1. Introduction
The Pliensbachian–Toarcian time span is marked in the Tethyan realm by palaeogeographic and palaeoenvironmental changes that had a profound impact on the marine biodiversity. The central region of the western Tethys was subject to a highly dynamic tectonic evolution driven by the Neotethyan rifting which caused the establishment of areas with pelagic/carbonate platform sedimentation (e.g. Channell, D’Argenio & Horváth, Reference Channell, D’Argenio and Horváth1979; Ziegler, Reference Ziegler1988; Nairn et al. Reference Nairn, Ricou, Vrielynck and Dercourt1996; Ziegler & Horváth, Reference Ziegler and Horváth1996; Dercourt et al. Reference Dercourt, Gaetani, Vrielynck, Barrier, Bi Ju-Duval, Brunet, Cadet, Crasquin and Sandulescu2000; Golonka, Reference Golonka2004, Reference Golonka2007; Santantonio & Carminati, Reference Santantonio and Carminati2011) and separate benthic faunas (e.g. Gatto & Monari, Reference Gatto and Monari2010; Gatto et al. Reference Gatto, Monari, Szabó and Conti2013). On the other hand, the stable European epicontinental shelf witnessed a period of environmental perturbations culminating with the widespread deposition of organic-rich sediments (‘black shales’) during early Toarcian time (Röhl et al. Reference Röhl, Schmid-Röhl, Oschmann, Frimmel and Schwark2001; McArthur et al. Reference McArthur, Algeo, van de Schootbrugge, Li and Howarth2008; Hermoso, Minoletti & Pellenard, Reference Hermoso, Minoletti and Pellenard2013). These sediments, such as the Posidonia Shale in southern Germany, the Jet Rock Beds in the Cleveland Basin of eastern England and the Schistes Cartons in the Paris and other French basins, have been interpreted as the expression of widespread marine anoxic conditions known as the Toarcian Oceanic Anoxic Event (T-OAE; Jenkyns, Reference Jenkyns1988). The controlling mechanisms and extension of the T-OAE are still debated (see reviews in Caruthers, Smith & Gröcke, Reference Caruthers, Smith and Gröcke2013; Harazim et al. Reference Harazim, de Schootbrugge, Sorichter, Fiebig, Weug, Suan and Oschmann2013; Hermoso, Minoletti & Pellenard, Reference Hermoso, Minoletti and Pellenard2013), as are the major environmental disturbances associated with it such as climatic oscillations (van de Schootbrugge et al. Reference van de Schootbrugge, Bailey, Rosenthal, Katz, Wright, Feist-Burkhardt, Miller and Falkowski2005; Dera et al. Reference Dera, Neige, Dommergues and Brayard2011; Dera & Donnadieu, Reference Dera and Donnadieu2012), the crisis of carbonate production (Suan et al. Reference Suan, Mattioli, Pittet, Mailliot and Lécuyer2008) and disruptions in biogeochemical cycles (Pearce et al. Reference Pearce, Cohen, Coe and Burton2008; Jenkyns, Reference Jenkyns2010; Gill, Lyons & Jenkyns, Reference Gill, Lyons and Jenkyns2011; Lézin et al. Reference Lézin, Andreu, Pellenard, Bouchez, Emmanuel, Fauré and Landrein2013). The event took place during a period of severe and protracted biotic crisis that assumed the character of a multi-phased mass extinction affecting both pelagic and benthic marine communities across the Pliensbachian–Toarcian boundary (Dera et al. Reference Dera, Neige, Dommergues, Fara, Laffont and Pellenard2010; Caruthers, Smith & Gröcke, Reference Caruthers, Smith and Gröcke2013). The extinction event at the base of the Toarcian is well documented in the European epicontinental shelf and has been intensely studied (e.g. Hallam, Reference Hallam1987; Little & Benton, Reference Little and Benton1995; Harries & Little, Reference Harries and Little1999; Vörös, Reference Vörös2002; Aberhan & Baumiller, Reference Aberhan and Baumiller2003; Cecca & Macchioni, Reference Cecca and Macchioni2004; Wignall, Newton & Little, Reference Wignall, Newton and Little2005; Caswell, Coe & Cohen, Reference Caswell, Coe and Cohen2009; Dera et al. Reference Dera, Neige, Dommergues, Fara, Laffont and Pellenard2010). Recently, research efforts have been devoted to investigate the tempo and mode of the post-crisis faunal recovery focusing on entire communities (Danise et al. Reference Danise, Twitchett, Little and Clémence2013) or on selected groups such as, for example, benthic foraminifers and calcareous nannofossils (Mailliot et al. Reference Mailliot, Mattioli, Bartolini, Baudin, Pittet and Guex2009), brachiopods (García Joral, Gómez & Goy, Reference García Joral, Gómez and Goy2011), ammonites (Dera et al. Reference Dera, Neige, Dommergues, Fara, Laffont and Pellenard2010; Neige, Dera & Dommergues, Reference Neige, Dera and Dommergues2013) and radiolarians (Goričan et al. Reference Goričan, Carter, Guex, O’Dogherty, De Wever, Dumitrica, Hori, Matsuoka and Whalen2013).
Studies on the relationship between the changes of gastropod diversity and the Toarcian crisis are very few (e.g. Gründel et al. Reference Gründel, Kaim, Nützel and Little2011; Monari & Gatto, Reference Monari and Gatto2013), the group being mostly considered as a component in analyses of multi-taxa benthic assemblages (Gahr, Reference Gahr2005; Caswell, Coe & Cohen, Reference Caswell, Coe and Cohen2009; Caswell & Coe, Reference Caswell and Coe2012; Danise et al. Reference Danise, Twitchett, Little and Clémence2013). In this context, the present study provides new data relevant to reconstruct the mode of recovery of this molluscan group based on a thorough systematic analysis of the gastropod fauna from the upper Pliensbachian – upper Toarcian succession cropping out in two sections of the Causses region (southern France). The Toarcian species recognized here belong to a faunal stock characteristic of several localities of the European shelf and represent the first repopulation of this Tethyan district after the T-OAE. Until now, knowledge of the Toarcian benthic molluscs from the Causses was limited to the study by Fürsich et al. (Reference Fürsich, Berndt, Scheuer and Gahr2001) who recorded few gastropods species. Most taxonomic contributions on Toarcian gastropods concern faunas from northern and southern Germany (Roemer, Reference Roemer1836, Reference Roemer1839; Goldfuss, Reference Goldfuss1841, Reference Goldfuss1844; Quenstedt, Reference Quenstedt1852, Reference Quenstedt1856, Reference Quenstedt1882, Reference Quenstedt1883; Oppel, Reference Oppel1854; Denckmann, Reference Denckmann1887; Schlosser, Reference Schlosser1901; Sieberer, Reference Sieberer1907; Brösamlen, Reference Brösamlen1909; Ernst, Reference Ernst1923; Kuhn, Reference Kuhn1935; Walther, Reference Walther1951; Klöcker, Reference Klöcker1966; Gründel, Reference Gründel1999a , Reference Gründel b , Reference Gründel2005, Reference Gründel2007, Reference Gründel2009; Gründel, Nützel & Schulbert, Reference Gründel, Nützel and Schulbert2009; Schulbert & Nützel, Reference Schulbert and Nützel2009, Reference Schulbert and Nützel2013). Studies on other areas of the European epicontinental shelf are much fewer and mostly outdated. They mainly refer to the Paris Basin (Eudes-Deslongchamps, Reference Eudes-Deslongchamps1842a , Reference Eudes-Deslongchamps b , Reference Eudes-Deslongchamps1849; d’Orbigny, Reference Orbigny1850, Reference Orbigny1851, Reference Orbigny1852, Reference Orbigny1853, Reference Orbigny1854, Reference Orbigny1855; Thevenin, Reference Thevenin1908; Cossmann, Reference Cossmann1913; Fischer & Weber, Reference Fischer and Weber1997), southern France (Dumortier, Reference Dumortier1874), England (Moore, Reference Moore1866; Hudleston, Reference Hudleston1889, Reference Koken1892, Reference Hudleston1894, Reference Hudleston1895, Reference Koken1896; Wilson & Crick, Reference Wilson and Crick1889; Gründel et al. Reference Gründel, Kaim, Nützel and Little2011) and Iberian Peninsula (Gahr, Reference Gahr2002). Few other species are recorded from areas that belonged to the central part of western Tethys during Jurassic time, that is, the central Apennine (central Italy; Mariotti & Schiavinotto, Reference Mariotti and Schiavinotto1977; Conti & Monari, Reference Conti, Monari and Mancinelli1995, Reference Conti and Monari2003), the western Sicily (southern Italy; Wendt, Reference Wendt1968) and the Transdanubian Central Range (Hungary; Szábo, Reference Szabó1982, Reference Szabó1995; Gálacz & Szábo, Reference Galácz and Szabó2001) and to its NE margin (Pčelincev, Reference Pčelincev1937). Finally, except for a little Toarcian fauna from Morocco described by Cossmann & Abrard (Reference Cossmann and Abrard1921), nothing is known about the gastropods of the southern margin of the western Tethys.
Being located at the southern margin of the European shallow-water shelf facing the central part of western Tethys, the Causses Basin might provide useful indications on the relationship between the benthic faunas inhabiting these palaeogeographic sectors during a crucial period of the history of the western Tethys.
2. Geographical and stratigraphical setting
The studied gastropods have been recovered from two sections, Tournadous and Cornus, located about 10 km apart in central-southern France (Fig. 1a). These localities have been the subject of several studies, mainly on account of their rich cephalopod faunas (e.g. Monestier, Reference Monestier1921a , Reference Monestier b , Reference Monestier1931; Guex, Reference Guex1972; Meister, Reference Meister1989; Morard, unpub. Ph.D. thesis, Université de Lausanne, 2004; Pinard et al. Reference Pinard, Weis, Neige, Mariotti and Di Cencio2014). Mailliot et al. (Reference Mailliot, Mattioli, Bartolini, Baudin, Pittet and Guex2009) analysed in detail the environmental changes at the Pliensbachian–Toarcian boundary in the Tournadous section. Both sections are situated on the southern part of the Causses Basin, a small intracratonic basin that during Jurassic time was located along the SW margin of the European epicontinental shelf (Fig. 1b). The basin was bordered by the Massif Central lands to the north and west and by the Montagne Noire lands to the south. Communication with adjacent basins was further limited by the Cévennes High on the eastern side of the basin. Spatial changes in thickness of the different lithostratigraphic units and even hiatuses are suggestive of a marked differential subsidence. Tectonics, stratigraphy and geochemistry aspects of the basin have been thoroughly investigated in a number of studies (e.g. Trümpy, Reference Trümpy1983; Graciansky et al. Reference Graciansky, Dardeau, Dommergues, Durlet, Marchand, Dumont, Hesselbo, Jacquin, Goggin, Meister, Mouterde, Rey, Vail, de Graciansky, Hardenbol, Jacquin and Vail1998; Mailliot et al. Reference Mailliot, Mattioli, Bartolini, Baudin, Pittet and Guex2009; Harazim et al. Reference Harazim, de Schootbrugge, Sorichter, Fiebig, Weug, Suan and Oschmann2013; van de Schootbrugge et al. Reference van de Schootbrugge, Bachan, Suan, Richoz and Payne2013).

Figure 1. (a) Locality map of the studied area. (b) Palaeogeographic setting of the Causses Basin at the Toarcian stage (simplified from Morard, unpub. Ph.D. thesis, Université de Lausanne, 2004); white stars indicate studied sections.
The Tournadous section is considered as very proximal (Mailliot et al. Reference Mailliot, Mattioli, Bartolini, Baudin, Pittet and Guex2009) and the studied interval (upper Pliensbachian – upper Toarcian; Fig. 2) includes three local formations. At the base of the section the Marnes de Villeneuve Formation is represented by dark marls. The top of the unit is characterized by a sequence of three nodular beds, the uppermost of which indicates the end of the Pliensbachian succession (Mailliot et al. Reference Mailliot, Mattioli, Bartolini, Baudin, Pittet and Guex2009). Fossils are generally well preserved, with cephalopods largely predominant and gastropods, bivalves (pectinids, plicatulids, nuculids and nuculanids) and small brachiopods rather frequent. The successive Schistes Cartons Formation is an organic-matter-rich shale occasionally containing silty sediments. At the very base of this formation, Mailliot et al. (Reference Mailliot, Mattioli, Bartolini, Baudin, Pittet and Guex2009) found a negative δ13C anomaly that has been tentatively correlated with the well-known negative carbon-isotope excursion (CIE) recorded in numerous sections in NW Europe and elsewhere (Hesselbo et al. Reference Hesselbo, Gröcke, Jenkyns, Bjerrum, Farrimond, Morgans Bell and Green2000, Reference Hesselbo, Jenkyns, Duarte and Oliveira2007; Caswell & Coe, Reference Caswell and Coe2012; Hermoso et al. Reference Hermoso, Minoletti, Rickaby, Hesselbo, Baudin and Jenkyns2012). The uppermost unit is represented by the Marnes de Fontaneilles Formation, a monotonous marl succession with rare intercalated carbonate beds that extends up to the Aalenian section. Ammonites, belemnites, gastropods and bivalves are frequent. The Cornus section (Fig. 2) has been studied in its well-exposed upper portion, equivalent to part of the Marnes de Fontaneilles Formation, which has been dated as being of upper Toarcian age. Gastropods are frequent, together with more rare bivalves (Nuculana–Palaeonucula group).
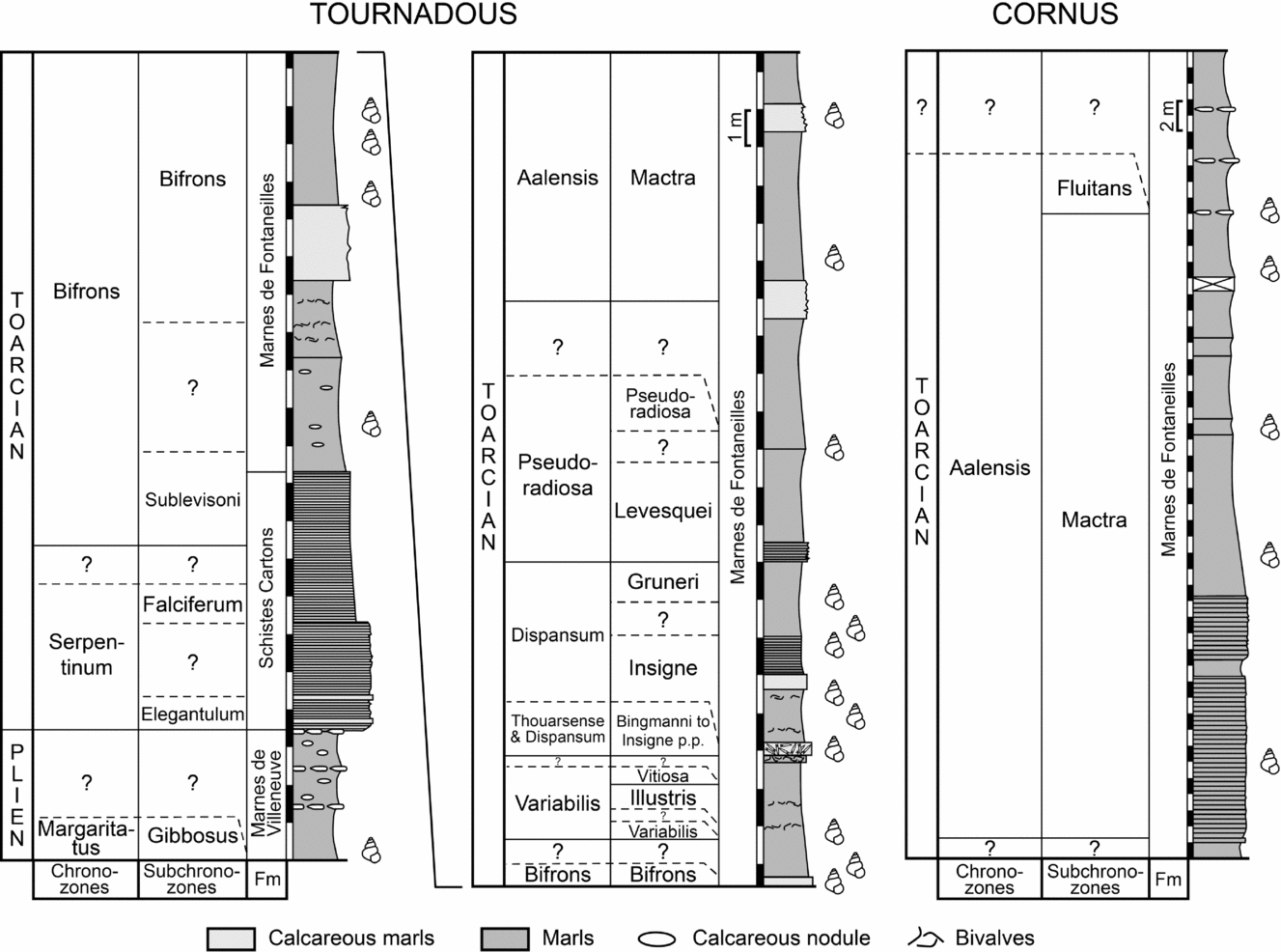
Figure 2. Stratigraphical columns of the late Pliensbachian – Toarcian succession cropping out at Tournadous and Cornus. Gastropod symbols: beds yielding gastropods. Question marks (?) indicate absence of stratigraphical markers. Standard zonation scheme from Page (Reference Page2003).
A detailed chronostratigraphic subdivision has been obtained by the study of the distribution of the ammonites (Pinard et al. Reference Pinard, Weis, Neige, Mariotti and Di Cencio2014). All zones have been recognized except the Spinatum Zone (uppermost Pliensbachian) and the Tenuicostatum Zone (lowermost Toarcian). In the Tournadous section a very distinctive calcareous bed (bed number 100 in Pinard et al. Reference Pinard, Weis, Neige, Mariotti and Di Cencio2014; Fig. 2) occurs at nearly 20 m above the base and represents a condensed interval characterized by the occurrence of a time-averaged fossil accumulation dated from the Thouarsense Zone to the base of the Dispansum Zone (Insigne Subzone). The base of the Cornus section contains ammonites from the lower part of the Aalensis Zone (Mactra Subzone), namely Pleydellia (Pleydellia) mactra (Dumortier, Reference Dumortier1874), Pleydellia (Pleydellia) aalensis (Zieten, Reference Zieten1832), Pleydellia (Cotteswoldia) paucicostata (Buckman, Reference Buckman1904), whereas the top of the section contains ammonites from the upper part of the Aalensis Zone (Fluitans Subzone) such as Pleydellia (Walkericeras) fluitans (Dumortier, Reference Dumortier1874) and Pleydellia (Walkericeras) sp.
Samples for the present study were recovered by surface collection. Although rather abundant, the gastropods do not occur throughout the sections (Fig. 2). They are present in the Margaritatus Zone, Gibbosus Subzone of the Marnes de Villeneuve Formation, mainly preserved as inner moulds. Gastropods have not been found in the Schistes Cartons Formation, although Morard (unpub. Ph.D. thesis, Université de Lausanne, 2004) reported Coelodiscus from nearby localities of Saint-Paul-des-Fonts and Saint-Beaulize. In the Marnes de Fontaneilles Formation, gastropods occur in several levels which are mainly concentrated in the Bifrons Zone, Dispansum Zone and Aalensis Zone, Mactra Subzone.
3. Systematic palaeontology
The distribution of the species reported below is exclusively based on the synonymy list of the species concerned and, more in detail, on the specimens figured by authors. Most of the morphological terms used in the systematic descriptions are in accordance with Cox (Reference Cox, Moore and Pitrat1960). The measurements of the specimens are reported in Table 1 and abbreviations for the dimensions are shown in Figure 3.
Table 1. Measurements of the most representative specimens studied.

Linear measurements are in millimetres. The asterisk (*) indicates measurements made on incomplete specimens. See Figure 3 for the abbreviations of the dimensions.

Figure 3. Measurements of the specimens reported in the systematic descriptions and in Table 1. H – height of the shell; HL – height of the last whorl; HA – height of the peristome; W – width of the shell; WA – width of the peristome; α – mean spire angle.
Institutional abbreviations are as follows: UBGD, University of Burgundy, Geology Dijon, France; MNHNL, National Museum of Natural History of Luxembourg, City of Luxembourg, Grand-Duchy of Luxembourg.
Superfamily EOTOMARIOIDEA Wenz, Reference Wenz and Schindewolf1938 Family GOSSELETINIDAE Wenz, Reference Wenz and Schindewolf1938 Genus Sisenna Koken, Reference Koken1896
Type species. Pleurotomaria turbinata Hörnes, Reference Hörnes1855. Carnian (Upper Triassic), Northern Calcareous Alps (Austria).
Sisenna canalis (Münster in Goldfuss, Reference Goldfuss1844) Figure 4a–l

Figure 4. Eotomarioidea and Ptychomphaloidea. (a–l) Sisenna canalis (Münster in Goldfuss, Reference Goldfuss1844): (a–c) dorsal, basal and apertural views, MNHNL QH580; (d–f) apical and dorsal views, and detail showing the pattern of growth lines, MNHNL QH577; (g–h) apertural and dorsal views, MNHNL QH578; (i–l) dorsal, basal, apertural and apical views, MNHNL QH579. Tournadous, lower Toarcian, Bifrons Zone. (m–r) Angulomphalus expansus (Sowerby, Reference Sowerby1821a ): apertural, lateral and dorsal views, detail of the selenizone of the penultimate whorl, apical view, and detail of the whorl surface, MNHNL QH619, Tournadous, upper Pliensbachian, Margaritatus Zone, Gibbosus Subzone; st – suture; sz – selenizone.
? 1836 Trochus helicinoides Roemer, p. 150, pl. 11, fig. 13.
1844 Turbo canalis Münster; in Goldfuss, p. 95, pl. 193, fig. 12a, b.
1854 Turbo canalis Goldfuss; Oppel, p. 103, pl. 3, fig. 20a, b.
1856 Turbo canalis Quenstedt, p. 155, pl. 19, figs 32, 33.
1866 Trochus carinatus n. s.; Moore, p. 207, pl. 4, figs 24, 25.
1876 Pleurotomaria helicinoides Roemer; Tate in Tate & Blake, p. 338, pl. 10, fig. 7.
1882 Turbo canalis Quenstedt, p. 427, pl. 201, figs 113, 114.
? 1889 Pleurotomaria helicinoides Roemer; Wilson in Wilson & Crick, p. 304, pl. 9, fig. 13a, b.
1889 Pleurotomaria (Turbo) canalis Münster; Wilson in Wilson & Crick, p. 304, pl. 9, fig. 14.
1909 Sisenna canalis Münster; Brösamlen, p. 200, pl. 17, fig. 2a–c.
1936 Sisenna canalis Münster; Kuhn, p. 281, pl. 8, fig. 8a, b.
1998 Sisenna canalis (Münster); Gründel & Nützel, p. 65, pl. 1, figs 3–5.
2008 Sisenna canalis (Münster); Nützel, p. 45, pl. 1, fig. 5.
Material. Four specimens: MNHNL QH577, MNHNL QH578, MNHNL QH579, MNHNL QH580, Tournadous, lower Toarcian, Bifrons Zone.
Dimensions. See Table 1.
Description. Shell very small (height about 5 mm), turbiniform, slightly higher than wide, composed of four teleoconch whorls with a distinctly gradate spire. Height of last whorl about 80% of shell height. Surface of early whorls strongly and evenly convex. Succeeding whorls with shoulder marked by spiral cord and subdividing whorl surface into slightly convex to almost flat ramp and vertical outer face. Fully adult whorls with second obtuse angulation on ramp closer to adapical suture than to shoulder and provided with spiral cord delimiting flat and horizontal subsutural shelf. Part of ramp between angulation and shoulder distinctly concave. Base strongly convex and swollen. Aperture roundedly trapezoidal. Additional spiral thread below shoulder scarcely visible on specimens described here. Base ornamented by regularly spaced spiral threads, seemingly more spaced abaxially. Growth lines prosocline and feebly prosocyrt on ramp, strongly opisthocline and opisthocyrt on outer face, widely prosocyrt on base, becoming slightly opisthocyrt on its axial region.
Remarks. The specimens are mostly preserved as inner moulds with shell remains of the main elements of the external ornament, including traces of the growth lines. According to Brösamlen (Reference Brösamlen1909), Sisenna canalis (Münster in Goldfuss, Reference Goldfuss1844) is most probably a junior synonym of Pleurotomaria helicinoides Roemer, Reference Roemer1836. In contrast, Wilson & Crick (Reference Wilson and Crick1889) maintained that P. helicinoides is a distinct species. The extremely poor information on this species prevents a safe relationship being established with S. canalis.
Distribution of the species. Upper Pliensbachian, Swabia and Franconia (southern Germany), Yorkshire (NE England); uppermost Pliensbachian – lowermost Toarcian, Leicestershire (central England) and Somerset (SW England); lower Toarcian, Causses Basin (southern France).
Superfamily PTYCHOMPHALOIDEA Wenz, Reference Wenz and Schindewolf1938 Family PTYCHOMPHALIDAE Wenz, Reference Wenz and Schindewolf1938 Genus Angulomphalus Gründel, Reference Gründel2011b
Type-species. Helicina expansa Sowerby, Reference Sowerby1821a . Hettangian – lower Sinemurian (exact stratigraphical level unknown), Dorset (SW England).
Angulomphalus expansus (Sowerby, Reference Sowerby1821a ) Figure 4m–r
1821a Helicina expansa; Sowerby, p. 129, pl. 273, figs 1–3.
1821a Helicina solarioides; Sowerby, p. 129, pl. 273, fig. 4.
? 1821b Helicina polita; Sowerby, p. 153, pl. 285.
? 1831 Turbo callosus nobis; Deshayes, p. 189, pl. 4, figs 5, 6.
1832 Helicina expansa Sowerby, Zieten, p. 45, pl. 33, figs 5a–c.
? 1836 Rotella polita Sow.; Bronn, p. 389, pl. 21, fig. 2a–c.
1844 Rotella expansa Sow.; Goldfuss, p. 102, pl. 195, figs 8a–c, ?9a–c.
1846 Helicina polita Sowerby; Schmidt, p. 62, pl. 16, fig. 5a–c (as Helicina expansa).
1849 Pleurotomaria suturalis E.D.; Eudes-Deslongchamps, p. 147, pl. 17, fig. 3a–d.
1849 Cochlicarina expansa Sowerby; Brown, p. 99, pl. 47, figs 1, 2.
1849 Cochlicarina solarioides Sowerby; Brown, p. 99, pl. 47, figs 3, 4.
? 1849 Cochlicarina polita Sowerby; Brown, p. 100, pl. 47, figs 5, 6.
1853 Pleurotomaria expansa Sow.; Chapuis & Dewalque, p. 97.
1853 Pleurotomaria expansa var. solarioides; Chapuis & Dewalque, p. 98, pl. 13, fig. 3a–d.
1853 Pleurotomaria expansa var. expansa; Chapuis & Dewalque, p. 99, pl. 13, fig. 3e–h.
? 1854 Pleurotomaria expansa Sowerby; d’Orbigny, p. 413, pl. 352, figs 1–4.
1856 Helicina expansa Sw.; Quenstedt, p. 153, 193, pl. 19, fig. 15, 16, pl. 24, fig. 19.
? 1856 Helicina expansa plicata; Quenstedt, p. 193, pl. 23, fig. 34.
1861 Pleurotomaria expansa Sow.; Stoliczka, p. 185, pl. 3, fig. 16a, b.
1869 Pleurotomaria expansa (Sowerby); Dumortier, p. 113, pl. 18, figs 11, 12.
1874 Pleurotomaria expansa Sow.; Gemmellaro, p. 114, pl. 13, fig. 20a, b.
1876 Cryptaenia solarioides Sowerby; Tate in Tate & Blake, p. 335, pl. 10, fig. 2a, b.
1876 Cryptaenia consobrina spec.nov.; Tate in Tate & Blake, p. 335, pl. 10, fig. 22a, b.
1882 Pleurotomaria expansa Sow.; Quenstedt, p. 331, pl. 197, figs 54, 55, ?56, 57–59, ?61–66.
? 1882 Pleurotomaria polita Sow.; Quenstedt, p. 332, pl. 197, fig. 60.
1888 Pleurotomaria (Cryptaenia) expansa Sowerby; Moberg, p. 60, pl. 2, figs ?32, 33–35.
1894 Pleurotomaria (Cryptaenia) expansa Sow.; Parona, p. 174, pl. 7, figs 7a–c.
1901 Cryptaenia aperta Burckhardt; Schlosser, p. 533, pl. 16, figs 20, 24.
1907 Cryptaenia expansa Sowerby; Sieberer, p. 25, pl. 1, fig. 5a–c.
? 1907 Cryptaenia nodosa nov. spec.; Sieberer, p. 26, pl. 1, fig. 7a–c.
1908 Ptychomphalus expansus (Sow.); Cossmann, p. 64, pl. 2, figs 25–27.
1909 Cryptaenia expansa Sow.; Dal Piaz, p. 7, pl. without number, fig. 7a–c.
1911 Cryptaenia expansa Sow.; Gemmellaro, p. 214, pl. 10, figs 13–16.
1911 Cryptaenia expansa var. subtilistriata; Gemmellaro, p. 215, pl. 10, figs 17–19.
1936 Ptychomphalus cirroidens Young & Bird; Kuhn, p. 280, pl. 8, figs ?2, 7, pl. 9, fig. 24a, b, pl. 12, fig. 25a, b.
1937 Cryptaenia expansa Sow.; Pčelincev, p. 24, pl. 1, fig. 25.
1964 Ptychomphalus expansus (Sow.); Sacchi Vialli, p. 3, pl. 1, fig. 1a, b.
1966 Cryptaenia expansa (Sowerby); Bourrouilh, p. 43, fig. 16a, b.
non 1980 Ptychomphalus expansus (Sowerby); Szabó, p. 55, pl. 1, fig. 9 ( = Ptychomphalus kericserensis Szabó, Reference Szabó2009).
? 1991 Ptychomphalus cfr. expansus (Sowerby); Conti & Monari, p. 262, pl. 7, fig. 15.
1997 Ptychomphalus expansus (Sowerby); Fischer & Weber, p. 160, pl. 26, figs ?1a, b, 2, 3.
1998 Ptychomphalus expansus (Sowerby); Gründel & Nützel, p. 66, pl. 1, figs 6–9.
2007 Ptychomphalus expansus (Sowerby); Conti et al., pl. 12, fig. 20a, b.
2008 Ptychomphalus expansus (Sowerby); Nützel, p. 45, pl. 1, fig. 5.
2008 Ptychomphalus expansus (Sowerby); Schubert et al., p. 20, fig. 2A–H.
2009 Ptychomphalus expansus (Sowerby); Szabó, p. 23, fig. 17A–D.
2011b Angulomphalus expansus (Sowerby); Gründel, p. 62, pl. 2, figs 4–7.
Material. One specimen: MNHNL QH619, Tournadous, upper Pliensbachian, Margaritatus Zone, Gibbosus Subzone.
Description. Shell sublenticular-discoidal, composed of six whorls. Apical spire turbiniform, slightly coeloconoid. Teleoconch whorls initially strongly and evenly convex with impressed suture, then progressively less convex, becoming flat or very slightly convex. Selenizone rather prominent and coinciding with peripheral bulge. Selenizone near abapical suture on early whorl, partially or completely covered by suture on adult whorls, except for final half of last whorl. Base only partially preserved, seemingly strongly convex and swollen. Regular pattern of very thin and dense spiral threads on whorl surface including peripheral bulge and selenizone. About 20 threads on surface of last whorl and others visible on preserved parts of base. Selenizone bordered by marginal threads slightly more prominent than those of whorl surface, with central spiral thread coinciding with shallow, obscure angulation of peripheral bulge. Lunulae scarcely visible, seemingly very thin and irregularly sized. Growth lines prosocline and prosocyrt on whorl surface and opisthocline and prosocyrt on peripheral region of base.
Remarks. The specimen is damaged by compaction affecting mainly the base and the last whorl. Helicina expansa Sowerby, Reference Sowerby1821a has been recently chosen by Gründel (Reference Gründel2011b ) as the type species of his genus Angulomphalus Gründel, Reference Gründel2011b . The species is among the most frequently recorded Lower Jurassic gastropods. It shows a stratigraphical, palaeogeographic and palaeoenvironmental distribution exceptionally wide for a benthic taxon. Its stratigraphical range corresponds to the Hettangian – upper Pliensbachian interval. Caswell, Coe & Cohen (Reference Caswell, Coe and Cohen2009) also listed the species in the lower Toarcian deposits (Falciferum Zone) of Yorkshire (NE England). The palaeogeographic distribution encompasses the whole western Tethys, from the European epicontinental shelf to the North African margin. It is associated with a range of very different facies, occurring in shallow-water mixed calcareous-terrigenous deposits, pelagic and carbonate platform sediments.
Most authors highlighted the wide morphological variability of A. expansus. Some of them instituted also different varieties (e.g. Chapuis & Dewalque, Reference Chapuis and Dewalque1853; Quenstedt, Reference Quenstedt1856; Gemmellaro, Reference Gemmellaro1911) or recognized different species (Sowerby, Reference Sowerby1821a ; Tate in Tate & Blake, Reference Tate and Blake1876; Schlosser, Reference Schlosser1901; Sieberer, Reference Sieberer1907; Szabó, Reference Szabó2009). The variability concerns several characters. Variations in the height of the spire and in the convexity of the base give rise to discoidal shells with swollen base to lenticular, symmetrical shells with the base almost as high as the spire. The surface of the last whorl commonly tends to become concave but more rarely it keeps its convexity up to the last peristome. The prominence and shape of the peripheral keel, and consequently of the selenizone, are also variable. In most cases the selenizone is convex and cord-like. In some shells it corresponds to a spiral groove edged by sharp threads within a somewhat prominent peripheral keel. The subsutural bulge is variably marked, reflecting the degree of overlap of the suture on the periphery of the previous whorl and the prominence of the periphery itself. It can be smooth or provided with nodes that commonly disappear in the fully adult whorls. These nodes vary from evenly spaced and sized tubercles to very irregularly distributed and sized subsutural wrinkles. The basal callus filling the umbilicus varies from thick and swollen to relatively thin and flat. In the latter case, it often draws an axial depression which reflects the presence of a wide umbilicus. Finally, the shell can be smooth or ornamented by sharp and dense spiral lines or threads.
Although several authors (e.g. Schubert, Gründel & Nützel, Reference Schubert, Gründel and Nützel2008; Szabó, Reference Szabó2009) did not exclude the possibility that A. expansus represents different species and suggested its revision, the analysis of the available literature does not evidence clear discontinuities mainly because the variable morphological characters are largely independent of each other. The only useful macroscopical tract could be represented by the presence/absence of subsutural nodes. Nodes were described and well illustrated by numerous authors (Deshayes, Reference Deshayes1831; Bronn, Reference Bronn1836; Goldfuss, Reference Goldfuss1844; d’Orbigny, Reference Orbigny1854; Quenstedt, Reference Quenstedt1856, Reference Quenstedt1882; Moberg, Reference Moberg1888; Sieberer, Reference Sieberer1907; Kuhn, Reference Kuhn1936; Fischer & Weber, Reference Fischer and Weber1997) and their presence was used by Quenstedt to distinguish the variety plicata. Sieberer (Reference Sieberer1907) erected a new species naming it as Cryptaenia nodosa Sieberer, Reference Sieberer1907. Gründel (Reference Gründel2011b ) synonymized these taxa under the name Angulomphalus plicatus (Quenstedt, Reference Quenstedt1856). Szabó (Reference Szabó2009) also maintained that the presence of nodes, together with the shape of the selenizone, could be useful to distinguish distinct species. However, when revising the rather rich material of d’Orbigny's collection, Fischer & Weber (Reference Fischer and Weber1997) found a continuity between smooth and nodose shells of A. expansus. As a matter of fact, nodes are the only distinctive character in otherwise indistinguishable shells. The question remains open and the extensive synonymy list compiled here represents a contribution for a future review.
Distribution of the species. Undifferentiated Hettangian – lower Sinemurian, Dorset (SW England); uppermost Hettangian – lowermost Sinemurian, Yorkshire (NE England); undifferentiated Hettangian–Sinemurian, southern Belgium; undifferentiated Sinemurian, NW Lombardian Basin (northern Italy); undifferentiated Sinemurian–Pliensbachian, SE Scania (south Sweden); upper Sinemurian, Caucasus (southern Russia), Northern Calcareous Alps (Austria) and eastern High Atlas (Morocco); undifferentiated Pliensbachian, Trento Plateau (Southern Alps, Italy); lower Pliensbachian, Vendée (western France), Saone-et-Loire (eastern France), eastern Sicily (Calabrian Arc, southern Italy) and Middle Atlas (Morocco); upper Pliensbachian, Yorkshire (NE England), Franconia and Swabia (southern Germany), Herford and Lower Saxony (northern Germany), Calvados (northern France), Causses Basin (southern France); upper? Pliensbachian, Sicani Mountains (western Sicily).
Superfamily PLEUROTOMARIOIDEA Swainson, Reference Swainson1840 Family PLEUROTOMARIIDAE Swainson, Reference Swainson1840 Genus Pleurotomaria Defrance, Reference Defrance and Cuvier1826
Type species. Trochus anglicus Sowerby, Reference Sowerby1818. Upper Pliensbachian, Somerset (SW England).
Pleurotomaria amalthei Quenstedt, Reference Quenstedt1856 Figure 5a–d

Figure 5. Pleurotomarioidea. (a–d) Pleurotomaria amalthei Quenstedt, Reference Quenstedt1856: dorsal, apical and apertural views, and detail of the whorl surface, UBGD 278805, Tournadous, upper Pliensbachian, Margaritatus Zone, Gibbosus Subzone. (e–h) Pleurotomaria escheri Goldfuss, Reference Goldfuss1844: dorsal, apical and subapertural views, and detail of the whorl surface, MNHNL QH581, Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone. (i–q) Bathrotomaria kronzwilmesorum sp. nov.: subapertural and apical view, detail of the early spire, dorsal, basal and lateral views, details of the fourth whorl, apex and last whorl, holotype MNHNL QH620, Tournadous, lower Toarcian, Bifrons Zone, Bifrons Subzone.
1844 Pleurotomaria anglica (Sowerby); Goldfuss, p. 69 pl. 184, fig. 8.
1854 Pleurotomaria anglica Goldfuss; Oppel, p. 99, pl. 3, fig. 15.
1856 Pleurotomaria amalthei Quenstedt, p. 191, pl. 23, figs 31, 32.
1882 Pleurotomaria amalthei Quenstedt; Quenstedt, p. 352, pl. 198, figs 48–50.
1907 Pleurotomaria amalthei Quenstedt; Sieberer, p. 17, text-fig. 2, pl. 2, fig. 1.
1907 Pleurotomaria amalthei var. elegans Sieberer; p. 19, pl. 1, fig. 4.
1936 Pleurotomaria amalthei Quenstedt; Kuhn, p. 273, pl. 8, fig. 13.
1998 Pleurotomaria amalthei Quenstedt; Gründel & Nützel, p. 64, pl. 1, figs 1, 2.
2008 Pleurotomaria amalthei Quenstedt; Nützel, p. 41, pl. 1, figs 1, 2.
2010 Pleurotomaria sp.; Lindström & Peel, p. 542, fig. 1C, F.
Material. One specimen: UBGD 278805, Tournadous, upper Pliensbachian, Margaritatus Zone, Gibbosus Subzone.
Dimensions. See Table 1.
Description. Shell trochiform and gradate, with rather high whorls. First teleoconch whorls strongly and evenly convex with impressed sutures. Fully adult whorls bearing quite obtuse angulation slightly above mid-line. Ramp quite inclined and slightly convex to almost flat. Outer face feebly convex and wider than ramp. Selenizone flat, slightly below the shoulder on early whorls, slightly above the middle of outer face on adult whorls. Selenizone initially very wide, covering slightly less than half of outer face, then narrowing to slightly less than one fourth of outer face. Periphery subangulated and with suture running on it. Base of last preserved whorl rather flat and with moderately wide umbilicus. Surface of teleoconch ornamented by sharp and densely packed growth threads, making the shell surface rough, gently waving the spiral ornament and forming granulations at intersection points. Ramp ornamented initially by one median spiral thread, then by up to three widely spaced and sharp threads. Shoulder bearing sharp and strong spiral cord with slightly pointed and widely spaced, spirally elongated nodes. Nodes more prominent on adult shell, about 20 on penultimate whorl. Outer face of early whorls bearing two sharp spiral threads delimiting selenizone and an equally sized spiral thread running between selenizone and abapical suture. Secondary spiral thread appearing during growth above and below selenizone. Suprasutural spiral thread with numerous, axially elongate nodes, less spaced than those of shoulder and forming short, shallow collabral rib-like swellings on periphery of last whorl. Selenizone ornamented by sharp, densely and evenly spaced lunulae and by median lira on adult whorls. On last whorls, median lira stronger than spiral threads delimiting selenizone. Peripheral band of base ornamented by strong spiral threads waved by collabral swellings. Growth lines slightly prosocline and feebly prosocyrt on ramp, more strongly prosocyrt on shoulder above selenizone, evenly opisthocyrt on selenizone, orthocline or feebly opisthocline and distinctly prosocyrt below selenizone and on peripheral band.
Remarks. Although poorly preserved, the specimen shows the shell shape and ornament characters typical of Pleurotomaria amalthei Quenstedt, Reference Quenstedt1856. These are the somewhat narrow and strongly sloping ramp, the rather wide selenizone, and the spiral cord on shoulder more prominent than the other spiral elements of the shell. The specimen from upper Pliensbachian strata of Dorset (SW England) illustrated by Lindström & Peel (Reference Lindström and Peel2010, fig. 1C, F) as Pleurotomaria sp. is considered here as belonging to P. amalthei, although it shows less prominent peripheral nodes.
Distribution of the species. Upper Pliensbachian, Swabia and Franconia (southern Germany), Dorset (SW England) and Causses Basin (southern France).
Pleurotomaria escheri Goldfuss, Reference Goldfuss1844 Figure 5e–h
1844 Pleurotomaria Escheri nobis; Goldfuss, p. 70, pl. 184, fig. 9a, b.
? 1844 Pleurotomaria tuberculato-costata Münster; Münster in Goldfuss, p. 70, pl. 184, fig. 10.
1844 Pleurotomaria Studeri Münster; Münster in Goldfuss, p. 70, pl. 184, fig. 11.
1907 Pleurotomaria Escheri Münster; Sieberer, p. 20, pl. 1, fig. 11a, b.
1935 Pleurotomaria studeri Münster; Kuhn, p. 128, pl. 9, fig. 19, pl. 10, figs 6, 34.
non 1964 Pleurotomaria escheri Goldfuss; Sacchi Vialli, p. 5, pl. 1, figs 5, 6.
2009 Pleurotomaria escheri Goldfuss; Schulbert & Nützel, p. 477, fig. 2A–C.
2013 Pleurotomaria escheri Goldfuss; Schulbert & Nützel, p. 728, fig. 5A–C.
Material. One specimen: MNHNL QH581, Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone.
Dimensions. See Table 1.
Description. Shell gradate, with somewhat high spire composed of strongly convex whorls separated by impressed sutures. Whorls subangulated by rounded, nodose shoulder. Ramp slightly inclined and rather narrow, about one-third of outer face. Outer face slightly and evenly convex. Selenizone flat, rather wide, placed at middle of outer face and delimited by two sharp spiral threads. Base and aperture not preserved. Ornament composed of spiral threads and collabral ribs. Ramp of last preserved whorls ornamented by 4 strong and evenly spaced spiral threads and about 16 strong and rounded collabral ribs forming weakly swollen and barely defined nodes on shoulder. Two stronger spiral threads running on shoulder. Periphery seemingly marked by a prominent and swollen bulge, limited below by suture. Selenizone with median lira and numerous, sharp and regularly spaced lunulae. Growth lines forming thin, sharp and evenly spaced threads on early shell, changing to packed and sharp striae on fully adult shell. Growth lines prosocline and feebly prosocyrt on ramp, opisthocline between selenizone and abapical suture.
Remarks. Although poorly preserved, the specimen shows the distinctive characters of Pleurotomaria escheri Goldfuss, Reference Goldfuss1844. They consist of a somewhat high, gradate early shell with strongly convex whorls, a rather narrow ramp ornamented by widely spaced, prominent and rounded ribs, barely defined nodes at the shoulder formed by a weak swelling of the collabral ribs and a rather wide selenizone. Due to the poor preservation, the peripheral bulge, which is another distinctive character of the species, is scarcely visible in the specimen described here. The type figured by Goldfuss (Reference Goldfuss1844) differs only in a slightly higher number of both collabral ribs and spiral threads on the ramp. Pleurotomaria tuberculatocostata Münster in Goldfuss, Reference Goldfuss1844, a poorly documented species from the lower Pliensbachian of Franconia (southern Germany) (Schlosser, Reference Schlosser1901; Trauth, Reference Trauth1908), is seemingly distinguished from P. escheri only by having a less prominent peripheral bulge.
Distribution of the species. Upper Pliensbachian, Swabia (southern Germany); upper Toarcian, Causses basin (southern France); upper Toarcian and lower Aalenian, Franconia (southern Germany).
Genus Bathrotomaria Cox, Reference Cox1956
Type species. Trochus reticulatus Sowerby, Reference Sowerby1821a . Kimmeridgian, Dorset (SW England).
Bathrotomaria kronzwilmesorum sp. nov. Figure 5i–q
Etymology. From Guy Kronz and his wife Liette Wilmes, scientific collaborators of the National Museum of Natural History of Luxembourg, who collected the material.
Holotype. MNHNL QH620 (Fig. 5i–q).
Type locality. Tournadous, Causses region (southern France).
Type level. Marnes de Fontaneilles Formation, early Toarcian, Bifrons Zone, Bifrons Subzone.
Material. A single specimen (holotype), MNHNL QH620.
Dimensions. See Table 1.
Diagnosis. Turbiniform earliest teleoconch with strongly convex whorls and prominent suprasutural cord. Distinctly gradate fully adult shell with whorls having a convex ramp and concave outer face. Ramp twice the width of the outer face. Selenizone of early teleoconch whorls wide, concave to flat and adjacent to the suprasutural cord. Selenizone of fully adult whorls corresponding to the angulation of the whorl surface and to a wide, very prominent spiral keel ornamented by thin spiral threads. Periphery of the fully adult shell marked by a swollen spiral bulge. Base anomphalous, with a deep axial cavity encircled by a sharp spiral keel. Early teleoconch ornamented by a sharp and regular network of spiral threads and collabral riblets. Fully adult whorls and base ornamented by marked spiral threads.
Description. Shell medium-sized, trochiform, composed of about eight whorls. Earliest spire turbiniform and slightly cyrtoconoid. Fully adult shell gradate and weakly coeloconoid. Protoconch with slightly depressed nucleus and composed of one and half, rounded and seemingly smooth volutions. First three teleoconch whorls strongly convex with somewhat impressed suture and cord-like periphery. Succeeding whorl angulated slightly above peripheral cord. Angulation more and more distinct during growth, shifting slightly abapically, and separating wide, oblique and convex ramp from narrow, concave and almost vertical outer face. Ramp about twice the width of outer face. Periphery quite prominent and bulged, partially covered by suture. Selenizone wide, concave and adjacent to peripheral cord on first teleoconch whorl, flat on subsequent whorl. Selenizone on adult shell corresponding to shoulder of whorl surface, forming wide, rounded and quite prominent spiral cord. Base low, with slightly convex surface, anomphalous and with deep axial cavity (pseudoumbilicus). Aperture subtrapezoidal with discontinuous peristome on parietal lip. Inner lip very stout, oblique and gently arched, reinforced by slightly outward reflected callus. Parietal lip covered by thin shell layer in continuity with columellar callus and extended to sutural corner. Outer lip sharp. Slit seemingly extended about one-quarter of last whorl. Ornament of early teleoconch consisting of a regular network of equally sharp spiral threads and collabral riblets with small granules at intersection points. Spiral threads increasing in number during growth; three to four on second teleoconch whorl and about eight on ramp of antepenultimate whorl. Spiral ornament becoming progressively dominant on last two whorls; collabral riblets persisting only as punctuations within thin interspaces of spiral threads. Last whorl ornamented by about 15 spiral threads on ramp and half a dozen on outer face. Few spiral threads on peripheral bulge, wider than those of outer face. Selenizone lacking spiral ornament at beginning of teleoconch and with sharp and evenly spaced lunulae. Selenizone having one median lira on second whorl. Lunule fading during growth. Spiral threads on selenizone increasing in number up to half a dozen on last whorl. Base sculptured by spiral threads progressively thicker towards axial region, some of them also duplicated. Axial cavity delimited by sharp and prominent spiral keel. Growth lines prosocline and widely prosocyrt on ramp, opisthocline and prosocyrt on outer face and on peripheral bulge, forming irregular wrinkles and distinctly sinuous on base, i.e. opisthocyrt on its abaxial half and prosocyrt on adaxial half.
Remarks. The single specimen is in a very good state of preservation. Bathrotomaria kronzwilmesorum sp. nov. is reminiscent of Bathrotomaria? turgidula (Eudes-Deslongchamps, Reference Eudes-Deslongchamps1849) (p. 125, pl. 10, fig. 4; d’Orbigny, Reference Orbigny1855, p. 427, pl. 356, fig. 4; Fischer & Weber, Reference Fischer and Weber1997, p. 163) from the upper Pliensbachian deposits of Calvados (northern France), in having a wide and prominent selenizone, a wide and convex ramp, and a similar ornament pattern. The species from Calvados is poorly known. It was instituted as a variety of Pleurotomaria hyphanta Eudes-Deslongchamps, Reference Eudes-Deslongchamps1849 and subsequently raised to species rank by d’Orbigny (Reference Orbigny1850). According to Fischer & Weber (Reference Fischer and Weber1997) the type material is missing and the species has not been recorded since d’Orbigny (Reference Orbigny1855). The original illustration shows a fragmentary specimen preserving only the last whorl. It differs from B. kronzwilmesorum in having a convex outer face, a more convex ramp, a roundly angulated periphery lacking spiral bulge, and a more swollen base. Moreover, B.? turgidula has a small umbilicus (Eudes-Deslongchamps, Reference Eudes-Deslongchamps1849; d’Orbigny, Reference Orbigny1855) whereas B. kronzwilmesorum is anomphalous.
Bathrotomaria gaudryana (d’Orbigny, Reference Orbigny1855) (p. 447, pl. 364, figs 11, 12; Fischer & Weber, Reference Fischer and Weber1997, p. 169, pl. 33, fig. 1a–c) from early Toarcian deposits of Rhône Basin (southern France) differs from B. kronzwilmesorum in having a higher and narrowly phaneromphalous shell, with a flat outer face and ramp. Moreover, it shows a finer ornament and a thinner selenizone which is also higher on the whorl surface. Pleurotomaria subtilis Münster in Goldfuss, Reference Goldfuss1844 (p. 71, pl. 185, fig. 4; Kuhn, Reference Kuhn1935, p. 129, pl. 9, fig. 27, pl. 10, figs 15, 41) from lower Aalenian deposits of Franconia (souther Germany) differs from B. kronzwilmesorum in having a wider apical angle and the outer face wider than the ramp. Moreover, the outer face is almost flat and oblique, and the selenizone is flush with a median lira and runs on the outer face. The specimen from lower Aalenian beds of Franconia described by Schulbert & Nützel (Reference Schulbert and Nützel2013, p. 726, fig. 4A–D) as Laevitomaria? cf. subtilis (Münster in Goldfuss, Reference Goldfuss1844) is distinguished from the species described here by having a flush selenizone ornamented by two spiral threads in the fully adult shell, a slightly less gradate shell and higher and less convex early teleoconch whorls.
Distribution of the species. Lower Toarcian, Causses Basin (southern France).
Superfamily TROCHOIDEA Rafinesque, Reference Rafinesque1815 Family NODODELPHINULIDAE Cox, Reference Cox, Moore and Pitrat1960 Genus Costatrochus Gründel, Reference Gründel, Nützel and Schulbert2009
Type species. Turbo subduplicatus d’Orbigny, Reference Orbigny1850. Lower Jurassic, Germany, stratigraphical level and locality uncertain (Gründel, Reference Gründel2009).
Costatrochus subduplicatus (d’Orbigny, Reference Orbigny1850) Figure 6a–w

Figure 6. (a–w) Costatrochus subduplicatus (d’Orbigny, Reference Orbigny1850): (a–b) dorsal and apertural views, MNHNL QH617; (c–e) dorsal, apertural and basal views, MNHNL QH618. Tournadous, lower Toarcian, Bifrons Zone; (f–h) basal, dorsal and apertural views, MNHNL QH595; (i–k) basal, dorsal and subapertural views, MNHNL QH597, Tournadous, upper Toarcian, Aalensis Zone, Mactra Subzone; (l–n) basal, dorsal and apertural views, MNHNL QH582; (o–q) apertural, basal and dorsal views, MNHNL QH584; (r–t) apertural, basal and dorsal views, MNHNL QH585; (u–w) sublateral, basal and dorsal views, MNHNL QH583, Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone.
1836 Trochus duplicatus Sow.; Bronn, 385, pl. 21, fig. 3a, b.
1844 Turbo duplicatus Sow.; Goldfuss, p. 95, pl. 179, fig. 2a–c.
1844 Turbo plicatus nobis; Goldfuss, p. 96, pl. 179, fig. 3.
1850 Turbo subduplicatus d’Orb.; d’Orbigny, p. 248.
1850 Turbo palinurus d’Orb.; d’Orbigny, p. 248.
1853 Turbo subduplicatus d’Orb.; d’Orbigny, p. 339, pl. 329, figs 1–6.
1856 Trochus duplicatus Goldfuss; Quenstedt, p. 314, pl. 43, figs 18, 19.
1882 Trochus duplicatus Sowerby; Quenstedt, p. 428, pl. 201, figs 120–122.
1894 Trochus subduplicatus, d’Orbigny; Hudleston, p. 375, pl. 31, figs 13, 14.
1909 Trochus subduplicatus Orbigny; Brösamlen, p. 211, pl. 17, figs 23, 24, pl. 18, fig. 1.
1935 Trochus subduplicatus d’Orb.; Kuhn, p. 133, pl. 9, fig. 35, pl. 10, fig. 1.
1935 Trochus subduplicatus d’Orb. var. münsteriana nov. var.; Kuhn, p. 133, pl. 10, fig. 2.
1966 Amphitrochus subduplicatus (d’Orbigny); Klöcker, p. 242, fig. 9.
1997 Amberleya subduplicata (d’Orbigny); Fischer & Weber, p. 135, pl. 21, figs 22, 23.
2001 Amphitrochus (Amphitrochus) subduplicatus (d’Orbigny); Fürsich et al., p. 176, fig. 4C.
2009 Amphitrochus subduplicatus (d’Orbigny); Schulbert & Nützel, p. 481, fig. 5A–D.
2009 Costatrochus subduplicatus var. subduplicatus (d’Orbigny); Gründel, p. 208, figs 2D, E–M, 4A–E, H, I.
2009 Costatrochus subduplicatus var. palinurus (d’Orbigny); Gründel, p. 211, fig. 2C, figs 3A–M, 4F, G.
2013 Costatrochus subduplicatus (d’Orbigny); Schulbert & Nützel, p. 735, fig. 10A–D.
Material. 179 specimens: MNHNL QH582, MNHNL QH583, MNHNL QH584, MNHNL QH585, MNHNL QH586, MNHNL QH587, MNHNL QH588, MNHNL QH589, MNHNL QH590, MNHNL QH591 (87 specimens), UBGD 278806 (10 specimens), UBGD 278807 (72 specimens), UBGD 278808, Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone. 23 specimens: MNHNL QH592, Cornus, upper Toarcian, Aalensis Zone, Lugdunensis Subzone. 7 specimens; UBGD 278809, Cornus, upper Toarcian, upper part of Aalensis Zone. 6 specimens: MNHNL QH593 (2 specimens), UBGD 278810 (2 specimens), UBGD 278815, UBGD 278816, Tournadous, lower Toarcian, Bifrons Zone, Bifrons Subzone. 4 specimens: MNHNL QH617, MNHNL QH618, UBGD 278811 (2 specimens), Tournadous, lower Toarcian, Bifrons Zone. 4 specimens: UBGD 278812, Tournadous, transition Bifrons Zone to Variabilis Zone. 4 specimens: MNHNL QH594, UBGD 278813 (3 specimens), Tournadous, upper Toarcian, condensed Thouarsense Zone to Dispansum Zone, Insigne Subzone. 3 specimens: UBGD 278814 (2 specimens), UBGD 278842, Tournadous, upper Toarcian, Dispansum Zone, Insigne Subzone. 4 specimens: UBGD 278817, Tournadous, upper Toarcian, Pseudoradiosa Zone. 16 specimens: MNHNL QH595, MNHNL QH596, MNHNL QH597, MNHNL QH598 (10 specimens), UBGD 278818 (3 specimens), Tournadous, upper Toarcian, Aalensis Zone, Mactra Subzone.
Dimensions. See Table 1.
Description. Shell trochiform, acute, with conical to slightly coeloconoid outline, composed of about eight moderately low whorls. Whorls width more than three times whorl height. Height of last whorl slightly more than half of shell height. Protoconch obtuse and seemingly depressed. Early teleoconch whorls distinctly concave, with strong peripheral bulge just above abapical suture. Adult whorls concave adapically, flat or feebly convex abapically. Base convex, swollen and anomphalous, with axial region narrowly depressed beside inner lip. Aperture subcircular, lying almost in one plane and moderately prosocline, discontinuous on parietal lip. Outer lip simple, angulated at periphery and evenly convex in basal part. Parietal lip covered by thin inductura. Columella stout. Inner lip with somewhat broad, comma-shaped and excavated outer columellar face limited by sharp outer rim. Callus covering partially or completely outer columellar face in fully adult shells. Two nodose spiral cords on first teleoconch whorls: first cord with small, collabrally elongate nodes just below adapical suture; second cord sharp, thicker and more prominent with spiny, very prominent nodes just above abapical suture. Whorl surface covered by about 10 fine, closely set spiral threads and by strongly prosocline, rather robust collabral ribs (20–25 per whorl) of constant thickness and regularly separated by slightly wider interspaces. Collabral ribs and spiral threads disappearing almost completely on adult whorls. Adapical cord changing into slightly elevated subsutural band on last whorls. Abapical cord changing into distinctly prominent peripheral bulge sculptured with rather strong and short collabral ribs crossed by 5–8 sharp and thin spiral threads with little nodes at intersections. Base with spiral threads of irregular strength and irregularly spaced, usually more marked abaxially. More or less prominent, sharp collabral wrinkles on axial region of base. Growth lines rather distinct, prosocyrt on subsutural band, then prosocline and gently opisthocyrt on whorl surface, reverting to prosocyrt on peripheral bulge. Growth lines sinuous on base, widely opisthocyrt abaxially and prosocyrt adaxially.
Remarks. Costatrochus subduplicatus (d’Orbigny, Reference Orbigny1850) has been widely reported and described, as evidenced by the synonymy list. Most authors remarked its high variability and Gründel (Reference Gründel2009) provided a detailed treatment to which the reader is referred. The material described here is more variable than that recorded by Gründel (Reference Gründel2009), especially in the spire angle. In the specimens with a wider spire angle, some sculptural elements, such as nodes of the cords and collabral wrinkles of the axial region of the base, are generally more pronounced.
Distribution of the species. Lower Toarcian, Burgundy (eastern France); lower and upper Toarcian, Causses Basin (southern France); upper Toarcian, Gloucestershire and Somerset (SW England); upper Toarcian and lower Aalenian, Lorraine and Alsace (eastern France), Swabia and Franconia (southern Germany).
Superfamily EUCYCLOIDEA Koken, Reference Koken1896 Family EUCYCLIDAE Koken, Reference Koken1896 Genus Eucyclus Eudes-Deslongchamps, Reference Eudes-Deslongchamps1860
Type species. Eucyclus obeliscus Eudes-Deslongchamps, Reference Eudes-Deslongchamps1860. Pliensbachian, Calvados (northern France).
Eucyclus escheri (Münster in Goldfuss, Reference Goldfuss1844) Figure 7a–e

Figure 7. Eucycloidea. (a–e) Eucyclus escheri (Münster in Goldfuss, Reference Goldfuss1844): (a–c) dorsal, apertural and basal views, MNHNL QH599; (d–e) dorsal and apertural views, MNHNL QH600, Tournadous, lower Toarcian, Bifrons Zone. (f–h) Eucyclus elegans (Münster in Goldfuss, Reference Goldfuss1844): dorsal, basal and apertural views, MNHNL QH601, Tournadous, lower Toarcian, Bifrons Zone. (i–k) Ooliticia? cyclostoma (Benz in Zieten, Reference Zieten1832) apertural, basal and dorsal views, UBGD 278819, Tournadous, upper Pliensbachian, Margaritatus Zone, Gibbosus Subzone. (l–s) Eucycloidea tenuistria (Münster in Goldfuss, Reference Goldfuss1844): (l–n) basal, dorsal and apertural views, MNHNL QH603; (o–p) dorsal and subapertural views, MNHNL QH604; (q–s) basal, apertural and dorsal views, UBGD 278820, Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone.
1844 Turbo escheri Münster; Goldfuss, p. 96, pl. 193, fig. 14.
1909 Eucyclus escheri Münster; Brösamlen, p. 259, pl. 20, fig. 11
1936 Eucyclus escheri Münster; Kuhn p. 290, pl. 9, fig. 25.
2008 Eucyclus ?escheri (Münster); Nützel, p. 45, pl. 1, fig. 7.
Material. Two specimens: MNHNL QH599, MNHNL QH600, Tournadous, lower Toarcian, Bifrons Zone.
Description. Shell moderately high-turbiniform. Early spire with slightly cyrtoconoid outline becoming pagodiform during growth. Periphery marked by sharp angulation running close to abapical suture on early teleoconch whorls and delimiting narrow suprasutural band. Ramp convex above periphery. Suprasutural band becoming progressively wider and forming outer face inclined in direction opposite to that of ramp. Ramp width about three times width of outer face. Lower edge of outer face marked by obtuse angulation less sharp than peripheral angulation and just hidden by suture. Ramp wide, feebly convex to almost flat and strongly sloping on fully adult whorls. Base strongly convex and anomphalous. Aperture elliptical and angulated at end of periphery on outer lip. Inner lip almost straight, forming rounded angle at junction with basal lip. Ornament mainly of densely and minutely nodose spiral cords. Cord on periphery more prominent and provided with larger nodes. Ramp ornamented by two, then three evenly sized and equally spaced, nodose spiral cords. Obtuse angulation at lower edge of outer face also marked by nodose cord less prominent than peripheral cord. Slightly nodose spiral thread seemingly present at middle of outer face. Base ornament by about 10 evenly sized and equally spaced, obscurely nodose spiral threads. Growth lines scarcely visible, seemingly prosocline and straight on whorl surface and widely prosocyrt on base.
Remarks. The shell wall of the specimens described here has been strongly altered by the recrystallization which exalted the most prominent features of the ornament, for example the primary spiral carinae and their nodes, and almost obliterated the finest sculptural elements. In spite of this, many of the distinctive characters of Eucyclus escheri (Münster in Goldfuss, Reference Goldfuss1844) are still recognizable. They include: the subpagodiform shell with conical early spire; the angulated periphery marked by a nodose carina running just besides the suture in the early spire and progressively further from the suture in the fully adult shell; the almost flat and wide ramp ornamented by two to three nodose spiral cords; and the base bearing numerous barely nodose spiral cords. The species is described by Münster (in Goldfuss, Reference Goldfuss1844) as having secondary spiral threads alternating with nodose spiral carinae. In the specimens here described the secondary ornament is not visible, most probably due to the poor preservation.
A number of coeval species shows close affinities with E. escheri. Eucyclus dunkeri (Goldfuss, Reference Goldfuss1844) (p. 95, pl. 193, fig. 11a, b; Kuhn, Reference Kuhn1936, p. 289, pl. 9, figs 30, 31; Gründel & Nützel, Reference Gründel and Nützel1998, p. 68, pl. 2, figs 6, 7; Nützel & Kiessling, Reference Nützel and Kiessling1997, p. 389, pl. 34, figs 1–4, as Eucyclus elegans), from upper Pliensbachian deposits of Franconia (southern Germany), differs from E. escheri in having a turbiniform shape due to more convex whorls. The adult whorls are ornamented by sharper, acute spiral carinae and by a dense pattern of thin growth riblets which form smaller and more packed nodes at the intersection points. Eucyclus gaudryanus (d’Orbigny, Reference Orbigny1853, p. 268, pl. 311, figs 4–7; Fischer & Weber, Reference Fischer and Weber1997, p. 105), from Pliensbachian sediments of Calvados (southern France), is similar in the shell shape and ornament pattern but the shell is almost twice as big and the carinate obtuse angulation below the peripheral keel is much less distinct. Eucyclus generalis (Münster in Goldfuss, Reference Goldfuss1844) (p. 98, pl. 194, fig. 4; Kuhn, Reference Kuhn1936, p. 291, pl. 9, fig. 50, pl. 12, fig. 23), a species from Pliensbachian deposits of Franconia (southern Germany), Turbo metis Münster in Goldfuss, Reference Goldfuss1844 (p. 96, pl. 193, fig. 13a, b) and Trochus cincinnus Moore, Reference Moore1866 (p. 207, pl. 4, figs 28, 29) from the Middle Lias of Somerset (SW England) might fall into the variability of E. escheri. The poor available information on these species prevents more detailed comparisons.
Distribution of the species. Lower and upper Pliensbachian, Franconia (southern Germany); lower Toarcian, Causses Basin (southern France); lower Aalenian, Swabia (southern Germany).
Eucyclus elegans (Münster in Goldfuss, Reference Goldfuss1844) Figure 7f–h
1844 Turbo elegans Münster; Münster in Goldfuss, p. 94, pl. 193, fig. 10a, b.
1844 Turbo venustus Münster; Münster in Goldfuss, p. 94, pl. 193, fig. 9a, b.
non 1876 Eucyclus elegans Münster; Tate in Tate & Blake, p. 346, pl. 9, fig. 30.
1909 Eucyclus elegans Münster; Brösamlen, p. 256, pl. 20, fig. 7a, b.
1936 Eucyclus elegans Münster; Kuhn, p. 288, pl. 9, fig. 32.
1936 Eucyclus venustus Münster; Kuhn, p. 288, pl. 9, figs 19, 20, 22, 33.
non 1997 Eucyclus elegans (Münster); Nützel & Kiessling, p. 389, pl. 34, figs 1–4.
1998 Eucyclus elegans (Münster); Gründel & Nützel, p. 67, pl. 2, figs 4, 5.
2008 Eucycloscala elegans (Münster); Schubert et al., p. 22, fig. 3J, K.
2008 Eucyclus elegans (Münster); Nützel, p. 45, pl. 1, fig. 6.
Material. Two specimens: MNHNL QH601, MNHNL QH602, Tournadous, lower Toarcian, Bifrons Zone.
Dimensions. See Table 1.
Description. Shell moderately high-turbiniform and pagodiform. About four teleoconch whorls observable, well rounded, slightly angulated at periphery and separated by impressed sutures. Periphery below middle of whorl surface. Base evenly convex and passing smoothly to whorl surface. Aperture elliptical and roundedly angulated at termination of periphery on outer lip. Ornament mainly of spiral elements. Peripheral subangulation marked by spiral thread. Surface of whorl ornamented by two widely and evenly spaced spiral threads above periphery and by one spiral thread below periphery next to suture. Thinner spiral thread visible on last whorl between peripheral and suprasutural threads. Base ornamented by half a dozen thinner and slightly less spaced spiral threads. Fine collabral threads forming granules at intersection with spiral ornament. Growth lines prosocline on spire and widely prosocyrt on base.
Remarks. The material is composed of inner moulds preserving traces of the shell that shows the ornament and the growth lines. The moderately high turbiniform shell, the strongly convex whorls, the roundedly angulated periphery and the ornament pattern correspond well to those of Eucyclus elegans (Münster in Goldfuss, Reference Goldfuss1844). The specimen figured by Tate (in Tate & Blake, Reference Tate and Blake1876) as E. elegans, from the latest Hettangian to earliest Sinemurian of Yorkshire (NE England), has a more acute, slender spire and less convex whorls with a more angulated periphery. The periphery is lower on the whorl surface and the spiral ornament is stronger.
The species was recorded by previous authors in upper Pliensbachian sediments. The material described here extends its stratigraphical distribution to lower Toarcian deposits.
Distribution of the species. Upper Pliensbachian, Franconia and Swabia (southern Germany), Herford (NW Germany); lower Toarcian, Causses Basin (southern France).
Genus Ooliticia Cossmann, Reference Cossmann1894
Type species. Turbo phillipsi Morris & Lycett, Reference Morris and Lycett1851. Bathonian, Yorkshire (NE England).
Ooliticia? cf. cyclostoma (Benz in Zieten, Reference Zieten1832) Figure 7i–k
cf. 1832 Turbo cyclostoma Benz; Benz in Zieten, p. 45, pl. 33, fig. 4a, b.
cf. 1852 Turbo cyclostoma Ziet.; Quenstedt, p. 420, pl. 33, fig. 35.
cf. 1857 Turbo cyclostoma Zieten; Dumortier, p. 213, pl. 10, figs 2, 2a, b.
? 1887 Amberleya callipyge, spec. nov.; Wilson, p. 8, pl. 5, figs 7, 7a.
cf. 1908 Littorina? chartroni nov. sp.; Cossmann, p. 56, pl. 2, figs 5, 6.
cf. 1909 Turbo cyclostoma Benz; Brösamlen, p. 230, pl. 19, fig. 2 (cum syn.).
cf. 1916 Ooliticia chartroni (Cossmann); Cossmann: 126, pl. 4, figs 1, 2.
cf. 1936 Turbo cyclostoma Benz; Kuhn, p. 286, pl. 8, fig. 9.
cf. 2008 Ooliticia? cyclostoma (Benz); Schubert et al., p. 23, figs 3L, M, 4A–C.
cf. 2011 Ooliticia? cyclostoma (Benz); Gründel et al., p. 489, fig. 6G, H.
Material. One specimen: UBGD 278819, Tournadous, upper Pliensbachian, Margaritatus Zone, Gibbosus Subzone.
Dimensions. See Table 1.
Description. Shell turbiniform, presumably composed of half a dozen whorls. Height of last whorl about three-quarters of shell height. Whorls strongly convex and with rounded periphery above suture, approximately at lower third of whorl surface. Base convex and passing smoothly to whorl surface. Aperture drop-shaped, pointed at sutural corner. Inner lip slightly arched and elongated downwards at junction with basal lip, perhaps forming a short outlet. Spiral ornament of about seven evenly spaced cord-like threads. Lowermost thread thicker and more prominent coinciding with suture. Spiral ornament superimposed to weak, slightly prosocline and feebly prosocyrt collabral wrinkles somewhat irregular in size and distribution. Two spiral threads visible on peripheral band of base.
Remarks. The material is represented by an inner mould reproducing the main characters of the ornament. These characters and the shape of the shell are comparable with those of Ooliticia? cyclostoma (Benz in Zieten, Reference Zieten1832). The species and its genus position have been discussed by Schubert, Gründel & Nützel (Reference Schubert, Gründel and Nützel2008), to which reference may be made for details. Analysis of the literature reveals a somewhat high variation of the height of the spire and of the density and strength of the spiral ornament. The specimen described here approaches better those having a low spire and less dense spiral ornament, such as the shells figured by Quenstedt (Reference Quenstedt1856, pl. 19, fig. 27 right side), Tate (in Tate & Blake, Reference Tate and Blake1876, pl. 9, fig. 20), and Schubert, Gründel & Nützel (Reference Schubert, Gründel and Nützel2008, fig. 4A, B).
The relationship of synonymy between Turbo cyclostoma and Phasianella paludinaeformis Schübler in Zieten, Reference Zieten1832 (p. 40, pl. 30, figs 12, 13) has been underlined by several authors (Quenstedt, Reference Quenstedt1843, Reference Quenstedt1856, Reference Quenstedt1882; Oppel, Reference Oppel1856–58; Brösamlen, Reference Brösamlen1909). Both species were introduced in the same year and in the same monograph. The first reviser of the species was Quenstedt (Reference Quenstedt1843, p. 551) who explicitly mentioned the synonymy and fixed the name cyclostoma (ICZN, 1999, art. 24.2).
In agreement with Gründel et al. (Reference Gründel, Kaim, Nützel and Little2011), Ooliticia chartroni (Cossmann, Reference Cossmann1908) from upper Pliensbachian deposits of Vendée (western France) is synonymous with O.? cyclostoma. Amberleya callipyge Wilson, Reference Wilson1887 from upper Pliensbachian beds of the Northamptonshire (central England) is also placed in synonymy with Benz's species by Gründel et al. (Reference Gründel, Kaim, Nützel and Little2011), but it shows a lower spire and a flatter whorl surface.
Distribution of the species. Ooliticia? cyclostoma occurs in upper Pliensbachian deposits of Franconia and Swabia (southern Germany), Herford (NW Germany), Yorkshire (NE England), Vendée (western France), Saône-et-Loire (eastern France), southern Belgium and possibly Causses Basin (southern France).
Genus Eucycloidea Hudleston, Reference Hudleston1888
Type species. Turbo bianor d’Orbigny, Reference Orbigny1850. Upper Bajocian, Calvados (northern France).
Eucycloidea tenuistria (Münster in Goldfuss, Reference Goldfuss1841) Figure 7l–s
1841 Rostellaria tenuistria Münster; Münster in Goldfuss, p. 16, pl. 169, fig. 9.
1841 Rostellaria nodosa Münster; Münster in Goldfuss, p. 16, pl. 169, fig. 10.
1844 Trochus sedgwicki Münster; Münster in Goldfuss, p. 53, pl. 179, fig. 4.
1844 Turbo subangulatus Münster; Münster in Goldfuss, p. 98, pl. 194, fig. 5.
1850 Turbo patroclus d’Orb.; d’Orbigny, p. 248.
1850 Turbo Hero d’Orb.; d’Orbigny, p. 266.
? 1853 Turbo sedgwickii Münster; d’Orbigny, p. 1853, p. 338, pl. 328, figs 9–11.
1853 Purpurina patroclus d’Orbigny; d’Orbigny, pl. 329, figs 9–11.
1856 Turbo subangulatus Quenstedt, p. 314, pl. 43, fig. 20.
1882 Turbo subangulatus Quenstedt, p. 429, pl. 202, figs 1–4.
1889 Nortonia (Purpurina) patroclus d’Orbigny; Wilson in Wilson & Crick, p. 299, pl. 9, fig. 1a, b.
1901 Amberleya tenuistria Münster; Schlosser, p. 543.
1906 Purpurina (Pseudalaria) patroclus d’Orbigny; Cossmann, p. 209, pl. 8, fig. 10.
1908 Turbo patroclus d’Orbigny; Thevenin, p. 190, pl. 14, figs 3, 4.
1909 Eucyclus subangulatus (Münster); Brösamlen, p. 258, pl. 20, fig. 10.
1913 Purpurina (Pseudalaria) patroclus d’Orbigny; Cossmann, p. 171, pl. 8, figs 27–30.
1935 Eucyclus subangulatus (Münster); Kuhn, p. 137, pl. 10, fig. 4.
1966 Amberleya (Eucyclus) tenuistria (Münster); Klöcker, p. 239, fig. 8.
? 1997 “Turbo” sp.; Fischer & Weber, p. 134.
1997 Eucycloidea (Pseudalaria) patroclus (d’Orbigny); Fischer & Weber, p. 135, pl. 24, figs 3a, b.
2001 Amberleya (Eucyclus) subimbricata (d’Orbigny); Fürsich et al., p. 176, fig. 4D.
2001 Eucycloidea (Pseudalaria)? subangulata (Münster); Conti & Monari, p. 198, figs 15.26–28.
2009 Eucyclus subangulatus (Münster); Schulbert & Nützel, p. 478, fig. 3.
2013 Eucycloidea tenuistria (Münster); Schulbert & Nützel, p. 731, fig. 8A–M.
Material. 7 specimens: MNHNL QH603, MNHNL QH604, MNHNL QH605 (2 specimens), UBGD 278820, UBGD 278821 (2 specimens), Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone. 2 specimens: MNHNL QH606, Cornus, upper Toarcian, Aalensis Zone, Lugdunensis Subzone. 1 specimen: UBGD 278824, Tournadous, lower Toarcian, Bifrons Zone, Bifrons Subzone. 8 specimens: MNHNL QH607 (3 specimens), UBGD 278822, UBGD 278823 (2 specimens), UBGD 278825, UBGD 278826, Tournadous, lower Toarcian, Bifrons Zone. 2 specimens: UBGD 278827, UBGD 278828, Tournadous, transition Bifrons Zone to Variabilis Zone. 5 specimens: MNHNL QH608, MNHNL QH609, MNHNL QH610, UBGD 278829 (2 specimens), Tournadous, upper Toarcian, condensed Thouarsense Zone to Dispansum Zone, Insigne Subzone. 4 specimens: UBGD 278830 (2 specimens), UBGD 278831 (2 specimens), Tournadous, upper Toarcian, Dispansum Zone, Insigne Subzone. 2 specimens UBGD 278832, Tournadous, upper Toarcian, Dispansum Zone. 5 specimens: UBGD 278833, Tournadous, upper Toarcian, Dispansum Zone, Gruneri Subzone. 1 specimen: UBGD 278834, Tournadous, upper Toarcian, Pseudoradiosa Zone.
Dimensions. See Table 1.
Description. Shell high-turbiniform to pagodiform, composed of 7–8 whorls. Height of last whorl about two-thirds of shell height. Whorls distinctly angulated and keeled. Peripheral keel a rather swollen spiral cord dividing whorl surface into wide and sloping ramp and narrower, concave outer face. Ramp width almost twice width of outer face, slightly convex on early teleoconch whorls, then concave. Outer face subvertical or slightly inclined abapically towards axis and limited abapically by second, less definite, keeled angulation bordering abapical suture or covered by it. Base convex, somewhat swollen. Aperture semicircular and discontinuous on parietal lip. Inner lip thick, almost straight and extended in its lower part to form outlet at junction with basal lip. Early teleoconch whorls ornamented by moderately strong, prosocline and slightly opisthocyrt collabral ribs starting at adapical suture and fading below peripheral keel. Ribs producing slightly pointed nodes at adapical suture and on keel (about 30 on third whorl). Sutural ramp and outer face covered by 5–6 spiral threads each, forming rather regular network with collabral ribs. Subsutural spiral thread slightly more robust but discontinuous. Small granules present at rib/thread intersections. Collabral ribs changing progressively into somewhat thin and sharp threads. Adult whorls bearing densely packed, very thin spiral threads, about 20 on ramp and about a dozen on outer face, separated by wider interspaces and also running over peripheral keel. Collabral threads forming small pointed nodes on keels and on subsutural spiral thread, and granules at intersections with other spiral threads. Nodes on peripheral keel larger than those on second angulation. Five very widely spaced, acute spiral cords on base between periphery and neck. Up to four more close-set and finer spiral cords on neck. Interspaces between cords showing same pattern of spiral and collabral threads as ramp and outer face. Growth lines prosocline and opisthocyrt on ramp, slightly prosocline to orthocline and gently prosocyrt on outer face, prosocyrt on base.
Remarks. Several authors (Schlosser, Reference Schlosser1901; Brösamlen, Reference Brösamlen1909; Klöcker, Reference Klöcker1966; Schulbert & Nützel, Reference Schulbert and Nützel2013) recognized the synonymy between Rostellaria tenuistria Münster in Goldfuss, Reference Goldfuss1841, Rostellaria nodosa Münster in Goldfuss, Reference Goldfuss1841, Trochus sedgwicki Münster in Goldfuss, Reference Goldfuss1844 and Turbo subangulatus Münster in Goldfuss, Reference Goldfuss1844 (see Klöcker, Reference Klöcker1966 and Schulbert & Nützel, Reference Schulbert and Nützel2013 for further details). According to the dates of publication of the different parts of Goldfuss’ monograph, the valid name of the species should be chosen between R. tenuistria and R. nodosa. In fact, both species were published in 1841 whereas T. sedgwiki and T. subangulatus were published three years later. The first reviser of the species was Schlosser (Reference Schlosser1901) who fixed the name tenuistria (ICZN, 1999, art. 24.2). As stated by Oppel (Reference Oppel1856–1858), Giebel (Reference Giebel1866) and Klöcker (Reference Klöcker1966), and suspected by Schlosser (Reference Schlosser1901), Brösamlen (Reference Brösamlen1909) and Conti & Monari (Reference Conti and Monari2001), Turbo patroclus d’Orbigny, Reference Orbigny1850 is another junior synonym of E. tenuistria. D’Orbigny (Reference Orbigny1853) ascribed to T. sedgwicki some specimens from Toarcian deposits in Alsace (eastern France). That material is lacking from the d’Orbigny collection and its species assignment cannot be verified (Fischer & Weber, Reference Fischer and Weber1997).
Distribution of the species. Undifferentiated Toarcian, Gard (southern France); lower Toarcian, Leicestershire (central England); lower–upper Toarcian, Causses Basin (southern France); upper Toarcian, Cher (central France); undifferentiated upper Toarcian – lower Aalenian and lower Aalenian, Franconia (southern Germany); lower Aalenian, Swabia (southern Germany); lower Bajocian, Central High Atlas (Morocco).
Superfamily NERITOPSOIDEA Gray, Reference Gray1847 Family NERITOPSIDAE Gray, Reference Gray1847 Genus Neritopsis Grateloup, Reference Grateloup1832
Type species. Neritopsis moniliformis Grateloup, Reference Grateloup1832. Lower Miocene, Aquitaine basin (western France).
Neritopsis philea d’Orbigny, Reference Orbigny1850 Figure 8a–g

Figure 8. (a–g) Neritopsis philea d’Orbigny, Reference Orbigny1851: lateral, basal, apical, lateral (opposite), apertural views, detail of the ornament and dorsal view, UBGD 278835, Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone.
1850 Neritopsis Philea d’Orb.; d’Orbigny, p. 247.
1852 Neritopsis Philea d’Orb. d’Orbigny, p. 222, pl. 300, figs 5–7.
non 1874 Neritopsis philea (d’Orbigny); Dumortier, p. 133, pl. 34, figs 8–10.
1894 Neritopsis philea d’Orbigny; Hudleston, p. 341, pl. 27, fig. 11a–c.
1909 Neritopsis opalina n.sp.; Brösamlen, p. 239, pl. 19, fig. 21a, b.
1997 Neritopsis philea d’Orbigny; Fischer & Weber, p. 85.
2013 Neritopsis opalina Brösamlen; Schulbert & Nützel, p. 737, fig. 11A–D.
Material. One specimen: UBGD 278835, Cornus, Upper Toarcian, Aalensis Zone, Mactra Subzone.
Dimensions. See Table 1.
Description. Shell with low but distinctly elevated spire and rather globose last whorl. Teleoconch preserving last two rapidly expanding whorls separated by impressed sutures. Whorls strongly convex and rounded at periphery. Subsutural part of whorl flat and forming ramp orthogonal to spire axis and delimited by obtuse, moderately sharp shoulder. Base strongly convex and swollen. Narrow groove, probably a false umbilicus, present on axial region of base and encircled by shallow spiral bulge. Aperture seemingly semicircular and peristome discontinuous on parietal lip. Outer lip not preserved. Inner lip sharp and gently arched, detached from surface of base. Ornament mainly of spiral threads. Ramp of penultimate whorl ornamented by six moderately strong spiral threads separated by somewhat narrow interspaces. Five–six widely spaced spiral threads intercalated by secondary spiral thread on rest of whorl. Third order of spiral threads appearing in the interspaces between the primary and the secondary threads on last whorl. Shell fully covered by dense and strong growth lines making surface rough and spiral ornament waved. Occasional thin collabral threads forming a sort of network with spiral threads. Most prominent growth lines forming also small, sparsely distributed tubercles at intersection with spiral ornament, especially on outer edge of ramp. Growth lines prosocline, gently and widely prosocyrt on whorl surface and on base, opisthocyrt on axial region of base.
Remarks. Neritopsis philea d’Orbigny, Reference Orbigny1851 is characterized by a flat subsutural ramp edged by an obtuse but sharp shoulder, a fully adult spiral ornament made of at least three orders of spiral threads progressively appearing during growth, and thin collabral threads or lines sparsely granulating the spiral threads. The same characters are present in Neritopsis opalina Brösamlen, Reference Brösamlen1909, a species originally instituted on a strongly fragmentary specimen and that is considered here as synonym of N. philea. This seems to be substantiated by the well-preserved specimen recently assigned by Schulbert & Nützel (Reference Schulbert and Nützel2013) to N. opalina which clearly shows the characters listed above. Rollier (Reference Rollier1918, p. 20) maintained that the material ascribed by Hudleston (Reference Hudleston1894) to N. philea belongs to a different species that he named Neritopsis abbas Rollier, Reference Rollier1918. Fischer & Weber (Reference Fischer and Weber1997) supported his opinion. However, Hudleston's specimen does not seem to show differences sufficient to justify a species distinction. In agreement with Fischer & Weber (Reference Fischer and Weber1997), the specimen coming from the Toarcian–Aalenian deposits of Isère (SE France) ascribed by Dumortier (Reference Dumortier1874) to N. philea most likely does not belong to that species because it lacks a flat subsutural ramp. The same difference distinguishes N. philea from Neritopsis spekei (Moore, Reference Moore1866) (p. 202, pl. 5, fig. 11), a species from the Toarcian deposits of Somerset (SW England).
Distribution of the species. Undifferentiated Toarcian, Burgundy (southern France); upper Toarcian, Causses Basin (southern France); lower Aalenian, Swabia and Franconia (southern Germany); upper Aalenian, Dorset (SW England).
Superfamily ZYGOPLEUROIDEA Wenz, Reference Wenz and Schindewolf1938 Family ZYGOPLEURIDAE Wenz, Reference Wenz and Schindewolf1938 Genus Katosira Koken, Reference Koken1892
Type species. Chemnitzia periniana d’Orbigny, Reference Orbigny1851. Pliensbachian, Saône-et-Loire (eastern France).
Katosira sp. Figure 9a, b

Figure 9. Zygopleuroidea, Cerithioidea and Ampullinoidea. (a–b) Katosira sp. apertural and dorsal views, MNHNL QH611, Tournadous, lower Toarcian, Bifrons Zone. (c–s) Procerithium pseudocostellatum (d’Orbigny, Reference Orbigny1850): (c–e) apertural, lateral and basal views, UBGD 278836; (f–h) basal, dorsal and apertural views, MNHNL QH612; (i–k) dorsal, apertural and lateral views, UBGD 278838; (l–o) detail of the ornament, dorsal, apertural and lateral views, UBGD 278837; (p–s) lateral, apertural, dorsal and basal views, UBGD 278839, Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone. (t–v) Cryptaulax armata (Goldfuss, Reference Goldfuss1844): (t–u) lateral and dorsal views, MNHNL QH614; (v) detail of the ornament, MNHNL QH615, Tournadous, lower Toarcian, Bifrons Zone, Bifrons Subzone. (w–z) Ampullospira pelops (d’Orbigny, Reference Orbigny1850): apical, apertural, basal and dorsal views, UBGD 278841, Tournadous, upper lower – upper Toarcian, exact stratigraphical level unknown.
Material. One specimen: MNHNL QH611, Tournadous, lower Toarcian, Bifrons Zone.
Dimensions. See Table 1.
Description. Shell high-spired, slightly cyrtoconoid, preserving last seven whorls. Whorls almost twice as wide as high, regularly convex, the convexity tending to attenuate slightly during growth. Suture distinctly inclined. Base anomphalous, slightly convex and seemingly encircled by rounded angulation. Aperture elliptical-ovate, angulated at sutural corner and apparently lacking siphonal outlet. Inner lip thin, gently arched and slightly detached from surface of base. Sparse shell remains with traces of about a dozen collabral ribs per whorl, roughly aligned to each other between adjacent whorls. Collabral ribs sharp, evenly spaced, slightly opisthocline and parasigmoidal, distinctly opisthocyrt on most of whorl surface and prosocyrt in its abapical band. Weak and narrow subsutural bulge (spiral thread?) on inner mould.
Remarks. The specimen is represented by an inner mould with sparse remains of the shell wall that does not consent a safe species attribution. It strongly resembles Scalaria liasica Quenstedt, Reference Quenstedt1852 (p. 418, pl. 33, fig. 27; Oppel, Reference Oppel1854, p. 98, pl. 3, figs 13, ?14; Quenstedt, Reference Quenstedt1856, p. 152, pl. 19, figs 5–12, Reference Quenstedt1882, p. 307, pl. 196, figs 66, 67, 69–73, ?68) from Pliensbachian deposits of Swabia and Franconia (southern Germany). Other similar species are Katosira carusensis (d’Orbigny, Reference Orbigny1850) (p. 226; d’Orbigny, Reference Orbigny1851, p. 34, pl. 237, figs 13–15; Fischer & Weber, Reference Fischer and Weber1997, p. 13, pl. 1, fig. 10) from lower Pliensbachian beds of Cher (central France) and Katosira corvaliana (d’Orbigny, Reference Orbigny1851) (p. 37, pl. 343, fig. 4; Fischer & Weber, Reference Fischer and Weber1997, p. 15, pl. 1, fig. 6a, b) from Pliensbachian levels of Saône-et-Loire (eastern France). Although Fischer & Weber (Reference Fischer and Weber1997) defined the collabral ribs of K. corvaliana as “orthocyrtes”, in the specimen described here they are parasigmoidal. Katosira? sp. described by Gründel (Reference Gründel2011a , p. 87, pl. 1, figs 5, 6) from the uppermost Pliensbachian deposits of the Franconian Jura differs in being bigger and in having a lower number of collabral ribs which are also more opisthocline.
Distribution of the species: Lower Toarcian, Causses Basin (southern France).
Superfamily CERITHIOIDEA Fleming, Reference Fleming1822 Family PROCERITHIIDAE Cossmann, Reference Cossmann1906 Genus Procerithium Cossmann in Chartron & Cossmann, Reference Chartron and Cossmann1902
Type species. Procerithium quinquegranosum Cossmann in Chartron & Cossmann, Reference Chartron and Cossmann1902. Hettangian, Vendée (western France).
Procerithium pseudocostellatum (d’Orbigny, Reference Orbigny1850) Figure 9c–s
? 1842b Melania undulata Var. b E.-D.; Eudes-Deslongchamps, p. 217, pl. 11, figs 59, 60, non figs 61, 62.
1844 Cerithium costellatum Münster; Münster in Goldfuss, p. 31, pl. 173, fig. 8.
1850 Cerithium pseudocostellatum d’Orb.; d’Orbigny, p. 250.
? 1850 Cerithium Jole d’Orb.; d’Orbigny, p. 250.
1874 Chemnitzia ferrea nov. sp.; Dumortier, p. 129, pl. 35, fig. 8.
1883 Cerithium costellatum Goldfuss; Quenstedt, p. 516, pl. 205, figs 40, 41.
? 1908 Cerithium Jole d’Orb.; Thevenin, p. 191, pl. 14, figs 7, 8.
1913 Procerithium (Rhabdocolpus) pseudocostellatum d’Orbigny; Cossmann, p. 71, pl. 3, figs 106–108.
? 1913 Procerithium (Rhabdocolpus) jole d’Orbigny; Cossmann, p. 71, pl. 4, figs 1, 18, 19.
1923 Cryptaulax subarmata sp. n.; Ernst, p. 70, pl. 1, fig. 20a, b.
? 1999a Procerithium compactum n.sp.; Gründel, p. 4, pl. 1, figs 7–9.
? 2009 Procerithium compactum Gründel; Schulbert & Nützel, p. 489, fig. 10.
? 2013 Procerithium compactum Gründel; Schulbert & Nützel, p. 742, fig. 14H–J.
Material. Six specimens: MNHNL QH612, MNHNL QH613, UBGD 278836, UBGD 278837, UBGD 278838, UBGD 278839, Cornus, upper Toarcian, Aalensis Zone, Mactra Subzone.
Dimensions. See Table 1.
Description. Shell turritelliform, slightly gradate and composed of about 10 whorls. Apical shell seemingly slightly coeloconoid, adult shell weakly cyrtoconoid. Whorls twice as wide as high. Height of last whorl slightly more than one-third of shell height. Whorls flat to very slightly convex, with narrow, scarcely defined ramp. Suture almost grooved. Base low-conical and slightly convex. Aperture not completely preserved. Inner lip seemingly slightly detached from surface of base. Ornament made by collabral ribs and spiral threads. Collabral ribs rather strong, weakly opisthocline, straight to gently opisthocyrt, about 12 per whorls on fully adult shell, separated by wider interspaces and roughly aligned to each other between adjacent whorls. Four–five equally spaced, quite marked spiral threads forming little nodes at intersection with collabral ribs. Subsutural spiral thread edging ramp and slightly more marked. Five–six evenly spaced and equally sized spiral threads visible on base. Growth lines slightly opisthocline and opisthocyrt on whorl surface, not observable on base.
Remarks. d’Orbigny (Reference Orbigny1850) replaced the name Cerithium costellatum Münster in Goldfuss, Reference Goldfuss1844 to Cerithium pseudocostellatum to avoid secondary homonymy with Nassa costellata Sowerby in Fitton, Reference Fitton1836, a species from Albian sediments of Blackdown Hills (SW England) that he considered as belonging to Cerithium. The type material of C. costellatum comes from Lower Jurassic deposits of Pretzfeld (Franconia). The main teleoconch characters of Procerithium pseudocostellatum (d’Orbigny, Reference Orbigny1850) are also present in Procerithium compactum Gründel, Reference Gründel1999a , a species from upper Toarcian – lower Aalenian deposits of Franconia (southern Germany). Gründel's species could be a synonym of P. pseudocostellatum, but the lack of information on the protoconch of the latter species does not allow the relationship between the two taxa to be examined.
Cryptaulax subarmata Ernst, Reference Ernst1923, which Gründel (Reference Gründel1999a ) tentatively included in the synonymy list of Cryptaulax armata (Goldfuss, Reference Goldfuss1844), does not seem to show differences sufficient to keep it distinct from P. pseudocostellatum. In contrast, as rightly established by Gründel (Reference Gründel1999a ), the material figured by Kuhn (Reference Kuhn1935, p. 144, pl. 8, fig. 6a, b) as C. subarmata belongs to Procerithium brandi (Walther, Reference Walther1951). That species differs from P. pseudocostellatum in having lower and more convex whorls, a slightly wider and sloping subsutural ramp and more numerous axial ribs per whorl (18–20) in the adult shell.
Procerithium jole (d’Orbigny, Reference Orbigny1850) is another possible synonym of P. pseudocostellatum. Cossmann (Reference Cossmann1913) also assigned to this species the specimen figured by Eudes-Deslongchamps (Reference Eudes-Deslongchamps1842b ) in pl. 11, fig. 59 as var. b of Melania undulata Eudes-Deslongchamps, Reference Eudes-Deslongchamps1842b . According to Cossmann (Reference Cossmann1913), P. jole differs from P. pseudocostellatum in having a smaller size, a less spiny subsutural spiral thread and an additional spiral thread on the whorl surface. Based on an analysis of the material studied here and in the literature, these differences could be accommodated within the morphological variability of P. pseudocostellatum.
Distribution of the species. Undifferentiated Toarcian, Franconia (southern Germany), Aude (southern France), Mont d’Or (central southern France), Alsace and Doubs (eastern France); upper Toarcian, Lower Saxony (northern Germany) and Causses Basin (southern France); lower Aalenian, Alsace (eastern France).
Genus Cryptaulax Tate, Reference Tate1869
Type species. Procerithium (Xystrella) protortile Cox, Reference Cox1969, nom. nov. pro Cerithium tortile Hébert & Eudes-Deslongchamps, Reference Hébert and Eudes-Deslongchamps1860 non Eudes-Deslongchamps, Reference Eudes-Deslongchamps1842a . Lower Callovian, Maine-et-Loire (western France).
Cryptaulax armata (Goldfuss, Reference Goldfuss1844) Figure 9t–v
1831 Turritella echinata Buch, pl. 7, fig. 1.
1836 Turritella echinata Buch; Bronn, p. 395, pl. 21, fig. 24.
1844 Cerithium armatum nobis; Goldfuss, p. 31, pl. 173, fig. 7.
1856 Cerithium armatum Goldfuss; Quenstedt, p. 315, pl. 43, fig. 22.
1883 Cerithium armatum Goldfuss; Quenstedt, p. 515, pl. 205, figs 37–39.
1887 Cerithium armatum Goldfuss; Denckmann, p. 83, pl. 9, figs 6, 6a.
1906 Cerithium (Xystrella) armatum Münster; Cossmann, p. 30, pl. 5, fig. 26.
1909 Cryptaulax armata Goldfuss; Brösamlen, p. 291, pl. 21, figs 27–29.
1913 Procerithium (Xystrella) armatum (Goldfuss); Cossmann, p. 85, pl. 4, figs 45–49, 51–53.
1935 Cryptaulax armata Goldfuss; Kuhn, p. 144, pl. 8, fig. 25a, b, pl. 9, fig. 4, pl. 10, fig. 36.
? 1937 Cryptaulax armata Goldfuss; Pčelincev, p. 37, pl. 5, fig. 9.
? 1937 Cryptaulax armata var. ornata Pčelincev; Pčelincev, p. 38, 68, pl. 2, fig. 61.
1951 Procerithium (Xystrella) echinatum (von Buch); Walther, p. 79, pl. 4, fig. 7a, b.
1997 Procerithium armatum (Goldfuss); Gründel, p. 96, pl. 6, figs 6–9.
1999a Cryptaulax armatum (Goldfuss); Gründel, p. 19, pl. 5, figs 1–4.
2001 Cryptaulax (Xystrella) armata (Goldfuss); Fürsich et al., fig. 4L.
2004 Cryptaulax armata (Goldfuss); Kaim, p. 37, fig. 22.
2009 Cryptaulax armatum (Goldfuss); Schulbert & Nützel, 488, fig. 9A–D.
2013 Cryptaulax armatum (Goldfuss); Schulbert & Nützel, 740, fig. 13A–D.
Material. Five specimens: MNHNL QH614, MNHNL QH615, MNHNL QH616 (three specimens), Tournadous, lower Toarcian, Bifrons Zone, Bifrons Subzone.
Dimensions. See Table 1.
Description. Shell small, high-spired and slender. Spire composed of about a dozen whorls. Whorls about twice as wide as high. Height of last whorl less than one-third of shell height. Whorls almost flat and limited by flush sutures. Base somewhat low, evenly convex and anomphalous. Ornament composed of three spiral threads persisting up to the last whorl. Upper and lower threads bearing prominent nodes and running near adapical and abapical suture, respectively. Lower thread marking periphery of whorl. Median thread closer to the upper thread and with smaller nodes. Nodes formed by intersection of spiral threads with slightly prosocline collabral ribs. Fourth thinner spiral thread sometimes between median and abapical threads. Fully adult whorls sculptured by 9–10 collabral ribs roughly aligned between adjacent whorls. Abaxial half of base ornamented by two spiral threads having nodes as prominent as those of median thread. Most abaxial thread of base covered by suture. Adaxial region of base not preserved.
Remarks. Cerithium armatum was apparently introduced by Goldfuss (Reference Goldfuss1844) as a replacement name for Turritella echinata Buch, Reference Buch1831. This last had been included in Cerithium by several authors (Roemer, Reference Roemer1836, p. 141, Reference Roemer1839, p. 3; Koch & Dunker,Reference Koch and Dunker1837, p. 10; Buch, Reference Buch1839, p. 104), thus becoming a secondary homonym of the Recent Cerithium echinatum Lamarck, Reference Lamarck1822. Subsequently, there has been considerable confusion about the nomenclatural status of these taxa. For example, Quenstedt (Reference Quenstedt1852, Reference Quenstedt1856, Reference Quenstedt1883) treated C. armatum and C. echinatum as distinct species. In his opinion the two taxa are extremely similar but could be differentiated based on stratigraphical distribution, that is, Braunen Jura α for armatum and Braunen Jura ε for echinatum. Brösamlen (Reference Brösamlen1909) and, more recently, Kaim (Reference Kaim2004) also kept the two species separate even if they report specimens with transitional morphologies. According to the first author (Brösamlen, Reference Brösamlen1909, p. 292), Cryptaulax echinata could be distinguished by the more slender shape, the higher whorls, the higher number of collabral ribs and the narrower sutural groove. Walther (Reference Walther1951) stated that the material referred to Buch's echinata by Quenstedt (Reference Quenstedt1856, Reference Quenstedt1883) and Brösamlen (Reference Brösamlen1909) is to be ascribed to a new taxon, Procerithium (Xystrella) quenstedti Walther, Reference Walther1951. He included armatum in the synonymy of Procerithium (Xystrella) echinatum arguing that Goldfuss’ name is invalid, being a younger synonym. As a matter of fact, Turritella echinata Buch, Reference Buch1831 and Cerithium armatum Goldfuss, Reference Goldfuss1844 are objective synonyms (ICZN, 1999, art. 72.7) and Buch's name is permanently invalid because it was replaced before 1961 (ICZN, 1999, art. 59.3), even if it is no longer considered congeneric with Lamarck's taxon.
A rather wide morphological variability of teleoconch ornaments, especially concerning the number, persistence and thickness of secondary spiral threads, has been described in C. armata (see descriptions in Brösamlen, Reference Brösamlen1909; Cossmann, Reference Cossmann1913; Gründel, Reference Gründel1999a ; Kaim, Reference Kaim2004). The median spiral thread can attain the same strength of the two main nodular spiral threads, but it can also disappear during the growth or be totally lacking. The number of collabral ribs is also variable. In the specimens described here, the median spiral thread is slightly less robust than the main threads and persists until the last whorl. A fourth intercalary thread is sometimes developed. The specimens are very similar to those figured by Cossmann (Reference Cossmann1913, pl. 4, figs 45, 46 and 51) from Toarcian sediments of Metz (Lorraine, eastern France) and by Schulbert & Nützel (Reference Schulbert and Nützel2013, fig. 13) from upper Toarcian – lower Aalenian deposits of Mistelgau (Franconia, southern Germany).
It is very difficult to differentiate Cryptaulax quenstedti (Walther) ( = C. echinata sensu Quenstedt and Brösamlen) from C. armata, especially taking into account the morphological variability reported for both species. Walther's taxon could well be a synonym of C. armata. Cryptaulax scobina (Eudes-Deslongchamps, Reference Eudes-Deslongchamps1842a ) (p. 196, pl. 10, figs 49, 50) from upper Pliensbachian deposits of Calvados (northern France) has the same ornamentation pattern of C. armata, but its shell is more slender and has a subcylindrical shape.
Distribution of the species. Undifferentiated Toarcian, Lorraine (eastern France) and Calvados (northern France); undifferentiated Toarcian and lower Aalenian, Alsace (eastern France); lower Toarcian, Causses Basin (southern France); upper Toarcian, Lower Saxony (northern Germany); upper Toarcian – lower Aalenian, Franconia (southern Germany); lower Aalenian, Swabia (southern Germany); middle Bathonian, Częstochowa region (SW Poland).
Superfamily AMPULLINOIDEA Cossmann in Cossmann & Peyrot, Reference Cossmann and Peyrot1919 Family AMPULLINIDAE Cossmann in Cossmann & Peyrot, Reference Cossmann and Peyrot1919 Genus Ampullospira Harris, Reference Hallam1897
Type species. Euspira canaliculata Morris & Lycett, Reference Morris and Lycett1851. Aalenian–Bajocian, Yorkshire (NE England).
Ampullospira pelops (d’Orbigny, Reference Orbigny1850) Figure 9w–z
1850 Natica Pelops d’Orb., d’Orbigny, p. 247.
1852 Natica Pelops d’Orb.; d’Orbigny, p. 188, pl. 288, figs 16, 17.
1874 Natica Pelops (d’Orbigny); Dumortier, p. 131, pl. 34, figs ?5, 6, 7.
1882 Natica pelops d’Orbigny; Quenstedt, p. 273, pl. 194, fig. 59.
1909 Natica pelops d’Orbigny; Brösamlen, p. 266, pl. 20, fig. 29.
1925 Ampullospira pelops (d’Orb.); Cossmann, p. 49, pl. 5, figs 1, 2.
non 1937 Natica pelops d’Orb.; Pčelincev, p. 33, pl. 2, figs 39, 40.
1997 Ampullospira pelops (d’Orbigny); Fischer & Weber, p. 69, pl. 17, fig. 5.
? 2002 Ampullospira (Ampullospira) cf. pelops (d’Orbigny); Gahr, p. 130, pl. 5, fig. 16.
Material. Two specimens: UBGD 278840, Tournadous, lower Toarcian, Bifrons Zone, Bifrons Subzone. One specimen: UBGD 278841, Tournadous, upper lower – upper Toarcian, exact stratigraphical level unknown.
Dimensions. See Table 1.
Description. Shell rather globose, composed of very convex whorls separated by strongly impressed sutures. Last whorl swollen, its height almost 90% of shell height. Upper band of whorl surface forming a slightly convex ramp almost orthogonal to spire axis. Ramp outer edge rounded on first observable whorls, becoming progressively more distinct on final half of last whorl. Base globose, high and downward elongated. Aperture ovate, wider in its upper half.
Remarks. The material consists of inner moulds preserving some remains of a thin shell wall. The outline of the shell and the rate of growth of the spire correspond to those of Ampullospira pelops (d’Orbigny, Reference Orbigny1850). However, the specimens are distinctly smaller and are probably represented by not fully adult shells. The material ascribed by Hudleston Reference Hudleston1892 (p. 259, pl. 20, figs 2–6) to Natica adducta Phillips, Reference Phillips1829 from the Aalenian–Bajocian of England differs in having a flatter ramp bordered by a sharper outer rim.
The specimen from the Toarcian deposits of Caucasus ascribed by Pčelincev (Reference Pčelincev1937) to Natica pelops differs from the type material and from the specimens described here in having a more elongated shell, less swollen whorls and a more oblique and less distinct ramp.
Distribution of the species. Undifferentiated Toarcian, Sarthe (NW France), Isère (SE France); lower and ?upper Toarcian, Causses Basin (southern France); upper Toarcian, Swabia (southern Germany).
4. Recovery after the early Toarcian anoxic event
The systematic analysis allowed the recognition of 15 species, two of which in open nomenclature. Two species, namely Angulomphalus expansus, Pleurotomaria amalthei and the material here dubitatively referred to Ooliticia? cyclostoma, come from upper Pliensbachian beds (Margaritatus Zone, Gibbosus Subzone) of the Marnes de Villeneuve Formation. All these species are typical of the western European gastropod communities of that age. The other species were collected from the Toarcian part of the Marnes de Fontaneilles Formation (Fig. 10). In their palaeoenvironmental analysis of some Toarcian sections of the Causses basin, Fürsich et al. (Reference Fürsich, Berndt, Scheuer and Gahr2001, table 1) recognized six gastropod species, namely Pleurotomaria sp., Amberleya subimbricata (=Eucycloidea tenuistria), Eucyclus capitaneus, Costatrochus subduplicatus, Procerithium sp. and Cryptaulax armata. Except for E. capitaneus, the taxa determined at species level have also been found in the sections of Tournadous and Cornus. In agreement with Fürsich et al. (Reference Fürsich, Berndt, Scheuer and Gahr2001), most probably the gastropods represented epifaunal mobile, soft bottom dwellers belonging to macrobenthic associations with low to moderate species diversity.

Figure 10. Stratigraphical distribution of the species studied in the Causses Basin and comparison with their overall stratigraphical distribution in other localities of the western European epicontinental shelf (see text for details).
The gastropod fauna described here adds further details to the reconstruction of the faunal recovery history after the early Toarcian anoxic event depicted by Fürsich et al. (Reference Fürsich, Berndt, Scheuer and Gahr2001) for the Causses Basin. The stratigraphical distribution of the species shows two peaks of relative diversity, i.e. in the Bifrons Zone, mainly Bifrons Subzone, and in the Aalensis Zone, Mactra Subzone (Fig. 10). Nine species occur in the Bifrons Zone. Five of them, namely Bathrotomaria kronzwilmesorum, C. subduplicatus, E. tenuistria, Cryptaulax armata and Ampullospira pelops, were collected in the Bifrons Subzone. The part of the Tournadous section yielding the four other species (Sisenna canalis, Eucyclus escheri, Eucyclus elegans and Katosira sp.) cannot be safely ascribed to a distinct subzone, although it is strongly suspected to represent the Bifrons Subzone. S. canalis, E. escheri and E. elegans are recorded in Pliensbachian localities of NW Europe and can be interpreted as survivors from the pre-event stock (Fig. 10). The first-known occurrence of the remaining species is subsequent to the anoxic event. In the beds including the Variabilis to Pseudoradiosa zones, gastropods are represented only by C. subduplicatus and E. tenuistria or are absent. The peak of diversification in the Aalensis Zone is much less pronounced, only represented by five species (Pleurotomaria escheri, C. subduplicatus, E. tenuistria, Neritopsis philea and Procerithium pseudocostellatum). The component inherited from the pre-event faunas is limited to a single species, namely P. escheri. This peak falls entirely within the Mactra Subzone, whereas in the Fluitans Subzone only C. subduplicatus and E. tenuistria occur. Except from the latter two species, the species of the Aalensis stock are different from those of the Bifrons stock.
According to Fürsich et al. (Reference Fürsich, Berndt, Scheuer and Gahr2001), the changes in the macrobenthic associations recorded in the Causses succession after the early Toarcian anoxic event are related to changes in oxygenation and substrate consistency. In the lower part of the sequence the low diversity reflects oxygen fluctuations, whereas the extremely soupy substrate resulting from the activity of burrowing organisms was the main controlling factor during late Toarcian time. In this scenario, the peaks of the Bifrons and Aalensis zones documented in the present study would reflect brief times during which the oxygen content and bottom consistency favoured the settlement of a relatively diversified fauna. The exclusive occurrence of C. subduplicatus and E. tenuistria in the interval between the Variabilis and Pseudoradiosa zones could indicate more severe and unstable environmental conditions, under which only gastropod taxa with wide adaptive capacities were able to survive. As a matter of fact, these two species show the typical features of the opportunistic taxa, e.g. wide morphological variability, long stratigraphical range, absolute dominance in low-diversity associations and accessory occurrence in diversified associations. Presumably, this low diversity depended mostly on the diffusion of soupy substrates, as indicated by Fürsich et al. (Reference Fürsich, Berndt, Scheuer and Gahr2001).
Additional information can be deduced from the comparison of the gastropod fauna of the Causses Basin with the upper Toarcian – lower Aalenian fauna of Mistelgau (Franconia, southern Germany) recently analysed by Schulbert & Nützel (Reference Schulbert and Nützel2009, Reference Schulbert and Nützel2013) with a comparable stratigraphical detail. The two faunas show substantial differences. In the Causses Basin the species diversity is much lower than at Mistelgau. This might be partly due to different sampling methods (bulk samples and surface collection in the case of Mistelgau), although the low diversity of gastropods in the Causses Basin is confirmed by the analysis based on quantitative sampling provided by Fürsich et al. (Reference Fürsich, Berndt, Scheuer and Gahr2001). Schulbert & Nützel (Reference Schulbert and Nützel2013) described 26 species in the upper Toarcian sediments, mainly Caenogastropoda and Heterobranchia; the most-represented taxa are Coelodiscus minutus and Toarctocera subpunctata. Those authors observed that, in general, the degree of diversity is comparable with that of the faunas preceding the anoxic event. In the Causses Basin the Vetigastropoda clearly prevail and the Heterobranchia are absent. C. minutus and T. subpunctata are lacking, whereas the most abundant species are C. subduplicatus and E. tenuistria. Both these species occur from the Bifrons Zone and persist throughout the sections up to the top of the Toarcian succession. At Mistelgau, E. tenuistria is only recorded in the lower Aalenian sediments. The dominance of C. subduplicatus and E. tenuistria has also been recognized by Fürsich et al. (Reference Fürsich, Berndt, Scheuer and Gahr2001, fig. 18) in other sections of the Causses Basin; it therefore seems to be a characteristic aspect of the Toarcian gastropod faunas of this basin. These differences do not seem to be related to significant facies differences. Both the Mistelgau and Causses successions consist mostly of homogenous clays and marls deposited in quiet environments with diffused soupy substrates, below storm wave base and in fully aerobic to dysaerobic conditions (Fürsich et al. Reference Fürsich, Berndt, Scheuer and Gahr2001; Schulbert & Nützel, Reference Schulbert and Nützel2013).
The trend of faunal recovery after the early Toarcian crisis also differs in the two areas. Schulbert & Nützel (Reference Schulbert and Nützel2013, fig. 3) showed a progressive increase of species diversity from the Levesquei Zone to the Aalensis and Opalinum zones. According to those authors, this diversity gradient was probably driven by a progressive rise of oxygen concentration and represents the recovery and turnover of gastropod communities after the anoxic event in Franconia. In contrast, this process was much slower and discontinuous in the Causses Basin. These contrasting recovery patterns could be better understood by considering local physiographic factors and the position of the two basins in a wider palaeogeographic setting (Fig. 11). During Toarcian time the Mistelgau area was part of a wide epicontinental seaway west of the Bohemian Massif (Schulbert & Nützel, Reference Schulbert and Nützel2013), with open communications and easy faunal exchanges with the other western European basins (Thierry, Reference Thierry, Dercourt, Gaetani, Vrielynck, Barrier, Ju-Duval, Brunet, Cadet, Crasquin and Sandulescu2000). Conversely, the Causses region was a small intracratonic basin at the southern margin of the European shallow-water shelf, tightly confined by exposed lands of the Montagne Noire and the Massif Central and with Cévennes High hampering eastwards communications (Baudrimont & Dubois, Reference Baudrimont and Dubois1977; Trümpy, Reference Trümpy1983; Mailliot et al. Reference Mailliot, Mattioli, Bartolini, Baudin, Pittet and Guex2009). The southern side of the basin was open towards the central part of western Tethys (Trümpy, Reference Trümpy1983), as also suggested by the relative mixing of NW Europe and Mediterranean pelagic faunas inhabiting the Causses Basin after the early Toarcian crisis (Guex, Reference Guex1972; Pinard et al. Reference Pinard, Weis, Neige, Mariotti and Di Cencio2014). However, repopulation of the benthic communities seems not to have benefited from the same Tethyan influx. Gastropod species of the Causses basin are very characteristic and exclusive components of the Toarcian – early Aalenian communities of the European epicontinental seas, and species from the central region of western Tethys are absent (Fig. 11). The Toarcian successions of central western Tethys are represented by pelagic sediments, such as red marly clays, nodular marls and condensed limestones, and by deposits of marginal and intra-oceanic carbonate platforms (Bernoulli & Jenkyns, Reference Bernoulli, Jenkyns, Dott and Shaver1974; Farinacci & Elmi, Reference Farinacci and Elmi1981; Winterer & Bosellini, Reference Winterer and Bosellini1981; Dercourt et al. Reference Dercourt, Gaetani, Vrielynck, Barrier, Bi Ju-Duval, Brunet, Cadet, Crasquin and Sandulescu2000; Marino & Santantonio, Reference Marino and Santantonio2010). They reflect environmental conditions very different from those of the Causses Basin, where the Toarcian facies are instead roughly comparable to those of other regions of the western European shelf. Speculating from this evidence, gastropods of the central western Tethys that in theory could easily have reached the Causses region by dispersal were probably unable to settle and colonize that area due to the very different environment. On the other hand, the relative geographic isolation and marginal location of the Causses Basin probably made the faunal exchange with the western European epicontinental seas difficult, determining the slow and discontinuous faunal recovery observed. Useful insights to test this interpretation might be derived from the study of the mode of recovery of other benthic groups and by extending the analysis to the Aalenian faunas.

Figure 11. Toarcian – early Aalenian palaeogeographic distribution of the species described. Map and depositional environments based on Dercourt et al. (Reference Dercourt, Gaetani, Vrielynck, Barrier, Bi Ju-Duval, Brunet, Cadet, Crasquin and Sandulescu2000). Asterisk indicates the studied area.
Acknowledgements
We thank Guy Kronz, Robert Haas, Andrea Di Cencio, Alain Faber (Palaeontological Department, National Museum of Natural History, Luxembourg) and Nino Mariotti (Department of Earth Sciences, University La Sapienza, Rome, Italy) who contributed to the field work. A. Nützel (Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany) and an anonymous reviewer provided valuable comments on the manuscript. The photographic work was executed by Stefano Castelli and the preparation of the material was carried out by Lorenzo Franceschin (both Department of Geosciences, University of Padua, Italy). This work was supported financially by the University of Padua (RG, SM grant number CPDA131234) and the Fonds National de la Recherche, Luxembourg (J-DP), and is a contribution to the INTERVIE (INSU) research program and to the BioME team of the Biogéosciences laboratory, Dijon, France (PN).
Declaration of interest
None.














