1. Introduction
Pteranodontid pterosaurs are best known from the Upper Cretaceous (Coniacian–Campanian) Niobrara and Pierre Shale formations of Kansas, USA, where they are represented by two species of the iconic toothless pterosaur Pteranodon (Marsh, Reference Marsh1871, Reference Marsh1872, Reference Marsh1876, Reference Marsh1884; Williston, Reference Williston1891, Reference Williston1892, Reference Williston1893, Reference Williston1895, Reference Williston1896, Reference Williston1897; Eaton, Reference Eaton1903, Reference Eaton1904, Reference Eaton1908, Reference Eaton1910; Wiman, Reference Wiman1920; Miller, Reference Miller1971, Reference Miller1972; Mateer, Reference Mateer1975; Schoch, Reference Schoch1984; Bennett, Reference Bennett, Currie and Koster1987, Reference Bennett1992, Reference Bennett1993, Reference Bennett1994, Reference Bennett2001a , Reference Bennett2018; Witton, Reference Witton, Moody, Buffetaut, Naish and Martill2010). Tethydraco regalis from the Maastrichtian of Morocco is the only other taxon referable to the Pteranodontidae (Longrich et al., Reference Longrich, Martill and Andres2018). Putative records of the Pteranodontidae were reported from the Albian Mowry Shale of Montana, USA (Bennett, Reference Bennett1989), the Albian (?) Chulec Formation of Peru (Bennett, Reference Bennett1989), the Santonian–Campanian Yezo Group of Hokkaido, Japan (Obata et al., Reference Obata, Hasegawa and Otsuka1972; Kellner et al., Reference Kellner, Costa, Wang and Cheng2016), the Campanian Selma Formation of Alabama, USA (Bennett, Reference Bennett1994), and the Campanian Merchantville Formation of Delaware, USA (Baird & Galton, Reference Baird and Galton1981; Bennett, Reference Bennett1994).
The first pterosaur bone found in Russia, the posterior fragment of a middle cervical vertebra, was found in the Campanian Rybushka Formation at Malaya Serdoba in Penza Province and described as a new species, Ornithostoma orientalis (Bogolyubov, Reference Bogolyubov1914). At that time, Ornithostoma was considered a senior subjective synonym of Pteranodon (Williston, Reference Williston1895, Reference Williston1896, Reference Williston1897). Now Ornithostoma is established as a valid taxon of non-azhdarchid azhdarchoids (Averianov, Reference Averianov2012). Later it was assumed that the vertebra from Malaya Serdoba belonged to an azhdarchid pterosaur, and the species ‘Ornithostoma’ orientalis was erected to a new genus, Bogolubovia (Nesov & Yarkov, Reference Nesov and Yarkov1989). Here, more than 100 years after the discovery of Pteranodon in the Late Cretaceous of Russia was announced, we describe several pterosaur specimens from the same Campanian Rybushka Formation that are attributable to the Pteranodontidae. These specimens come from the Beloe Ozero locality in Saratov Province, which is now the biggest pterosaur locality in Russia. All previously described pterosaur specimens from the Beloe Ozero locality were referred to the Azhdarchidae (Averianov, Reference Averianov2007; Averianov & Panteleyev, Reference Averianov, Panteleyev, Shishkin and Tverdokhlebov2009; Averianov & Popov, Reference Averianov and Popov2014; Averianov et al., Reference Averianov, Arkhangelsky and Merkulov2016). Some of these and newly collected specimens are now referred to the Pteranodontidae and described in this paper. Volgadraco bogolubovi, described from the Rybushka Formation at the Shyrokii Karamysh locality and initially referred to Azhdarchidae (Averianov et al., Reference Averianov, Ivakhnenko and Kurochkin2008), is reassigned here to the Pteranodontidae. Bogolubovia orientalis might also be a pteranodontid. We also review other putative records of the Pteranodontidae worldwide.
2. Materials and methods
The first pterosaur bones from the Beloe Ozero locality were collected by EV Popov in 2003. In 2017–19, large-scale excavations at this locality were conducted by Saratov State Technical University under licence SRT 01881 PD. Most pterosaur bones described in this paper were found during these excavations. All pterosaur specimens from Beloe Ozero are housed in the Zoological Institute, Russian Academy of Sciences, in Saint Petersburg (collection ZIN PH 43). The anatomical nomenclature generally follows that in Averianov (Reference Averianov2010). For description and measurements, the wing bones are oriented in flight position.
2.a. Geological setting
The locality Beloe Ozero is situated in the Lysogorskii District of Saratov Province, 78 km SW of Saratov city (Fig. 1). The locality is a natural outcrop of Upper Cretaceous sediments, which are exposed on the right side of the Golyi Ravine, extending from the village of Beloe Ozero, in a SE direction to a distance of c. 4 km. The Upper Cretaceous deposits at Beloe Ozero are attributed to the Rybushka Formation of a local stratigraphic scale.
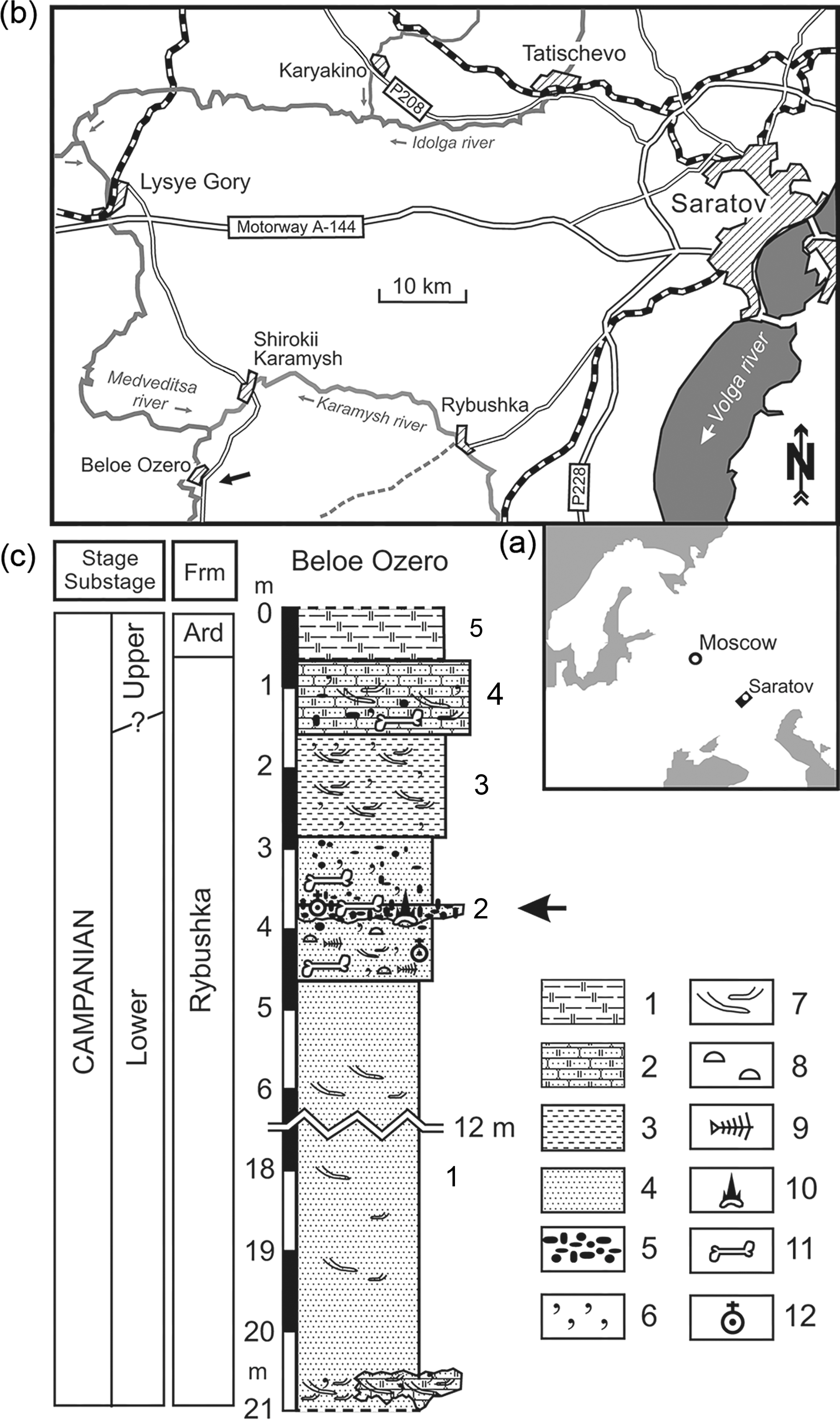
Fig. 1. Geographic position and geological section of Beloe Ozero locality. (a) Map of Eastern Europe showing position of the inset (b). (b) Map of vicinity of Saratov city, with Beloe Ozero locality indicated by arrow. (c) Geological section at Beloe Ozero locality. The fossil-bearing level is indicated by arrow. Legend to the section: (1) siliceous clay, (2) siliceous sandstone, (3) silty sand, (4) inequigranular sand, (5) phosphorite nodules, (6) glauconite, (7) bioturbation, (8) thin-walled shells of linguliform brachiopods, (9) scales and bones of small teleostean fishes, including those in (?) decapod burrows, (10) chondrichthyan (sharks and chimaeroids) remains, (11) marine tetrapod remains; (12) pterosaur remains. Abbreviation: Ard, Ardym Formation. Modified after Zverkov et al. (Reference Zverkov, Averianov and Popov2018).
The Rybushka Formation has yielded belemnites (Belemnellocamax mammillatus, Belemnitella mucronata), ammonites (Hoplitoplacenticeras sp.), bivalves (Oxytoma psilomonica, O. tenuicostata, Cataceramus balticus, C. regularis), and foraminifera of the regional zone Brotzenella monterelensis and subzone Cibicidoides aktulagayensis. The Rybushka Formation also produced teeth and bones of bony fishes (Acipenseridae, Enchodontidae), shark teeth (Cretolamna appendiculata, Squalicorax kaupi, Pseudocorax laevis, Archaeolamna kopingensis, Eostriatolamia sp., Heterodontus sp., Squatina hasei, Squatirhina sp.), and dental plates of chimaeras (Ischyodus bifurcatus, Amylodon karamysh, Edaphodon sp., Elasmodus sp.). Rare bones of reptiles (plesiosaurs, mosasaurs, sea turtles, pterosaurs) and birds (Hesperornis) are also known from this formation (Averianov et al., Reference Averianov, Arkhangelsky, Pervushov and Ivanov2005, Reference Averianov, Arkhangelsky and Merkulov2016; Olferiev & Alekseev, Reference Olferiev and Alekseev2005; Arkhangelsky et al., Reference Arkhangelsky, Averianov and Pervushov2007; Averianov, Reference Averianov, Ivakhnenko and Kurochkin2008; Seltser & Ivanov, Reference Seltser and Ivanov2010; Averianov & Popov, Reference Averianov and Popov2014; Grigoriev et al., Reference Grigoriev, Arkhangelsky and Merkulov2015; Zelenkov et al., Reference Zelenkov, Panteleyev and Yarkov2017; Danilov et al., Reference Danilov, Obrazstova, Arkhangelsky, Ivanov, Averianov and Hirayama2018; Zverkov et al., Reference Zverkov, Averianov and Popov2018).
Within the locality, facial changes of the layers and their thickness are recorded. Deposits of the Rybushka Formation are represented here by five layers (description from bottom to top).
Layer 1. Green-grey, quartz–glauconitic, medium-grained sand. Some levels are strongly compacted to the state of loose sandstone. In the layer are very rarely found light brown, rounded phosphorites up to 1 cm in size. In places, the layer is stained brown with iron hydroxide. Rare weakly phosphatized bones of marine reptiles and fish are found in levels of loose sandstone. The apparent thickness of the layer is c. 13 m.
Layer 2. Phosphorite horizon. Brown and dark brown, poorly sabulous, irregularly shaped phosphorite nodules up to 3.5 cm in size. Along the strike, phosphorite inclusions are distributed non-uniformly, forming in places lenticular aggregations or, on the contrary, wedging out. In some sites, the horizon is non-uniformly stained with brownish-red spots of iron hydroxide. Rare silicified dolomite pebbles up to 8 cm in size are found in the layer. The horizon is cemented in places. Cementing material – ferruginous and psammitic components. The lower surface of the layer is uneven and pit-like. Numerous teeth and vertebras of sharks, fin spikes and dental plates of chimaeras, remains of a large sturgeon and salmoniform fishes, bones and teeth of reptiles, and numerous shark coprolites reaching lengths of up to 8 cm are found in the layer. Layer thickness up to 0.2 m.
Layer 3. Green-grey, quartz–glauconitic, medium-grained sand. Over the entire layer, dark brown phosphorite inclusions up to 2 cm in size and ferriferous inclusions up to 10 cm in size are regularly scattered. Rare, weakly phosphatized bones of marine reptiles and fish are found at the base of the layer. The thickness of the layer is c. 1.5 m.
Layer 4. Green-grey, quartz–glauconitic, calcareous, fine-grained sandstone. Subhorizontally oriented moulds of crustacean burrows occur at the base of the layer. The contact with the underlying layer is gradual. Rare weakly phosphatized bones of marine reptiles and fish come across in the layer. The thickness of the layer is c. 1.2 m.
Layer 5. Dark grey with light grey areas clayey silicite, with a small admixture of quartz–glauconitic fine-grained sand. Contact with the underlying layer is prominent. The thickness of the layer is c. 1 m.
The thalweg of the ravine is composed of varying degrees of rounded sandstone fragments and sand. It contains abundant moulds of crustacean burrows, teeth and coprolites of fishes, and less frequent reptile bones.
All the specimens described in the paper were collected in the headwaters of the ravine, in the abandoned quarry for the extraction of flask and sandstone. The bones come from the phosphorite horizon (layer 2).
2.b. Institutional abbreviations
FHSM: Fort Hays State Museum, Fort Hays State University, Hays, Kansas, USA.
HMG: Hobetsu Museum, Hobetsu, Hokkaido, Japan.
LINHM: Long Island Natural History Museum, New York, USA.
MTM: Hungarian Natural History Museum, Budapest, Hungary.
NHMUK: Natural History Museum, London, United Kingdom.
NSM PV: National Science Museum, Tokyo, Japan.
PU: Museum of Natural History, Princeton University, New Jersey, USA.
TMM: Texas Memorial Museum, Austin, Texas, USA.
TMP: Royal Tyrell Museum of Paleontology, Drumheller, Canada.
USNM: United States National Museum, Washington, USA.
ZIN PH: Palaeoherpetological collection, Zoological Institute, Russian Academy of Sciences, Saint Petersburg, Russia.
3. Systematic palaeontology
Pterosauria Kaup, Reference Kaup1834
Pterodactyloidea Plieninger, Reference Plieninger1901
Pteranodontidae Marsh, Reference Marsh1876
Pteranodontidae indet.

Fig. 2. Pteranodontidae indet., ZIN PH 64/43, incomplete cervical 3, in anterior (a), dorsal (b), posterior (c), lateral (d) and ventral (e) views. Photographs and explanatory drawings. Beloe Ozero, Saratov Province, Russia; Rybushka Formation, Upper Cretaceous (Campanian). Abbreviations: ep, epipophysis; hy, hypapophysis; nc, neural canal; pf, pneumatic foramen; poz, postzygapophysis. Scale bar equals 10 mm.

Fig. 3. Pteranodontidae indet., ZIN PH 72/43, distal fragment of right scapula, in anterior (a), dorsal (b), posterior (c), ventral (d) and distal (e) views. Photographs and explanatory drawings. Beloe Ozero, Saratov Province, Russia; Rybushka Formation, Upper Cretaceous (Campanian). Abbreviations: ms, muscle scar; st, scapular tubercle. Scale bar equals 10 mm.

Fig. 4. Pteranodontidae indet., ZIN PH 75/43, deltopectoral crest of right humerus, in dorsal (a), anterior (b), proximal (c) and ventral (d) views. Photographs and explanatory drawings. Beloe Ozero, Saratov Province, Russia; Rybushka Formation, Upper Cretaceous (Campanian). Abbreviations: ms, muscle scar. Scale bar equals 10 mm.

Fig. 5. Pteranodontidae indet., ZIN PH 74/43, right proximal syncarpal, in anterior (a), distal (b), posterior (c), proximal (d), dorsal (e) and ventral (f) views. Photographs and explanatory drawings. Beloe Ozero, Saratov Province, Russia; Rybushka Formation, Upper Cretaceous (Campanian). Abbreviations: aas, accessory articulation surface; dar, articulation surface for radius; dau, facet for dorsal articulation surface of ulna; fov, fovea, facet for ulnar tuberculum; ftp, flexor tendon process; isa, intersyncarpal articulation surface; pf, pneumatic foramen; tub, tuberculum for the ulnar fovea; veu, facet for ventral articulation surface of ulna. Scale bar equals 10 mm.

Fig. 6. Pteranodontidae indet., ZIN PH 76/43, left preaxial carpal, in dorsal (a), distal (b), ventral (c) and posterior (d) views. Photographs and explanatory drawings. Beloe Ozero, Saratov Province, Russia; Rybushka Formation, Upper Cretaceous (Campanian). Abbreviations: art, articulation surface for preaxial carpal process of distal syncarpal; pf, pneumatic foramen; pt, facet for pteroid; trp, terminal process. Scale bar equals 10 mm.

Fig. 7. Pteranodontidae indet., ZIN PH 66/43, left femur, in posterior (a), medial (b), anterior (c), anterolateral (d), lateral (e), proximal (f) and distal (g) views. Photographs and explanatory drawings. Beloe Ozero, Saratov Province, Russia; Rybushka Formation, Upper Cretaceous (Campanian). Abbreviations: gt, greater trochanter; h, femoral head, is, intercondylar sulcus; lc, lateral condyle; ltr, lesser trochanter; mc, medial condyle; ms, muscle scar; pf, pneumatic foramen; pof, popliteal fossa. Scale bar equals 50 mm (for (a)–(e)). (f) and (g) are not to scale.
Azhdarchidae indet.: Averianov & Popov (Reference Averianov and Popov2014: fig. 2).
3.a. Material
ZIN PH 64/43, middle cervical vertebra. ZIN PH 55/43, free dorsal vertebra. ZIN PH 72/43, left scapula distal fragment. ZIN PH 75/43, right deltopectoral crest; ZIN PH 74/43, right proximal syncarpal. ZIN PH 76/43, left preaxial carpal. ZIN PH 66/43, left femur.
3.b. Description
In the middle cervical vertebra ZIN PH 64/43, the cotyle is pentagonal, with a straight dorsal margin and a ventral angle extending onto the very large hypapophysis (Fig. 2). The cotyle width is almost twice its height. The preexapophyses are not preserved. The missing prezygapophyses were placed mostly lateral to the cotyle. On the ventral centrum surface, there is a short ridge extending posteriorly from the hypapophysis. Lateral to the hypapophysis there is a paired pneumatic foramen close to the anterior margin of the centrum. The ventral centrum surface lateral to the midline is flat or slightly concave. The neural arch (excluding the unknown neural spine) is low anteriorly and very high posteriorly, with the postzygapophyses placed higher than the prezygapophyses (missing). There is a relatively large oval-shaped pneumatic foramen on the lateral side of the neural arch anteroventral to the postzygapophysis. The neural canal is almost round; its anterior and posterior openings are similar in size. On the posterior side, the pneumatic foramina lateral to the neural canal are relatively large, almost half the size of the neural canal. They are oval-shaped. The left lateral pneumatic foramen is oriented horizontally, while the right one is almost vertical. The posterior opening of the neural canal extends dorsal to the left lateral pneumatic foramen, but it is ventral to the dorsal margin of the right lateral pneumatic foramen. The posterior opening of the neural canal and lateral pneumatic foramina are situated in a flat triangular area facing posteroventrally. This area is limited dorsally by a faint ridge which extends laterally towards the postzygapophysis. Above this triangular area and dorsal to the posterior opening of the neural canal, there is a small concave area delimited laterally by a vertical ridge. The postzygapophysis is almost round, slightly concave and faces posterolaterally. The epipophysis projects posterolaterally. It has a bump on its posterior side. The neural arch length is 35.1 mm; the minimum width of the neural arch is 28.2 mm.
The free dorsal vertebra ZIN PH 55/43 is described in Averianov & Popov (Reference Averianov and Popov2014: pp. 327–8, fig. 2).
The scapular distal fragment ZIN PH 72/43 is flattened dorsoventrally (Fig. 3). Its distal part is somewhat twisted to the proximal part. The distalmost part of the scapular blade has a triangular cross-section, with a flat dorsal side and a longitudinal ridge on the ventral side. The anterior margin of the scapula is thick. Posterior to the median ventral ridge, the depth of the scapula steadily decreases and the posterior end is very thin. In dorsal/ventral view the scapula widens proximally. At the beginning of the proximal widening on the dorsal surface, there is a distinct longitudinal depression. At the anteroproximal end of the preserved fragment, there is a large bump-like scapular tubercle. In the middle of the anteroventral margin of the scapular blade, there is a cleft-like longitudinal depression. There is a prominent longitudinal muscle scar proximal to this depression. There is also an oblique muscle scar on the ventral side of the scapular blade. The supraneural plate articulation is set at an angle to the scapular blade. The scapular blade needs to be oriented dorsomedially to have the supraneural articulation surface vertical. The supraneural plate articulation surface is D-shaped, with flat dorsal margin and convex ventral margin. The articulation surface is slightly convex.
The deltopectoral crest ZIN PH 75/43 belongs to a large specimen. The deltopectoral crest is relatively short, deflecting ventrally and distally at its anterior end (Fig. 4). The proximal margin is convex, converging towards the anterior end. The distal margin is concave. The proximal margin is very thin at the humeral shaft but distinctly increases in thickness towards the anterior end. The anterior end is occupied by the flattened area facing anterodorsally. This flattened area apparently served for the insertion of m. pectoralis (Bennett, Reference Bennett, Buffetaut and Mazin2003: fig. 3). The thickness of the distal margin is more constant. On the ventral side, there is a prominent short ridge for the attachment of m. deltoides scapularis (Bennett, Reference Bennett, Buffetaut and Mazin2003: fig. 3).
The proximal syncarpal ZIN PH 74/43 is trapezoidal in the proximal/distal view (Fig. 5). In the centre of the proximal side, there is a large, round and deeply concave circular fovea for articulating with the tubercle of the ulna. Anterodorsal and anteroventral to this fovea there are two elongated and less concave articular surfaces. The dorsal facet is for the dorsal articulating facet of the ulna, and the ventral facet is for the dorsal articulation surface of the radius. The dorsal facets for the ulna and the fovea are confluent, while the ventral facet for the radius is separated from both surfaces by a high ridge. The ventral facet continues posteriorly into a smaller articulation surface apparently for the ventral part of the ulna. The two surfaces are partially separated dorsally by the fovea, and between them and the fovea there is a small pneumatic foramen. Posterior to the fovea and dorsal to the ventral ulna facet there is a round distinct flat eminence: the tuberculum articulating with the fovea on the distal surface of the ulna. Posterior to the dorsal part of the dorsal facet there is a prominent ridge directing posteroventrally and ending in the flexor tendon process. This ridge forms the most elevated surface of the proximal side of the proximal syncarpal. It is separated from the fovea by a flat area. The distal side of the bone is largely occupied by the intersyncarpal articulation facet which is separated by an oblique ridge into larger posterior and smaller anterior parts. This ridge is oriented anterodorsally–posteroventrally and consists of two portions separated by a sulcus. The larger dorsal portion is part of the rim surrounding the whole anterior intersyncarpal facet. The smaller ventral portion is a short ridge forming a prominent tubercle at the end. Dorsal and ventral to this tubercle there are two large pneumatic foramina (the dorsal one is somewhat larger and oval). The dorsal portion of the ridge separating the intersyncarpal articulation surfaces terminates anteriorly in a much larger tubercle. Ventral to this tubercle, on the dorsal margin of the anterior intersyncarpal articulation surface, there is a large round pneumatic foramen. The anterior intersyncarpal articulation surface is teardrop-shaped, with a rounded ventral margin and converging dorsally anterior and posterior margins. A prominent subhorizontal ridge bordering the posterior intersyncarpal articulation surface dorsally divides in its posterior part into two ridges. The ventral ridge extends posteroventrally and borders the posterior intersyncarpal articulation surface for some distance (there is no ridge along its short posterior margin). The dorsal ridge extends posterodorsally onto the flexor tendon process. Between the two ridges, there is a deep posteriorly widening groove. Within this groove, close to the ventral ridge, there is a large pneumatic foramen. The anterior and posterior sides of the proximal syncarpal are very narrow. The dorsal side of the proximal syncarpal is more or less flat. The larger ventral side of the proximal syncarpal is convex anteroposteriorly and concave proximodistally. There are four pneumatic foramina of different sizes in the anterior half of this ventral side. The ventral side is bordered by a slight ridge from the anterior side of the bone. The anteroposterior diameter 60.4 mm; dorsoventral height 83.7 mm.
The preaxial carpal ZIN PH 76/43 is complete, with some bone surface eroded on the ventral surface (Fig. 6). The bone is triangular in dorsal/ventral view, with the anterior end deflecting distally. The proximal margin is convex and the distal margin is concave. Most of the posterior surface is occupied by an oval-shaped concave articulation surface for the preaxial carpal process of distal syncarpal. The proximodistal diameter of this surface is about twice its dorsoventral height. There is also a short distal process on the posterior side of the bone. On the ventral side, there is a large pneumatic foramen adjacent to the articulation surface and subdivided by bony septa. There are also several slit-like small pneumatic foramina along the distal bone margin on the ventral surface. The anterior half of the bone dorsal surface is occupied by the elongated concavity of the terminal process. This surface is separated from the rest of the bone by a groove on the proximal side. The poorly defined facet for the pteroid is at the distal margin of the ventral surface. The preserved dorsal surface is flat. The anteroposterior diameter 22.7 mm; mediolateral width 13.5 mm; dorsoventral height 33.4 mm.
ZIN PH 66/43 is an almost complete femur; only a small piece of the diaphysis near the distal end is missing (Fig. 7). The femoral head is globular, with a round articulation surface. It is placed on a relatively long neck which is similar in width to the head anteroposteriorly but distinctly narrower mediolaterally. The neck is oriented at an angle of 39° to the longitudinal axis of the diaphysis proximal portion. The greater trochanter is relatively low. On the posterior side between the greater trochanter and the femoral neck, there is intertrochanteric depression with a large pneumatic foramen in the centre. This depression is bordered laterally by a prominent crest that is connected with the greater trochanter dorsally. The lateral side of the diaphysis adjacent to this crest is depressed.
There is a prominent rugosity along the anterolateral margin of the diaphysis distal to the greater trochanter (‘lesser trochanter’ for insertion of m. iliofemoralis internus). On the anterior side of the diaphysis, medial and parallel to this rugosity, there is a linear muscle scar which extends further distally on the diaphysis.
The diaphysis is sigmoidal in both anterior–posterior and medial–lateral views. The diaphysis in the middle has a slightly convex anteromedial side and a more convex opposite side. The diaphysis widens distally where it has a triangular cross-section with a flat anterior side. There is a prominent longitudinal muscle scar along the posteromedial margin of the middle diaphysis. The mediolaterally narrowest point of the diaphysis is situated well dorsal to its middle height.
The distal epiphysis is asymmetrical in distal view, with the medial condyle extending more anteriorly and the lateral condyle extending more laterally and posteriorly. Both condyles are separated anteriorly and distally by a very shallow intercondylar sulcus. There is a round depression within the intercondylar sulcus in the middle of the distal epiphysis. There are three small pneumatic foramina within this depression. Posteriorly the two condyles are separated by two depressions with a longitudinal ridge between them. The popliteal fossa is very shallow and poorly defined. On the medial condyle near the anterior end, there is a small circular depression. The articulation surface of the medial condyle is convex distally and concave posteriorly. The articulation surface of the lateral condyle is flat distally and posteriorly. On the lateral surface of the lateral condyle, there is a short crest, which is oriented anteroposteriorly. The total length 238 mm; proximal anteroposterior diameter15.5 mm; proximal width 32.2 mm; distal anteroposterior diameter 30.9 mm; distal width 40.0 mm.
3.c. Comparison
Cervical ZIN PH 64/43 is similar in proportions to the cervical 3 of Pteranodon (Bennett, Reference Bennett2001a: fig. 33B), but has a more posterior position of the lateral pneumatic foramen and relatively higher postzygapophysis, which more closely match the condition of more elongated middle cervicals of Pteranodon (Bennett, Reference Bennett2001a: fig. 37B). In Pteranodon the postaxial cervical vertebrae have a pair of pneumatic foramina lateral to the neural canal and a median pneumatic foramen above the neural canal on both anterior and posterior sides of the neural arch (Bennett, Reference Bennett2001a: p. 43). On the posterior side of the neural arch in the axis of Pteranodon, the lateral pneumatic foramina are small and placed mostly dorsal to the neural canal, and the median pneumatic foramen is absent (Bennett, Reference Bennett2001a: figs 34B, 35B). In ZIN PH 64/43, one lateral pneumatic foramen extends dorsally to the neural canal and the median pneumatic foramen is absent.
ZIN PH 64/43 is similar to the cervical 3 in the pteranodontid Volgadraco (Averianov et al., Reference Averianov, Ivakhnenko and Kurochkin2008: pl. 5, fig. 2) by general proportions (short and wide), by straight dorsal margin of the cotyle, by large hypapophysis, by postzygapophyses placed on the neural arch higher than the prezygapophyses, and by a pneumatic foramen on the lateral side of the neural arch. It differs by pits on the ventral centrum side lateral to the hypapophysis, by smaller pneumatic foramina lateral to the posterior opening of the neural canal (larger than the neural canal in Volgadraco), by a much weaker and more oblique ridge above the posterior opening of the neural foramen and collateral pneumatic foramina, and by lack of a strong vertical ridge dorsal to the posterior opening of the neural canal.
A pteranodontid cervical HMG 1052 from the Campanian Hakobuchi Group at Enbetsu, Hokkaido, Japan (Chitoku, Reference Chitoku1996: figs 2–4), is very similar to ZIN PH 64/43 in short and wide proportions, postzygapophysis placed higher than the prezygapophysis, pneumatic foramen on the lateral side of the neural arch, shape of the anterior and posterior openings of the neural canal, and size and relative position of the pneumatic foramina lateral to the posterior opening of the neural canal.
A pteranodontid middle cervical vertebra PU 21820 from the Campanian Merchantville Formation at Marshalltown, Delaware, USA (Baird & Galton, Reference Baird and Galton1981: fig. 2; Bennett, Reference Bennett1994), is relatively longer than ZIN PH 64/43 but similarly wide. It likely had a similarly large hypapophysis which is mostly destroyed. The hypapophysis continues posteriorly into a median ridge, as in ZIN PH 64/43. The dorsal side of the cotyle is convex (straight in ZIN PH 64/43). There is a pneumatic foramen on the lateral side of the neural arch, as in ZIN PH 64/43.
The middle cervical vertebra NHMUK R16479a, b from the Coniacian Chalk Formation at Hope Point, England, United Kingdom (Martill et al., Reference Martill, Witton and Gale2008: figs 3–6), was identified initially as belonging to a non-azhdarchid azhdarchoid. This specimen is very similar to ZIN PH 64/43 in proportions (short and wide), in having a large hypapophysis extending posteriorly into a median ridge, a pneumatic foramen on the lateral side of the neural arch, and lateral pneumatic foramina at the level of the middle height of the posterior opening of the neural canal. NHMUK R16479a, b differs from ZIN PH 64/43 by a concave dorsal margin of the cotyle.
In the azhdarchid Azhdarcho, there is no cervical vertebra that would match ZIN PH 64/43 in proportions (Averianov, Reference Averianov2010). This specimen differs additionally from the postaxial vertebrae of Azhdarchidae by the presence of the pneumatic foramen on the lateral side of the centrum.
An elongated middle cervical vertebra LINHM 014 of an azhdarchid from the Cenomanian Kem Kem beds of Morocco (Rodrigues et al., Reference Rodrigues, Kellner, Mader and Russell2011: fig. 4) is likely a cervical 4. It is much longer and narrower than ZIN PH 64/43, with a lower neural arch. It is similar to ZIN PH 64/43 in having a paired pneumatic foramen on the ventral centrum side lateral to the hypapophysis, although in the specimen from Beloe Ozero the foramen is smaller. This foramen has not been recorded in other pterodactyloids and its systematic value is unclear.
The free dorsal vertebra ZIN PH 55/43 was differentiated from the dorsal vertebrae in Pteranodon by transversely wider centrum, presence of hypapophysis, and the larger pneumatic foramen at the base of the prezygapophysis. However, all these characters are present in a three-dimensionally preserved free dorsal vertebra USNM 11642 of Pteranodon longiceps from the Niobrara Formation, Kansas, USA (personal observation by A.A.). These two specimens are nearly identical. In ZIN PH 55/43 there is a slit-like foramen on the dorsal side of the centrum. This foramen is characteristic of the free dorsal vertebrae of Pteranodon (Bennett, Reference Bennett2001a: p. 51). This foramen is slit-like in ZIN PH 55/43 but round in USNM 11642. In Azhdarcho there is a large depression that occupies most or all of the ventral floor of the neural canal (ZIN PH collection). Averianov & Popov (Reference Averianov and Popov2014) noted that ZIN PH 55/43 is almost identical to the posterior centrum surface of the notarium fragment of Volgadraco bogolubovi from the Rybushka Formation of the Shyrokii Karamysh locality (Averianov et al., Reference Averianov, Ivakhnenko and Kurochkin2008: pl. 6, fig. 1b) and concluded that this specimen likely belongs to V. bogolubovi. This conclusion is followed here, taking into account that Volgadraco is reassigned here to the Pteranodontidae.
The distal fragment of scapula ZIN PH 72/43 is similar in proportions to the scapula of an adult specimen of Pteranodon, including the position of the scapular tubercle and the orientation of the articulation facet for the notarium (Bennett, Reference Bennett2001a: fig. 65). Distal scapula morphology is poorly known in the Azhdarchidae.
Padian (Reference Padian1984) and Bennett (Reference Bennett1989) considered the form of the deltopectoral crest of the humerus to be diagnostic for the Pteranodontidae. Because of the flattened preservation of most Pteranodon specimens, these authors referred to the condition of three-dimensionally preserved proximal humerus fragment USNM 13804 from the Turonian Eagle Ford Group in Texas, USA (Gilmore, Reference Gilmore1935; Bennett, 1989: fig. 2, 1-5; Reference Bennett1994). However, this specimen is a non-istiodactylid ornithocheiroid rather than a pteranodontid (Andres & Myers, Reference Andres and Myers2013). Unwin (Reference Unwin, Buffetaut and Mazin2003) considered humerus with warped deltopectoral crest a synapomorphy for the Ornithocheiroidea. The deltopectoral crest ZIN PH 75/43 is markedly different from that crest in the Azhdarchidae. In azhdarchids, the deltopectoral crest is much longer and has subparallel proximal and distal margins for most of its length (Lawson, Reference Lawson1975: fig. 1b, c; Buffetaut et al., Reference Buffetaut, Grigorescu, Csiki, Buffetaut and Mazin2003: fig. 6; Averianov, Reference Averianov2010: fig. 23G, H; Hone et al., Reference Hone, Habib and Therrien2019: fig. 2).
ZIN PH 74/43 is the largest proximal syncarpal known for the Pterosauria. The shape of the proximal syncarpal and distribution of the articulations surfaces is quite variable in the Pterodactyloidea (Fig. 8a). ZIN PH 74/43 matches closely in proportions the proximal syncarpal of Pteranodon (Fig. 8b). It differs from the proximal syncarpal in Pteranodon mostly by the relatively larger fovea. In ornithocheirids, the dorsal portion of the proximal syncarpal is distinctly narrower and the flexor tendon process is less prominent (Fig. 8c–f). In the Dsungaripteridae the flexor tendon process is further reduced, the fovea is larger, the radius and ventral ulna articulation surfaces are merged and there are no pneumatic foramina (Fig. 8g). The proximal syncarpal of azhdarchids is similar to that bone in the Dsungaripteridae but has a large pneumatic foramen ventral to the fovea (Fig. 8h).

Fig. 8. Right proximal syncarpal in proximal view. (a) Pteranodontidae indet., ZIN PH 74/43. (b) Pteranodon sp., after Bennett (Reference Bennett2001a: fig. 79C), flipped horizontally. (c) Santanadactylus pricei, after Wellnhofer (Reference Wellnhofer1991: fig. 29d). (d) Santanadactylus araripensis, after Wellnhofer (Reference Wellnhofer1985: fig. 11f). (e) Anhanguera santanae, after Wellnhofer (Reference Wellnhofer1985: fig. 38f). (f) Anhanguera piscator, after Kellner & Tomida (Reference Kellner and Tomida2000: fig. 36b). (g) Dsungaripterus weii, PIN 3953/1, flipped horizontally. (h) Azhdarcho lancicollis, ZIN PH 94/44. Abbreviations: dar, articulation surface for radius; dau, facet for dorsal articulation surface of ulna; fov, fovea, facet for ulnar tuberculum; ftp, flexor tendon process; tub, tuberculum for ulnar fovea; veu, facet for ventral articulation surface of ulna. Not to scale.
The preaxial carpal ZIN PH 76/43 is generally similar to that bone in Pteranodon (Bennett, Reference Bennett2001a: fig. 81). The preaxial carpal is unknown for the Azhdarchidae. ZIN PH 183/44, identified previously as a preaxial carpal of Azhdarcho (Averianov, Reference Averianov2010: fig. 29), is an ulna fragment.
The femur ZIN PH 66/43 is similar to that bone in Pteranodon (Bennett, Reference Bennett2001a: fig. 107) in having asymmetrical distal condyles. It differs by more pronounced sigmoidal curvature of the bone, lack of internal trochanter, and distribution of muscle scars. ZIN PH 66/43 is almost identical to the distal femur fragment NSM PV15005 of Pteranodontidae indet. from the Santonian–Campanian Yezo Group in Hokkaido, Japan (Kellner et al., Reference Kellner, Costa, Wang and Cheng2016: fig. 4A). It differs by slightly deeper round depression with pneumatic foramen in the centre of the intercondylar sulcus.
ZIN PH 66/43 differs from the femur in Azhdarcho (Averianov, Reference Averianov2010: fig. 34) by lack of the internal trochanter and by asymmetrical distal diaphysis, with the lateral condyle extending more laterally and posteriorly. A distal femur fragment TMP 91.36.616 from the Campanian Provincial Park Formation at Dinosaur Provincial Park, Alberta, Canada (Godfrey & Currie, Reference Godfrey, Currie, Currie and Koppelhus2005: fig. 16.10 B–D), is similar to that of Azhdarcho in having symmetrical distal condyles of roughly similar size but differs in the distal end more expanded anteroposteriorly than mediolaterally. Femora of Azhdarcho and TMP 91.36.616 differ from ZIN PH 66/43 by more distinct popliteal fossa.
ZIN PH 66/43 differs from the proximal femur in Quetzalcoatlus (TMM 42422-27) by femoral head less expanded mediolaterally, femoral neck relatively longer and gracile, and by the pneumatic foramen on the posterior side placed more proximally and having a round shape (cleft-like in Quetzalcoatlus). The femur of Cryodrakon (Godfrey & Currie, Reference Godfrey, Currie, Currie and Koppelhus2005: fig. 16.10A) is similar to Azhdarcho and ZIN PH 66/43 in proportions of the proximal end and similar position of the proximal pneumatic foramen.
The distal femur of Quetzalcoatlus (TMM 41544-27) is nearly symmetrical in distal view, but, in contrast to Azhdarcho, the medial condyle is distinctly larger than the lateral condyle (both condyles are of nearly the same size in Azhdarcho). In this respect, the femur of Quetzalcoatlus is markedly different from ZIN PH 66/43, where the distal end is asymmetrical and the lateral condyle is distinctly larger than the ventral condyle.
The bone fragment MTM V2010.99.1 from the Santonian Csehbanaya Formation at Inarkut Mine, Hungary, identified initially as a possible distal end of wing metacarpal of Pterodactyloidea indet. (Ősi et al., Reference Ősi, Buffetaut and Prondvai2011: fig. 3C–E), is a right femur distal fragment. It is similar to ZIN PH 66/43 in having an asymmetrical distal end, with distal condyle extending laterally. A pterosaur femur with a similarly asymmetrical distal end is known also from the Albian Alamyshik Formation at Kylodzhun, Kyrgyzstan (Averianov, Reference Averianov2004: fig. 1).
4. Discussion
At least two pterodactyloid taxa are present in the Campanian Rybushka Formation at the Beloe Ozero locality: Azhdarchidae and Pteranodontidae. This makes Beloe Ozero the most diverse pterosaur assemblage for the Late Cretaceous of Europe. The bones of the Pteranodontidae are described in this paper. The free dorsal vertebra ZIN PH 55/43, referred previously to the Azhdarchidae (Averianov & Popov, Reference Averianov and Popov2014), is attributed here to the Pteranodontidae. The uncontested specimens of the Azhdarchidae are coracoid fragments (Averianov & Panteleyev, Reference Averianov, Panteleyev, Shishkin and Tverdokhlebov2009) and undescribed ilium postacetabular process (ZIN PH 73/43). There are some pterosaur bones from the Beloe Ozero locality that cannot currently be attributed to either the Pteranodontidae or Azhdarchidae: edentulous jaw fragment, scapulocoracoid fragment with glenoid, humerus proximal fragments, wing metacarpal distal fragments, and wing phalanx 1 proximal fragments. Some of these fragments were referred previously to the Azhdarchidae (Averianov, Reference Averianov2007; Averianov et al., Reference Averianov, Arkhangelsky and Merkulov2016); the other specimens are undescribed. The most intriguing findings are the scapulocoracoid fragment with flat glenoid (ZIN PH 71/43) and fragments of the humerus with a convex head (ZIN PH 59/43 and others) that fit this glenoid surface. These specimens differ significantly from the morphology of the scapulocoracoid and humerus known previously for the Pteranodontidae and Azhdarchidae.
Volgadraco bogolubovi from the Rybushka Formation at Shyrokii Karamysh locality in Saratov Province, Russia, is based on edentulous rostrum fragment, cervical vertebrae 3 and 9 and posterior notarium fragment (Averianov et al., Reference Averianov, Ivakhnenko and Kurochkin2008). The rostrum fragment (holotype) is consistent in morphology with either the Pteranodontidae or Azhdarchidae. The cervical 3 differs from the third cervical in Azhdarcho by proportions (short and wide), high neural arch, and pneumatic foramen on the lateral centrum side. In these characters and overall proportions, it is very close to the cervical 3 in Pteranodon (Bennett, Reference Bennett2001a: fig. 33B). The cervical 9 of V. bogolubovi is also very similar to that vertebra in Pteranodon (Bennett, Reference Bennett2001a: figs 42–43) in proportions and in having the large depression on the lateral side posterior to the prezygapophysis. As was noted above, the morphology of notarium is also consistent with that bone in Pteranodon. Based on these similarities, Volgadraco is referred here to the Pteranodontidae (Fig. 9). Previously, this taxon was recovered as a pteranodontoid in a phylogenetic analysis presented by Longrich et al. (Reference Longrich, Martill and Andres2018). The Beloe Ozero pteranodontid might well be the same taxon as Volgadraco bogolubovi, but currently there are no overlapping skeletal elements between the Beloe Ozero and Shyrokii Karamysh localities to prove this.

Fig. 9. Hypothethical reconstruction of a pteranodontid from the Campanian Rybushka Formation of Saratov Province, Russia, by Andrey Atuchin.
Bogolubovia orientalis from the Rybushka Formation at Malaya Serdoba locality in Penza Province, Russia, is known from a single specimen, a posterior fragment of the cervical vertebra (Bogolyubov, Reference Bogolyubov1914; Nesov & Yarkov, Reference Nesov and Yarkov1989; Bakhurina & Unwin, Reference Bakhurina and Unwin1995). The morphology of the posterior side of the cervical vertebrae is poorly known for the Pteranodontidae (Bennett, Reference Bennett2001a). The holotype of B. orientalis is similar to the cervical 3 of V. bogolubovi by spindle-like condyle, high postexapophyses, large neural canal, large pneumatic foramina lateral to the neural canal, lack of the median pneumatic foramen dorsal to the neural canal and strong horizontal ridge dorsal to the neural canal and side pneumatic foramina. It is possible that the holotype of B. orientalis belongs to the Pteranodontidae, as was initially suggested (Bogolyubov, Reference Bogolyubov1914), and even to V. bogolubovi. This question can be solved after the discovery of complete pterosaur cervical vertebrae from the Malaya Serdoba locality and other localities of the Rybushka Formation. Additional pterosaur specimens from the Malaya Serdoba locality, an edentulous jaw fragment and wing metacarpal distal fragment (Averianov, Reference Averianov2007), cannot be distinguished between the Pteranodontidae and Azhdarchidae.
The Late Cretaceous Pteranodontidae with a single genus Pteranodon was long considered to be endemic for North America. Tethydraco from the Maastrichtian of Morocco is the second pteranodontid genus described so far (Longrich et al., Reference Longrich, Martill and Andres2018). Pteranodontidae indet. were also known previously from the Santonian–Campanian of Japan (Obata et al., Reference Obata, Hasegawa and Otsuka1972; Kellner et al., Reference Kellner, Costa, Wang and Cheng2016). The other putative records of Pteranodontidae are briefly discussed below.
The middle cervical PU 21820 from the Campanian Merchantville Formation at Marshalltown, Delaware, USA (Baird & Galton, Reference Baird and Galton1981: fig. 2), is similar to middle cervicals of Pteranodon and differs from those in the Azhdarchidae by proportions (relatively short and wide) and presence of a pneumatic foramen on the lateral side of the centrum. It likely belongs to a pteranodontid or even to Pteranodon as was previously suggested (Bennett, Reference Bennett1994).
The cervical HMG 1052 from the Campanian Hakobuchi Group at Enbetsu, Hokkaido, Japan (Chitoku, Reference Chitoku1996: figs 2–4), is very similar to ZIN PH 64/43 from Beloe Ozero in short and wide proportions, lateral pneumatic foramen on centrum, and size and position of the pneumatic foramina lateral to the posterior opening of the neural canal. By these characters, this specimen is also similar to Pteranodon and likely belongs to the Pteranodontidae. This group was present in the Late Cretaceous of Hokkaido as it is known by material from another locality (Kellner et al., Reference Kellner, Costa, Wang and Cheng2016).
A short and wide middle cervical NHMUK R16479a, b with lateral pneumatic foramen on the centrum from the Coniacian Chalk Formation at Hope Point, England, United Kingdom (Martill et al., Reference Martill, Witton and Gale2008: figs 3–6), also can be attributed to the Pteranodontidae.
The distal femur fragment MTM V2010.99.1 from the Santonian Csehbanaya Formation at Inarkut mine, Hungary (Ősi et al., Reference Ősi, Buffetaut and Prondvai2011: fig. 3C–E), has an asymmetrical distal end with distal condyle extending laterally. A similar morphology is present in a distal femur fragment ZIN PH 1/43 from the Albian Alamyshik Formation at Kylodzhun, Kyrgyzstan (Averianov, Reference Averianov2004: fig. 1). This morphology is found in the Ornithocheiridae, Pteranodon and Nyctosaurus (Williston, Reference Williston1903: pl. 43, figs 3, 10). Ornithocheiridae, however, are not known in the fossil record after the Cenomanian–Turonian faunal turnover (Averianov, Reference Averianov2014). While the femur from Kyrgyzstan can still belong to the Ornithocheiridae, the femur from Hungary is more likely attributable to the Pteranodontidae.
The pteranodontid pterosaur from the Beloe Ozero locality was quite a large pterodactyloid. The femur is somewhat smaller than the pteranodontid femur NSM PV15005 from the Santonian–Campanian of Japan (Kellner et al., Reference Kellner, Costa, Wang and Cheng2016): the distal femur width is 40.0 and 46.2 mm in the Russian and Japan specimens, respectively. The Japanese pteranodontid with an estimated wingspan of 6.8 m was considered the largest flying reptile from Asia (Kellner et al., Reference Kellner, Costa, Wang and Cheng2016). Using the data on the distal femur width from the cited paper, the wingspan of the Beloe Ozero pteranodontid would be 6.31 m. Pteranodon sp. FHSM 184 from the Niobrara Formation of Kansas, USA, with a wingspan of 6.1 m, has femur length 278 mm (Bennett, Reference Bennett2001b: tab. 10). By comparison with this specimen, the Beloe Ozero pteranodontid with a femur length of 238 mm would have a wingspan of 5.22 m. The measurements of the proximal syncarpal are available for the two large specimens of an ornithocheiroid Anhanguera with a wingspan of 4.15 and 5.00 m (Wellnhofer, Reference Wellnhofer1991; Kellner & Tomida, Reference Kellner and Tomida2000). Based on these measurements, the proximal syncarpal ZIN PH 74/43 from Beloe Ozero belongs to an individual with a wingspan of 6.48 m.
Identification of the Pteranodontidae in the Campanian Beloe Ozero locality in Saratov Province, Russia, is in line with the previous finding of North American vertebrate taxa in this locality, which include the chelonioid sea turtle Protostegidae indet. close to Protostega gigas (Danilov et al., Reference Danilov, Obrazstova, Arkhangelsky, Ivanov, Averianov and Hirayama2018) and the mosasaurid Clidastes propython (Grigoriev et al., Reference Grigoriev, Arkhangelsky and Merkulov2015). The other localities within the Rybushka Formation also produce remains of hesperornithiform birds (Zelenkov et al., Reference Zelenkov, Panteleyev and Yarkov2017). These data, together with the recent identification of the Pteranodontidae in the Santonian–Campanian of Japan (Kellner et al., Reference Kellner, Costa, Wang and Cheng2016 and this report), suggest a much more pronounced cosmopolitanism of the Late Cretaceous pterosaurs in the northern continents than was previously thought.
Conflict of interest
Authors declare no competing interests.
Acknowledgements
We thank SC Bennett and an anonymous reviewer for reading the paper and providing useful comments that improved the paper. We are grateful to AV Ivanov for help in organizing the fieldwork, and to DD Andreev, NN Afonkov, IT Sabirov, RM Medzhidov, MS Pustovalov, SV Lipatov, DO Gribkov, AA Shetinkin, MA Zenkin, MD Kudryashov, NYu Zozyrev and DV Grigoriev for assistance in excavations in 2017–19. We thank DV Grigoriev for taking photographs of ZIN PH 66/43. A.A. thanks H-D Sues for assistance during the study of the pterosaur collection in USNM. The laboratory research by A.A. received support from the Russian Science Foundation (19-14-00020) and the Zoological Institute, Russian Academy of Sciences (project АААА-А19-119032590102-7).











