1. Introduction, geological setting and sampling sites
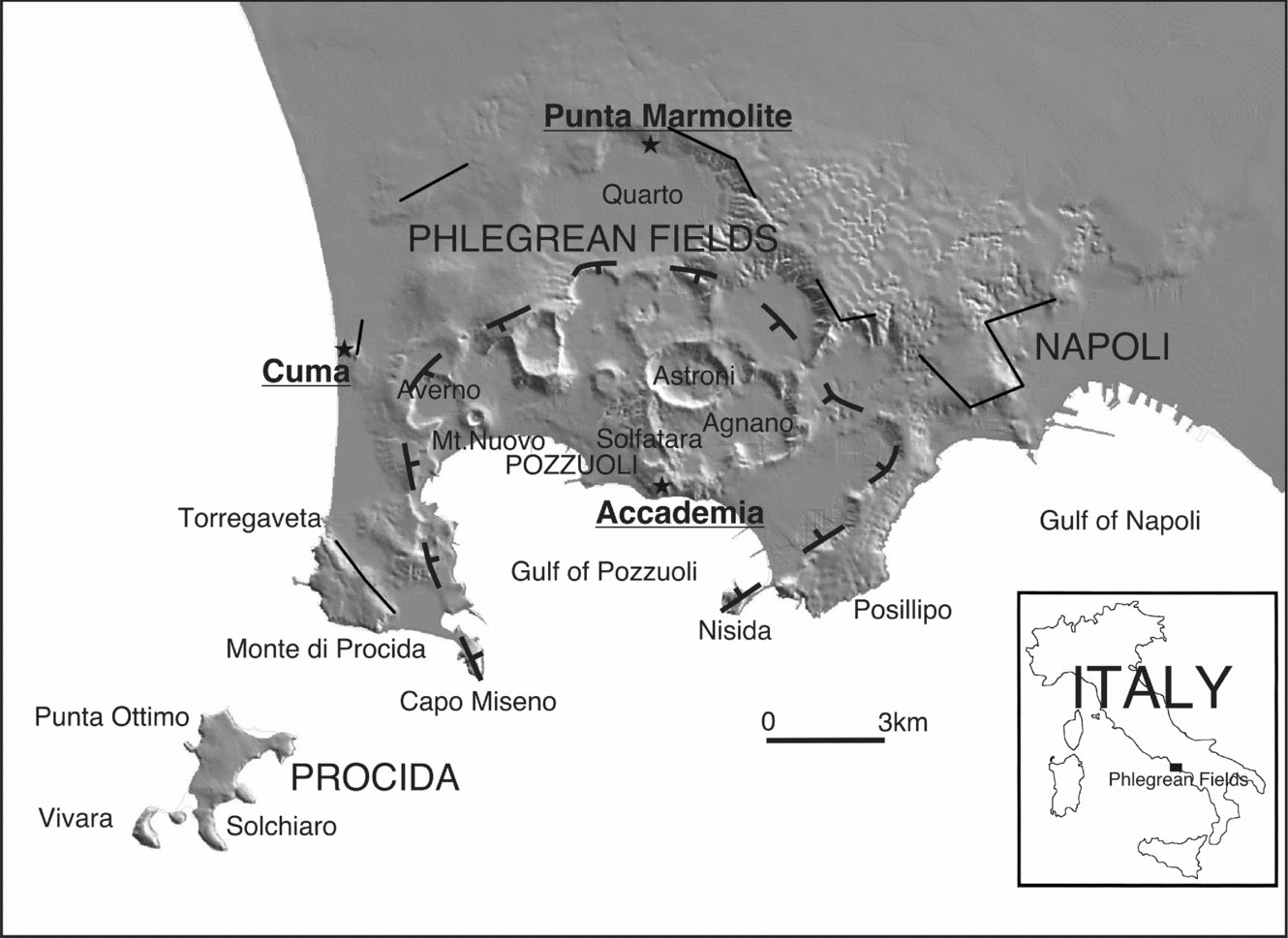
The Phlegrean Fields (Campi Flegrei) is a volcanic area that includes the urban area of Naples and its western outskirts (Fig. 1), and is part of the Campanian district, which includes Ischia, Procida and Somma-Vesuvius. The Phlegrean Fields are characterized by a great number of explosive eruptions, the most powerful of which produced the Campanian Ignimbrite (CI; ~ 39 ka; De Vivo et al. Reference De Vivo, Rolandi, Gans, Calvert, Bohrson, Spera and Belkin2001; Fedele et al. Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008) and the Neapolitan Yellow Tuff (NYT; ~ 15 ka; Deino et al. Reference Deino, Orsi, de Vita and Piochi2004), the two most important events in the volcanic history of the area which determined the development of a nested caldera (see Perrotta et al. Reference Perrotta, Scarpati, Luongo, Morra and De Vivo2006). The CI and NYT deposits are the main chronostratigraphic markers for the reconstruction of Phlegrean activity, which can be broadly subdivided into three periods: (1) pre-CI; (2) between CI and NYT and (3) post-NYT. The post-NYT ‘recent’ activity has been commonly subdivided into three epochs of active volcanism separated by quiescent intervals (Orsi et al. Reference Orsi, Di Vito, Selva and Marzocchi2009 and references therein), although there is evidence that the subdivision is more complex (Insinga et al. Reference Insinga, Calvert, Lanphere, Morra, Perrotta, Sacchi, Scarpati, Saburomaru, Fedele and De Vivo2006; Fedele et al. Reference Fedele, Insinga, Calvert, Morra, Perrotta and Scarpati2011). The Phlegrean Fields are potentially active, as testified by the last eruption of Monte Nuovo (ad1538), the 1970–72 and 1982–84 bradyseismic episodes, and intense fumarolic activity.
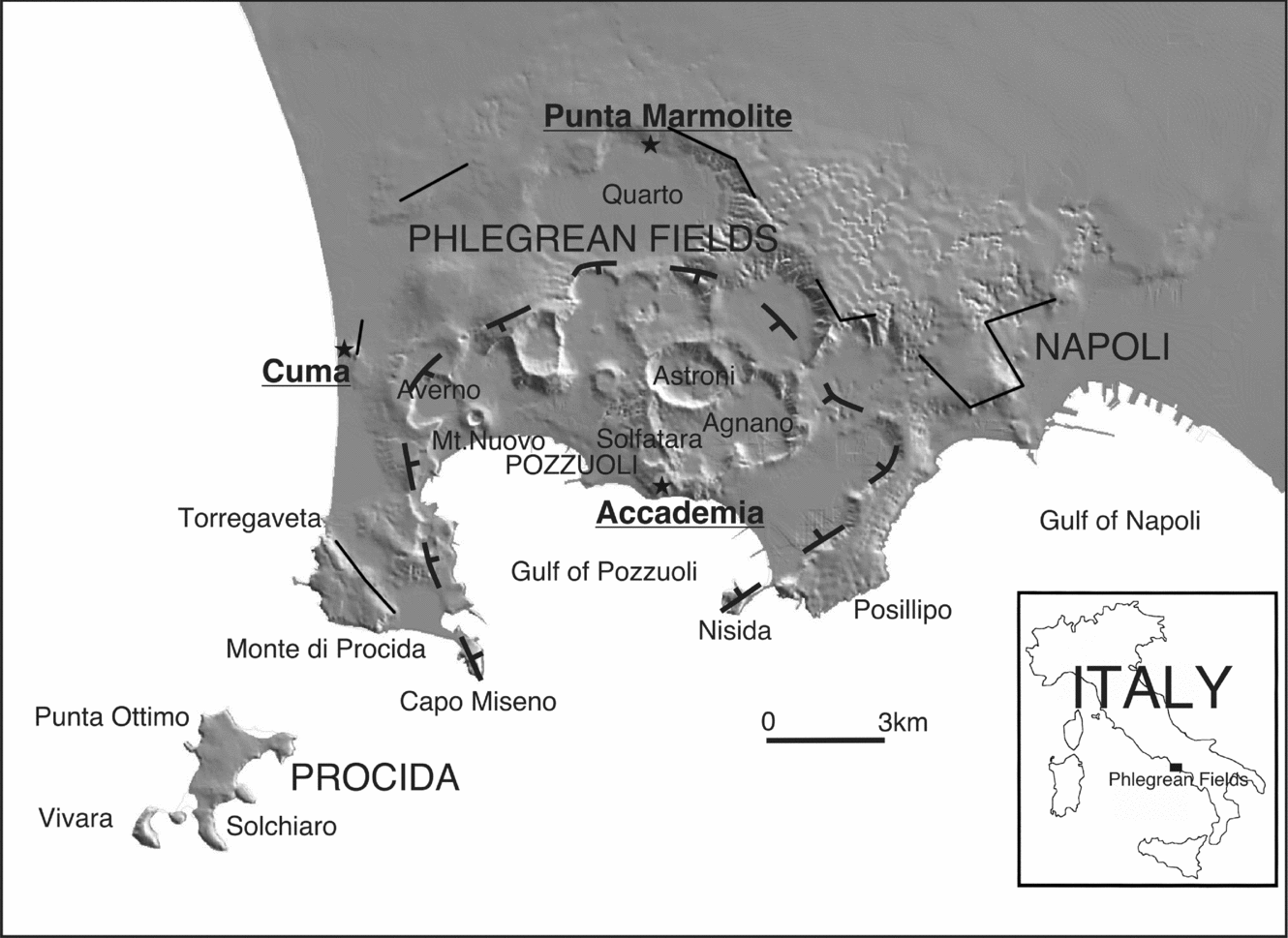
Figure 1. Sketch map of Phlegrean Fields, with main localities and sampling sites (image after Vilardo et al. Reference Vilardo, Terranova, Bronzino, Giordano, Ventura, Alessio, Gabriele, Mainolfi, Pagliuca and Veneruso2001).
Phlegrean rock compositions range from shoshonitic basalt through latite, to trachyte, trachyphonolite and phonolite, with the chemically evolved products predominating. The least-evolved compositions have geochemical and isotopic signatures commonly interpreted as indicative of subduction-related magmatism, generated by melting mantle metasomatized by fluids and melts from the still seismically active subducting slab (e.g. high LREE/HREE and LILE/HFSE ratios and Sr-, Nd-, Pb- and B-isotopic signature; LREE = light lanthanides; HREE = heavy lanthanides; LILE = large-ion lithophile elements; HFSE = high-field-strength elements; e.g. Beccaluva, Di Girolamo & Serri, Reference Beccaluva, Di Girolamo and Serri1991; Beccaluva et al. Reference Beccaluva, Coltorti, Di Girolamo, Melluso, Milani, Morra and Siena2002; D'Antonio et al. Reference D'Antonio, Tonarini, Arienzo, Civetta, Di Renzo, Beccaluva, Bianchini and Wilson2007; D'Antonio, Civetta & Di Girolamo, Reference D'Antonio, Civetta and Di Girolamo1999; Avanzinelli et al. Reference Avanzinelli, Lustrino, Mattei, Melluso and Conticelli2009).
The evolution of Phlegrean magmas is generally thought to be driven mainly by fractional crystallization processes (e.g. Di Girolamo, Reference Di Girolamo1970; Villemant, Reference Villemant1988; Melluso et al. Reference Melluso, Morra, Perrotta, Scarpati and Adabbo1995; Fowler et al. Reference Fowler, Spera, Bohrson, Belkin and De Vivo2007; Fedele et al. Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008, Reference Fedele, Zanetti, Morra, Lustrino, Melluso and Vannucci2009; D'Antonio, Reference D'Antonio2011), although open-system processes (crustal contamination, magma mixing/mingling) also seem to have been active, as testified by several lines of evidence, including (a) the occasional presence of xenocryst phases (e.g. Beccaluva et al. Reference Beccaluva, Di Girolamo, Morra and Siena1990; Civetta et al. Reference Civetta, Orsi, Pappalardo, Fisher, Heiken and Ort1997; Fedele et al. Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008, Reference Fedele, Zanetti, Morra, Lustrino, Melluso and Vannucci2009) and (b) significant isotopic variability in the juvenile products (e.g. Civetta et al. Reference Civetta, Orsi, Pappalardo, Fisher, Heiken and Ort1997; D'Antonio et al. Reference D'Antonio, Civetta, Orsi, Pappalardo, Piochi, Carandente, de Vita, Di Vito and Isaia1999, Reference D'Antonio, Tonarini, Arienzo, Civetta, Di Renzo, Beccaluva, Bianchini and Wilson2007; Pappalardo et al. Reference Pappalardo, Civetta, D'Antonio, Deino, Di Vito, Orsi, Carandente, de Vita, Isaia and Piochi1999, Reference Pappalardo, Piochi, D'Antonio, Civetta and Petrini2002; Pabst et al. Reference Pabst, Wörner, Civetta and Tesoro2008; Di Renzo et al. Reference Di Renzo, Arienzo, Civetta, D'Antonio, Tonarini, Di Vito and Orsi2011).
The samples of the present study belong to some of the relatively limited effusive products of the Phlegrean Fields (mainly explosive) activity: the lava domes of Cuma, Punta Marmolite and Accademia (also known as Mt Olibano). The Cuma and Punta Marmolite domes, with unspiked K–Ar ages of 37 and 47 ka, respectively (Cassignol & Gillot, Reference Cassignol, Gillot and Odin1982), have been emplaced on sectors involved in the caldera collapse related to the CI eruption, whereas the Accademia dome was emplaced at c. 3.9 ka (K–Ar; Di Girolamo et al. Reference Di Girolamo, Ghiara, Lirer, Munno, Rolandi and Stanzione1984) in the central part of the Phlegrean volcanic area, close to the Solfatara crater, the Agnano plain and in front of the roughly coeval Nisida tuff cone (3.9 ka Ar–Ar; Fedele et al. Reference Fedele, Insinga, Calvert, Morra, Perrotta and Scarpati2011).
This paper is the first report of the occurrence and compositional range for most minerals in Phlegrean Fields magmatic rocks. Therefore, this study provides information on the complete crystallization history of the Phlegrean Fields magmas to date, and helps to shed light on the processes that generate agpaitic rocks in silica-undersaturated trachytic systems in Italy and elsewhere. Moreover, the occurrence of chromiferous spinel, forsterite-rich olivine and other high-temperature minerals in the Accademia latite is also another undescribed and intriguing feature of the plumbing system.
The detailed study of the mineral assemblages and the mineral compositions of the almost unknown lava domes of the Phlegrean Fields (see the very few data reported by Di Girolamo et al. Reference Di Girolamo, Ghiara, Lirer, Munno, Rolandi and Stanzione1984; Rosi & Sbrana, Reference Rosi and Sbrana1987; D'Antonio et al. Reference D'Antonio, Civetta, Orsi, Pappalardo, Piochi, Carandente, de Vita, Di Vito and Isaia1999; Pappalardo et al. Reference Pappalardo, Civetta, D'Antonio, Deino, Di Vito, Orsi, Carandente, de Vita, Isaia and Piochi1999; Isaia, Marianelli & Sbrana, Reference Isaia, Marianelli and Sbrana2009; Morra et al. Reference Morra, Calcaterra, Cappelletti, Colella, Fedele, de’ Gennaro, Langella, Mercurio, de’ Gennaro, Beltrando, Peccerillo, Mattei, Conticelli and Doglioni2010) indicates a very prolonged crystallization history, unlike the Phlegrean pyroclastic rocks, and provides useful information on the following topics: (a) closed-system crystallization of highly evolved silica-undersaturated trachytic-trachyphonolitic compositions; (b) recharge in shallow magma reservoirs, with recycling of xenocrysts derived by distinct, often very magnesian, magma batches; (c) relevance for the Phlegrean Fields feeder system.
The magmatic assemblages of peralkaline undersaturated rocks have been studied in the classic settings of the Oslo rift in Norway and the Gardar Province of south Greenland (Sørensen, Reference Sørensen1974; Larsen & Sørensen, Reference Larsen, Sørensen, Fitton and Upton1987; Andersen et al. Reference Andersen, Erambert, Larsen and Selbekk2010 and references therein), and are of economic interest: studies on mineral assemblages and evolution trends in peralkaline oversaturated rocks are far more abundant (see White, Ren & Parker, Reference White, Ren and Parker2005; Ronga et al. Reference Ronga, Lustrino, Marzoli and Melluso2010; Macdonald et al. Reference Macdonald, Baginski, Leat, White and Dzierzanowski2011 and references therein). Our study adds a piece of knowledge on the final stages of crystallization in silica-undersaturated magmatic systems, where the main volatiles involved are Cl and F, as well as H2O.
2. Analytical techniques
Samples of the three lava domes, together with scorias of the Nisida tuff cone (Fedele et al. Reference Fedele, Insinga, Calvert, Morra, Perrotta and Scarpati2011) were collected, cut with a saw and crushed in a jaw crusher. Powders were produced in a low-blank agate mortar from clean chips first washed in distilled water. Major and trace elements were analysed by ICP-MS (inductively coupled plasma mass spectrometry) at ACTLABS, Ancaster, Ontario (see www.actlabs.com for details of the analytical procedures and Table 1 for analyses of reference standards). A few trace elements were analysed by XRF (X-ray fluorescence) at University of Napoli Federico II using pressed powder pellets and an Axios Panalytical instrument (see Ronga et al. Reference Ronga, Lustrino, Marzoli and Melluso2010 for details). Some 950 mineral compositions were obtained with Energy Dispersive Spectrometry at CISAG, University of Napoli Federico II, utilizing an Oxford Instruments Microanalysis Unit, equipped with an INCA X-act detector and a JEOL JSM-5310 microscope operating at a 15 kV primary beam voltage, 50–100 μA filament current, variable spot size and 50 s net acquisition time. Measurements were done with an INCA X-stream pulse processor. The following standards were used for calibration: diopside (Mg), wollastonite (Ca), anorthoclase (Al, Si), albite (Na), rutile (Ti), almandine (Fe), Cr2O3 (Cr), rhodonite (Mn), orthoclase (K), apatite (P), fluorite (F), barite (Ba), strontianite (Sr), zircon (Zr, Hf), ilmenite (Nb), synthetic Smithsonian orthophosphates (La, Ce, Nd, Sm, Y), pure vanadium (V), Corning glass (Th and U), sphalerite (S, Zn), sodium chloride (Cl) and pollucite (Cs). Backscattered electron (BSE) images were obtained with the same instrument (see Melluso, Conticelli & de'Gennaro, Reference Melluso, de’ Gennaro and Rocco2010 and Melluso, de'Gennaro & Rocco, Reference Melluso, de’ Gennaro and Rocco2010 for details).
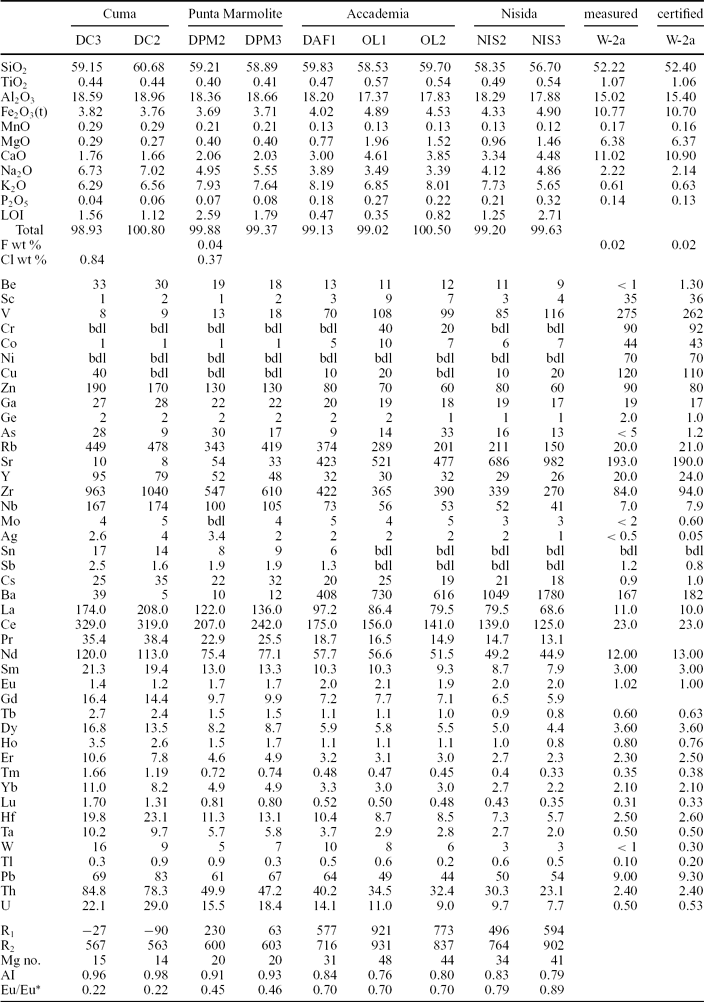
Table 1. Major oxides (in wt%), F and Cl (in wt%), trace elements (in ppm) and other parameters for whole-rock samples from the Cuma, Punta Marmolite and Accademia lava domes and for two samples from the Nisida tuff cone.
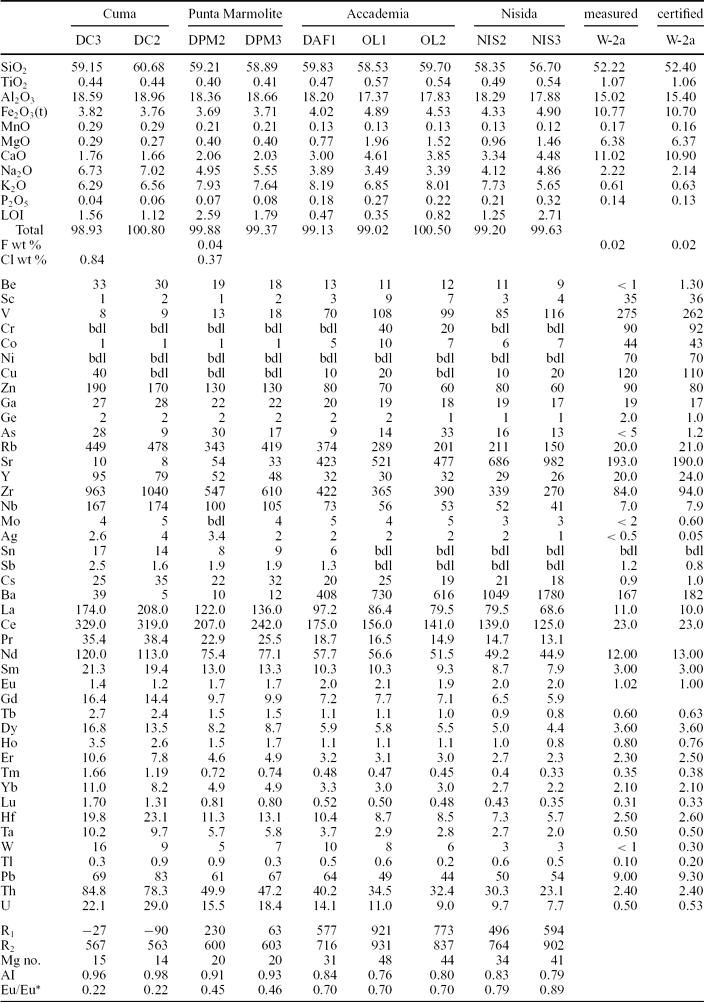
Loss on ignition (LOI, in wt%) was determined by standard gravimetric techniques. The analyses of reference standard W-2a are reported for comparison. R1 = 1000(6Ca + 2Mg + Al); R2 = 1000(4Si − 11(Na + K) − 2(Fe + Ti)); Eu/Eu* = EuN/(SmNGdN)1/2 (the subscript N means chondrite-normalized; chondrite of Boynton, Reference Boynton and Henderson1984); AI = molar (Na2O + K2O)/Al2O3; Mg no. = molar MgO*100/(MgO + FeO), with Fe2O3/FeO = 0.2. bdl – below detection limit.
3. Petrography and bulk-rock compositions
The samples of the massive facies of the Cuma dome (Fig. 2a–d; samples DC2, DC3) are porphyritic, with sparse phenocrysts of zoned sanidine, set in a fine-grained fluidal holocrystalline mesostasis made of sanidine and sodalite, with clinopyroxene, amphibole, olivine, oxides, fluorite, apatite and other phases. Plagioclase is absent. Yellow olivine is common as microlite and encloses feldspar, apatite and magnetite.

Figure 2. Typical petrographic features of the analysed samples as seen with a polarizing microscope or backscattered electron images (BSE): (a) Cuma dome, BSE: idiomorphic sanidine (alk), magnetite (mt), sodalite (sod) and zoned apatite/britholite (ap, bri) in a poikilitic olivine (ol) and accessory pyrochlore (pcl); (b) Cuma dome, BSE: strongly zoned apatite/britholite, magnetite and alkali feldspar included in poikilitic amphibole (amph); (c) Cuma dome, BSE: poikilitic rosenbuschite (ros), fluorite, olivine (ol) and idiomorphic anorthoclase (alk) in the groundmass; (d) Cuma dome, BSE: idiomorphic britholite, sanidine in poikilitic olivine and late aegirine (aeg); magnetite has a core of gahnite (Mn-gahn); (e) Punta Marmolite dome, parallel nicols: sodalite microphenocryst in a fluidal groundmass (gm); (f) Punta Marmolite dome, BSE: pollucite microlites with sanidine (san) and clinopyroxene (cpx). (g) Accademia dome, parallel nicols: groundmass fayalite (ol), sanidine and sodalite; (h) Accademia dome, crossed nicols: glomeroporphyritic cluster of sanidine with cores of highly Ca-rich plagioclase (plg); (i) Accademia, crossed nicols: the same feature with sparse phenocrysts; (j) Accademia dome, parallel nicols: clinopyroxene with amphibole rims and inclusions of sanidine (alk) and magnetite; (k) Accademia dome, BSE: idiomorphic, zoned olivine with high-Mg core, high-Fe rims (bright) and chromite inclusions (chr); (l) Nisida tuff cone, BSE: idiomorphic sanidine (san), with bright, Ba–Sr-rich cores, included in idiomorphic analcime (anl), likely former sodalite. gm – groundmass.
The samples of the massive facies of the Punta Marmolite dome (Fig. 2e, f; samples DPM2, DPM3) are weakly porphyritic, with sanidine (sometimes mantling plagioclase) and sodalite phenocrysts, rare subhedral nepheline, magnetite and clinopyroxene microphenocrysts in a holocrystalline, strongly fluidal groundmass with the same minerals and accessories, among which apatite-britholite, fluorite and baddeleyite are the most abundant.
The samples of the Accademia dome (Fig. 2g–k) have at least two different facies. Samples from the front of the ‘Cava Regia’ quarry (samples OL1, OL2) have phenocrysts of plagioclase, colourless to pale-green clinopyroxene, sanidine, resorbed phlogopite and olivine with spinel inclusions, often arranged in glomeroporphyritic clusters of clinopyroxene, plagioclase and phlogopite, or plagioclase and sanidine, and set in a groundmass of sanidine, Fe-rich olivine, clinopyroxene and magnetite. Baddeleyite and fluorite are very frequent accessory phases. Plagioclase is frequently and abruptly mantled by sanidine (never the reverse), and crystallized earlier than, or contemporaneously with, Mg-clinopyroxene. The other facies, represented by very large blocks of several tens of cubic metres at the base of the quarry and along the ‘La Pietra’ coastline (sample DAF1) and in the surrounding areas, is devoid of olivine phenocrysts, has sanidine phenocrysts, with plagioclase inclusions, small amounts of green-brown amphibole surrounding greenish clinopyroxene (Fig. 2j), and a more interstitial green clinopyroxene, with microlites of yellow olivine, found interstitial to sanidine and in the groundmass (Fig. 2g)
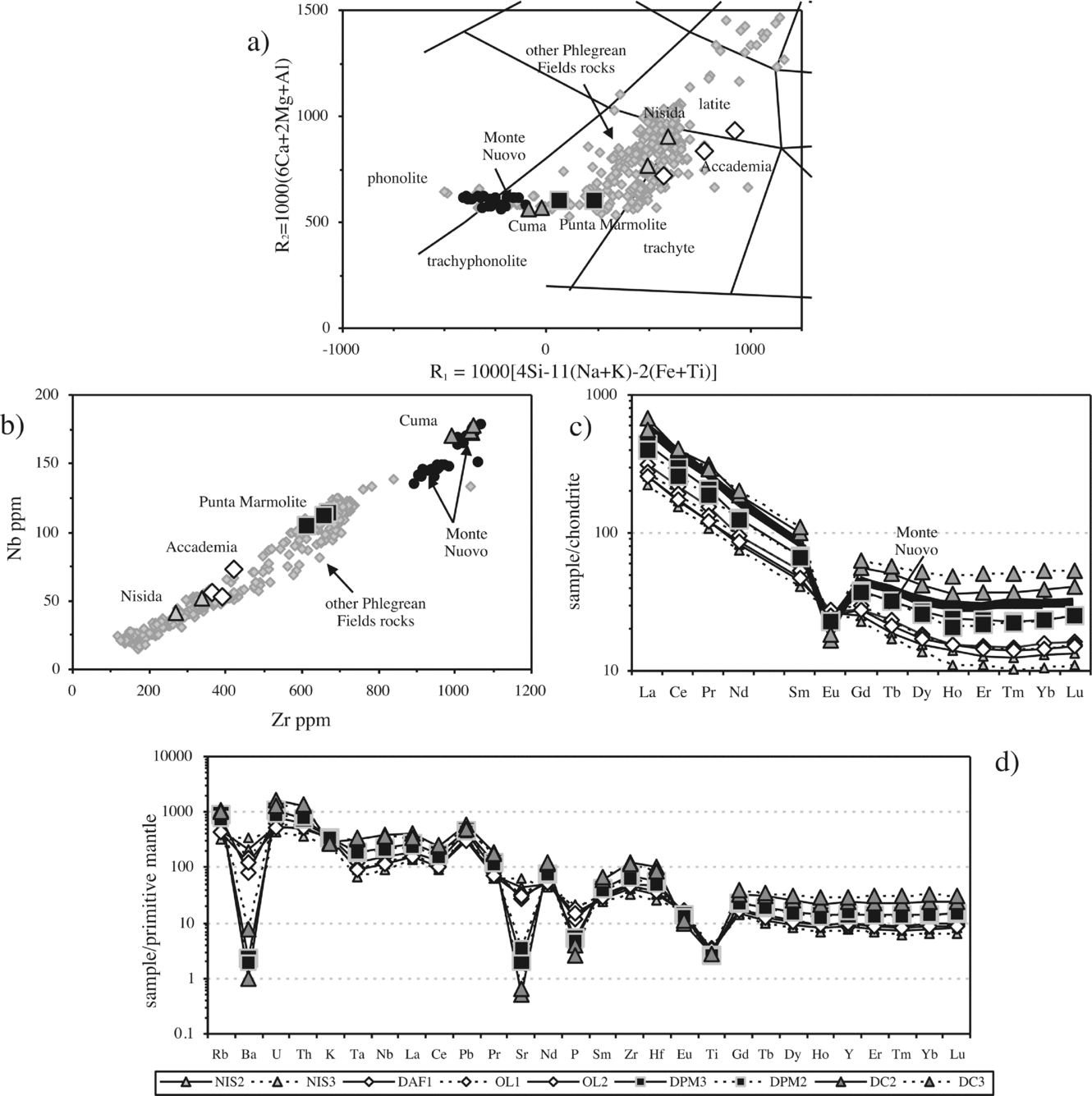
The Cuma samples are trachyphonolites, the Punta Marmolite samples are trachytes and trachyphonolites, and the Accademia samples are latites and trachytes, according to the R1–R2 classification scheme (Fig. 3a). Major oxides have mild compositional ranges: SiO2 ranges from 56.7 to 60.7 wt%, MgO ranges from 0.27 to 2 wt%, CaO from 1.7 to 4.6 wt% and Fe2O3t from 3.8 to 4.9 wt%. The increasing Na2O/K2O from Accademia (Na2O/K2O = 0.42–0.51), through the Punta Marmolite (Na2O/K2O = 0.73) to Cuma (Na2O/K2O = 1.07) rocks indicates that the degree of magmatic evolution is accompanied by an increasing sodic character. The Cuma sample reaches 0.84 wt% Cl, whereas the Punta Marmolite sample has Cl = 0.37 wt% and F = 0.04 wt% (Table 1).
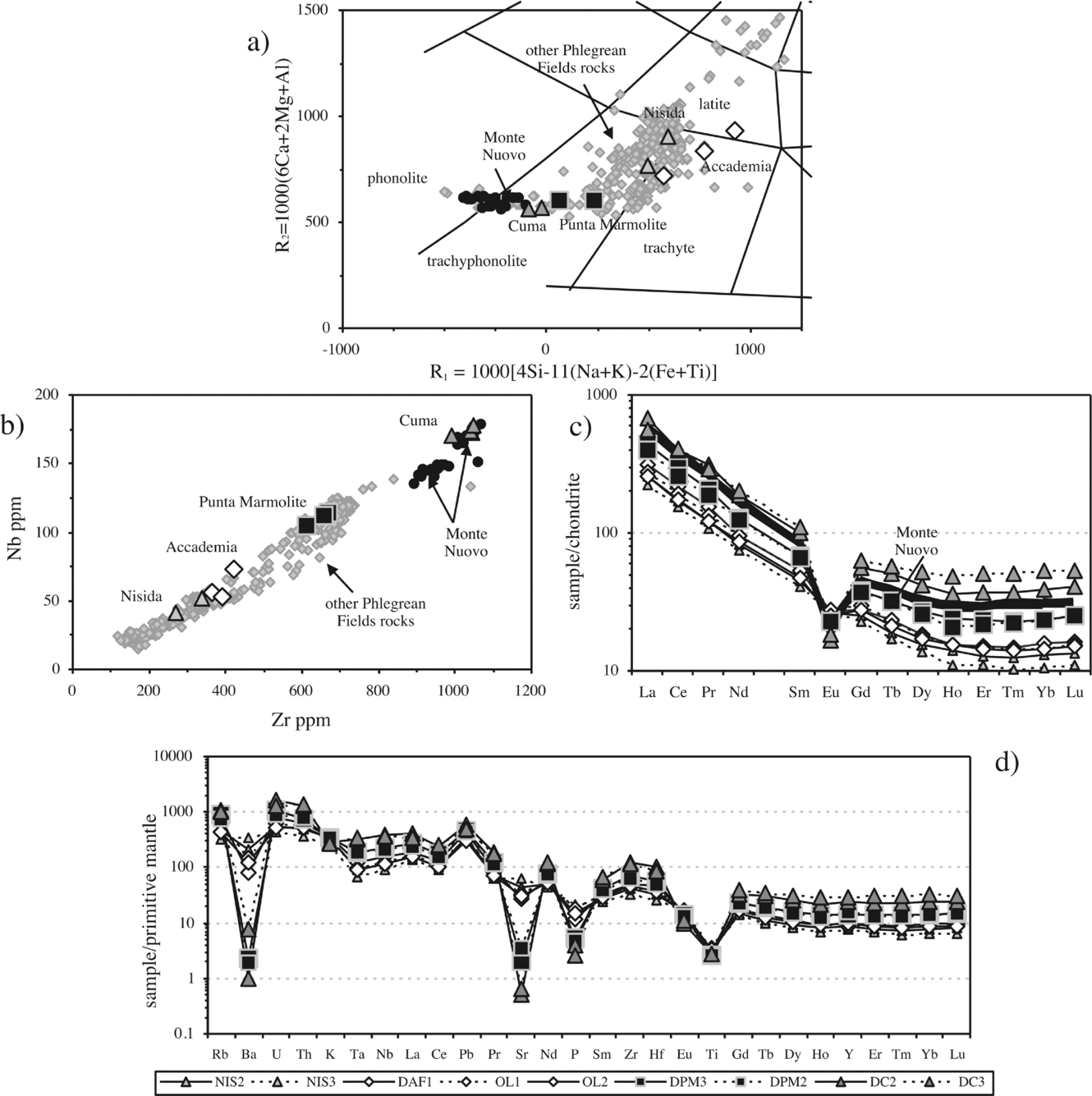
Figure 3. (a) R1–R2 classification diagram of the Phlegrean Fields volcanic rocks (after De La Roche et al. Reference De La Roche, Leterrier, Grandclaude and Marchal1980) with the samples from this study and literature data from D'Antonio et al. (Reference D'Antonio, Civetta, Orsi, Pappalardo, Piochi, Carandente, de Vita, Di Vito and Isaia1999), Pappalardo et al. (Reference Pappalardo, Piochi, D'Antonio, Civetta and Petrini2002) and Fedele et al. (Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008); (b) Zr–Nb diagram with the Phlegrean Fields rock compositions and the samples of this study; the Monte Nuovo analyses are taken from Pappalardo et al. (Reference Pappalardo, Piochi, D'Antonio, Civetta and Petrini2002) and D'Oriano et al. (Reference D'Oriano, Poggianti, Bertagnini, Cioni, Landi, Polacci and Rosi2005); (c) Lanthanide chondrite-normalized diagrams: the Monte Nuovo lanthanide compositions are taken from D'Oriano et al. (Reference D'Oriano, Poggianti, Bertagnini, Cioni, Landi, Polacci and Rosi2005). Normalization values of Boynton (Reference Boynton and Henderson1984); (d) mantle-normalized multi-element patterns. Normalization values of Lyubetskaya & Korenaga (Reference Lyubetskaya and Korenaga2007).
The Cuma samples have an agpaitic index (AI, molar (Na2O + K2O)/Al2O3) of 0.96–0.98, while Punta Marmolite and Accademia samples have an AI of 0.91–0.93 and 0.76–0.84, respectively. The Cuma bulk-rock compositions reach among the highest concentrations of Mn (0.29 wt% MnO), Zn (170 ppm), Rb (480 ppm), Zr (1040 ppm) and Nb (174 ppm) reported for the Phlegrean rocks (see Fig. 3b). These values are coupled with very low MgO (0.27 wt%), P2O5 (0.06 wt%), Sr and Ba (both < 10 ppm). Cr and Ni contents are below the detection limits in the Cuma and Punta Marmolite samples, whereas Cr concentration reaches 20–40 ppm in samples OL1 and OL2. Compositional zoning is observed at the Accademia dome, with the samples from the ‘Cava Regia’ being slightly more mafic with respect to lava blocks from the outskirts of the dome (Table 1).
Total lanthanide (ΣREE) concentrations of the Cuma dome reach about 750 ppm, and are coupled with the deepest Eu troughs (Eu/Eu* = 0.22; Table 1). At Punta Marmolite, ΣREE is 530 ppm, with a slightly less pronounced Eu trough (Eu/Eu* = 0.46). The samples of the Accademia dome have ΣREE = 320–384 ppm and moderate Eu troughs (Eu/Eu* = 0.70). Worth noting is the parallel shape of the chondrite-normalized patterns (Fig. 3c), with the exception of Eu. On multielemental mantle-normalized diagrams (Fig. 3d), we note that, apart from the similar pattern of all the samples, including those of the Nisida juvenile samples (data in Table 1), the patterns are characterized by very small Ta and Nb troughs, a more marked trough at Ti, peaks at Pb and increasing element abundances towards the rocks of the Cuma dome. The relative concentrations of P, Ba, Sr and Eu decrease towards the most evolved rocks, whereas the Ti, K and Pb abundances are roughly constant.
4. Mineral compositions
4.a. Clinopyroxene and aenigmatite
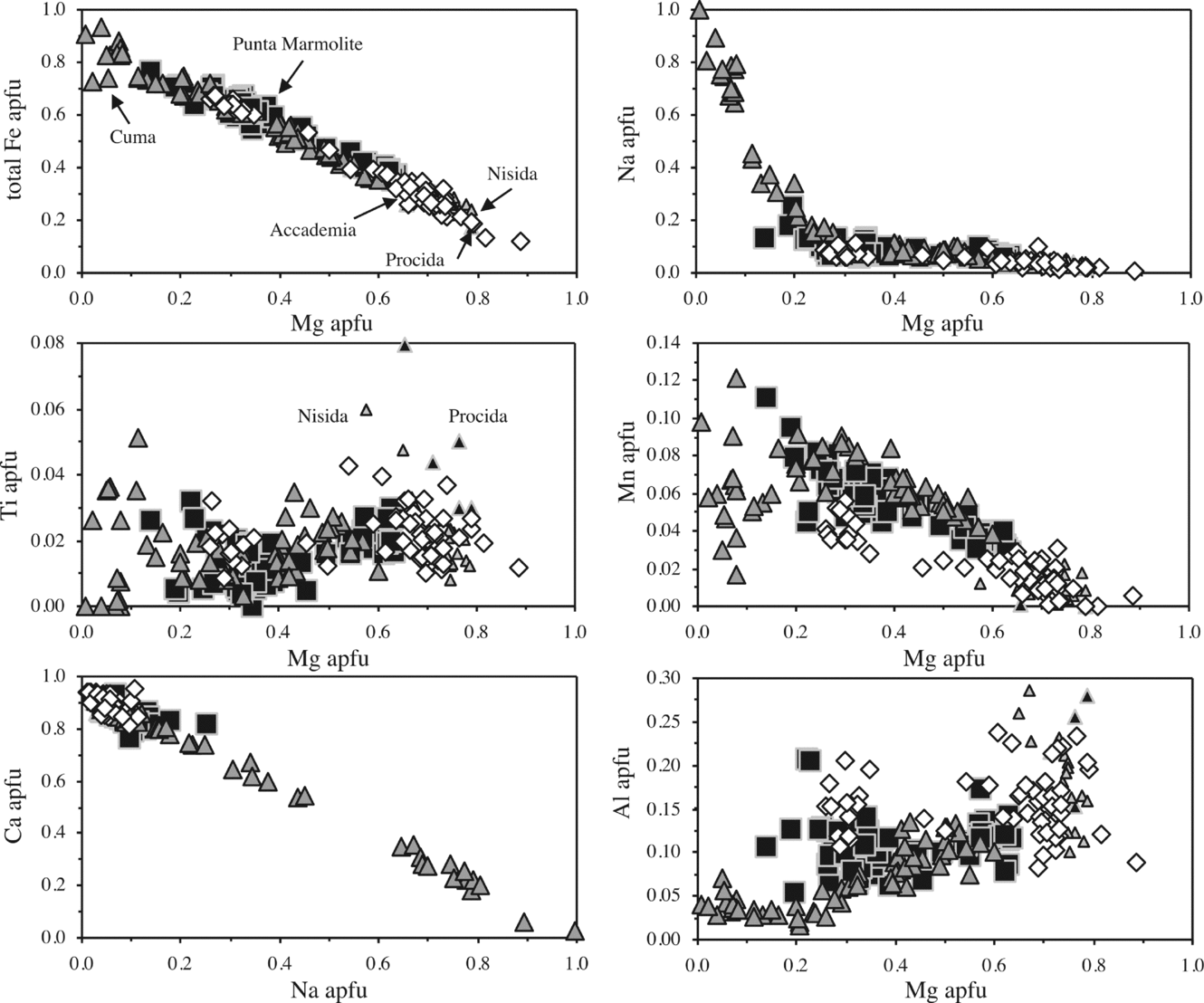
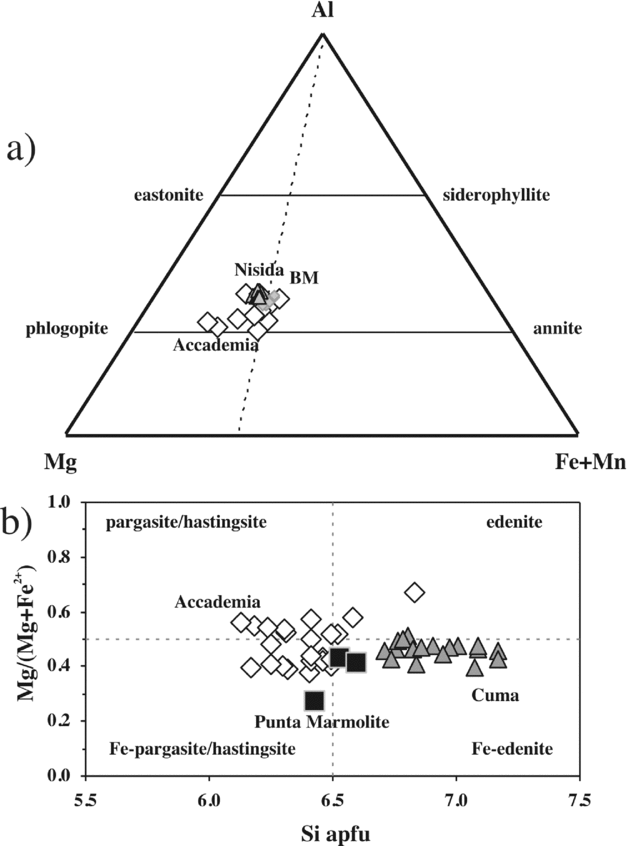
Clinopyroxene in the Cuma samples ranges continuously from Fe-rich diopside (salite; Ca48Mg30Fe22, end-members in atoms %) to almost pure aegirine (Na2O = 13.3 wt%; CaO = 0.7 wt%), the latter present interstitially and occasionally with Zr-rich compositions (up to 3.1 wt% ZrO2; Table S1 in the online Appendix at http://www.journals.cambridge.org/geo). In the Punta Marmolite samples, clinopyroxene ranges from diopside (salite; Ca45Mg34Fe21) to Na-rich hedenbergite (ferrosalite; Ca45Mg11Fe44; Na2O = 3.3 wt%). In the Accademia samples, clinopyroxene ranges from Mg-rich diopside (Ca48Mg45Fe7) to hedenbergite (Ca48Mg19–14Fe34–38; Na2O up to 1.45 wt%), showing evident bimodal distribution (Fig. 4). The compositional variation, which covers the complete spectrum from Mg- to Fe-rich compositions, and therefore is thought to be representative of the whole Phlegrean magmatic system, indicates decreasing Al and increasing Fe with decreasing Mg. Ti broadly behaves as Al, with the exception of the most Na-rich pyroxenes, where variable amounts of Ti-rich, Ca–Al-free molecules (such as NaTi0.5Fe0.5Si2O6 and, for Zr, NaZr0.5Fe0.5Si2O6) can be sporadically present. Na gently increases to the most Fe-rich compositions and then abruptly reaches pure aegirine compositions in the Cuma samples, whereas Mn increases with Fe, and then decreases in Fe-rich crystals from Cuma (a likely effect of co-crystallization of Mn-olivine and Mn-oxides, see below).
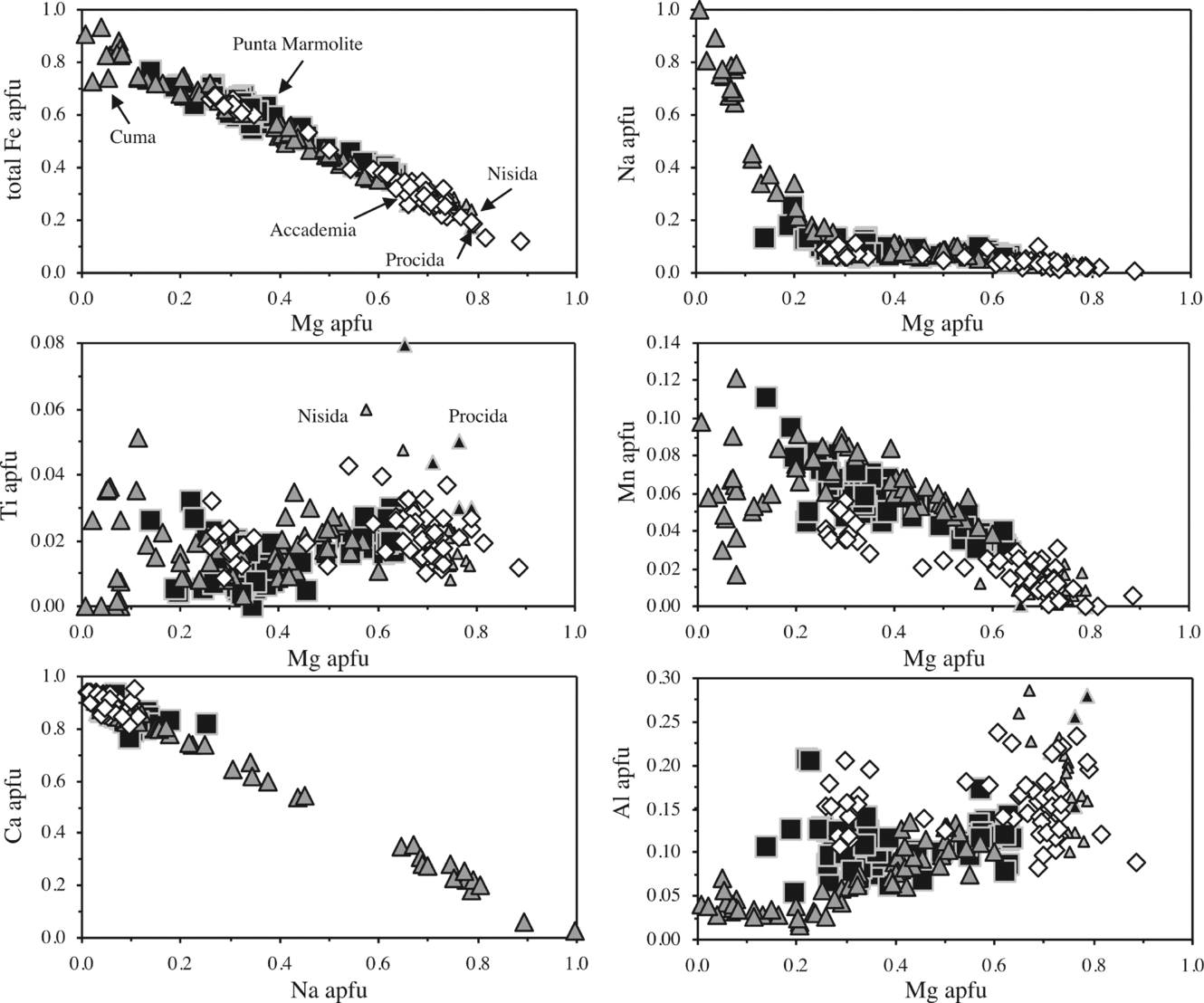
Figure 4. Binary diagrams with analysed clinopyroxene compositions (elements in apfu, atoms for 6 oxygens and 4 cations). The pyroxenes of Nisida and Procida (small triangles) are authors’ unpublished data. Note that the marked increase in the aegirine component occurs only at low Mg contents. This corresponds also to a scattered increase in Ti and to the lowest Al contents. The tight correlation between Mg and total Fe suggests that Mg→Fe (regardless of the oxidation state) is the main substitution in the M2 site, and that Fe2+ and Fe3+ cannot be treated as different cations.
Rare interstitial crystals of aenigmatite (Na2Fe5TiSi6O20) have been found in the Cuma dome. The compositions have relatively high Mn (1.8–2.6 wt% MnO; Table S1), higher than the type aenigmatites from Pantelleria (< 1.5 wt% MnO; see White, Ren & Parker, Reference White, Ren and Parker2005 for reference compositions).
4.b. Feldspar
Low-Ca sodic sanidine-anorthoclase (Ca1–5Na45–63K55–32, end-members in atoms %) is the main feldspar of the Cuma dome, with the exception of one relatively Ca-rich composition (Ca20Na62K18; Fig. 5). A slightly more potassium- and calcium-rich sanidine (Ca2–6Na28–65K75–29) is found in the Punta Marmolite samples rarely associated with very Ca-rich plagioclase (Ca84–79Na16–20K0–1) with more sodic rims (Ca47–38Na49–55K5–8). Sodium-rich pollucite ((Cs, Na)AlSi3O8) grains have been found in the groundmass of the Punta Marmolite dome (Fig. 2j; Table S1).
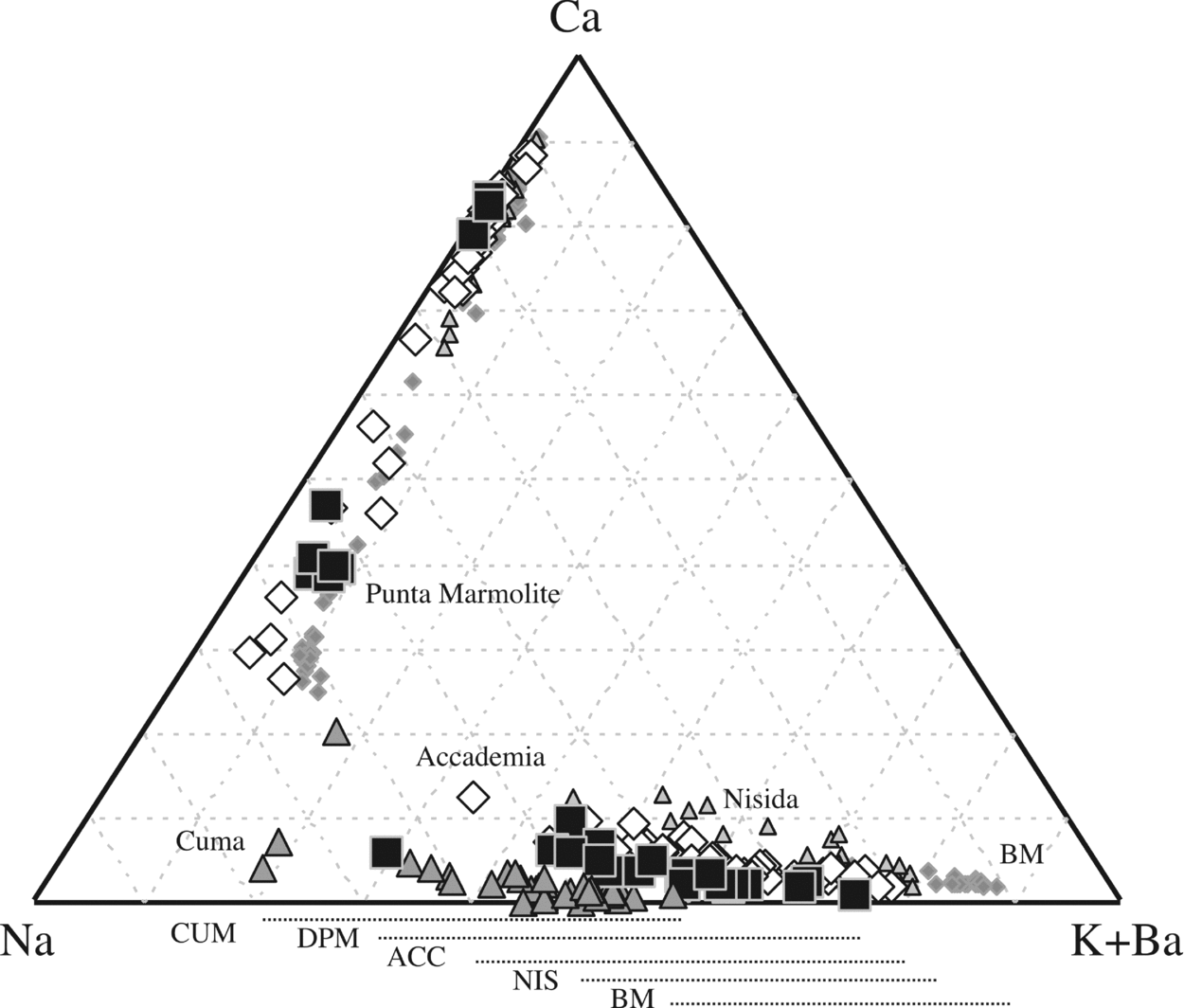
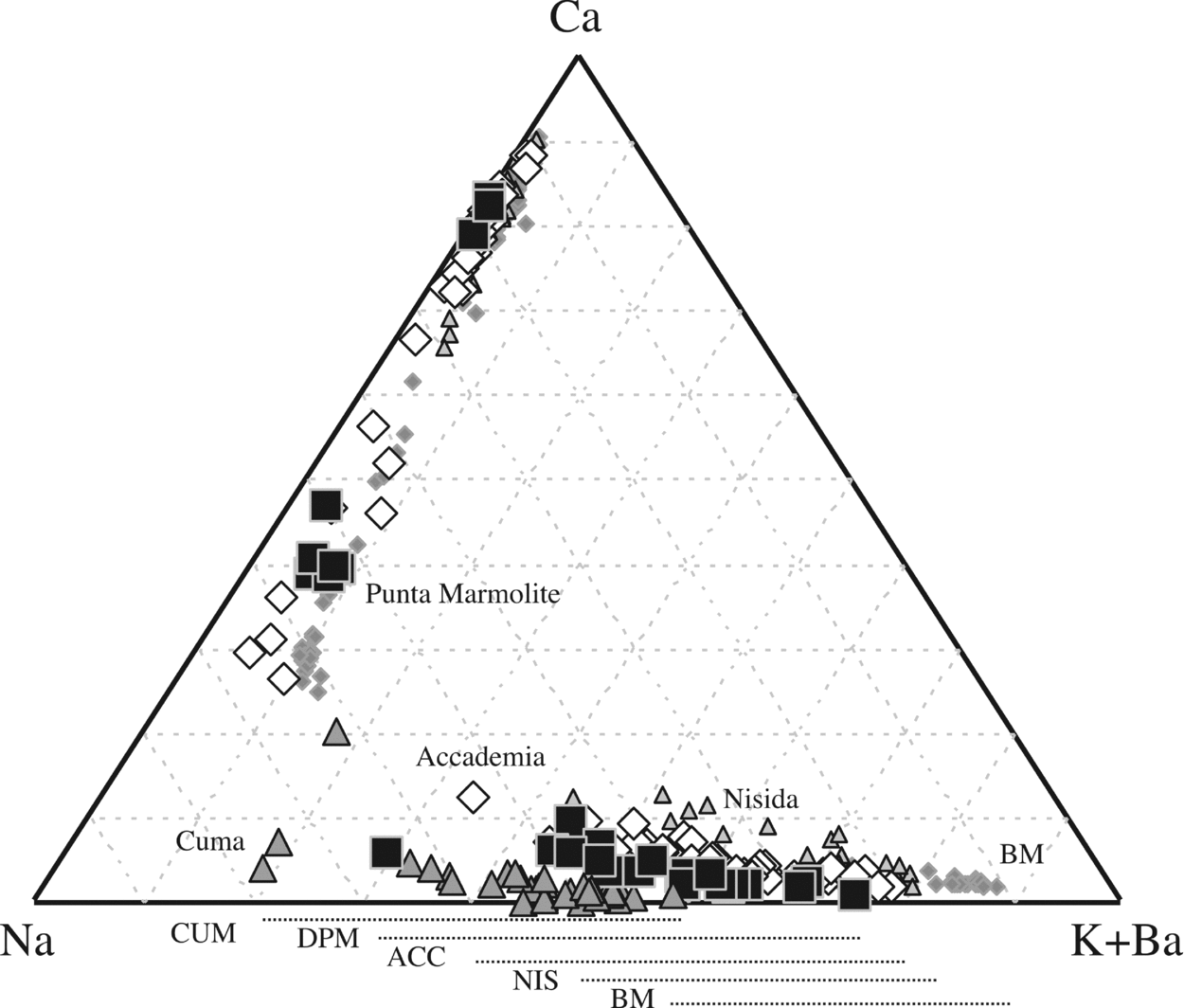
Figure 5. Ca–Na–K+Ba diagram for feldspar compositions. The feldspars of the Breccia Museo (grey diamonds) are from Fedele et al. (Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008), those of Nisida are authors' unpublished data. The different ranges of alkali feldspar compositions are evidence of crystallization from differently evolved magmas, and that the increasing Na contents of the most potassic feldspars tend towards the minimum of the alkali feldspar loop (Ab65Or35), actually reached only in the Cuma samples.
Ca-to-Na-rich plagioclase (Ca87–26Na11–64K2–10) and K-rich sanidine (Ca2–13Na20–54K78–34) are found as phenocrysts in the Accademia dome. Sr and Ba contents are relatively low throughout (up to 2 wt% SrO and 1.3 wt% BaO, respectively), as expected from the host-rock concentrations.
4.c. Sodalite, nepheline and analcime
Sodalite is the main feldspathoid of the Phlegrean rocks. It is idiomorphic in the Punta Marmolite samples, with a typical hexagonal shape, resembles analcime in other Phlegrean rocks (Fig. 2e, l) and typically occurs as an interstitial phase in the samples from the Cuma and Accademia domes. As usual, it shows very limited solid solution to the other volatile-rich feldspathoids, thus is almost devoid of K, Ca and SO3 (see Melluso, Morra & Di Girolamo, Reference Melluso, Morra and Di Girolamo1996; Melluso, Morra & de’ Gennaro, Reference Melluso, Morra and de’ Gennaro2011).
Nepheline has been found in Punta Marmolite samples. It ranges in composition from Ne79Ks13Sil8 to Ne78Ks9Sil13 (end-members in wt%), displaying high Na/(Na + K) (= 0.87–0.91), relatively high silica excess and low Ca contents (0.6–1.5 wt% CaO; Table S1). Similar nephelines have been found in the Toppo S. Paolo phonolite, whereas the bulk of the Mt Vulture nephelines have a much lower silica excess than the Punta Marmolite compositions (Melluso, Morra & Di Girolamo, Reference Melluso, Morra and Di Girolamo1996; Melluso, Morra & de’ Gennaro, Reference Melluso, Morra and de’ Gennaro2011).
4.d. Amphibole and phlogopite
Brown-greenish amphibole is common in the groundmass of Cuma samples, is rare in the Punta Marmolite samples, but is typically found in the samples of the Accademia dome, both as the classical rim on clinopyroxene (Fig. 2j) and as a late-crystallized groundmass phase. The compositions are distinct, with pargasite, Fe-pargasite and Fe-pargasitic hornblende (with a few edenites) found in the Accademia samples, and Fe-edenite in the Cuma samples (Fig. 6). The rare amphibole in the Punta Marmolite dome is a ferro-edenitic to ferro-pargasitic hornblende (Table S1). The range of Mg nos is similar for the Cuma and Accademia samples (Mg no. = 40–51 at Cuma; Mg no. = 38–67 at Accademia) and slightly lower in the Punta Marmolite samples (Mg no. = 27–43). The TiO2 content never exceeds 1.9 wt%, and MnO reaches similar values (up to 2.1 wt%; Table S1). No alkali amphiboles were positively identified: therefore, amphibole crystallized before the late-stage liquid reached peralkaline compositions.
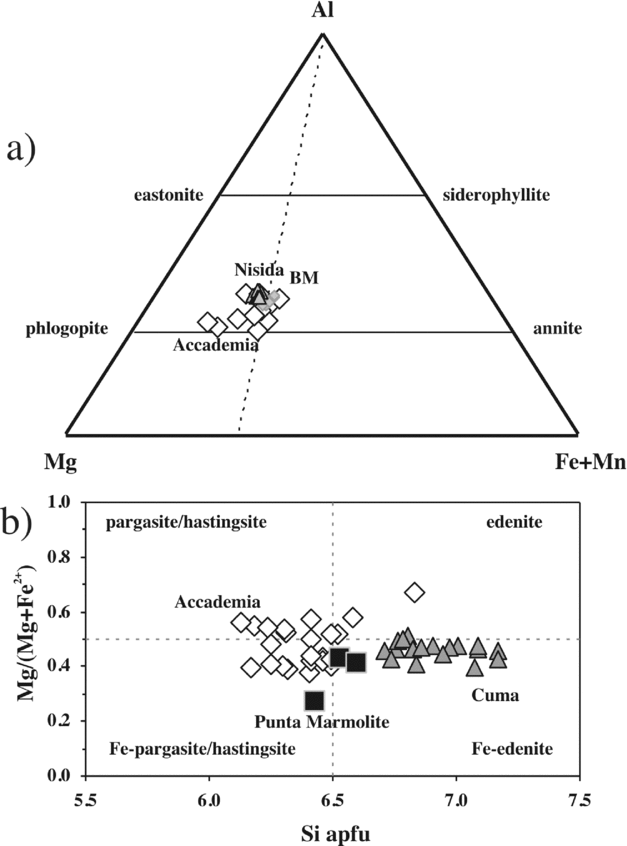
Figure 6. (a) Phlogopite and (b) amphibole classification diagrams. Phlogopite compositions of Breccia Museo (BM) are taken from Fedele et al. (Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008). The phlogopite compositions of the Nisida tuff cone are authors’ unpublished data.
Phlogopite is rare or absent in the Cuma and Punta Marmolite samples, and more common in the Accademia samples in the form of corroded phenocrysts and in glomeroporphyritic clusters with plagioclase, clinopyroxene and magnetite. It has a Mg no. from 64 to 74, F from 0.6 to 6.4 wt%, and moderate Ba and Ti contents (up to 1.2 wt% BaO and 5.5 wt% TiO2, respectively; Table S1).
4.e. Olivine
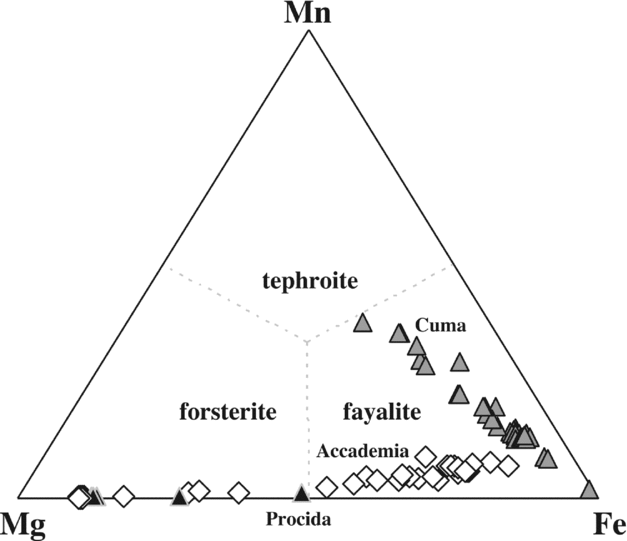
Olivine of the Cuma samples is exclusively found as microlites in the groundmass, often in contact with aegirine (Fig. 2d), and ranges in composition from pure fayalite (Mg1Fe96Ca2Mn2, in atoms %) to tephroite-rich olivine (Mg22Fe40Ca2Mn37; Fig. 7). In the Accademia samples (e.g. OL2), idiomorphic, zoned olivine phenocrysts (Fig. 2k) range from Mg89Fe10Ca1Mn0 to Mg61Fe37Ca1Mn1, while groundmass microlites in samples OL1, OL2 and DAF1 range from Mg45Fe52Ca0Mn2 to Mg12Fe79Ca2Mn7, with a small compositional gap from the most to the least Mg-rich compositions, and with no resemblance to the olivine compositions found in the groundmass of the Cuma rocks. Olivine is absent in the Punta Marmolite dome (Table S1). The most Fe-rich olivines of the Cuma samples also carry small amounts of Zn (up to 0.5 wt% ZnO).
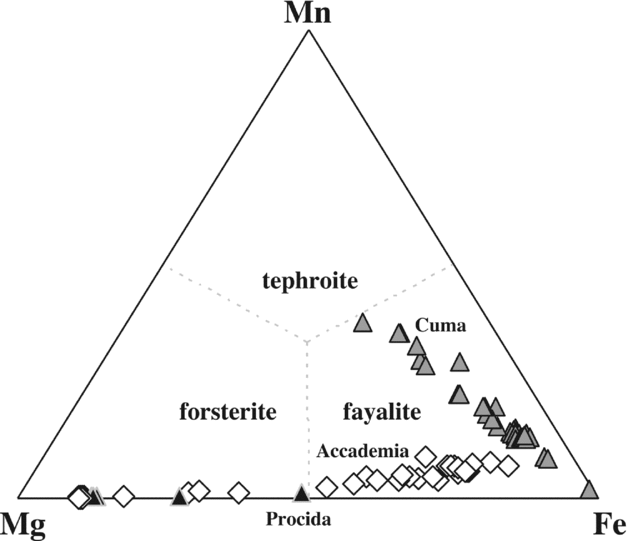
Figure 7. Fe–Mg–Mn diagram for the analysed olivine compositions. Olivine compositions of Procida (Solchiaro eruption) are unpublished data of the first author.
The Mg-rich crystals of the Accademia lava dome include chromiferous spinels (Fig. 2k and see next Section). In the groundmass, olivine matches the compositions found in evolved trachytic-rhyolitic magmas (see Ronga et al. Reference Ronga, Lustrino, Marzoli and Melluso2010 for examples with similar compositional ranges).
4.f. Chromiferous spinel, magnetite and other spinels
Magnetite is the main Fe–Ti oxide in the Cuma and Punta Marmolite domes. The compositions are quite different, with the Cuma spinels showing the highest ulvöspinel content (62 mol% ulvöspinel, corresponding to 20 wt% TiO2). Mn contents in magnetite reach relatively high values (4.5 wt% MnO). Additional manganoan gahnite (MnO = 12.8–13.7 wt%; ZnO = 28.6–29.2 wt%; Al2O3 = 57.3–60 wt%) has been found in the Cuma samples as an inclusion in altered magnetite (Fig. 2d) and other Mn-spinels in the Punta Marmolite samples (Table S1). In the Accademia samples, magnetite of the groundmass has TiO2 up to15 wt% and MnO up to 3.6 wt%. No ilmenite was found.
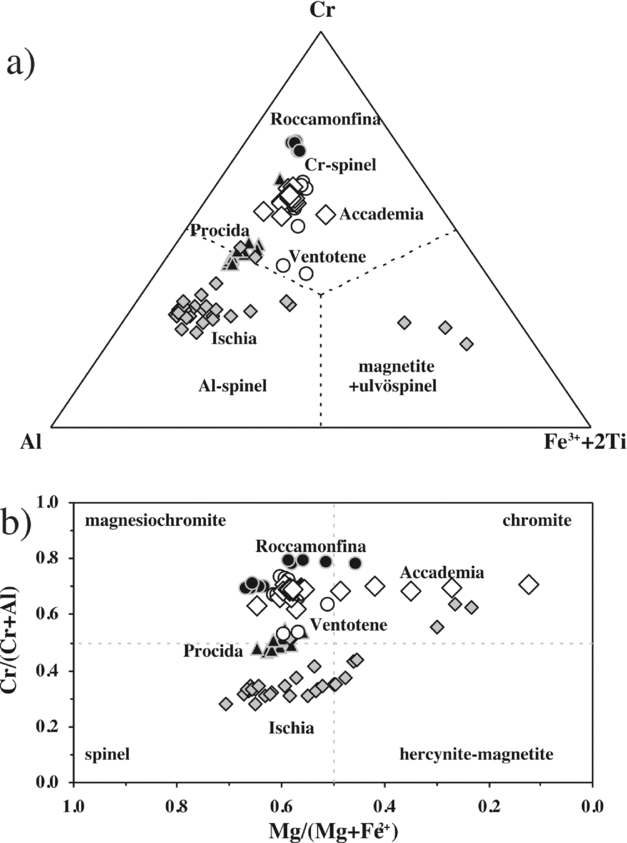
Chromiferous spinel has been found in Mg-rich olivine phenocrysts and microphenocrysts of the Accademia dome (Fig. 2k). It ranges from magnesiochromite to chromite, with a significant range in Mg/(Mg + Fe2+), from 0.12 to 0.65, and minor in Cr/(Cr + Al), from 0.62 to 0.71 (Fig. 8). The Ti concentration is low (0.5–1.5 wt% TiO2; Table S1), as typical of spinels crystallized in primitive basalts and found in olivines with high forsterite content (see Melluso, de’ Gennaro & Rocco, Reference Melluso, Conticelli and de’ Gennaro2010; S. Conticelli & L. Melluso, unpub. data).
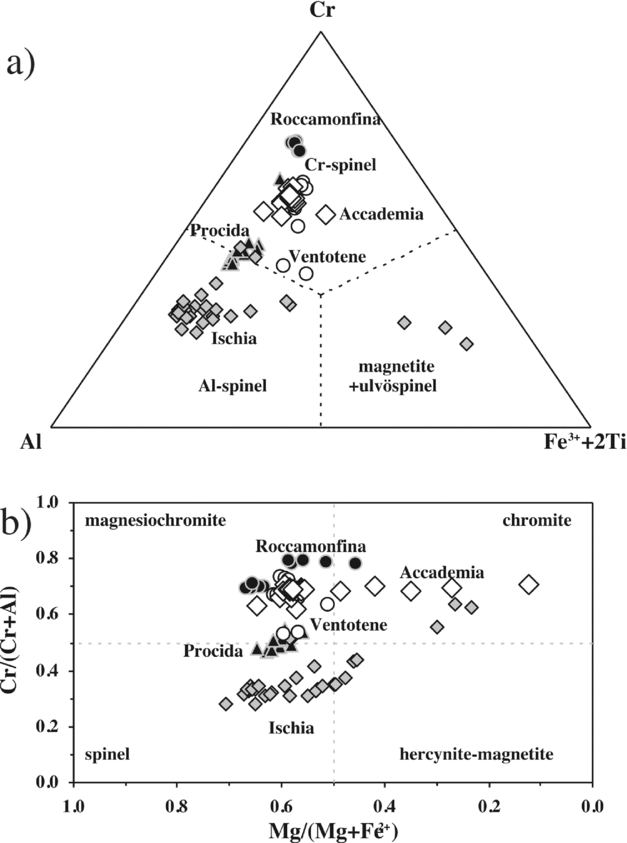
Figure 8. (a) Cr–Al–Fe3+ + 2Ti and (b) Mg/(Mg + Fe2+) v. Cr/(Cr + Al) diagrams for the chromiferous spinel compositions of Accademia. Spinels from Ischia, Procida, Ventotene and Roccamonfina are taken from D'Antonio & Di Girolamo (Reference D'Antonio and Di Girolamo1994), Di Girolamo et al. (Reference Di Girolamo, Melluso, Morra and Secchi1995) and L. Melluso & S. Conticelli, unpub. data.
4.g. Baddeleyite, fluorite, pyrochlore, rosenbuschite, thorite, zircon and zirconolite
The three lava domes have small amounts of groundmass baddeleyite (ZrO2), occasionally U-rich (1–3.7 wt% UO2). In the Cuma samples, baddeleyite is accompanied by zircon. Pyrochlore ((Ca,Na)2Nb2O6 (OH,F)) is common in the Cuma rocks, and rare in the other outcrops. It is very rich in U (10.5–17.7 wt% UO2), and almost devoid of Th (Table S1). A few Mn-rich compositions are also found. Fluorite (CaF2) is another very common groundmass mineral, often inglobated by rosenbuschite in the Cuma dome. It is completely devoid of solid solutions to Na–Al or Sr fluorides (Table S1). Rosenbuschite, a typical Zr–F-rich disilicate ((Ca,Na)3(Zr,Ti)Si2O7(OH,F)2), is a featuring accessory phase in the groundmass of the Cuma dome. The composition is widely variable relative to reference compositions (e.g. Christiansen, Johnsen & Makovicky, Reference Christiansen, Johnsen and Makovicky2003; Andersen et al. Reference Andersen, Erambert, Larsen and Selbekk2010), and has a high concentration of Na (7.6–12.5 wt% Na2O), Mn (2.6–8.1 wt% MnO) and Fe (4–9.9 wt% FeO), widely variable Ca (7–25 wt% CaO) and low Ti (0.7–2.1 wt% TiO2), at ZrO2 ranging from 16.8 to 21.3 wt% and Nb2O5 from 1.7 to 3.7 wt% (Table S1). Fluorine varies from 5.6 to 8.4 wt%. Na and Ca have a marked negative correlation, and so is the relationship between Zr and Nb, and Ca and Fe, but the substitutions are certainly complex, given also the high concentration of Fe and Mn and the substantially unknown oxidation state of these two elements. The REE, Th and U contents are very low (Table S1). Zirconolite (CaZrTi2O7) has been found in the Accademia dome, sometimes as rims of baddeleyite. The composition has high Nb (10.3 wt% Nb2O5; Table S1). Thorite (ThSiO4) has been found at Cuma.
We did not find titanite, which is the main Ti-rich mineral found in Phlegrean juvenile rocks and intrusive syenitic ejecta.
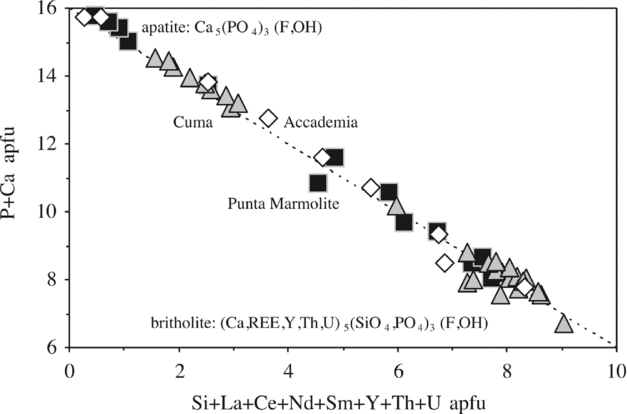
4.h. Apatite-britholite and monazite
Fluorapatite (Ca5(PO4)3(F,OH)), almost systematically zoned to britholite (REE5(SiO4)3(OH,F); Fig. 2a, b), is a common mineral of all the samples of this study. The compositions vary from pure apatite, relatively poor in lanthanides, Th and U, to compositions very rich in a britholite component (e.g. SiO2 = 20.5 wt%; P2O5 = 8.2 wt%; atomic Si*100/(Si + P) = 72–75), which carry notable concentrations of lanthanides, Th and U (Table S1; Fig. 9). The marked and systematic (core to rim) negative correlation between apatite and britholite components (Table S1; Fig. 9) suggest that inclusion of lanthanides, Th and U at the expense of Ca generates this substitution mechanism. Judging from Figure 9, it is possible that there is a compositional gap between apatite and britholite. A few crystals of britholite also contain locally significant amounts of arsenic (up to 7–18 wt% AsO4), pointing to additional solid solution with P and Si involving johnbaumite and svabite end-members (nomenclature of Pasero et al. Reference Pasero, Kampf, Ferraris, Pekov, Rakovan and White2010). Fluorine decreases with increasing britholite components (from 5 wt% to nearly 0 wt%). Monazite (nominally (La,Ce,Nd)PO4) has been found in the Cuma rocks (Table S1). It has La2O3, Ce2O3 and Nd2O3 reaching contents as high as 55–56 wt%, high Th (5–5.5 wt% ThO2) and low Ca (0–3 wt% CaO).
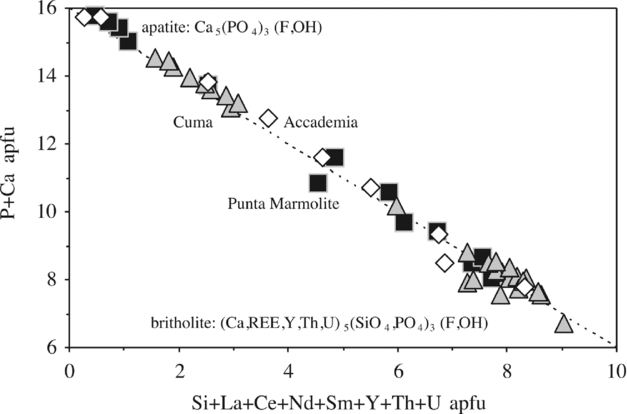
Figure 9. Apatite–britholite compositions as plotted in the Ca + P (apatite) v. Si + REE + U + Th (britholite) diagram (16 cations for 26 oxygens).
5. Discussion
5.a. Conditions of crystallization
The weakly peralkaline character of the most evolved rocks of the Phlegrean Fields was recognized long ago (e.g. Armienti et al. Reference Armienti, Barberi, Bizouard, Clocchiatti, Innocenti, Metrich, Rosi and Sbrana1983; Di Girolamo et al. Reference Di Girolamo, Ghiara, Lirer, Munno, Rolandi and Stanzione1984; see also Poli et al. Reference Poli, Chiesa, Gillot, Gregnanin and Guichard1987 for the Ischian volcanic rocks), but no mineral assemblages typical of peralkaline products were identified; therefore, the evidence of a true peralkaline nature has previously been elusive. The Cuma rocks, having the most extreme mineral assemblages typical of peralkaline volcanic rocks, are among the most evolved compositions of the Phlegrean Fields, at roughly the same level as those of the ad1538 Monte Nuovo eruption (Figs 3, 10) and more evolved than the most evolved compositions of the Campanian Ignimbrite (Melluso et al. Reference Melluso, Morra, Perrotta, Scarpati and Adabbo1995; Fedele et al. Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008). Mineral assemblages roughly similar to that of the Cuma dome were found in sanidinite facies ‘syenitic’ ejecta at Procida and Monte di Procida (see Mazzi & Munno Reference Mazzi and Munno1983; Fedele et al. Reference Fedele, Tarzia, Belkin, De Vivo, Lima, Lowenstern and De Vivo2007; L. Melluso, unpub. data), but not yet in Phlegrean magmatic rocks. Zr-silicates of the wöhlerite group were described in trachytes of Ischia by Rittmann (Reference Rittmann1948).
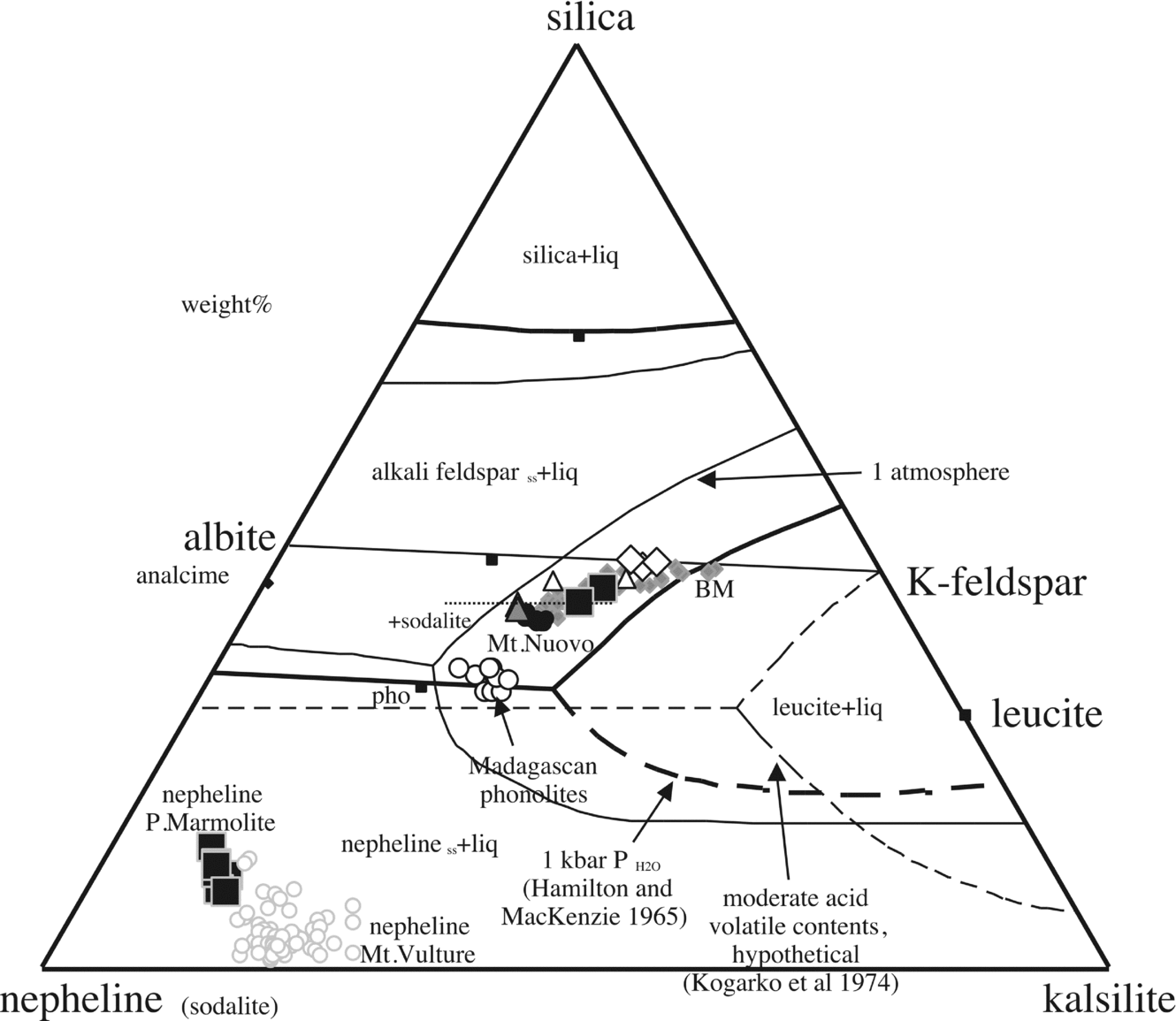
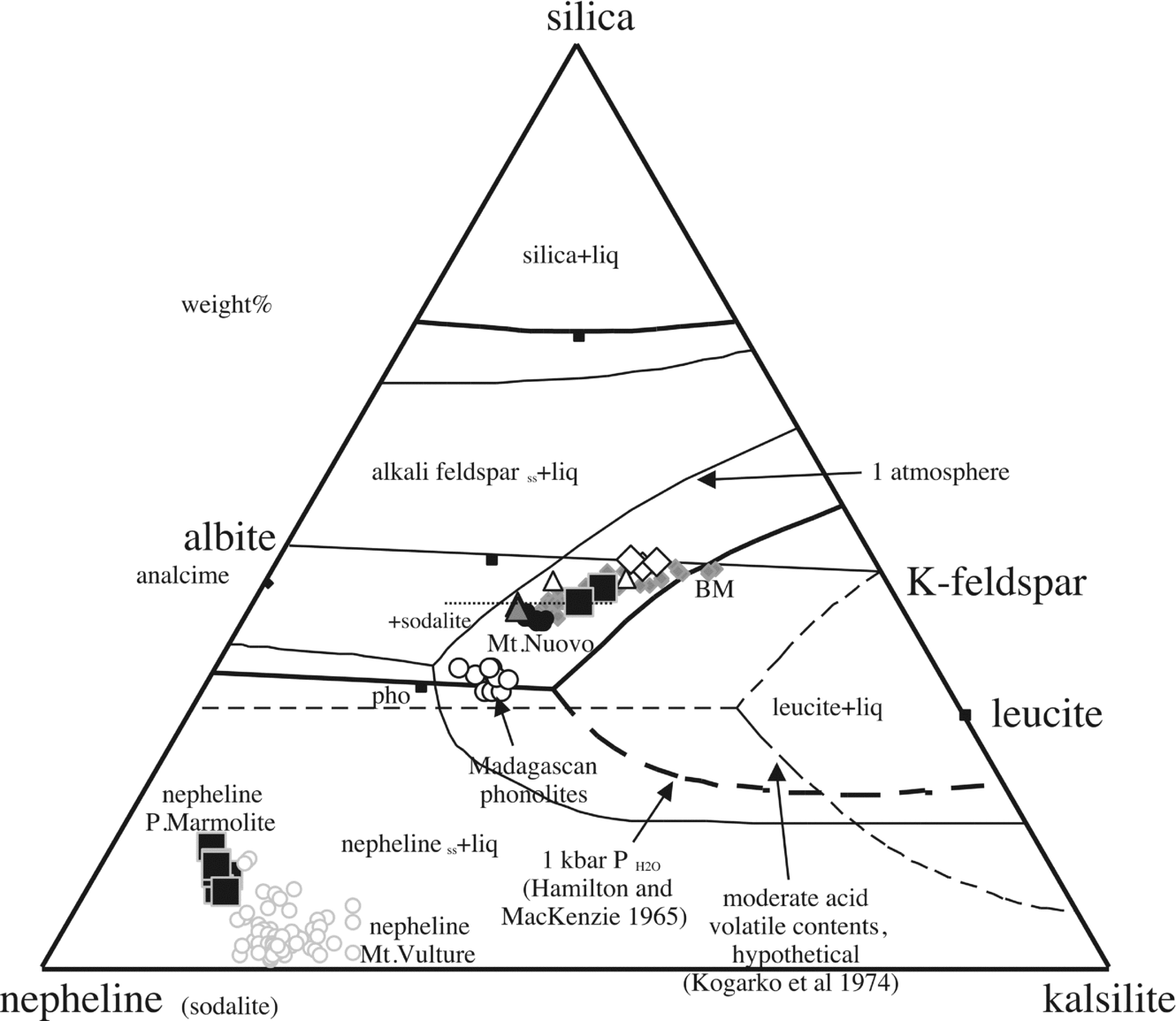
Figure 10. Nepheline compositions, bulk-rock compositions and reference nepheline–kalsilite–silica diagram at 1 atmosphere and 1 kbar PH2O after Hamilton & MacKenzie (Reference Hamilton and MacKenzie1965). Other inferred boundary lines are from Kogarko, Ryabchikov & Sørensen (Reference Rønsbo1974). Data from D'Oriano et al. (Reference D'Oriano, Poggianti, Bertagnini, Cioni, Landi, Polacci and Rosi2005; Monte Nuovo); Melluso et al. (Reference Melluso, Morra, Riziky, Veloson, Lustrino, Del Gatto and Modeste2007; Madagascan phonolites); Fedele et al. (Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008; Breccia Museo (BM)). The nephelines of Mt Vulture are also shown for comparison (data from Melluso, Morra & Di Girolamo, Reference Melluso, Morra and Di Girolamo1996; Melluso, Morra & de’ Gennaro, in press). The inferred appearance of sodalite in the crystallization sequence is shown with a dashed line on the phase diagram.
In terms of classification (e.g. Sørensen, Reference Sørensen1974, p. 22), the Cuma trachyphonolites are not peralkaline (having AI ≤ 1), but they have a groundmass assemblage made up of minerals typical of peralkaline undersaturated rocks, specifically peralkaline rocks with agpaitic affinity (i.e. having an AI > 1.2, with sodic pyroxenes or amphiboles, and Na–Ca–Zr–Ti silicates), as opposed to assemblages of typical miaskitic (e.g. with titanite ± zircon assemblages), non-peralkaline silica-undersaturated rocks. Trachytic/phonolitic magmas and intrusive equivalents (alkali and nepheline syenites) commonly cross the miaskitic/agpaitic crystallization stage (e.g. Larsen & Sørensen, Reference Larsen, Sørensen, Fitton and Upton1987; Brotzu et al. Reference Brotzu, Gomes, Melluso, Morbidelli, Morra and Ruberti1997, Reference Brotzu, Melluso, Bennio, Gomes, Lustrino, Morbidelli, Morra, Ruberti, Tassinari and D'Antonio2007; Markl et al. Reference Markl, Marks, Schwinn and Sommer2001; Ridolfi et al. Reference Ridolfi, Renzulli, Macdonald and Upton2006; Bailey et al. Reference Bailey, Sørensen, Andersen, Kogarko and Rose-Hansen2006; Melluso et al. Reference Melluso, Morra, Riziky, Veloson, Lustrino, Del Gatto and Modeste2007; Andersen et al. Reference Andersen, Erambert, Larsen and Selbekk2010; Marks et al. Reference Marks, Hettmann, Schilling, Frost and Markl2011), so this high degree of evolution cannot be considered as unexpected in the Phlegrean Fields magmatism, particularly if we consider holocrystalline facies products, where the complete mineral assemblages can be easily studied.
The Punta Marmolite and Accademia domes have groundmass compositions with only a weak peralkaline trend (cf. the highly Fe-rich, but relatively Na-poor clinopyroxene compositions of the Punta Marmolite samples), and typical minerals indicating agpaitic affinity are lacking. The silica-undersaturated assemblage of Cuma, made up of anorthoclase, sodalite, Na-rich clinopyroxene and Zr-silicates, closely resembles the assemblages of the Ilimaussaq intrusion (see fig. 4a of Larsen & Sørensen, Reference Larsen, Sørensen, Fitton and Upton1987; Markl et al. Reference Markl, Marks, Schwinn and Sommer2001; Bailey et al. Reference Bailey, Sørensen, Andersen, Kogarko and Rose-Hansen2006), with the main difference being the occurrence of F-bearing Ca–Zr-silicates (rosenbuschite) rather than Cl-bearing analogues (eudialyte and so on). In the Cuma lava dome, it appears that crystallization of sodalite scavenged substantial amounts of Cl from the melts, strongly increasing the F/Cl ratio and allowing subsequent stabilization of F-rich phases, including fluorite. After crystallization of sodalite, Cl-free feldspathoids, such as the nepheline of the Punta Marmolite, can crystallize. The presence of fluorite indicates the attainment of the maximum allowed fluorine fugacity, in the same way as sodalite indicates the maximum allowed chlorine fugacity (see Dolejs & Baker, Reference Dolejs and Baker2004; Andersen et al. Reference Andersen, Erambert, Larsen and Selbekk2010). Lacking quantitative studies of the mineral assemblages found (and allowed) in similar rocks, the association fluorite–rosenbuschite–pyrochlore found at Cuma indicates relatively high activity of Na2Si2O5 (peralkaline component) and HF, and relatively low H2O (Andersen et al. Reference Andersen, Erambert, Larsen and Selbekk2010). Indeed, aegirine, the most typical indicator of peralkaline compositions, is one of the latest minerals to crystallize (see Fig. 2d). Nonetheless, the crystallization order of the various accessory phases in the Cuma samples, excluding favourable situations, cannot be established with certainty.
The position of the samples of this study in the nepheline–kalsilite–silica phase diagram is highly indicative. The Accademia dome latites plot close to the alkali feldspar join (as well the samples from the Nisida volcano; Fig. 10), whereas the Punta Marmolite and Cuma samples plot towards the phonolite minimum (reflecting increasing feldspathoid components), but without reaching it. Feldspar compositions become more sodic and thus migrate towards the minimum of the alkali feldspar loop, as typical of low temperature compositions of residual melt compositions worldwide (see Nash, Carmichael & Johnson, Reference Nash, Carmichael and Johnson1969). The Punta Marmolite rocks represent one of the rare cases in magmatic systems where nepheline, sanidine and sodalite co-crystallized (see the hypothetical phase diagram in Kogarko, Ryabchikov & Sørensen, Reference Kogarko, Ryabchikov, Sørensen and Sørensen1974; see also Barker, Reference Barker1976; Bailey et al. Reference Bailey, Sørensen, Andersen, Kogarko and Rose-Hansen2006; Andersen et al. Reference Andersen, Erambert, Larsen and Selbekk2010). High F (> 3000 ppm) and Cl (> 5000 ppm) are known in trachytic samples of the Phlegrean Fields (Ricci, Reference Ricci2000; Signorelli & Carroll, Reference Signorelli and Carroll2002 and references therein; this study). Furthermore, it is well known that fluorine has high solubility in depolymerized and silica-undersaturated magmas (e.g. Carroll & Webster, Reference Carroll and Webster1994; Dolejs & Baker, Reference Dolejs and Baker2004; Mysen, Cody & Smith, Reference Mysen, Cody and Smith2004). Chlorine can have a lower, but roughly similar degree of solubility (see Signorelli & Carroll, Reference Signorelli and Carroll2002), as indicated by the occurrence of sodalite in the three domes, and even as phenocrysts in the Punta Marmolite samples. Therefore, the abundance of Cl (and F as well) in the Phlegrean (and Ischian) evolved magmas, testified by the occurrence of sodalite, affects the position of the minimum melt compositions, increasing the stability field of a feldspathoid such as sodalite at the expense of nepheline and alkali feldspar (see Sharp et al. Reference Sharp, Helffrich, Bohlen and Essene1989; Fig. 10). High F and Cl fugacity in the melts is well known to heavily shrink the leucite liquidus volume (see Kogarko, Ryabchikov & Sørensen, Reference Kogarko, Ryabchikov, Sørensen and Sørensen1974; Fig. 10), and all these rocks plot within the leucite stability field in the nepheline–kalsilite–silica diagram at one atmosphere (Fig. 10). There is little doubt that all these domes crystallized their groundmass at conditions close to atmospheric pressure, but none of them has any trace of leucite. It is worth remembering that leucite also has a different morphology (rounded or pseudo-octagonal), compared to that typical of sodalite, and that leucite is never systematically hexagonal in shape, when idiomorphic. Sodalite is never a replacement phase, and is always interstitial to sanidine; on the other hand, it can be easily replaced by analcime, as it is observed, for instance, in the products of the Nisida tuff cone (Fig. 2l). We additionally note that hydrous minerals (amphibole and phlogopite) are rare in the Phlegrean rocks, including the holocrystalline facies. Therefore, the use of anhydrous or hydrous versions of the nepheline–kalsilite–silica phase diagram (see Arienzo et al. Reference Arienzo, Moretti, Civetta, Orsi and Papale2010) is only a very rough approximation of the actual crystallization conditions, as volatiles more soluble in magmas, like F and Cl, can more effectively influence the crystallization behaviour of the evolved Phlegrean magmas, also at depth, and these two elements are indeed present in significant amounts of the volatile budget of evolved trachytic magmas, as clearly shown in this and other papers. The appearance of sodalite in the mineral assemblages could be one of the several causes of the absence of genuine phonolites (i.e. evolved, low-Mg volcanic rocks with anorthoclase and feldspathoid phenocrysts, Fe-rich minerals, and bulk-rock SiO2 around 55–56 wt%) at Phlegrean Fields, considering also that these volatile-rich melts are low-mass residues of slightly silica-undersaturated basaltic compositions, different from the Somma-Vesuvius phonolites, or typical (sodic) basanite–phonolite magmatic lineages (cf. typical phonolites shown in Fig. 10).
Olivines of the Cuma dome have by far the most Mn-rich fayalite compositions found in magmatic rocks of the Roman Province (see Melluso, Conticelli & de’ Gennaro, Reference Melluso, Conticelli and de’ Gennaro2010 for further Fe-rich groundmass olivines in other Roman Province rocks). The coexistence of these Fe2+-rich olivines (± aenigmatite) with Fe3+-rich aegirine (see Fig. 2d) is a rare and noteworthy feature (see Marks et al. Reference Marks, Hettmann, Schilling, Frost and Markl2011). Furthermore, Mg–Mn–Fe-olivines should form complete solid solutions (see Uchida, Kitamura & Imai, Reference Uchida, Kitamura and Imai1997), but the stability field of such high-Fe, high-Mn olivine is substantially unknown and, given that aegirine can crystallize also in reduced environments (e.g. Mitchell & Fareeduddin, Reference Mitchell and Fareeduddin2009), clear links between mineral assemblages, composition of minerals, oxygen fugacity and silica activity still need much experimental work. The silica activity of some of the Phlegrean Fields trachyphonolites seems to be constrained between the baddeleyite–zircon and albite–nepheline equilibrium (see Barker, Reference Barker2001), and may indicate an aSiO2< 0.6–0.55 at 1100°K. It cannot be excluded that the lack of zircon indicates a slightly lower silica activity, at least in the groundmass, but the well-known high solubility of zirconium in peralkaline melts has also to be taken into account. At any rate, the late-crystallized assemblage of the Cuma rocks shows evidence for the expected increasing degree of silica undersaturation.
5.b. The role of other accessory phases
High-silica and REE, low-Ca–P britholite is an extremely common (though highly underestimated) accessory mineral of the Italian potassic-ultrapotassic volcanic rocks (e.g. Melluso, Conticelli & de’ Gennaro, Reference Melluso, Conticelli and de’ Gennaro2010; Melluso, Morra & de’ Gennaro, 2011 and unpub. data), and it occurs also in other volcanic rocks elsewhere (e.g. Ilimaussaq: Rønsbo, Reference Rønsbo2008; Olkaria: Macdonald et al. Reference Macdonald, Baginski, Belkin, Dzierzanowski and Jezak2008; our unpub. data). Britholite is with no doubt the most important host for lanthanides, and, possibly, U and Th, of evolved holocrystalline volcanic rocks. The systematic occurrence of a zoned phosphate-silicophosphate in the three lava domes is interesting for at least two aspects: (1) the environment where apatite crystallized was increasingly poorer in P and Ca, and (2) the other included elements, particularly lanthanides, Th and U, were strongly incompatible in the early crystallized phases (feldspar, spinel, clinopyroxene and olivine), so they were heavily concentrated in residual melt fractions, and eventually fixed into the late-crystallized accessory phases. A direct evidence of such crystallization processes in the Phlegrean magmas is noted in the parallel patterns of the lanthanides, with the highest values found in the Cuma samples (Fig. 3c). This indicates no significant involvement of accessory phases in the fractional crystallization processes. Eu, Ba and Sr are low because they were scavenged by earlier feldspar fractionation (as an example, the cores of sanidine phenocrysts at Nisida reach 3 wt% BaO and 1.2 wt% SrO; authors’ unpub. data; see the bright sanidine cores in the BSE image of Fig. 2l).
It is also interesting to note the variable partitioning behaviour among elements with generally similar geochemical behaviour (e.g. Th in monazite and thorite, U in pyrochlore and baddeleyite, U and Th in britholite, REE, Zr and Nb in various minerals), which still require compelling explanation.
5.c. High-temperature phases in trachytes: phenocrysts or xenocrysts?
Olivine with a high forsterite content (Fo88–90) is commonly observed in primitive shoshonitic basalts of the nearby Procida island and in mafic shoshonitic inclusions at Ischia (Beccaluva, Di Girolamo & Serri, Reference Beccaluva, Di Girolamo and Serri1991; D'Antonio & Di Girolamo, Reference D'Antonio and Di Girolamo1994; Di Girolamo et al. Reference Di Girolamo, Melluso, Morra and Secchi1995; our unpub. data). The same composition (Fo89) cannot be expected in an evolved rock such as the Accademia lava dome. Indeed, the bulk-rock Mg nos of samples OL1 and OL2 are low (Mg no. = 44–48), and the bulk-rock MgO and Ni (and Cr) concentrations are so low that they cannot be in equilibrium with such a forsterite-rich olivine including chromite. Analogous reasoning can be inferred for plagioclase as calcic as An87, Mg-rich diopside as is a Ca48Mg45Fe7 composition (Mg no. = 88), and chromiferous spinel with Cr2O3 as high as 45–46 wt%. Therefore, all these phases must be considered as magmatic xenocrysts in any Phlegrean trachyte and latite (Fedele et al. Reference Fedele, Zanetti, Morra, Lustrino, Melluso and Vannucci2009; cf. Fowler et al. Reference Fowler, Spera, Bohrson, Belkin and De Vivo2007 for an alternative view). The crystallization of a Ca-rich plagioclase is even earlier than, or contemporaneous with, Mg-rich clinopyroxene, as again observed in mafic rocks of Procida and Ischia (see D'Antonio & Di Girolamo, Reference D'Antonio and Di Girolamo1994; Di Girolamo et al. Reference Di Girolamo, Melluso, Morra and Secchi1995), thus demonstrating that calcic plagioclase cannot be a late-crystallized phase. Similar considerations can be made for the Ca-rich plagioclase sporadically found in Punta Marmolite samples, which testifies to recycling of phases derived from more mafic magma batches.
5.d. Relevance to the Phlegrean magmatism
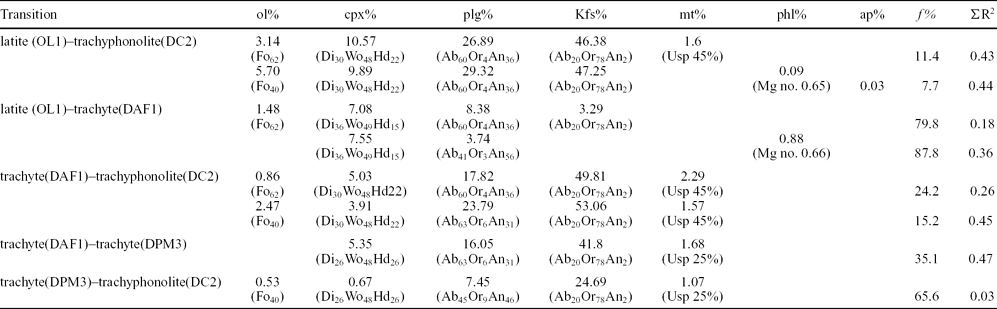
The lava domes of this study allow inferences to be made on the evolution of the Phlegrean magmatic system. The samples from the Cuma dome give evidence of a substantially closed-system evolution process, such as: (1) weakly peralkaline agpaitic parageneses and compositions, indicating a prolonged differentiation history; (2) absence of high-temperature xenocrysts (such as those of the Accademia and Punta Marmolite domes); (3) absence of reversely zoned crystals, again suggesting that the evolving magmas did not experience significant interaction with less-evolved melts. The attainment of such extremely evolved compositions can be modelled by means of least-squares major element mass balance calculations (Table 2), performed following the method of Stormer & Nicholls (Reference Stormer and Nicholls1978). Whole-rock and mineral compositions, used respectively for initial and final magma compositions and fractionated phases, are those reported in this work. The obtained results indicate that the transition from latite (sample OL1 from the Accademia lava dome) to trachyphonolite (DC2, Cuma dome) can be adequately modelled (i.e. with a ΣR2 of 0.43–0.44; ΣR2 = sum of square residuals) assuming ~ 89–92% removal of an assemblage mainly made of alkali feldspar (46–47%), plagioclase (27–29%) and clinopyroxene (10–11%), accompanied by olivine (3.4–5.7%) and accessory magnetite or apatite + phlogopite. Similar results can be obtained by splitting the above transition in two steps (ΣR2 = 0.18–0.36 and 0.26–0.45, respectively): (1) from latite to trachyte (DAF1, Accademia dome), implying 12–20% removal of clinopyroxene and plagioclase (plus accessory olivine/phlogopite), and then (2) from trachyte to trachyphonolite, assuming 76–85% removal of alkali feldspar (50–53%), plagioclase (18–24%), clinopyroxene (4–5%) and accessory magnetite and olivine. The transition from Punta Marmolite to Cuma has been successfully modelled with c. 34% removal of sanidine, plagioclase and mafic phases as accessories (Table 2). The results, together with the increasing Eu troughs from the least to the most evolved compositions (Fig. 3; Table 1) again point out the essential role of feldspar (first plagioclase and then alkali feldspar) removal in the formation and evolution of trachytic magmas (e.g. Melluso et al. Reference Melluso, Morra, Perrotta, Scarpati and Adabbo1995; Fedele et al. Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008, Reference Fedele, Zanetti, Morra, Lustrino, Melluso and Vannucci2009). This transition towards the most evolved magmas took place at all the stages of activity (i.e. pre-CI, CI–NYT and post-CI) of the Phlegrean Fields, as widely documented (e.g. Armienti et al. Reference Armienti, Barberi, Bizouard, Clocchiatti, Innocenti, Metrich, Rosi and Sbrana1983; Di Girolamo et al. Reference Di Girolamo, Ghiara, Lirer, Munno, Rolandi and Stanzione1984; Rosi & Sbrana, Reference Rosi and Sbrana1987; Villemant, Reference Villemant1988; Ghiara, Reference Ghiara1989–1990; Beccaluva et al. Reference Beccaluva, Di Girolamo, Morra and Siena1990; Civetta et al. Reference Civetta, Carluccio, Innocenti, Sbrana and Taddeucci1991, Reference Civetta, Orsi, Pappalardo, Fisher, Heiken and Ort1997; Scarpati, Cole & Perrotta, Reference Scarpati, Cole and Perrotta1993; Melluso et al. Reference Melluso, Morra, Perrotta, Scarpati and Adabbo1995; Orsi et al. Reference Orsi, Civetta, D'Antonio, Di Girolamo and Piochi1995; D'Antonio et al. Reference D'Antonio, Civetta, Orsi, Pappalardo, Piochi, Carandente, de Vita, Di Vito and Isaia1999; de Vita et al. Reference de Vita, Orsi, Civetta, Carandente, D'Antonio, Deino, di Cesare, Di Vito, Fisher, Isaia, Marotta, Necco, Ort, Pappalardo, Piochi and Southon1999; Pappalardo et al. Reference Pappalardo, Civetta, D'Antonio, Deino, Di Vito, Orsi, Carandente, de Vita, Isaia and Piochi1999, Reference Pappalardo, Piochi, D'Antonio, Civetta and Petrini2002; D'Oriano et al. Reference D'Oriano, Poggianti, Bertagnini, Cioni, Landi, Polacci and Rosi2005; Fedele et al. Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008; Arienzo et al. Reference Arienzo, Moretti, Civetta, Orsi and Papale2010).
Table 2. Major oxide mass balance calculations for the transition from Accademia to Cuma and Punta Marmolite lava dome samples
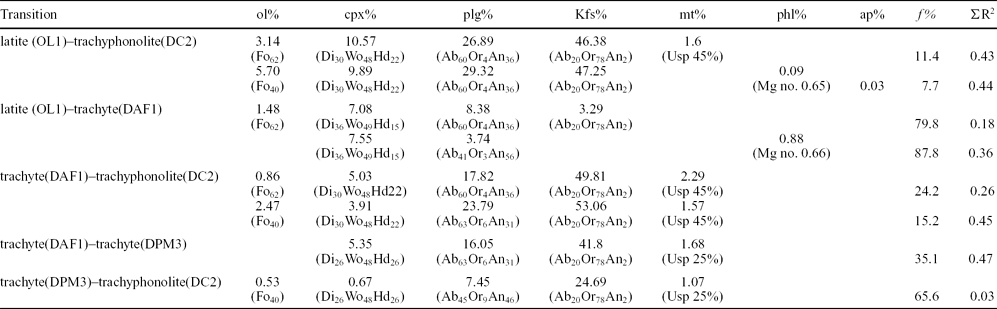
For each of the modellized transitions the wt% of subtracted olivine (ol%), clinopyroxene (cpx%), plagioclase (plg%), K-feldspar (Kfs%), magnetite (mt%), phlogopite (phl%) and apatite (ap%), the fraction of residual liquid (f %) and the sum of the square residuals (ΣR2) are reported.
Assuming a single magma chamber at least for the recent Phlegrean volcanic rocks, we are confident that the modelled transition from latite to trachyphonolite, as observed by comparing compositions of Accademia dome and Nisida tuff cone (3.9 ka) with those of Monte Nuovo (0.5 ka), took place in a closed system within a few thousand years. Although this is only a qualitative estimate, we note that these timescales are shorter (even allowing for a narrower differentiation step) than the ~ 200 ka estimated on the basis of thermodynamic modelling for the production of the trachytic magmas of the Campanian Ignimbrite starting from a more primitive basaltic trachyandesite (latite) (Fowler et al. Reference Fowler, Spera, Bohrson, Belkin and De Vivo2007).
The Accademia and, to a lesser extent, the Punta Marmolite lava domes are petrographically characterized by xenocrysts, testifying to open-system processes. The relatively abundant Accademia xenocrysts, clearly derived from primitive magmas, may have been picked up from a cumulitic layer in the shallow feeder system of the Phlegrean Fields or introduced into trachytic magma chambers by primitive magma recharge. The latter seems to be more reasonable, as the presence of a cumulitic (gabbroic/ultramafic?) layer at shallow depths in the Phlegrean area seems unlikely, given the negative buoyancy of basaltic magmas and their crystallization products (sensu lato), owing to their higher density with respect to wall rocks. It thus appears much more likely that sporadic batches of primitive magmas locally found favourable tectonic conditions that allowed them to reach the shallowest magma chambers, where they interacted with the residing volumes of evolved liquids. Therefore, olivine, chromite, plagioclase and clinopyroxene compositions of the Accademia dome record interaction of an evolved trachytic melt residing in a magma reservoir with a primitive, mantle-derived composition, as indicated by the relative proportion of ‘trachytic’ and ‘basaltic’ phenocrysts in the different facies and by the uniform trachytic texture and composition of the groundmass. This feature is also observed in the mineral composition of the nearby (and almost identical in age) Nisida tuff cone (e.g. Fig. 4), and in the Breccia Museo (CI) pyroclastic deposits as well (see Fedele et al. Reference Fedele, Scarpati, Lanphere, Melluso, Morra, Perrotta and Ricci2008, Reference Fedele, Zanetti, Morra, Lustrino, Melluso and Vannucci2009). Di Girolamo et al. (Reference Di Girolamo, Melluso, Morra and Secchi1995) described blobs of very primitive basaltic compositions in Ischian trachytes, thus suggesting that open-system behaviour is a common feature of the Campanian volcanic rocks.
It is finally noted that the chemical composition of the chromiferous spinel found in the olivines of the Accademia dome does not overlap with the chromiferous spinel compositions found in the olivines at Procida (Solchiaro), recent lavas of Ischia (Zaro and Arso lava flows) and Roccamonfina and Ventotene island shoshonitic basalts and leucite-basanites (Fig. 8), clearly suggesting that the shoshonitic basalt magma batches of the volcanic fields in western Campania, from which those spinels grew, crystallized in independent reservoirs with independent crystallization histories.
Both closed-system and open-system evolution processes are evident in the magmatic history of the Phlegrean Fields, and this is observed in rocks erupted throughout its entire time span. This strongly argues in favour of a somewhat cyclic behaviour of the Phlegrean magmatic system, as also previously documented mainly on the basis of isotopic evidence (e.g. D'Antonio et al. Reference D'Antonio, Civetta, Orsi, Pappalardo, Piochi, Carandente, de Vita, Di Vito and Isaia1999, Reference D'Antonio, Tonarini, Arienzo, Civetta, Di Renzo, Beccaluva, Bianchini and Wilson2007; Pappalardo et al. Reference Pappalardo, Civetta, D'Antonio, Deino, Di Vito, Orsi, Carandente, de Vita, Isaia and Piochi1999, Reference Pappalardo, Piochi, D'Antonio, Civetta and Petrini2002; Di Renzo et al. Reference Di Renzo, Arienzo, Civetta, D'Antonio, Tonarini, Di Vito and Orsi2011). This cyclic behaviour can be the consequence of a relatively random arrival of hot mafic magma from depth, recharging the magmatic reservoir(s) and triggering new eruptive periods.
6. Conclusions
The crystallization histories of three Phlegrean Fields lava domes show cooling of trachytic magma batches having different degrees of magmatic evolution in a closed-to-open magmatic system. Extreme closed-system fractional crystallization generated peralkaline compositions with appropriate mineral assemblages, such those found at Cuma. Closed-system fractional crystallization had the most important role in the magmatic evolution of the Phlegrean magmas, ultimately leading to Cl and F-rich trachyphonolites with peralkaline affinity, very poor in P, Sr and Ba and with mineral assemblages comprising anorthoclase, sodalite, aegirine, Mn–Fe-rich olivine, britholite, rosenbuschite, fluorite and other Zr-, Nb- and F-rich phases, thus reaching the agpaitic stage.
Open-system process are indicated by the occurrence of Ca-rich plagioclase, Mg-rich clinopyroxene, forsteritic olivine and chromiferous spinel in chemically evolved rocks. These phases crystallized in primitive, Mg-rich basalt compositions, rather than in the trachytic or latitic composition where they were found. The phases were introduced into the evolved compositions during magma chamber recharge, a possible trigger of the eruption.
Acknowledgements
We thank P. Cappelletti, S. Conticelli, D. Cosentino, C. Cucciniello, M. P. D'Albora and M. de' Gennaro for supplying samples, help in the field and laboratory work, for sharing unpublished data and for useful suggestions. Sergio Bravi is gratefully thanked for his skilled ability and patience in thin-section preparation. Initial versions of this manuscript benefitted from the useful comments and advice of Ray Macdonald and David Pyle. Two anonymous journal reviewers and Philip Leat provided further constructive comments and advice. Grants for this study were provided by Regione Campania (Legge Regionale n.5 to L. M.: Petrological study of the magmatic feeding systems of Somma-Vesuvius, Phlegrean Fields, and Ischia, similarities, differences and implications for the eruptive styles).