1. Introduction
Fruit-mediated seed dispersal can take many forms, for example winged fruits aiding wind dispersal or sticky fruits allowing dispersal by attachment to animal coats. A widespread seed dispersal regime is endozoochory, where fruits are eaten by animals, causing the ingested seeds to be dispersed in the animal's droppings (Tiffney, Reference Tiffney2004). Endozoochory is of vital importance in ecosystem function and drives co-evolutionary interactions between plant and animal species (Wenny, Reference Wenny2001; Hardesty et al. Reference Hardesty, Hubbell and Bermingham2006).
Both mammals and birds are common vectors for endozoochory in modern ecosystems. Mammalian seed dispersal (mammalochory) has occurred since Mesozoic times (Tiffney, Reference Tiffney2004), while some Early Cretaceous fossils provide possible evidence for avian endozoochory (ornithochory) (Zheng et al. Reference Zheng, Martin, Zhou, Burnham, Zhang and Miao2011). Saurochory, the role of reptiles as seed dispersal agents by ingestion of fruits (Moll & Jansen, Reference Moll and Jansen1995; Valido & Olesen, Reference Valido and Olesen2007), is generally considered to be of lesser importance than dispersal by mammals or birds, mainly due to the lesser abundance of reptilian herbivores in most modern ecosystems (Cobo & Andreu, Reference Cobo and Andreu1988). In Mesozoic ecosystems, however, saurochory was likely more significant as the dominant large herbivores were archosaurs. The earliest fossil evidence for endozoochory is that of fossil seeds from the gut cavities of Protorosaurus from the Permian (Tiffney, Reference Tiffney2004). Saurochory remains important in modern ecosystems when reptiles are the dominant herbivore group, such as giant tortoises on the Galapagos or Aldabaran islands (Hnatiuk, Reference Hnatiuk1978; Marlow, Reference Marlow1989).
There are ways to determine trophic interactions, such as frugivory and endozoochory, from fossil evidence. One method is to search for features of the dentition and mouthparts characteristic of certain dietary regimes, for example, patterns of tooth crown wear (Mihlbachler et al. Reference Mihlbachler, Rivals, Solounias and Semprebon2011), micro-scale scratches and pits on tooth enamels (Solounias & Semprebon, Reference Solounias and Semprebon2002; Semprebon et al. Reference Semprebon, Janis and Solounias2004) or quantified dental crown complexity (Wilson et al. Reference Wilson, Evans, Corfe, Smits, Fortelius and Jernvall2012). A more direct approach is the use of coprolites and trace fossils to reconstruct diet, such as dinosaur feeding behaviour (Barrett & Willis, Reference Barrett and Willis2001; Prasad et al. Reference Prasad, Strömberg, Alimohammadian and Sahni2005). The most conclusive evidence comes from enterolites (fossilized intestinal contents) however, preserving the final meal of the animal and allowing identification of trophic interactions at the species level (Zhu et al. Reference Zhu, Vannier, van Iten and Zhao2004; Kear, Reference Kear2006; Zheng et al. Reference Zheng, Martin, Zhou, Burnham, Zhang and Miao2011).
This paper describes evidence for the preservation of enterolites within the remains of large terrestrial tortoises from the Early Oligocene Scenic Member of the Brule Formation from Badlands National Park, South Dakota. The enterolites have preserved hackberry seeds and, by sedimentological and taphonomic analysis, we show that these seeds are very unlikely to have arrived in position within the tortoise shells by abiotic means. These fossils are the oldest reliable evidence of endozoochory in tortoises and point towards an ancient relationship between hackberry plants and their animal dispersal agents.
2. Fossils
2.a. Locality
The White River Group is a series of Eocene–Oligocene reworked volcaniclastic sediments that crops out across the Great Plains of North America. The specimens described here were collected from adjacent to the Saddle Pass Marker in the middle Scenic Member of the Brule Formation, Badlands National Park (BADL), South Dakota (Evanoff et al. Reference Evanoff, Terry, Benton and Minkler2010). This level is equivalent to Mudstone III of Clark et al. (Reference Clark, Beerbower and Kietzke1967) and marker horizon 2 of Benton et al. (Reference Benton, Evanoff, Herbel and Terry2001). The Scenic Member contains the top of magnetochron C13n and much of C12r (Prothero & Whittlesey, Reference Prothero and Whittlesey1998), dating it conservatively between 33.705 and 31.014 Ma (Gradstein et al. Reference Gradstein, Ogg, Schmitz and Ogg2012): early Oligocene in age (Orellan North American Land Mammal Age). The Scenic Member is composed of a sequence of mudstone-dominated intervals interbedded with silty sandstone-dominated intervals (Evanoff et al. Reference Evanoff, Terry, Benton and Minkler2010). The Saddle Pass Marker interval is a prominent c. 2.2 m thick sequence of palaeosol-overprinted mudstones that is visible and unbroken throughout Badlands National Park (Evanoff et al. Reference Evanoff, Terry, Benton and Minkler2010). These palaeosols have been identified as a mix of Alfisols, Entisols and Inceptisols (Retallack, Reference Retallack1983), corresponding to a sub-humid to semi-arid forested environment. The grain size and lateral persistence of the Saddle Pass Marker interval, combined with the absence of any major channels within the interval throughout its outcrop area, suggests that these sediments were deposited in a stable low-energy distal floodplain environment by fluvial or possibly aeolian processes.
The White River Group is noted for its well-preserved fossils, with chelonians being common (Corsini et al. Reference Corsini, Smith and Leite2006). The first discovery of a non-marine chelonian in North America was in the White River Group (Hay, Reference Hay1908) and currently there are 33 genera known from the group (Hutchison, Reference Hutchison, Prothero and Berggren1992). The most common terrestrial chelonian is the genus Stylemys (Wall & Maddox, Reference Wall, Maddox, Santucci and McClelland1998) a relative of the modern genus Gopherus (Reynoso & Montellano-Ballesteros, Reference Reynoso and Montellano-Ballesteros2004). Stylemys are interpreted as large herbivores that roamed in the semi-arid forests of the Oligocene South Dakota. Smaller individuals may have dug burrows similar to modern gopher turtles, but larger individuals are thought to have had lifestyles more like those of Galapagos giant tortoises (Hansen et al. Reference Hansen, Donlan, Griffiths and Campbell2010).
Hackberry seeds (genus Celtis) are relatively frequently preserved in Scenic Member sediments. There are 80 extant species of Celtis, found as large shrubs or trees in the Americas, Eurasia and Africa (Demir et al. Reference Demir, Doğan, Özcan and Haciseferoğullari2002). Modern Celtis species are mainly found in semi-arid environments (Jahren et al. Reference Jahren, Gabel and Amundson1998), much like those seen in the Scenic Member. Celtis seeds are commonly preserved as fossils due to the aragonite biomineralization of their endocarps (Wang et al. Reference Wang, Jahren and Amundson1997; Jahren et al. Reference Jahren, Gabel and Amundson1998; Shillito & Almond, Reference Shillito and Almond2010). This may be an adaptation to the low pH experienced during the passage through the digestive tract, as Celtis seeds are primarily dispersed by frugivorous animals.
2.b. Specimens
Two articulated tortoise specimens, BADL 43535 and BADL 43378 (Fig. 1), were collected in 2005 as part of a palaeoecological and taphonomic survey of the Scenic Member of the Brule Formation (Moore & Norman, Reference Moore and Norman2009). The specimens were identified based on descriptions by Hay (Reference Hay1908), Hutchison (Reference Hutchison, Prothero and Berggren1992) and Wall & Maddox (Reference Wall, Maddox, Santucci and McClelland1998). BADL 43378 was identified as being a Stylemys nebrascensis on the basis of its size, plastron dimensions and the morphology of shell elements and scutes. BADL 43535 could not be identified to species level, but was assigned to the genus Stylemys on the basis of its size and its shell lobe dimensions.
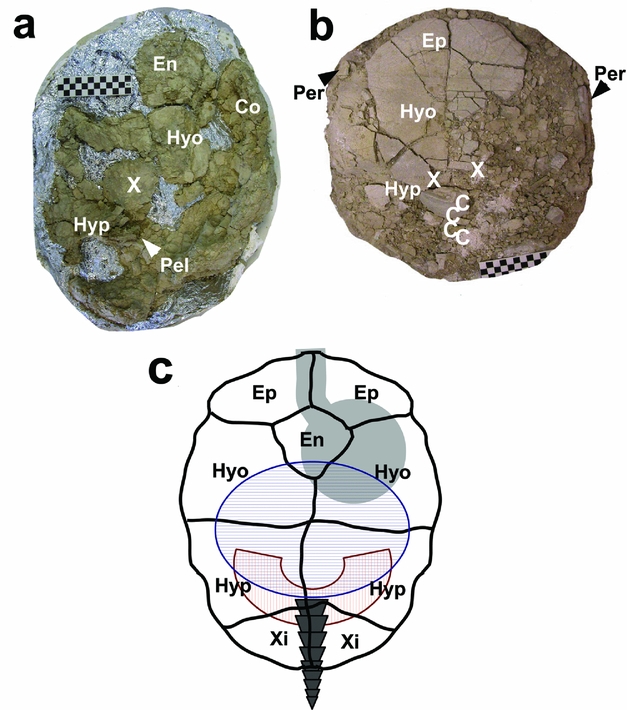
Figure 1. Prepared tortoise specimens showing locations where fossil seeds were found and corresponding locations on an outline of a living tortoise. In all figures the tortoises are orientated with the anterior end at the top. (a) Specimen BADL 43535 (Stylemys sp.) viewed through the removed carapace shell elements. (b) Specimen BADL 43378 (Stylemys nebrascensis) viewed from the outside of the intact plastron shell elements. (c) Outline schematic of the corresponding view of a living tortoise based on McArthur et al. (Reference McArthur, Meyer, Innis and Wilkinson2008), giving the approximate location of the oesophagus and stomach region (light grey), intestinal region (blue horizontal hatching), pelvic region (red vertical hatching) and caudal vertebrae (dark grey). Plastron elements are overlaid and labelled in black. Ep: epiplastron; En: endoplastron; Hyo: hyoplastron; Hyp: hypoplastron; Xi: Xiphiplastron; Co: costal (carapace element); Per: peripheral (carapace element); Pel: pelvis; C: location of caudal vertebrae; X: location of fossil hackberry seed. Scale bar in (a) and (b) is one square = 1 cm across.
The mineralized endocarps of several hackberry seeds (Celtis) were found preserved within the matrix filling the shells of BADL 43378 and BADL 43535. One seed was found in BADL 43535 (Fig. 2a), while two seeds were present inside BADL 43378 (Fig. 2b). The seed from BADL 43535 appears to be more poorly preserved, lacking surface detail in comparison to the seeds found within BADL 43378.
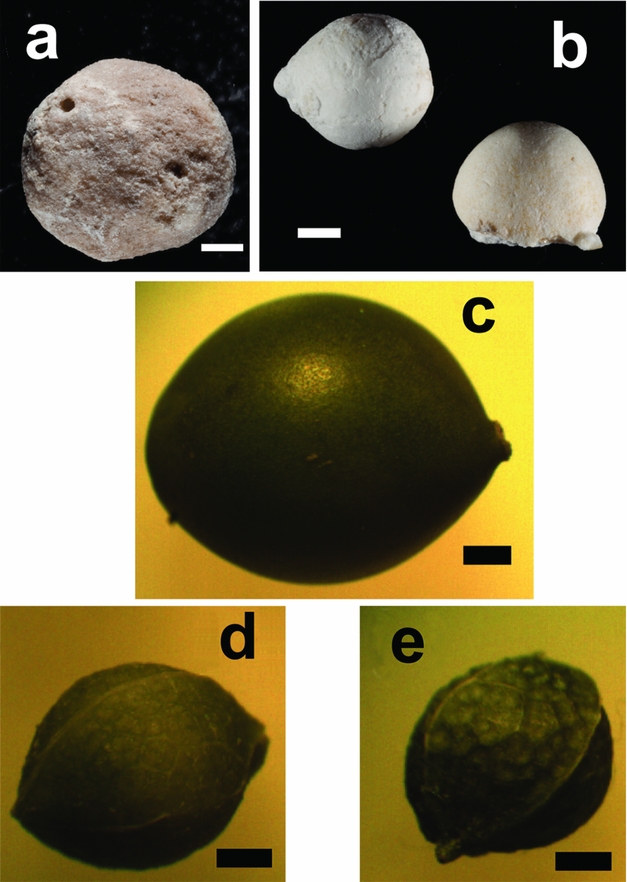
Figure 2. Modern and fossilized hackberry (Celtis) fruit and seeds. Shown are fossil seeds isolated from the matrix within the fossilized tortoise shells: (a) within the carapace of Stylemys specimen BADL 43535 and (b) underneath the plastron of Stylemys specimen BADL 43378. Also shown are examples of modern C. occientalis seeds and fruits: (c) fresh intact fruit; (d) fresh seed isolated from this fruit; and (e) the same seed after drying treatment. Scale bars are 1 mm across.
There are two possible scenarios by which these seeds could have been preserved within the tortoise skeletons. One is that the seeds or fruits may have been transported post mortem into the cavity within the shell by abiotic means. This essentially means that the seeds entered in the same fashion as the sediment that buried the carcass. The other scenario is that the seeds entered by biotic means. The biotic method of entry that we propose is that the tortoises ingested the fruits of the Celtis plants while they were alive and died before egesting the seeds. In this case, the seeds were fossilized along with the tortoise and represent preserved stomach contents (enterolites).
3. Evidence for enterolites
3.a. Taphonomic evidence
It is necessary to reconstruct the taphonomic history of both tortoise specimens to determine the likelihood of biotic versus abiotic emplacement of the Celtis seeds. BADL 43535 was preserved as an incomplete but articulated carapace and plastron. The anterior of the shell was missing and, in preparation, parts of the costals were removed (Fig. 1a). The pelvis was found intact and in life position, with only slight damage to the ischium and pubis (Pel, Fig. 1a). A Celtis seed was found within the carapace of BADL 43535 at position X indicated on Figure 1a.
BADL 43378 was preserved as an almost-complete carapace. The plastron was incomplete due to modern erosion; only elements of the epiplastron, entoplastron and hypoplastron were present. Four caudal vertebrae were found at positions indicated on Figure 1b (see Fig. 3 for images of each vertebra). The caudal vertebrae were positioned in a linear array, implying that they were preserved in near-life position (cf. Fig. 1b, c). Their position in relation to the plastral elements is consistent with them being the in situ remains of the tail of BADL 43378. Two Celtis seeds were found at the positions X indicated on Figure 1b.

Figure 3. Caudal vertebrae found in BADL 43378. Vertebrae are labelled in order of recovery position, with (a) being the anterior-most and (d) being the posterior-most. The locations on specimen BADL 43378 where the vertebrae were found are given in Figure 1b. Scale bars are 0.5 cm across.
Whereas some of the non-shell skeletal elements from each tortoise specimen are preserved in place, the skeletons are not complete; many elements have been lost. While some of these may have been lost to modern erosion, there was limited evidence for this from field observations. The remaining elements therefore must have been removed either by fluvial transport or by scavenger action. Unless bound by soft tissue, the skeletal elements within a tortoise carapace can be used to suggest the flow regime to which the carcass was subject. Taphonomic experiments by Blob (Reference Blob1997) on freshwater turtle remains demonstrated that pelvic elements are late intermediate dispersal elements requiring a competent velocity of 0.375 m s−1 to transport. While Blob (Reference Blob1997) did not directly measure the transportability of caudal vertebrae, it is reasonable to assume that they would behave similarly to cervical vertebrae; these early intermediate dispersal elements require a competent velocity of 0.292 cm s−1 to transport. For these elements to be present within the tortoise carapaces, the specimens cannot have been subject to competent velocities significantly greater than these measured values. The absence of specimens that are more difficult to transport than pelvic elements and caudal vertebrae, combined with the life positions of the preserved elements, suggests that loss of the remaining elements of the skeletons can be attributed to scavenger modification.
The presence of skeletal elements within the tortoise carapace provides evidence regarding the speed of burial of the carcass. Brand et al. (Reference Brand, Hussey and Taylor2004) have documented the break-up of turtles in arid terrestrial environments. Their findings showed that the pelvis disarticulates after an average of 30 weeks, while the caudal vertebrae disarticulate after an average of 35 weeks. In comparison, the carapace and plastron remain intact up to 90 weeks and have an average disarticulation time of 90–150 weeks. The relative lack of damage to the skeletal elements from erosion or weathering is consistent with the completeness of the shells of the specimens (Corsini et al. Reference Corsini, Smith and Leite2006) and their rapid burial. The intact plastron and carapace would have protected the internal contents from damage or erosion.
Given these data we propose that the specimens were buried rapidly and, if internal soft tissue was lost prior to burial, they were not subject to significant transport energies. Burial is estimated to have occurred within 30–35 weeks while the shells were still largely intact. For the seeds to be present abiotically, they would have had to infiltrate the shells within this time. The chance infiltration of three separate seeds into two separate tortoise carcasses is considered highly unlikely, particularly as all seeds were found posterio-medially within the shells, consistent with an intestinal location (Fig. 1c), with intestinal contents protected by the surrounding shell. The ornamentation retained on the seeds in BADL 43378 also suggests that the seeds were protected from pre-burial abrasion by being within the tortoise shell. Consequently, the taphonomic histories of both specimens favour the enterolite hypothesis.
3.b. Sedimentological evidence
In order to further test the likelihood of the seeds having been transported by abiotic or biotic agents, a sedimentological analysis was conducted. The settling velocity of the seeds was estimated using modern hackberry seeds. This was then compared to the reconstructed transport speed and mode of the sediment to determine if the seeds could have been deposited within the shells during post-mortem burial.
Celtis occientalis fruits (Fig. 2c) were collected from plants growing in the Cambridge University Botanic Garden. Five fruits were weighed and measured, and an average fruit weight and diameter calculated. The flesh was then stripped from the fruits to isolate the fresh seeds (Fig. 2d). The average fresh weight and diameter was determined from the five isolated seeds. The seeds were dried in an oven at 65 °C for 150 minutes. The dried seeds (Fig. 2e) were then used to calculate an average dry seed diameter and weight. Density was calculated by assuming the seeds to be spherical (see Table 1 and Supplementary Material available online at http://journals.cambridge.org/geo).
Table 1. Diameter, density and settling velocities of modern hackberry fruits and seeds and the sediment found within the tortoise specimen shells. Fruit and seed measurements were calculated from the average size and density (n = 5) of the C. occientalis samples. The sediment measurements were taken from samples of matrix found surrounding the fossil seeds. Sediment grain size was calculated by Coulter Counter. The density of the sediment grains was taken to be that of quartz. Settling velocities were calculated using Stoke's equation and the method of Heywood (Reference Heywood1962) (see Supplementary Material available online at http://journals.cambridge.org/geo).

A sample of matrix that was found surrounding the seeds in BADL 43378 was disaggregated using Calgon. A Coulter Counter was then used to determine average grain size. The grain density was taken to be that of quartz, consistent with the known mineralogy of the sediments of the Saddle Pass Marker interval.
Settling velocities were calculated using Stoke's equation for the sediment grains. For the fruits and seeds, settling velocities were determined using Heywood's tables (Heywood, Reference Heywood1962). The results and calculations can be seen in Table 1 (also see Supplementary Material available online at http://journals.cambridge.org/geo).
Given that the Saddle Pass Marker interval sediments could have been deposited by fluvial or aeolian processes, it is necessary to consider both cases here. If the sediment was aeolian in nature, the wind speeds required to deposit the sediment grains in the matrix are realistic (0.03 m s−1); however, the wind speeds needed to transport and carry either the seeds or fruits are much greater. For the seeds or fruits to be wind deposited it would be expected that larger grains would also be transported into the matrix, yet this is not the case. The shapes of the seeds, especially those in BADL 43378 which retain ornamentation, are contrary to the smooth rounded shape expected following wind abrasion. There is a similar incongruity between the sediment and seed settling velocities in water. If the sediment was fluvial, however, it would be possible for fruits (although not seeds alone) to float into the tortoise carcass together with the very fine sediment. It is considered unlikely that this not only occurred three times in two specimens, but that all seeds were left in anatomically correct positions in the carcasses each time.
With abiotic transport having been ruled out using sedimentological evidence, the only alternative abiotic method would be in situ placement of the seeds (i.e. falling directly from the parent plant). However the probability of this event occurring three separate times between two separate skeletons, both of which have intact carapaces, is expected to be extremely low. Therefore the most parsimonious explanation is that the seeds represent intestinal contents preserved in situ (i.e. enterolites).
3.c. Ecological evidence from modern analogues
Comparisons with extant relatives and ecological analogues of both Celtis and Stylemys nebrascensis provide support for the fossilized seeds representing preserved gut contents, and point towards tortoises acting as seed dispersal agents for Celtis plants. It is suggested that the Scenic Member tortoises would have had a lifestyle similar to that of modern giant tortoises (Hansen et al. Reference Hansen, Donlan, Griffiths and Campbell2010). On their respective island ecosystems, these tortoises are major herbivores and important seed dispersal agents. Geochelone nigrata, found on Santa Cruz Island in the Galapagos, shows evidence of co-evolution with Psidium plants based on seed dispersal (Marlow, Reference Marlow1989). Measurements of BADL 43378 indicate that it would have been over 70 cm long in life, while BADL 43535 was estimated at over 60 cm. This is typical of adult Stylemys fossils from the White River Group (Corsini et al. Reference Corsini, Smith and Leite2006), with Stylemys being the largest and most common chelonian genus. It should be noted however that the Scenic Member had several large mammalian herbivores present (e.g. Hyracodon, Mesohippus and Poebrotherium) and therefore Stylemys herbivory cannot be directly paralleled with that of modern giant tortoises in their unique island ecosystems. Dental microwear analyses (Dewar, Reference Dewar2008) show a limited number of mammalian frugivores within the White River Group ecosystem, albeit with weak support.
Nevertheless, unlike ungulates modern tortoises do not chew fruit, and so seeds are more likely to pass through their digestive tract intact (Strong & Fragoso, Reference Strong and Fragoso2006; Traveset, Reference Traveset1998; Jerozolimski et al. Reference Jerozolimski, Ribeiro and Martins2009). This behaviour makes tortoises useful as seed dispersal agents, and may have made Stylemys a more efficient dispersal agent in contrast to the ungulate and rodent species also present in the ecosystem of the White River Group.
Gopherus polyphemus is an extant relative of Stylemys that is found in arid areas of North America. In the SE pine savannah of the US, G. polyphemus is a significant dispersal agent of several plants (Birkhead et al. Reference Birkhead, Guyer, Hermann and Michener2005). This is partly due to the good chance of a seed passing through the digestive system and partly due to the burrowing behaviour of Gopherus species. In other modern ecosystems tortoises are significant dispersers of seeds. Saurochory has been noted as being important in Brazilian forests (Strong & Fragoso, Reference Strong and Fragoso2006; Jerozolimski et al. Reference Jerozolimski, Ribeiro and Martins2009), and also in the Karoo (Milton, Reference Milton1992).
Modern Celtis species are mainly dispersed by animals (Jahren et al. Reference Jahren, Gabel and Amundson1998). Varela & Bucher (Reference Varela and Bucher2002) specifically investigated saurochory in Celtis plants in the Chaco Dry Woodland of Argentina. They found that germination success of Celtis pallida increased greatly when eaten and dispersed by the tortoise Chelodonis chilensis. This situation is notable as a modern analogue to the White River Group, with similar plant and terrestrial tortoise species living in a semi-arid environment.
4. Conclusions
The evidence from the taphonomy of the specimens, sedimentology of the area and ecology of modern analogues supports the hypothesis that the seeds found in the two White River Group tortoise specimens are preserved stomach contents. This indicates that Stylemys was, at least in part, frugivorous. It also provides a basis for the idea that in Oligocene ecosystems Celtis seeds were dispersed by endozoochory, and that giant tortoises were among the dispersal agents.
This is the oldest record of terrestrial chelonian gut contents (cf. Kear, Reference Kear2006). These specimens are the oldest confirmed evidence of tortoise frugivory, with previous claims for seed-containing Cretaceous era coprolites being assigned to chelonians only on the basis of size (Rodríguez-de la Rosa et al. Reference Rodríguez-de la Rosa, Cevallos-Ferriz and Silva-Pineda1998).
This study supports the hypothesis that the use of endozoochory for seed dispersal has been present in the Celtis genus since the Oligocene. Today hackberry species are widespread (Demir et al. Reference Demir, Doğan, Özcan and Haciseferoğullari2002) and the use of animal dispersal agents is one factor in their prevalence within semi-arid environments. The advantages of endozoochory are manifold (Eriksson et al. Reference Eriksson, Friis and Löfgren2000; Wenny, Reference Wenny2001) and it can be speculated that, with the drying climate of the Oligocene in North America, these ecological advantages may have contributed to the spread of the Celtis genus range across the Great Plains region during Cenozoic times (Jahren et al. Reference Jahren, Gabel and Amundson1998).
Our findings also highlight the role played by reptiles in seed dispersal. Saurochory is often overlooked in palaeoecological and palaeobotanical reconstructions (Tiffney, Reference Tiffney2004; Valido & Olesen, Reference Valido and Olesen2007). Its importance may be greater in certain situations such as: where reptiles are the dominant herbivores; where seeds require passage through the digestive tract undamaged; or where the environment is unfavourable and seed dispersal contained in reptilian dung or around reptile burrows provides a significant competitive advantage for germination (Marlow, Reference Marlow1989; Moll & Jansen, Reference Moll and Jansen1995; Varela & Bucher, Reference Varela and Bucher2002; Birkhead et al. Reference Birkhead, Guyer, Hermann and Michener2005). Stylemys and the closely related Gopherus saw an expansion of range and increased prevalence in the increasingly arid conditions of the Early Oligocene (Corsini et al. Reference Corsini, Smith and Leite2006), possibly promoting their importance as seed dispersal agents.
Acknowledgements
The authors would like to thank Sarah Finney, Jennifer Clack and the staff of the Segdwick Museum, Cambridge for their help and support with this work. Images of fossil seeds in Figure 2a and b and caudal vertebrae in Figure 3 are credit of Jamie Gundry, University Museum of Zoology, Cambridge. We also thank the staff of the Cambridge University Botanical Garden for providing fruit samples from C. occientalis. Rachel Benton and the staff at Badlands National Park provided assistance with the original collection of the specimens and Emmett Evanoff gave valuable sedimentological insight. The fieldwork for this project was funded by the Wingate Foundation and the research by the Ian Karten Charitable Trust.






