype SAM P46332. (a) Counterpart, SAM P46332a. (b) Part, SAM P46332b. Arrowheads indicate margins of hypostome. Abbreviations as in Figure 1. Scale bar 3 mm (same scale for both images).

Figure 4. Reconstruction of Eozetetes gemmelli gen. et sp. nov.
Etymology. After Mike Gemmell, a regular member of our field team, for moving a vast amount of Emu Bay Shale and finding many superb specimens, including the type specimens of this taxon.
Diagnosis. As for the genus.
Description. Holotype 29.5 mm in length, from anterior margin of cephalic shield to preserved (incomplete) posterior tip of tailspine; cephalon 4.9 mm (sag.), 13.5 mm wide across genal angles; trunk 11.7 mm long excluding tailspine; preserved extent of tailspine 13.0 mm long.
Anterior and lateral margins of cephalic shield evenly curved; posterior margin weakly arched anteriorly. Genal angle acute, blunt, lacking spine. Narrow marginal rim present along at least anterior and anterolateral margins of cephalic shield (Fig. 2a). Doublure moderately wide, its position indicated by strong paradoublural line in both specimens (Figs 2a, 3a, b); doublure gently narrowing posteriorly. Hypostomal suture indistinct; hypostome directly attached to doublure based on paradoublural line sharply flexed posteriorly, confluent with anterolateral margin of hypostome. Hypostome widening posteriorly; posterolateral angle rounded; posterior margin transverse; maximum width (tr.) of hypostome c. 25% width of posterior margin of cephalic shield; doublure and hypostome extending 90% length (sag.) of cephalic shield.
Antenna known only from one side in holotype (Figs 1, 2a); elongate flagelliform, with only slight tapering along preserved extent from its insertion at side of hypostome to where it curves inwards and is concealed by cephalic shield; articulations between a few articles preserved in a section a short distance from where antenna is exposed outside the cephalic shield, these articles all being of about equal length and width.
Trunk consists of 18 tergites with tergopleurae, tergite 2 being the broadest; trilobation distinct, axis set off from tergopleurae by break in slope, more pronounced in posterior part of trunk (Fig. 2c); tergopleurae broad along most of trunk, with straight posterior margins, curved anterolateral margins, and acute tips, markedly narrowing (tr.) from c. tergite 10 and becoming more strongly curved and pointed; broad overlap between adjacent tergites abaxially (Fig. 2b); pair of longitudinally ovate nodes on tergites 1–13 grading into more elongate carina-like ridges on tergites 14–18 (Figs 1, 2c). Tergite 19 (= caudal tergite) a short (sag.) ring lacking nodes, tergopleurae apparently lacking (Fig. 2c).
Tailspine as wide as caudal tergite at its base, rapidly tapering in its proximal c. 3 mm and then maintaining an even, narrow width; wider proximal part bearing a median carina (Figs 1b, 2c).
Discussion. The shape of the cephalic shield (especially the nearly transverse/weakly arched posterior margin), lack of dorsal eyes, proportions of the antenna, size of the hypostome, distinct trilobation, and shape and length of the tailspine relative to the rest of the trunk (Fig. 4) are reminiscent of Emeraldella (Walcott, Reference Walcott1912; Bruton & Whittington, Reference Bruton and Whittington1983; Stein, Church & Robison, Reference Stein, Church and Robison2011; Stein & Selden, Reference Stein and Selden2012). Distinction of Eozetetes is justified based on its greater number of trunk tergites (18 with tergopleurae versus 10 or 11 in Emeraldella), greater relative width of the tergopleurae compared to the axis, absence of articulating ridges in the trunk, shorter caudal tergite and apparent absence of articulations in the tailspine. The caudal tergite of Emeraldella bears a prominent pair of caudal flaps, which are not known in Eozetetes. However, it is possible that the absence of these structures in the available material of Eozetetes is taphonomic, with the holotype being preserved in dorsal rather than ventral view. Given the diminutive size of the caudal tergite in Eozetetes compared to that of Emeraldella, it might be expected that associated flaps, if present, would be smaller than those of Emeraldella. Transverse articulations on the tailspine have been observed in Emeraldella brutoni and E. brocki (Stein, Church & Robison, Reference Stein, Church and Robison2011). No articulations can be discerned in the tailspine of Eozetetes gemmelli, and given the quality of preservation in the holotype, we consider this to be a real absence.
Eozetetes displays a general similarity to Aglaspidida as well, and some results of phylogenetic analyses (discussed in Section 4 below) are consistent with a close relationship between these taxa. Membership in Aglaspidida s.s. (Van Roy, Reference Van Roy2006; Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013; Ortega-Hernández, Van Roy & Lerosey-Aubril, Reference Ortega-Hernández, Van Roy and Lerosey-Aubril2015) is opposed by Eozetetes having a non-biomineralized exoskeleton (versus phosphatic in Aglaspidida), broadly overlapping rather than edge-to-edge tergite articulations, a markedly greater number of trunk segments compared to Cambrian aglaspidids, and lacking dorsal eyes; however, it should be noted that aglaspidid morphology varies within this clade with respect to the last three characters (Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013; Ortega-Hernández, Van Roy & Lerosey-Aubril, Reference Ortega-Hernández, Van Roy and Lerosey-Aubril2015). A roughly ovate bulge at the right edge of the hypostome in SAM P48369 (Fig. 2a) could be compared in its position to the eyes of various aglaspidids, but we regard it more likely to be sediment injected into the cephalic cavity limited by the doublure; the lack of a similar feature on the other side of this specimen and in SAM P46332 (Fig. 3) supports this interpretation. One of the key autapomorphies of Aglaspidida s.s., postventral plates (Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013; Ortega-Hernández, Van Roy & Lerosey-Aubril, Reference Ortega-Hernández, Van Roy and Lerosey-Aubril2015), is not observed in Eozetetes, and the preservation of the holotype compared to similarly preserved articulated aglaspidids suggests they may be truly absent.
4. Phylogenetic affinity
Comparison with Emeraldella and Aglaspidida above signals membership in Vicissicaudata, a putative clade that includes Emeraldella and other artiopodans with a postabdomen that lacks walking legs but bears other paired ventral structures, at least some of which are convincingly regarded as appendicular (Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013). The case for a caudal tergite in Eozetetes is inconclusive owing to the absence of associated appendicular derivatives, but evidence at hand is indicative of such a structure. The holotype allows for tergopleurae to be associated with the axial rings of the first 18 trunk segments, all of which bear paired axial nodes (Figs 1, 2c); the curved tergopleurae of tergite 18 run almost immediately against the base of the tailspine (Fig. 2c). The tergite immediately anterior to the tailspine (tergite 19) lacks the paired nodes that are present on all other tergites and has no associated tergopleurae. As such, we consider it most probable that the short, ring-like structure identified as tergite 19 corresponds to a caudal tergite, resembling that of Xenopoda in having reduced (apparently absent) tergopleurae. As discussed above in comparison with Emeraldella, no caudal flaps or other ventral structures are associated with tergite 19 in Eozetetes, though the limited amount of available material provides a weak case for this apparent absence being real. An alternative interpretation of what we identify as tergite 19 would be that it represents an articulating structure at the anterior margin of the tailspine, but this is inconsistent with its distinct elevation relative to the tailspine.
Recent cladistic analyses of relevant taxa provide a more explicit basis for inferring the systematic position of Eozetetes. The character matrices of Ortega-Hernández, Legg & Braddy (Reference Ortega-Hernández, Legg and Braddy2013) and Stein et al. (Reference Stein, Budd, Peel and Harper2013) were used unmodified (see online Supplementary Material available at http://journals.cambridge.org/geo). In both matrices, many characters are scored as missing (all appendage characters apart from a few antennal characters) or inapplicable for Eozetetes.
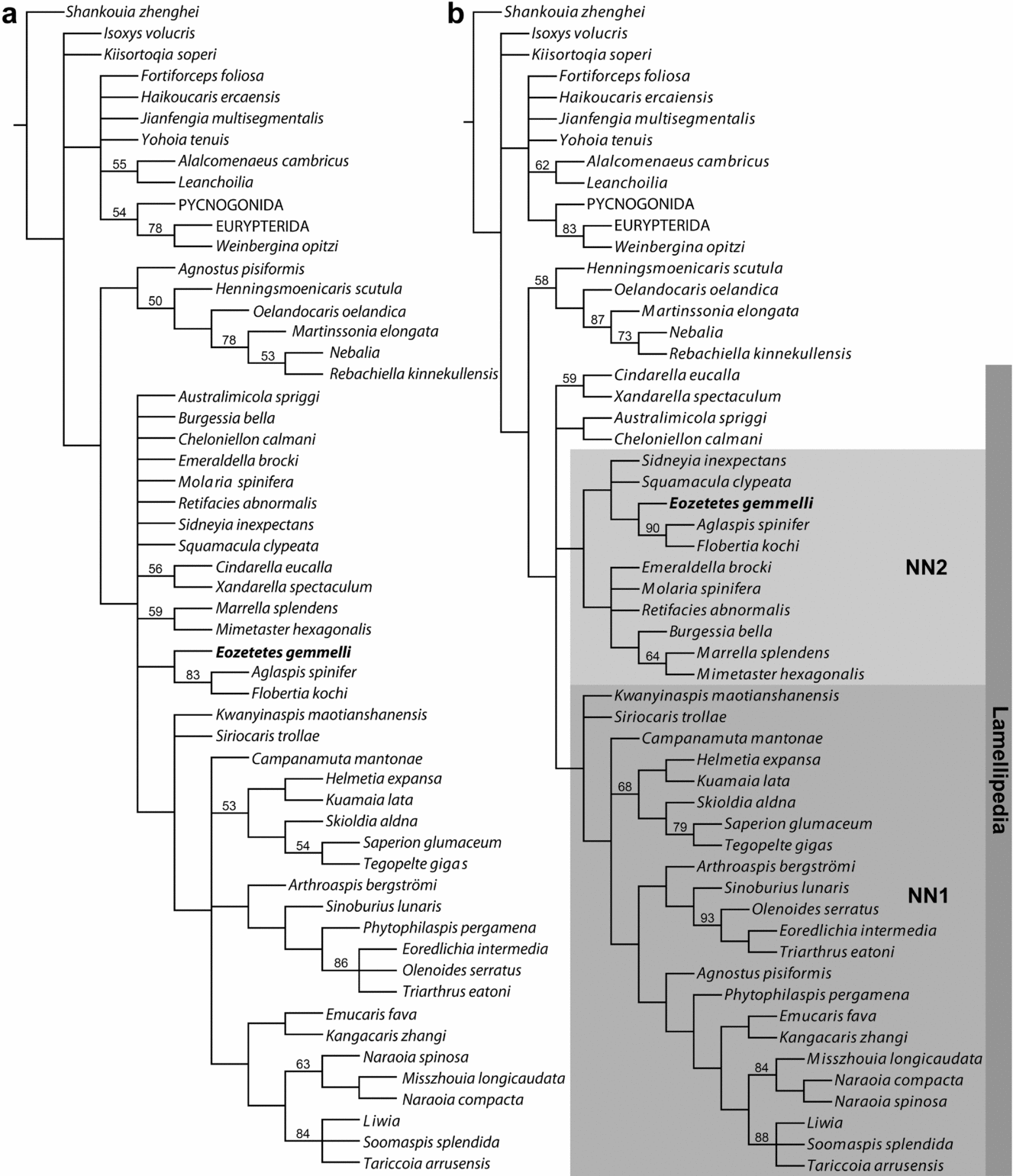
The Ortega-Hernández, Legg & Braddy (Reference Ortega-Hernández, Legg and Braddy2013) matrix resolves Eozetetes as most closely related to Emeraldella under either equal or implied character weights (Fig. 5a, b). In the context of implied weights (Fig. 5b), Eozetetes and Emeraldella are members of a ‘xenopodan’ grade allied to Cheloniellida within a monophyletic Vicissicaudata. However, as in the original analyses of Ortega-Hernández, Legg & Braddy (Reference Ortega-Hernández, Legg and Braddy2013, their fig. 6), Vicissicaudata is sensitive to character weighting, not being retrieved under equal weights or more extreme concavity constants. In the implied weighted tree, Eozetetes and Emeraldella are united by their lack of lateral eyes and long tailspine (characters 18 and 70, respectively, of Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013). They are more broadly united with Cheloniellida based on a single segment in the preabdomen. Although this latter state was not coded for Eozetetes, discussion of tergite 19 above is consistent with the same coding as for Emeraldella. Membership of Eozetetes + Emeraldella within a ‘xenopodan’–cheloniellid clade resolved precisely as in Figure 5b is stable across a range of concavity constants (k = 2, 3, 4, 5 and 6).

Figure 5. Cladograms with Eozetetes gemmelli coded in the 82-character matrix of Ortega-Hernández, Legg & Braddy (Reference Ortega-Hernández, Legg and Braddy2013). (a) Strict consensus of 532 shortest cladograms (237 steps, Consistency Index 0.45, Retention Index 0.75) under equal character weights. Numbers at nodes are jackknife frequencies >50%. (b) Strict consensus of 90 shortest cladograms under implied character weights (k = 3). Numbers at nodes are GC values >50%.

Figure 6. Cladograms with Eozetetes gemmelli coded in the 74-character matrix of Stein et al. (Reference Stein, Budd, Peel and Harper2013). (a) Strict consensus of 2820 shortest cladograms (180 steps) under equal character weights. Numbers at nodes are jackknife frequencies >50%. (b) Strict consensus of 63 shortest cladograms under implied character weights (k = 2–20). Numbers at nodes are GC values >50% calculated for k = 3. NN1 and NN2 refer to two unnamed clades in the analysis of Stein et al. (Reference Stein, Budd, Peel and Harper2013).
Including Eozetetes in the Stein et al. (Reference Stein, Budd, Peel and Harper2013) matrix, parsimony analysis with implied weights across concavity constants k = 2–20 in TNT (see Section 2 above) finds 63 cladograms that invariantly place Eozetetes within unnamed clade NN2 of Stein et al. (Reference Stein, Budd, Peel and Harper2013, fig. 16) as sister taxon to Aglaspidida (Fig. 6b). All relationships depicted in the strict consensus for all analyses are identical to those presented by Stein et al. (Reference Stein, Budd, Peel and Harper2013, fig. 16A) in their sets of 63 cladograms apart from two different resolutions of Agnostus and Phytophilaspis relative to Trilobita. In all shortest cladograms, including those retrieved under equal character weights (Fig. 6a), Eozetetes and Aglaspidida are united by the shared presence of a cephalic marginal rim (character 42 of Stein et al. Reference Stein, Budd, Peel and Harper2013).
Hence, determining the closest relative of Eozetetes – whether a ‘xenopodan’ or aglaspidids – is sensitive to differences in taxonomic and character sampling in available data matrices. In either case, however, Eozetetes is apparently allied to taxa that have been informally grouped as ‘aglaspidid-like (eu)arthropods’ (table 1 of Van Roy, Reference Van Roy2006; table 2 of Ortega-Hernández, Legg & Braddy, Reference Ortega-Hernández, Legg and Braddy2013). Some of these taxa have subsequently been corroborated as close relatives of Aglaspidida (e.g. Kodymirus Chlupáč & Havlicek, Reference Chlupáč and Havliček1965: Lamsdell, Stein & Selden, Reference Lamsdell, Stein and Selden2013), although excluded from Aglaspidida sensu stricto.
Acknowledgements
The Emu Bay Shale project has been supported by grants from the Australian Research Council (LP0774959, DP120104251, FT120100770, FT130101329), Spanish Research Council (CGL2009-07073, CGL2013-48877-P) and National Geographic Society Research & Exploration (#8991-11), with additional financial assistance from Beach Energy Ltd and the South Australian Museum, and logistical support from SeaLink. We are grateful to the Buck family for access to the field area, and to our regular collaborators J. Gehling, J. Jago and M. Lee. We thank R. Atkinson, K. Bailey, M. A. Binnie, G. Brock, A. Camens, A. Daley, R. Gaines, M. Gemmell, J. Holmes, K. Kenny, P. Kruse, J. Laurie, B. McHenry, M. Mills, L. Reid, D. Rice, N. Schroeder, E. Thomson, and members of the South Australian Museum Waterhouse Club for assistance in the field and lab, and for discussions. R. Lerosey-Aubril provided insightful comments on the material and our interpretations, as did the journal's referees. TNT was made available courtesy of the Willi Hennig Society.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0016756815001053.