1. Introduction
The Geisel Valley of central Germany (Fig. 1) is infilled with fossil-bearing coal layers of mostly middle Eocene age. These fossils have been exquisitely preserved, including many different animals from this extinct ecosystem such as fossil beetles still containing iridescent epicuticular interference reflectors (Krumbiegel, Rüffle & Haubold, Reference Krumbiegel, Rüffle and Haubold1983). The local climate at this time was very warm, and the presence of fossil crocodylians at the nearby Messel fossil locality led to a crocodylian-derived temperature estimate of 20°C (Berg, Reference Berg1964). A more recent study based on palaeobotanical evidence estimated a mean annual temperature of 22.9–25°C for the Geiseltal fossil locality (Mosbrugger, Utescher & Dilcher, Reference Mosbrugger, Utescher and Dilcher2005). These values are both higher than the mean annual temperature estimate derived from fossil teeth and bones of the nearby Messel-Fossillagerstätte of similar age, c. 18±2.5°C (Tütken, Reference Tütken2014). However, the estimates are similar to lake surface water temperature of Messel estimated at c. 25±3°C (Tütken, Reference Tütken2014). Crocodylians were not only present at Geiseltal, but abundant. No less than five genera are recognized: Diplocynodon, Asiatosuchus, Boverisuchus (until recently called Pristichampsus; Brochu, Reference Brochu2013), Allognathosuchus, and Bergisuchus (Berg, Reference Berg1966; Rauhe & Rossmann, Reference Rauhe and Rossmann1995; Hellmund, Reference Hellmund2007). All but Bergisuchus have well-preserved skulls within the impressive Geiseltal collection (Geiseltalsammlung), housed at Martin-Luther-Universität, Halle-Wittenberg in Halle (Saale), Germany.
Figure 1. Map of Germany with the Geiseltal-Fossillagerstätte marked with a star. Fossils were recovered from the Geisel Valley of southern Sachsen-Anhalt.
Modern crocodylians typically have a single genus in a given area. Overlap between two genera is not uncommon, particularly with the globally widespread genus Crocodylus (Ross, Reference Ross1989). The greatest amount of potential overlap between living genera of crocodylians is four. However, this is only reconstructed from geographic ranges and no observations of four genera in a single modern area have been published. This potential is between three different caimanine genera (Caiman, Melanosuchus and Paleosuchus) and potential overlap with the Orinoco Crocodile (Crocodylus intermedius; Ross, Reference Ross1989). Crocodylian species within a given area are known to spatially separate, as observed in neighbouring populations of caimanines in the Amazon, living in different bodies of water within the forested environment (Magnusson, Vieira da Silva & Lima, Reference Magnusson, Vieira da Silva and Lima1987). Spatial separation has been hypothesized for large-bodied predatory non-avian theropod dinosaurs as explaining occurrence of multiple species in one geological formation (Farlow & Pianka, Reference Farlow and Pianka2003). However, these fossils were distributed across a much larger area than Geiseltal and one of the large species was notably less common than the other (Farlow & Pianka, Reference Farlow and Pianka2003). There is also typically a significant size difference between neighbouring crocodylian species, such as the Australian fresh- and saltwater crocodiles that occasionally overlap in territory (Crocodylus johnstoni and C. porosus, respectively; Webb, Manolis & Sack, Reference Webb, Manolis and Sack1983). In the case of the Geiseltal crocodylians, however, each was found to co-occur within a very limited geographic area, at times from a single pit located within a single stratigraphic horizon, severely limiting any potential spatial and temporal differences between genera.
In regions with significant overlap in habitat between two genera, it has been proposed that these crocodylians specialize by having different prey preferences (Brochu, Reference Brochu2001; Pearcy, Reference Pearcy2011). In the case of Australian varanids, six species are known to co-occur within the Great Victoria Desert and differ in size, method of prey acquisition and microhabitat (Pianka, Reference Pianka1994; Farlow & Pianka, Reference Farlow and Pianka2003). Sympatric ceratopsian species have exhibited key differences in skull structure, indicating differences in dietary preference (Henderson, Reference Henderson, Ryan, Chinnery-Allgeier and Eberth2010). With five crocodylian genera at Geiseltal, specialization may also have occurred between these crocodylians. Given the number of genera, they may even have exceeded the current range of morphospace seen in modern crocodylians.
Crocodylian skull shape has a strong connection to its range of preferred prey (Pierce, Angielczyk & Rayfield, Reference Pierce, Angielczyk and Rayfield2008; Sadleir & Makovicky, Reference Sadleir and Makovicky2008). A seminal study of skull structure investigated the benefits of different shapes in extant crocodylian skulls for different feeding methods and found ‘adaptive measures that strengthened the skull, reflecting different feeding behaviors’ (Busbey, Reference Busbey and Thomason1994). A more recent study utilizing geometric morphometrics and finite element analysis came to the conclusion that ‘. . . it appears that ecological specializations, related to feeding and foraging strategies, are major controls on the morphological diversity within extant crocodilian skulls’ (Pierce, Angielczyk & Rayfield, Reference Pierce, Angielczyk and Rayfield2008). Phylogenetic studies have found similar correlation between function and skull shape in fossil and modern crocodylians (Brochu, Reference Brochu2001). Differences in skull shape between genera of crocodylians can therefore be informative of their prey preference and their role within the ecosystem.
Structural stability is largely controlled by shape: shorter snouts deliver force without incurring a large amount of torsional strain, and narrow snouts deliver greater speed but have greater hydrodynamic drag and less resistance to strain (McHenry et al. Reference McHenry, Clausen, Daniel, Meers and Pendharkar2006; Pierce, Angielczyk & Rayfield, Reference Pierce, Angielczyk and Rayfield2008; Cuff & Rayfield, Reference Cuff and Rayfield2013). Short-snouted species of living crocodylians are in turn able to take advantage of harder foods such as bivalves, crustaceans, gastropods and testudines (Delany & Abercrombie, Reference Delany and Abercrombie1986; McHenry et al. Reference McHenry, Clausen, Daniel, Meers and Pendharkar2006; Borteiro et al. Reference Borteiro, Gutiérrez, Tedros and Kolenc2009). However, their snouts are less adapted for swift prey such as certain forms of fish. Long, narrow-snouted crocodylians, such as the Indian Gharial, Gavialis gangeticus, are very well adapted for catching swift, unarmored prey, mostly fish (Piras et al. Reference Piras, Colangelo, Adams, Buscalioni, Cubo, Kotsakis, Meloro and Raia2010, Reference Piras, Buscalioni, Teresi, Raia, Sansalone, Kotsakis and Cubo2014; Stevenson & Whitaker, Reference Stevenson, Whitaker, Manolis and Stevenson2010), but lack the structural stability to regularly consume hard prey items. Long-snouted crocodylians were found to have adapted different musculature along different evolutionary lines to adduct the jaws, implying the function of primary driver of adaptation (Endo et al. Reference Endo, Aoki, Taru, Kimura, Sasaki, Yamamoto, Arishima and Hayashi2002). Species with intermediate snouts tend to be generalists, not adapted particularly towards one end of the spectrum or the other (Brochu, Reference Brochu2001). Crocodylians eating much larger prey, such as the Nile Crocodile (Crocodylus niloticus), have rather long snouts that are wide and wedge-shaped, well-suited for a very strong bite (McHenry et al. Reference McHenry, Clausen, Daniel, Meers and Pendharkar2006). This is particularly useful in an animal incapable of true mastication. All crocodylian skull shapes are capable of delivering comparable levels of force, as measured in extant forms on land (Erickson et al. Reference Erickson, Gignac, Steppan, Lappin, Vliet, Brueggen, Inouye, Kledzik and Webb2012, Reference Erickson, Gignac, Lappin, Vliet, Brueggen and Webb2014). The only significant correlation with non-aquatic bite force has been body size, showing that in each skull shape bite force increases with size (Erickson et al. Reference Erickson, Gignac, Lappin, Vliet, Brueggen and Webb2014).
A study of the relationship between crocodylian morphological disparity and functional performance was conducted by Piras et al. (Reference Piras, Luciano Teresi, Buscalioni and Cubo2009) by comparing snout shape and structural stability in the form of beam mechanics. This study focused on differences between the crocodile lineage (Crocodyloidea) and the alligator lineage (Alligatoroidea). Their results suggested ‘that the geometry of skull rostra is tightly linked to performance in prey capture . . . in Crocodyloidea’. The researchers found more of a phylogenetic connection to structure in Alligatoroidea. The team suggested that the ‘evolutionary history of the clade Alligatoroidea is marked by its tightness to the ecosystems’ and that they are less likely to branch out from their preferred ecological role (Piras et al. Reference Piras, Luciano Teresi, Buscalioni and Cubo2009). Despite these differences in morphological adaptability, the animals still maintain a connection between morphology and mode of predation. This study and a more recent one addressing the issue of modularity within crocodylian skulls (Piras et al. Reference Piras, Buscalioni, Teresi, Raia, Sansalone, Kotsakis and Cubo2014) used only recent alligatoroids, and did not include any fossil alligatoroid taxa, which represent potentially greater occupation of morphospace.
Shape can be quantified using geometric morphometrics that utilizes a series of landmarks (Bookstein, Reference Bookstein1991; Zelditch, Swiderski & Sheets, Reference Zelditch, Swiderski and Sheets2012). Each landmark represents a point of morphology that can be found in each specimen of the dataset, and can be represented in either two or three dimensions (Bookstein, Reference Bookstein1991; Zelditch, Swiderski & Sheets, Reference Zelditch, Swiderski and Sheets2012). One example of a landmark used in crocodylians is the anterior-most extension of the premaxillary suture (Landmark 9 of Piras et al. Reference Piras, Luciano Teresi, Buscalioni and Cubo2009). Following the principle of anatomical homology, digitizing these landmarks creates a simplified digital version of each specimen (Mitteroecker & Gunz, Reference Mitteroecker and Gunz2009). When many specimens have been digitized in this manner, they form a dataset of specimens that can be analysed together. Procrustes superimposition is the method by which these landmarks are centred, scaled and rotated to a single orientation (Mitteroecker & Gunz, Reference Mitteroecker and Gunz2009). The differences between the spatial relationships of each specimen's landmarks can be studied by means of a principal components analysis or PCA (Slice, Reference Slice2007). This kind of analysis isolates different linear combinations of the original dataset that ‘most efficiently reproduce sample variability’ (Slice, Reference Slice2007). In a PCA, a consensus configuration is generated from the dataset and deviation from this configuration is summarized as a series of axes, with the first summarizing more variation than any of the following axes (Bookstein, Reference Bookstein1991; Mitteroecker & Gunz, Reference Mitteroecker and Gunz2009; Polly et al. Reference Polly, Lawing, Fabre and Goswami2013). The majority of variation within the dataset is often covered by the first and second axes, but not always (Polly et al. Reference Polly, Lawing, Fabre and Goswami2013). Plotting the PCA axes provides a visual method for evaluating similarities and differences between specimens (Polly et al. Reference Polly, Lawing, Fabre and Goswami2013).
This study focuses on the application of geometric morphometrics to fossil and modern crocodylians in order to address the question of prey partitioning within a high-crocodylian-diversity site. Geometric morphometric methods have been applied to other crocodylian groups in order to address other questions regarding evolution, ecology and taxonomy (e.g. Pierce, Angielczyk & Rayfield, Reference Pierce, Angielczyk and Rayfield2008; J. Martin, unpub. Ph.D. thesis, Univ. Lyon, 2009; Piras et al. Reference Piras, Luciano Teresi, Buscalioni and Cubo2009, Reference Piras, Colangelo, Adams, Buscalioni, Cubo, Kotsakis, Meloro and Raia2010, Reference Piras, Buscalioni, Teresi, Raia, Sansalone, Kotsakis and Cubo2014; Young et al. Reference Young, Brusatte, Ruta and de Andrade2010). Similar methods were applied to the extinct crocodylomorphs, the thalattosuchians, in order to assess factors affecting morphological variation (Pierce, Angielczyk & Rayfield, Reference Pierce, Angielczyk and Rayfield2008, Reference Pierce, Angielczyk and Rayfield2009; Young et al. Reference Young, Brusatte, Ruta and de Andrade2010; Young, Bell & Brusatte, Reference Young, Bell and Brusatte2011a; Wilberg, Reference Wilberg2015). These studies found correlation between dietary preference and skull structure in thalattosuchians (Pierce, Angielczyk & Rayfield, Reference Pierce, Angielczyk and Rayfield2009; Young et al. Reference Young, Brusatte, Ruta and de Andrade2010; Young, Bell & Brusatte, Reference Young, Bell and Brusatte2011a). Other recent studies of thalattosuchians specifically addressed the coexistence of two related taxa and found adaptations for specialization towards different preferred prey (Young et al. Reference Young, Brusatte, de Andrade, Desojo, Beatty, Steel, Fernández, Sakamoto, Ruiz-Omeñaca and Schoch2012; Buchy, Young & de Andrade, Reference Buchy, Young and de Andrade2013). A recent study showed some degree of cranial separation between sympatric crocodylian taxa using traditional morphometrics from a Miocene site in Peru (Salas-Gismondi et al. Reference Salas-Gismondi, Flynn, Baby, Tejada-Lara, Wesselingh and Antoine2015).
Geiseltal is not the only site which has recovered high crocodylian diversity. A middle Miocene site in Peru and a late Miocene site in Venezuela have both recovered a high diversity, reaching up to seven sympatric species within six genera (Scheyer et al. Reference Scheyer, Aguilera, Delfino, Fortier, Carlini, Sánchez, Carillo-Briceño, Quiroz and Sánchez-Villagra2013; Salas-Gismondi et al. Reference Salas-Gismondi, Flynn, Baby, Tejada-Lara, Wesselingh and Antoine2015). However, the number of well-preserved crocodylian specimens is greater at the Geiseltal site than the number of well-figured specimens from either of the other sites. Although the Geiseltal crocodylian taxa also appear at the nearby Messel fossil site (Keller & Schaal, Reference Keller, Schaal, Schaal and Ziegler1988; Morlo et al. Reference Morlo, Schaal, Mayr and Seiffert2004), we have included only material from Geiseltal since the main focus of the study is where these crocodylians would have lived together. As a result, all fossils were chosen only from the Geiseltal brown-coal (lignite) deposit. As species-level diversity in crocodylians typically involves subtle morphologic differences, we instead focus on the taxonomic level of genera for this study where differences in skull shape are greater between taxa.
2. Materials and methods
2.a. Crocodylian skulls
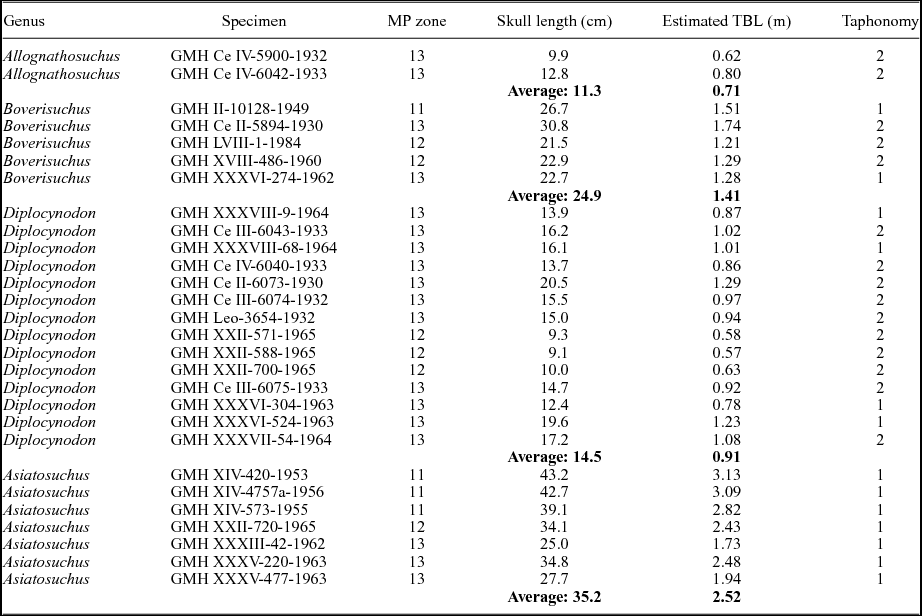
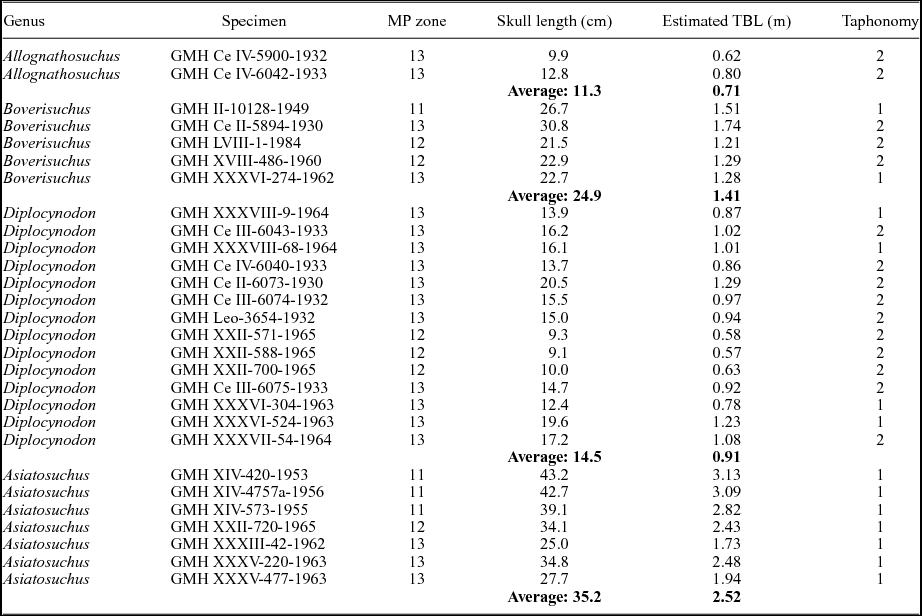
A total of 28 skulls representing four of the five crocodylian genera of Geiseltal were included in the analysis (see examples of each in Fig. 2). As Bergisuchus from Geiseltal only has mandibular rami preserved (Rauhe & Rossmann, Reference Rauhe and Rossmann1995; Rossmann, Rauhe & Ortega, Reference Rossmann, Rauhe and Ortega2000), it could not be included in the present analysis. Even the holotype of this taxon from Messel only includes a single mandibular ramus and a partial rostrum (Rauhe & Rossmann, Reference Rauhe and Rossmann1995; Rossmann, Rauhe & Ortega, Reference Rossmann, Rauhe and Ortega2000). Some taxa were better represented than others at Geiseltal, and as a result this dataset includes: 14 Diplocynodon; 7 Asiatosuchus; 5 Boverisuchus; and 2 Allognathosuchus. We note here that the Asiatosuchus specimens pertain to A. germanicus, although recent work has shown this genus is potentially non-monophyletic (Delfino & Smith, Reference Delfino and Smith2009). This could lead to a potential revision of the generic name (Delfino & Smith, Reference Delfino and Smith2009), but given that this is not the aim of the study, we refer to these specimens as Asiatosuchus. The skulls used in this analysis have at least one complete side preserved and are visible in dorsal view, resulting in the necessary exclusion of very-well-preserved material that was prepared in ventral view only. Only skulls of adults were used for this study. Very few well-preserved juveniles were collected from the locality and did not represent a large enough sample size for meaningful addition to the analysis. Furthermore, their morphology is consistently different enough that they would not be expected to place within the same morphospace as the adults. Adult status was determined based primarily on sutural closure of the vertebral column (sensu Brochu, Reference Brochu1996). Fossil specimens with noticeable taphonomic shear affecting both sides of the skull were not included in the analysis. A list of specimens used for the analysis can be found in Table 1.

Figure 2. Examples of the skulls of the four fossil crocodylian genera included in the present study, all collected from Geiseltal, Germany. (a) Boverisuchus, GMH Ce II-5894-1930; scale bar 10 cm. (b) Diplocynodon, GMH Ce III-6075-1933; scale bar 10 cm. (c) Asiatosuchus, GMH XIV-420-1953; scale bar 4 cm. (d) Allognathosuchus, GMH Ce IV-5900-1932, scale bar 5 cm.
Table 1. List of Geiseltal fossil crocodylian specimens used for geometric morphometric analysis (n = 28). Ages are presented as mammal Palaeogene (MP) zones. Skull lengths were measured for this study. Estimated total body lengths (TBL) were calculated for Allognathosuchus and Diplocynodon using data from Alligator mississippiensis (Woodward, White & Linda, Reference Woodward, White and Linda1995; Young et al. Reference Young, Bell and Brusatte2011 b). Estimates for Asiatosuchus were calculated using data from Crocodylus porosus (Sereno et al. Reference Sereno, Larsson, Sidor and Gado2001). Estimates for Boverisuchus were generated from this study. Taphonomy is based on compression as either having little to no compression (1) or having notable dorsoventral compression (2). Sheared specimens were not used in this study. GMH – Geiseltalsammlung, Zentralmagazin Naturwissenschaftlicher Sammlungen, Martin-Luther-Universität Halle-Wittenberg, Halle (Saale), Germany.
For extant crocodylia, complete osteological specimens were used from the Florida Museum of Natural History at the University of Florida (UF), the American Museum of Natural History (AMNH), the Herpetological Collections of the Universidad Nacional in Bogotá, Colombia (ICN), the Smithsonian United States Natural History Museum (USNM) and the Carnegie Museum of Natural History (CMNH). Again, only adult specimens were used. This sample represented all living species and individuals from all over the world to achieve a full-picture view of living crocodylians with a total sample size of 218. Some species are represented better than others. A list of the extant specimens used in the analysis can be found in online supplementary Table S1 (available at http://journals.cambridge.org/geo).
2.b. Photography and landmark assignment
All specimens were photographed in dorsal view with a scale bar. Specimens were oriented so that the tip of the snout was raised up until the skull table was horizontal. For each specimen a camera was mounted directly above the skull, with the focus centred over the nasal-nasal suture as described by Pearcy and Wijtten (Reference Pearcy and Wijtten2010). By maintaining an appropriate distance between the specimen and the camera lens and by standardizing the method of photography, we endeavoured to minimize the potential effects of parallax. A past study recognized that even when slight parallax has affected a dataset, it affects all specimens very similarly as long as photography is standardized and therefore does not significantly affect the results of the analyses (Mullin & Taylor, Reference Mullin and Taylor2002).
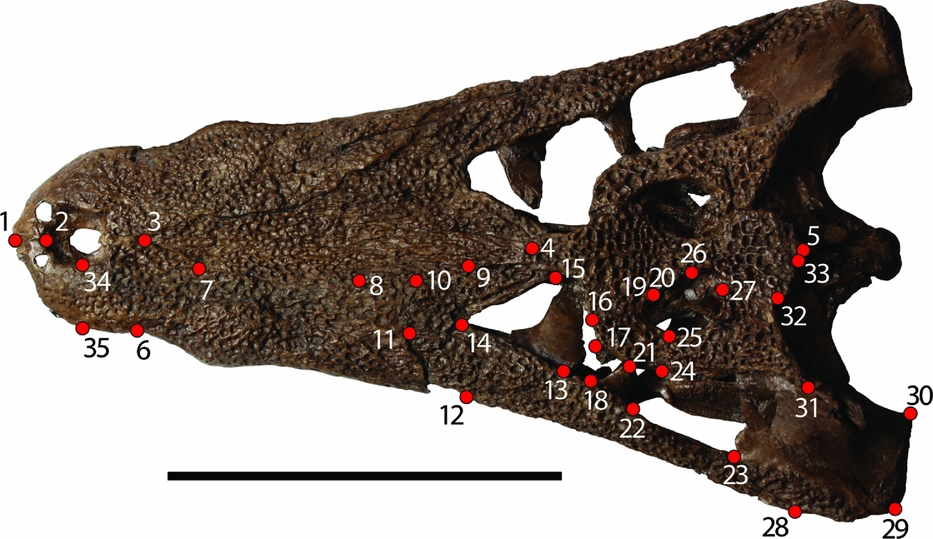
A total of 35 landmarks (Fig. 3) were used to encompass skull shape following the method of Pierce, Angielczyk & Rayfield (Reference Pierce, Angielczyk and Rayfield2008). This method focused on one half of the skull in order to take advantage of more of the fossil record. Had the procedure required complete skulls, the sample size would have been much smaller. The left side was arbitrarily chosen for the analysis. In cases where fossils were better preserved on the right side, the images were digitally flipped so that it would not offset the analysis. Images were merged into a TPS file using the program tpsUtil (F. J. Rohlf, unpub. program, 2013: http://life.bio.sunysb.edu/morph/soft-utility.html). Landmarks were assigned using the program tpsDIG2 (F. J. Rohlf, unpub. program, 2013: http://life.bio.sunysb.edu/morph/soft-dataacq.html) from in-person comparison of the images to the specimens. Landmarks were chosen from the dorsal cranial surface (mostly on the snout and skull table) in order to minimize potential preservational effects of comparison between fossil and modern taxa. We used landmarks that were consistently applied in both fossil and modern crocodylian studies. The landmarks of this study are either similar or identical to those of other geometric morphometric fossil crocodylomorph studies (J. Martin, unpub. Ph.D. thesis, Univ. Lyon, 2009; Pierce, Angielczyk & Rayfield, Reference Pierce, Angielczyk and Rayfield2009; Young et al. Reference Young, Brusatte, Ruta and de Andrade2010).
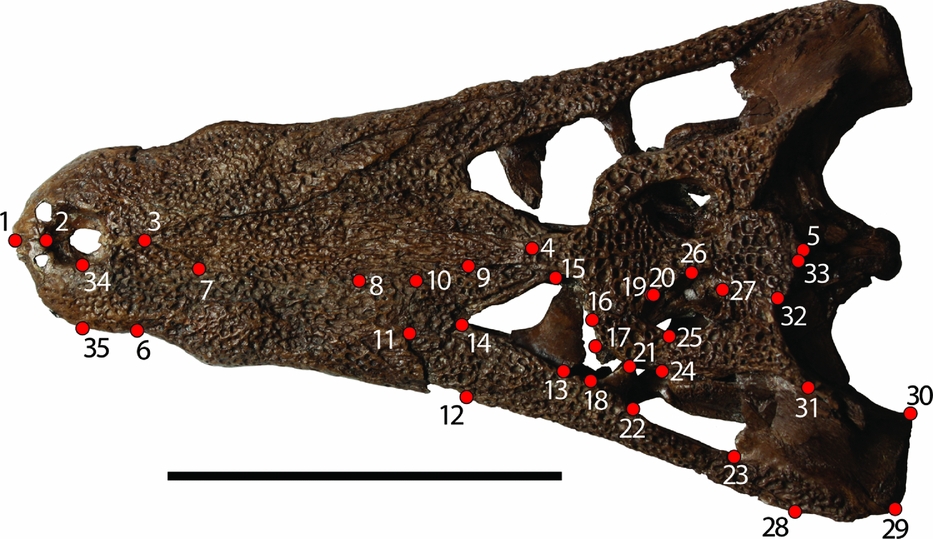
Figure 3. Landmarks used in the current study from Pierce, Angielczyk & Rayfield (Reference Pierce, Angielczyk and Rayfield2008). Specimen is an adult Diplocynodon (GMH XXXVI-524-1963). Scale bar 10 cm. All landmarks are assigned in dorsal view. (1) Anterior-most tip of the premaxillary suture. (2) Anterior point of the narial opening at the premaxillary suture. (3) Anterior-most point of the nasal. (4) Contact of the left and right nasal with the frontal. (5) Posterior-most extent of the supraoccipital, on the midline. (6) Contact of the premaxilla and maxilla along the lateral margin. (7) Contact of the premaxilla, nasal and maxilla. (8) Contact of the nasal, maxilla and prefrontal. (9) Contact of the nasal, frontal and prefrontal. (10) Contact of the maxilla, prefrontal and lacrimal. (11) Contact of the maxilla, lacrimal and jugal. (12) Contact of the maxilla and jugal along the lateral margin. (13) Contact of the jugal and lacrimal along the orbital margin. (14) Contact of the lacrimal and prefrontal along the orbital margin. (15) Contact of the prefrontal and frontal along the orbital margin. (16) Contact of the frontal and postorbital along the orbital margin. (17) Anterodorsal tip of the postorbital bar. (18) Anteroventral tip of the postorbital bar. (19) Contact of the frontal, parietal and postorbital. (20) Contact of the parietal and postorbital along the margin of the supratemporal fenestra. (21) Posterodorsal tip of the postorbital bar. (22) Posteroventral tip of the postorbital bar. (23) Contact of the jugal and quadratojugal along the infratemporal fenestra. (24) Contact of the postorbital, squamosal and quadratojugal. (25) Contact of the postorbital and squamosal along the margin of the supratemporal fenestra. (26) Midpoint of the supratemporal fenestra along the parietal. (27) Contact of the parietal and squamosal along the margin of the supratemporal fenestra. (28) Contact of the jugal and quadratojugal along the lateral skull margin. (29) Contact of the quadratojugal and quadrate along the lateral skull margin. (30) Medial condyle of the quadrate. (31) Posterolateral tip of the dorsal extension of the squamosal on the skull table. (32) Contact of the parietal and squamosal along the posterior margin of the skull table. (33) Lateral contact of the parietal and supraoccipital. (34) Mid-lateral point on the external narial opening. (35) Point on lateral margin of the premaxilla corresponding to Landmark 34, the mid-lateral point on the external narial opening.
2.c. Analysis
The TPS file from tpsDIG2 was loaded into tpsRelw (F. J. Rohlf, unpub. program, 2013: http://life.bio.sunysb.edu/morph/soft-tps.html) in order to generate relative warps, a process very similar to principal components analyses or PCA (Rohlf, Reference Rohlf1993). The difference between these methods is whether or not a factor (alpha) is used to multiply partial warps before computing principal components, and therefore differentially weighting them in the analysis (Rohlf, Reference Rohlf1993; Zelditch, Swiderski & Sheets, Reference Zelditch, Swiderski and Sheets2012). Since alpha in this study was left in the default position of 0, our study is effectively a PCA. Morphospace is represented in these analyses by XY scatter plots of the relative warps axes, referred to as the principal components of a PCA. The morphospace is identified by the relative warps analysis, showing distribution of variation relative to a consensus configuration, placed at the centre of the axes (Rohlf, Reference Rohlf1993; F. J. Rohlf, unpub. program, 2013: http://life.bio.sunysb.edu/morph/soft-tps.html). The program tpsRelw can also generate deformation grids that are determined by calculating the deviation of landmarks from the consensus configuration at a given point in the relative warps (Bookstein, Reference Bookstein1991; F. J. Rohlf, unpub. program, 2013: http://life.bio.sunysb.edu/morph/soft-tps.html). This method is based on the position of landmarks relative to each other, so that specimens group together due to similarity of shape and not similarity of size (Bookstein, Reference Bookstein1991; Rohlf, Reference Rohlf1993, Zelditch, Swiderski & Sheets, Reference Zelditch, Swiderski and Sheets2012). By using two-dimensional (2D) methods of landmark assignment, we minimized the spatial difference between landmarks of compressed and uncompressed fossil specimens. This would have been more of a problem had our study used 3D methods of analysis. Dorsoventral compression of some specimens may have moved landmarks from their original location, but the resultant difference in position of landmarks as determined from 2D images is likely very small. Also, the study only used specimens where bones were still in their same relative positions (i.e. quadrate condyle ventral to skull table). In order to determine whether or not the compression of some specimens affected the geometric morphometric data, we conducted additional correlation statistics. Each fossil was assigned a ‘1’ if it had little to no compression or ‘2’ if it had notable compression. We then tested for correlation between these specimens across the entire dataset and within Diplocynodon and Boverisuchus. There was no variation of taphonomy within Asiatosuchus (all uncompressed, n = 7) or Allognathosuchus (all compressed, n = 2).
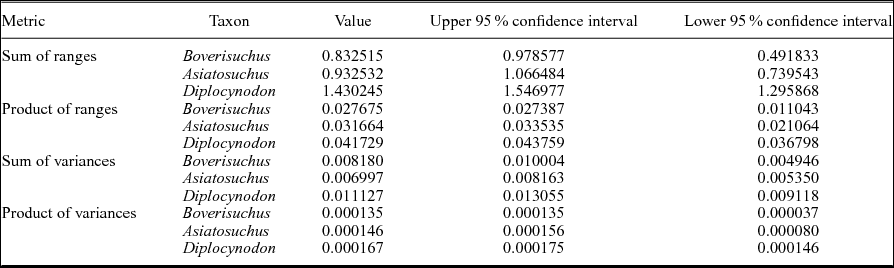
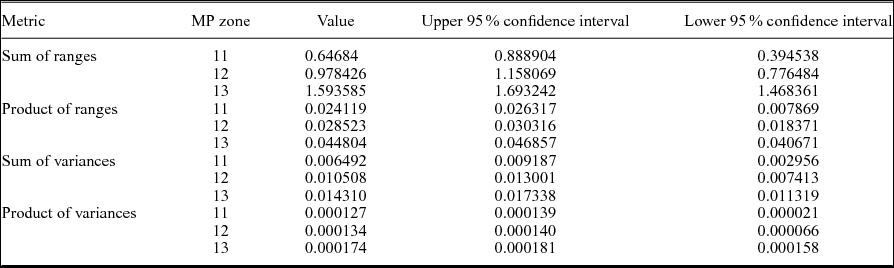
In order to evaluate relative variation within the dataset, disparity metrics were generated including sum of ranges, product of ranges, sum of variances and product of variances (Tables 2, 3) using Rare 1.2 (Wills, Reference Wills1998). This program code was run using Chipmunk Basic version 3.6.6b0 (R Nicholson, unpub. program, 2012: http://www.nicholson.com/rhn/basic) and used to rarefy the data with 1000 replicates using bootstrapping, generating 95% confidence intervals and utilizing all relative warps axes. Variance metrics are best suited for discerning between different forms and are comparatively less sensitive to differences in sample size, but are in turn more sensitive to inconsistent taxonomy (Wills, Reference Wills1998). Range metrics are more sensitive to sample size, but are better suited for estimating the total extent of morphological differences within a group relative to another (Wills, Reference Wills1998). Statistical differences in range and variance can be determined by overlap or non-overlap of 95% confidence intervals.
Table 2. Disparity metrics for the Geiseltal crocodylian geometric morphometric analysis using all 27 relative warps axes. Sample sizes are: Boverisuchus (n = 5), Asiatosuchus (n = 7) and Diplocynodon (n = 14). Due to the low sample size of Allognathosuchus (n = 2), this taxon could not be included in these metrics. Products were normalized by taking their 27th root.
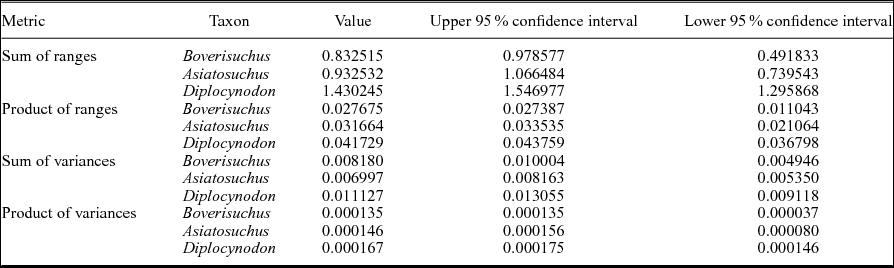
Table 3. Disparity metrics from the geometric morphometric analysis of Geiseltal crocodylians in terms of mammal Palaeogene (MP) zone, using all 27 relative warps axes. Sample sizes are: MP 11 (n = 4), MP 12 (n = 6), MP 13 (n = 18). Products were normalized by taking their 27th root.
2.d. Stratigraphic resolution of crocodylians and potential prey
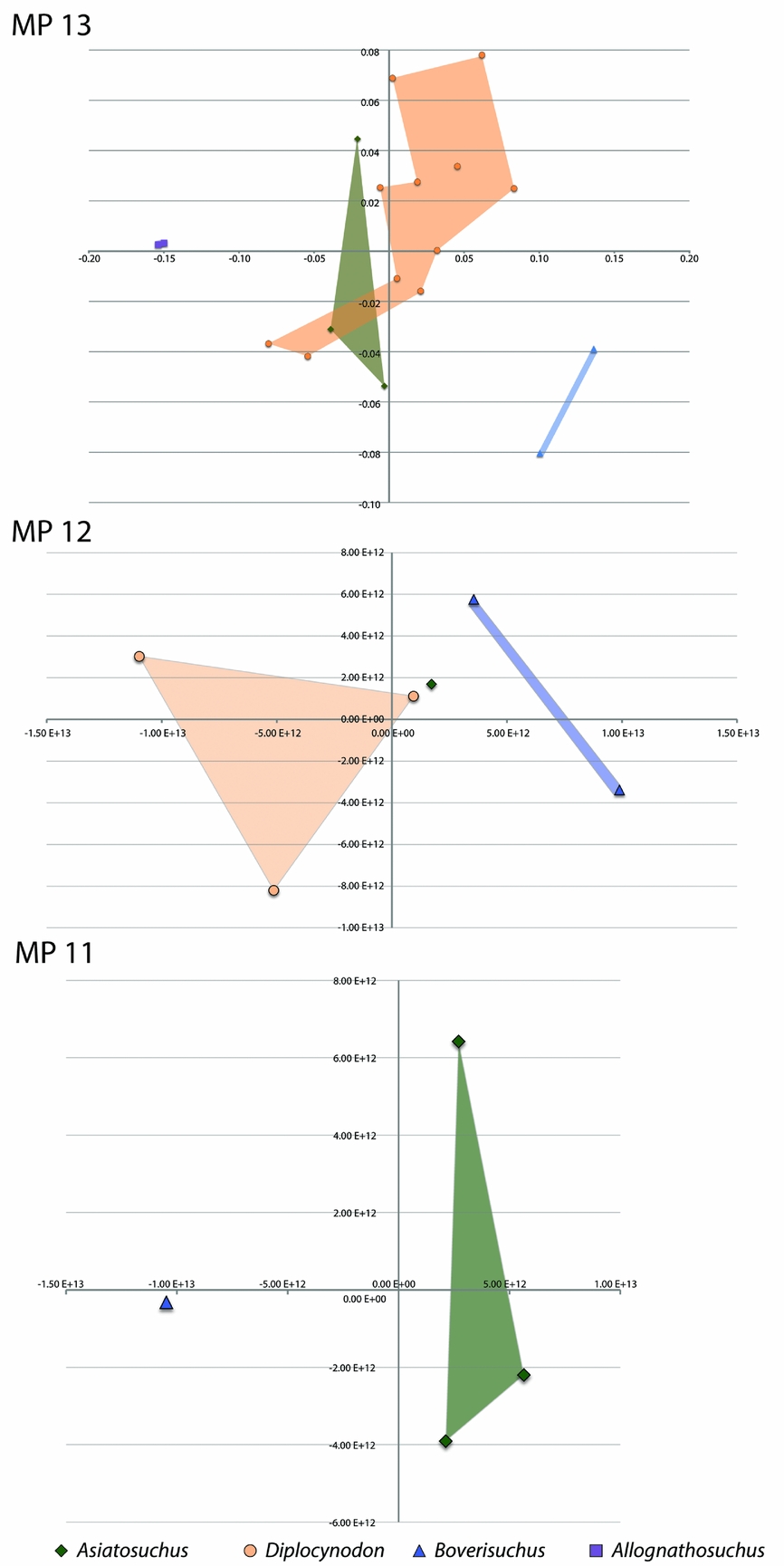
Fossils from Geiseltal were collected over a series of decades and many different researchers have contributed their provenance data over this time (Haubold & Krumbiegel, Reference Haubold and Krumbiegel1984). In general, ages of the fossils have been determined based on their stratigraphic section and mammalian biostratigraphy. The crocodylian fossils of Geiseltal span the entire ‘Geiseltalian’ European land mammal age, falling into one of three mammal Palaeogene zones, MP 11 through MP 13 (Franzen & Haubold, Reference Franzen and Haubold1987; Schmidt-Kittler, Reference Schmidt-Kittler1987; Haubold & Thomae, Reference Haubold and Thomae1990; Aguilar, Legendre & Michaux, Reference Aguilar, Legendre and Michaux1997; Hellmund, Reference Hellmund2007). We determined the MP zone (MP 11, 12, or 13) for each of the 28 specimens in the study from original locality data (Table 1).
It should be mentioned that due to the sedimentary (non-volcanic) nature of the site, radiometric data are not directly available from the Geiseltal site. However, an approximation of the absolute age of Geiseltal can be made by comparison with two similar terrestrial fossil sites in central Germany. The beginning of the fossil record of Geiseltal during MP 11 is also identified for the Messel fossil site (Franzen, Reference Franzen2005). The Messel fossil deposit lies atop a basalt dated to 47.8±0.2 Ma (Mertz & Renne, Reference Mertz and Renne2005). Younger fossils of Geiseltal were deposited during MP 13, the same stratigraphic zone as Eckfeld, a maar lake deposit from Eifel, Germany (Lutz & Kaulfuß, Reference Lutz and Kaulfuß2006). Basaltic ejecta from Eckfeld during a coeval eruption were dated to 44.3±0.4 Ma (Mertz et al. Reference Mertz, Swisher, Franzen, Neuffer and Lutz2000). This creates a roughly 3.5 Ma time span of fossilization at Geiseltal that can be divided into three stratigraphic zones (MP 11–13).
Interestingly, the four crocodylian taxa from Geiseltal discussed here are also reported from both the Messel fossil locality (MP 11; Morlo et al. Reference Morlo, Schaal, Mayr and Seiffert2004) and the Eckfeld fossil locality (MP 13; Neuffer et al. Reference Neuffer, Gruber, Lutz and Frankenhäuser1996). This shows that the interactions of these taxa likely occurred in central Germany throughout Geiseltalian time. Only at the Geiseltal site are all three MP zones recorded in a nearly continuous sequence at a single location, providing a more inclusive picture of interactions between these four different crocodylian genera. Allognathosuchus is relatively uncommon at all three localities, and only recorded from MP 13 at Geiseltal (Hellmund, Reference Hellmund2007). The incomplete specimen of Bergisuchus was only recorded from MP 11 at Geiseltal and Messel (Rossmann, Rauhe & Ortega, Reference Rossmann, Rauhe and Ortega2000). Asiatosuchus, Diplocynodon and Boverisuchus can all be found in each MP zone at Geiseltal (Hellmund, Reference Hellmund2007). As an example of their co-occurrence at Geiseltal, fossil teeth collected from one location within a single stratigraphic layer represent all four of these crocodylian species (XXXV within the ‘Neumark-Süd’ pit; MP 13). The dentition of these isolated teeth matches very well in terms of size and shape with teeth preserved in diagnostic jaws and skulls from other localities at Geiseltal. Furthermore, these teeth correspond to other teeth figured of Boverisuchus and Asiatosuchus (Prasad & de Lapparent de Broin, Reference Prasad and de Lapparent de Broin2002) and Diplocynodon (Delfino & Smith, Reference Delfino and Smith2012; Martin et al. Reference Martin, Amiot, Lécuyer and Benton2014).
The fossils from the different-aged deposits of Geiseltal change throughout the Geiseltalian; certain taxa-like fish, amphibians, snakes and lizards do not appear at all during MP 11, are rare or absent in MP 12 and common during MP 13 (Hellmund, Reference Hellmund2007). The reason for the absence of these taxa from the ‘Unterkohle’ seam (MP 11) and their rarity or absence from the lower ‘Mittelkohle’ seam (MP 12) can only be reasonably explained as a geological or geochemical setting lacking suitable parameters such as hydrocarbon inflow for successful fossil preservation (Krumbiegel, Reference Krumbiegel1977). Considering the lengthy and thorough means of collection over so many years, it is unlikely that fish and similar aquatic life were simply not collected; their lack of record from these layers more likely indicates a difference in fossilization and/or environment between the MP zones (G. Krumbiegel, pers. comm., 2013).
Ungulates are found at each MP zone at Geiseltal, and are the most common fossils discovered. Most notable of these are the relatively common primitive horses Propalaeotherium that include four valid species (the smallest now attributed to Eurohippus; Franzen, Reference Franzen2006). These early horses mainly differ in size, with some slight variation in tooth morphology (Hellmund, Reference Hellmund2013 a). The quantity of propalaeothere fossils in some locations is remarkably high, especially for site ‘XIV’ from MP 11 (Franzen & Haubold, Reference Franzen and Haubold1986; Hellmund, Reference Hellmund2013 a).
3. Results
3.a. Geiseltal crocodylians
The relative warps analysis of Geiseltal crocodylians resulted in 27 axes (online supplementary material Table S2 and Fig. S1; available at http://journals.cambridge.org/geo). The greatest amount of variation within the dataset was unsurprisingly composed of Diplocynodon specimens, which had the largest sample size (online supplementary Fig. S1). However, the largest portion of variation was within the first two axes for all taxa (online supplementary Fig. S1). Since the Procrustes analysis is generated within Kendall's shape space, a curved surface, data must be projected orthogonally onto a plane tangential to the consensus in order to be viewed in 2D space (Dryden & Mardia, Reference Dryden and Mardia1998; Stayton & Ruta, Reference Stayton and Ruta2006). We tested for potential distortion of this effect by regressing Procrustes distance against Euclidean distance using the program tpsSmall 1.28 (F. J. Rohlf, unpub. program, 2013: http://life.bio.sunysb.edu/morph/soft-tps.html). The resulting high correlation between these (0.999999) indicates that there is very strong agreement between the two projections (Stayton & Ruta, Reference Stayton and Ruta2006).
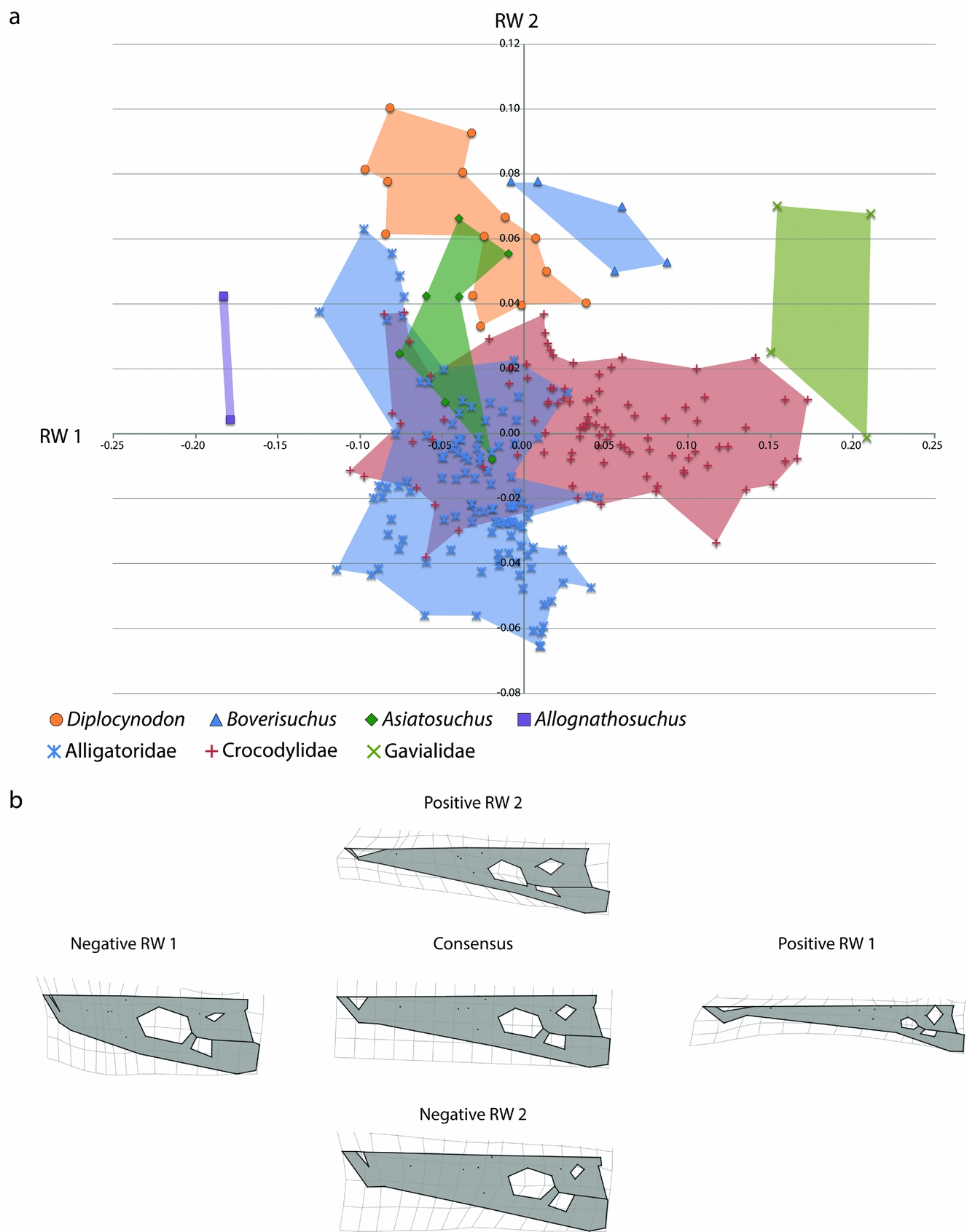
The first relative warp axis (RW 1; Fig. 4) primarily summarizes the transition from the brevirostrine condition of Allognathosuchus (negative) and the more longirostrine condition of Boverisuchus (positive). Landmarks of negative RW 1 configurations have: (1) more widely separated landmarks around the orbit, representing a relatively large orbit; (2) lateral margin landmarks that are relatively further from the midline, resulting in a ‘bowed out’ margin that creates a rounder, less straight lateral margin; (3) anterior landmarks that are relatively closer to other snout landmarks, resulting in a shorter snout; and (4) the landmark representing the anterior extent of the nasal is relatively closer to the anterior-most landmarks. Landmarks of positive RW 1 configurations have instead: (1) more constricted landmarks around the orbit, resulting in a relatively small orbit; (2) landmarks on the lateral skull margin that are relatively closer to the midline, resulting in a modestly ‘bowed in’ outline to the lateral margin; (3) anterior landmarks that are relatively further from the orbital landmarks, resulting in a longer snout; and (4) landmarks representing the anterior extent of the nasal bones and suture between the premaxilla and maxilla that are relatively closer to the orbital landmarks, resulting in a more elongated premaxilla.
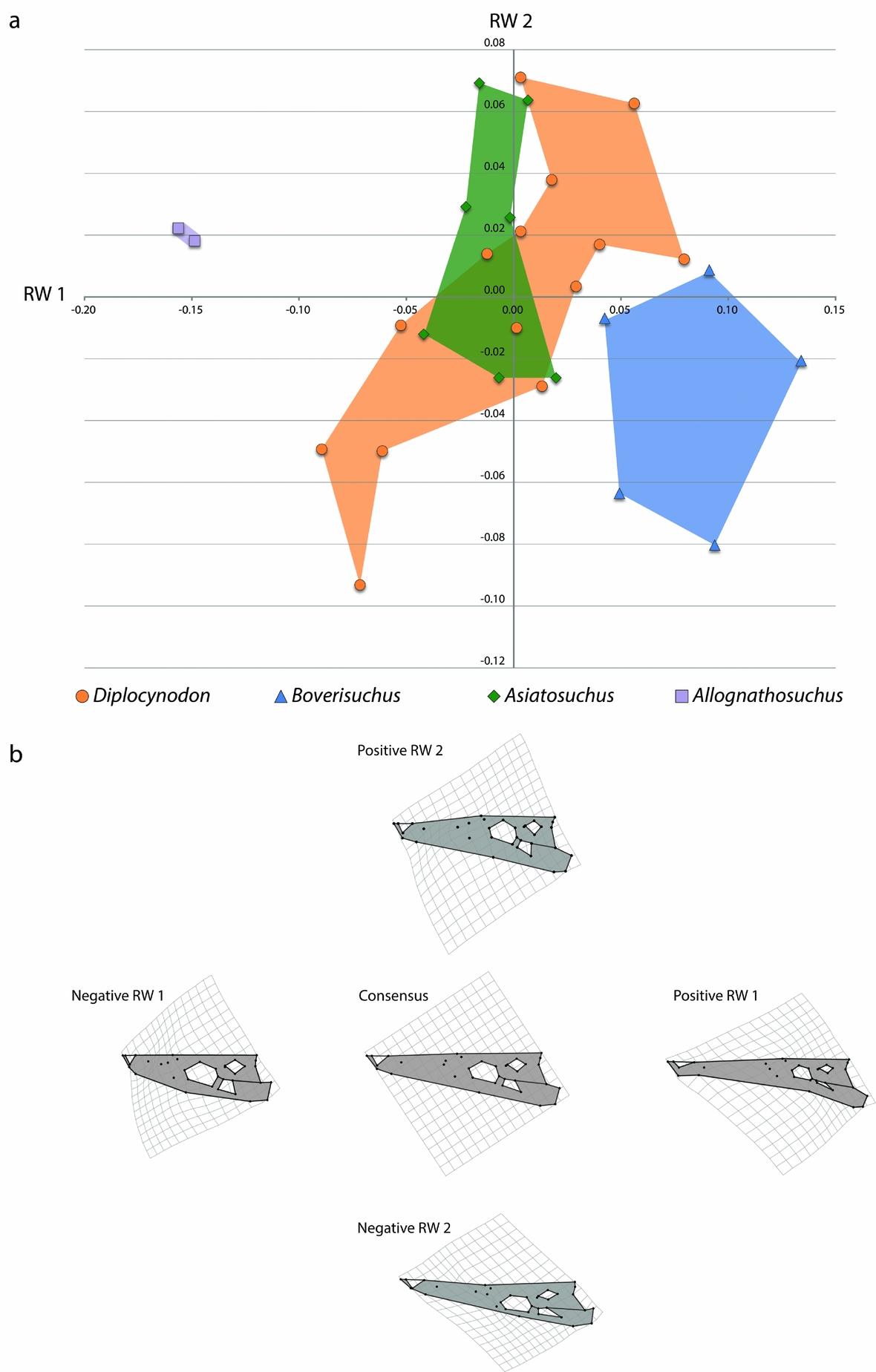
Figure 4. (a) Morphospace of 28 skulls representing four genera from Geiseltal, Germany. The horizontal axis is relative warp 1; the vertical axis is relative warp 2. (b) Deformation grids representing landmark placement centred on the consensus configuration and deviating in positive and negative directions for relative warps 1 and 2.
The landmarks of the second relative warp axis (RW 2; Fig. 4) appear to encompass a transition in skull width, mostly between certain members of Asiatosuchus and Diplocynodon. The landmarks of negative RW 2 have: (1) orbital landmarks that are relatively closer to the lateral margin; (2) landmarks of the lateral margin that are relatively closer to those of the midline, resulting in a narrower snout; and (3) a slightly more posterior relative position of the landmark representing the posterior extent of the squamosal, resulting in a larger skull table in dorsal view. The landmarks of positive RW 2 have instead: (1) orbital landmarks placed relatively closer to the midline, resulting in an orbit that is more centrally placed; (2) landmarks of the lateral margin that are relatively further from the midline, resulting in a wider snout; and (3) a slightly more anterior position of the landmark representing the jugal-quadratojugal suture, resulting in a slightly reduced infratemporal fenestra in dorsal view.
Morphospace of the Geiseltal crocodylians shows fairly distinct and coherent grouping within each of the four genera included in the analysis, in terms of RW 1 v. RW 2 (Fig. 4). The most distinct of these are Boverisuchus and Allognathosuchus which both exhibited no overlap with any other genus. Although with a small sample size, Allognathosuchus plotted very distinctly from other taxa. The landmarks of this taxon are characterized by strongly negative RW 1 traits, and both specimens were very similar in relative landmark positions. The Allognathosuchus lateral skull margin landmarks were marginally farther from the midline, resulting in a slightly positive RW 2. On the other extreme of RW 1 is Boverisuchus with anterior landmarks more distant from orbital landmarks, resulting in a longer snout. Orbital landmarks of Boverisuchus are more closely set, also matching with a more positive RW 1 position. Specimens of Boverisuchus trended towards more negative RW 2 traits, with orbital landmarks still more closely set to each other but also more closely set to the landmarks of the lateral skull margin. Variation in Diplocynodon was primarily along the RW 2 axis, with relatively less variation in the relative positions of anterior premaxillary landmarks and orbital landmarks. Asiatosuchus variation was closer to the consensus despite not having the largest representation within the dataset (7 of 28 specimens). Specimens of this taxon varied mostly in RW 2 values, but with less variation than Diplocynodon. Asiatosuchus and Diplocynodon overlapped in portions of their RW 1 v. RW 2 morphospace occupation. However, both genera had specimens outside the overlap zone.
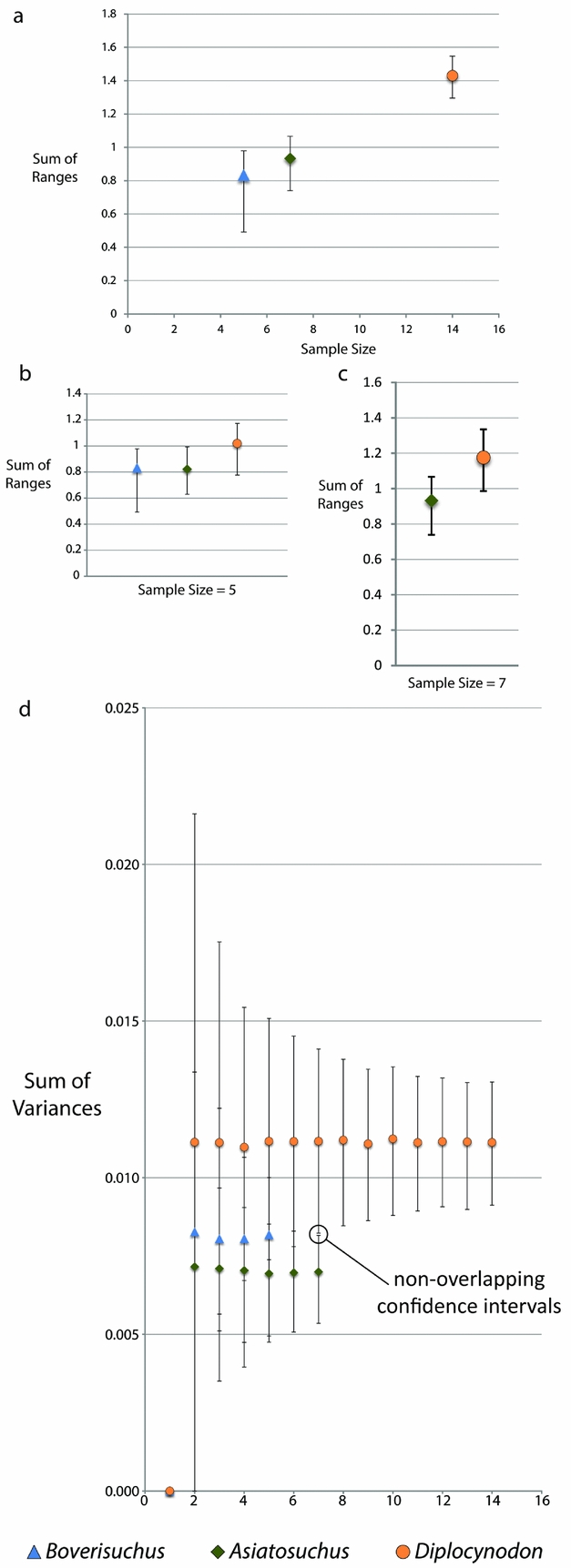
Due to the small sample size of Allognathosuchus, disparity metrics could not be calculated for this taxon. Variance, which is less sensitive to sample size differences (Wills, Reference Wills1998), was highest in Diplocynodon followed by Boverisuchus and Asiatosuchus (Table 2). However, these differences were not statistically different when the data were rarefied due to overlapping confidence intervals at the lowest common sample number of five individuals. However, comparison between the smallest common sample size of Asiatosuchus and Diplocynodon (seven individuals) did recover statistically significant, non-overlapping confidence intervals (Fig. 5). Range metrics indicate total extent of morphological differences, but are more sensitive to small sample size (Wills, Reference Wills1998). On the whole, Diplocynodon had significantly higher disparity than Boverisuchus or Asiatosuchus (Table 2; Fig. 5) but this difference is no longer significant when the data are resampled at the lowest common sample number (both five and seven; Fig. 5).
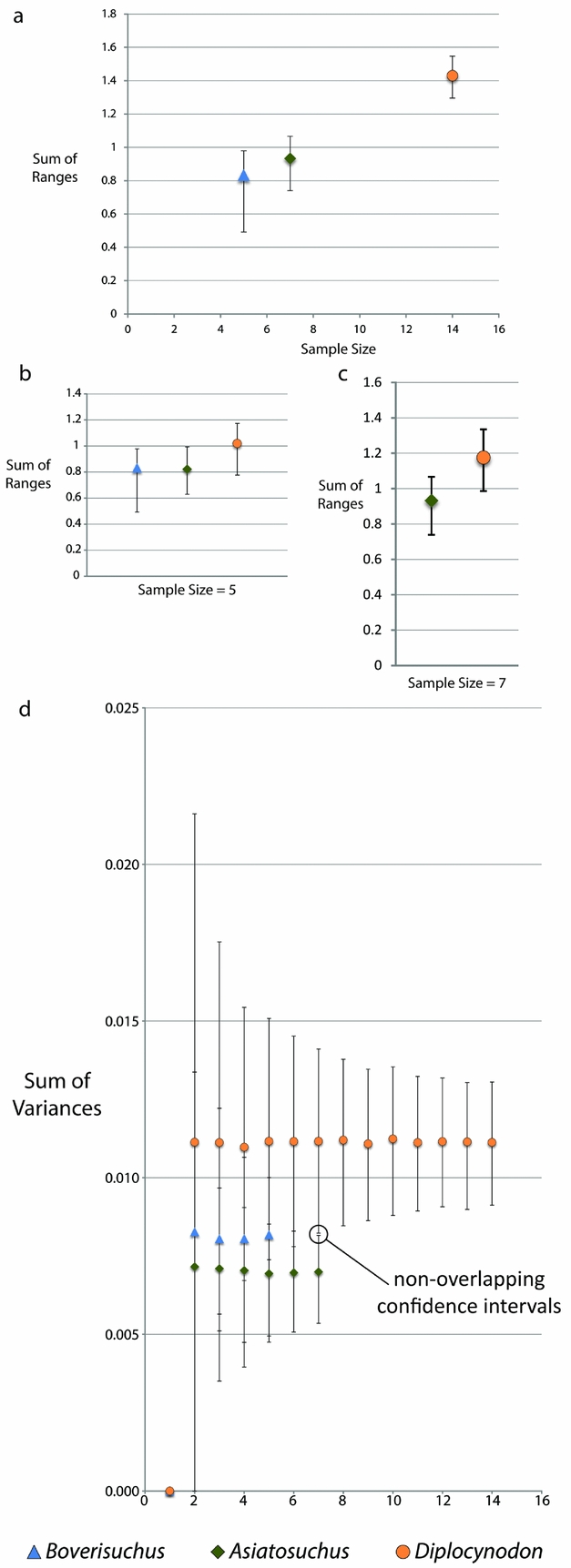
Figure 5. Disparity metrics with 95% confidence intervals from the Geiseltal geometric morphometric analysis encompassing all 27 relative warps axes. Data were generated using Rare 1.2 (Wills, Reference Wills1998). Key at bottom refers to all XY scatter plots. Allognathosuchus could not be included due to its small sample size (n = 2). (a) Sum of ranges for each taxon. (b) Sum of ranges data resampled by bootstrapping rarefaction to the smallest common sample size (n = 5). (c) Sum of ranges data resampled by bootstrapping rarefaction to the next smallest common sample size (n = 7). (d) Sum of variances for Geiseltal taxa, with indication of statistically independent values between rarefied Diplocynodon and Asiatosuchus due to non-overlapping confidence intervals at equal sample size.
We calculated Pearson's correlation coefficients and p-values for each of the 27 relative warps axes with respect to centroid size, stratigraphic age and taphonomy. These were conducted both as a whole for the Geiseltal dataset and within each taxon (except Allognathosuchus, which had too small of a sample size, n = 2), with the aim of determining statistically significant correlation (online supplementary Tables S2–S6, available at http://journals.cambridge.org/geo). There was a statistical correlation of centroid size for RW 3 and RW 10 in all Geiseltal specimens (online supplementary Table S2). When these data were subdivided into taxa, there was no significant correlation between size and the relative warps of Diplocynodon (online supplementary Table S3) or Boverisuchus (online supplementary Table S5). Asiatosuchus had statistically significant correlation in RW 6, 9, 13 and 15 (online supplementary Table S4). Centroid size had a significant inverse correlation with stratigraphic age for Geiseltal crocodylians as a whole and within Diplocynodon and Asiatosuchus (online supplementary Table S6). In all three cases, size decreased with stratigraphic age. No such correlation was found in Boverisuchus.
In terms of taphonomic effect, we assessed whether compression had a statistical correspondence with any of the relative warps axes. When treated as a whole, the Geiseltal crocodylians only showed a statistically significant correlation between taphonomy and RW 6 and 7 (online supplementary Table S2). These two axes account for a total of 10.85% of the variation within the dataset. When subdivided into taxa, taphonomy had a statistical correlation with RW 7 and 10 in Diplocynodon, accounting for a total of 8.51% of the variation within the taxon (online supplementary Table S3). There was no correlation within Boverisuchus (online supplementary Table S5). Due to the lack of taphonomic variation within Asiatosuchus (all specimens were uncompressed), this correlation statistic could not be calculated. Taphonomy had a statistically significant correlation with centroid size for Geiseltal as a whole (online supplementary Table S6), which is not surprising given the lack of compression in the large Asiatosuchus and the presence of compression in the smaller Diplocynodon and Allognathosuchus (Table 1).
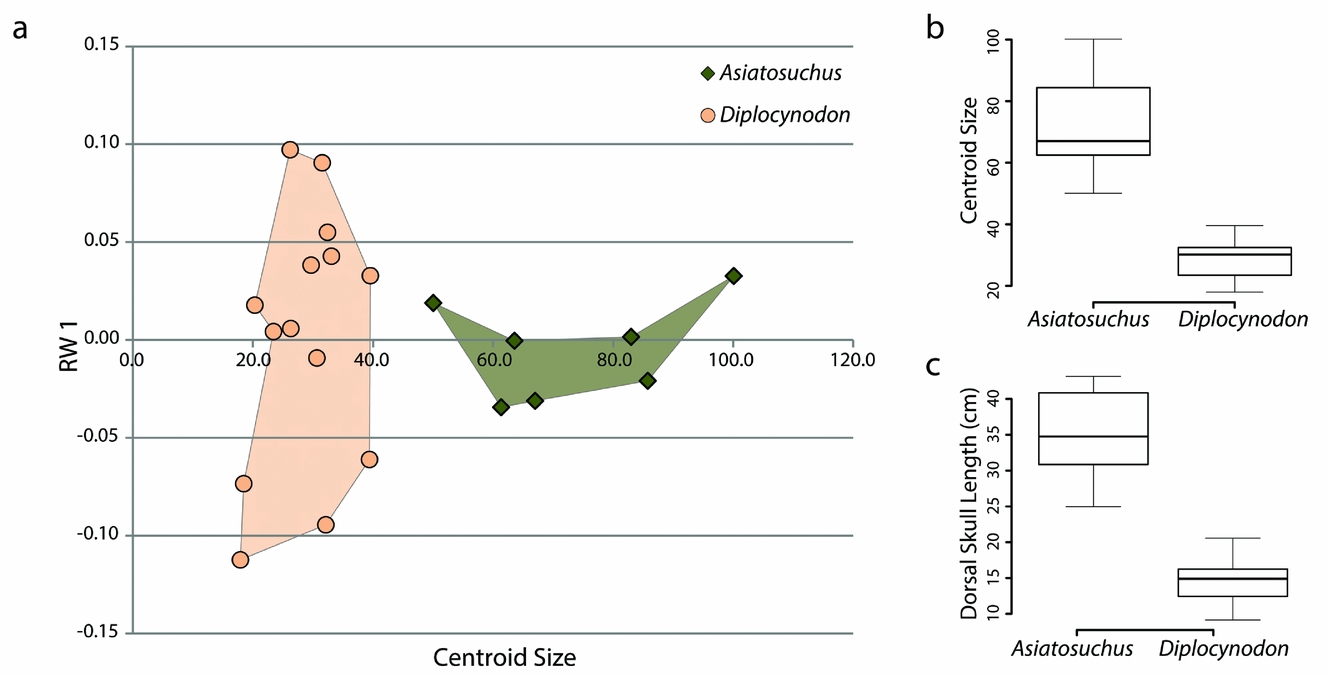
The most notable difference between skulls of Diplocynodon and Asiatosuchus is that those of adult Asiatosuchus are invariably much larger (Table 1). We conducted an independent analysis using only these two taxa and calculated centroid size. Centroid size was statistically independent between the two samples (p-value = 0.0003253) using a Welch Two Sample t-test in the statistics program R (see box plot Fig. 6; R Core Team, unpub. program, 2013: http://www.R-project.org). Dorsal skull length, measured from the tip of the snout to the posterior wall of the skull table, was entered into a second Welch Two Sample t-test and was also found to be statistically independent (p-value = 0.0001101; see box plot in Fig. 6). When centroid size was plotted against the first order of variation (relative warp 1), there was a distinct separation in size despite partial overlap in shape (Fig. 6). Pearson's correspondence analyses comparing each axis of the relative warps analysis to centroid size recovered only one (relative warp 2, comprising 10.75% of total variance) with statistically significant correlation (Pearson's correlation: 0.6859, p-value: 0.0006). However, when the data from this same geometric morphometric analysis are separated into Asiatosuchus and Diplocynodon, statistical correspondence is significant in different axes (Diplocynodon: RW 2, 17; Asiatosuchus: RW 5). This may imply that the growth of the two species follows separate trajectories, with different parameters affecting shape with increasing size during ontogeny. Without a full ontogenetic series of these taxa, it is impossible to say whether or not young Asiatosuchus would overlap in shape and size with adult Diplocynodon. However, the sizes of these taxa are statistically distinct as adults.
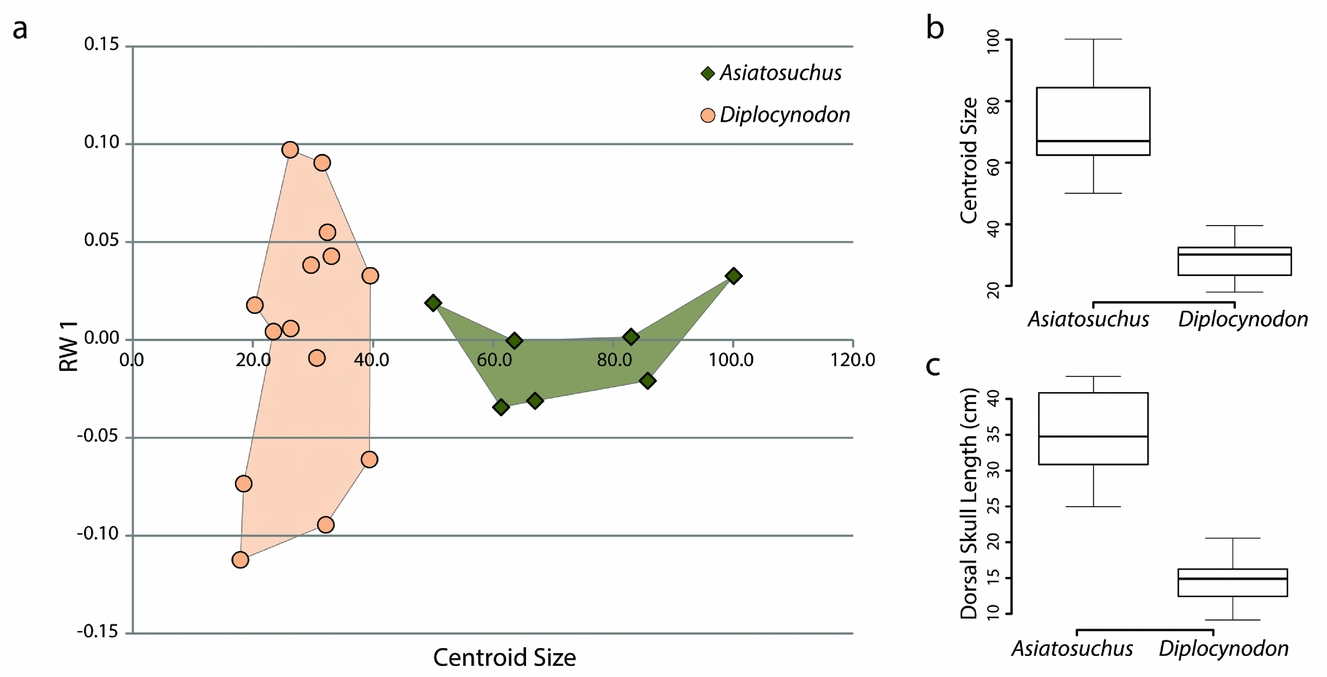
Figure 6. Data plots comparing Asiatosuchus and Diplocynodon from Geiseltal, Germany. (a) XY scatter plot incorporating the first order of variation from geometric morphometric analysis (relative warp 1, the vertical axis) with centroid size (horizontal axis). (b) Box plot of centroid sizes. (c) Box plot of dorsal skull lengths.
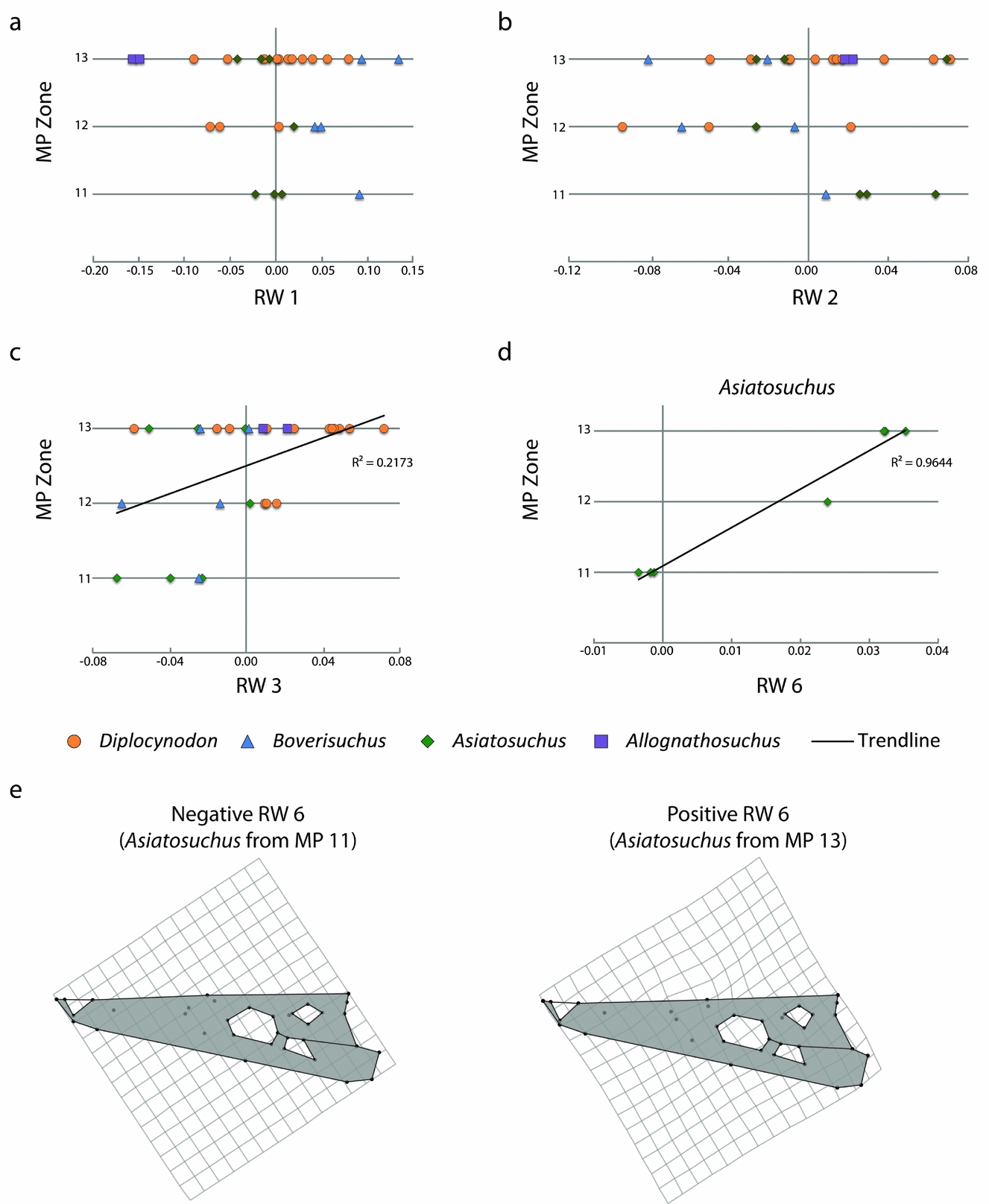
The crocodylian fossil skulls of Geiseltal span the middle Eocene MP zones 11–13. We compared the data from the 27 relative warps axes against time, both for the Geiseltal crocodylians and for each taxon (online supplementary Tables S2–S6; available at http://journals.cambridge.org/geo). As a whole, only RW 3 had a statistically significant correlation when plotted against time (online supplementary Table S2), although the correlation is weak (R 2 = 0.21731; Fig. 7). Relative warps 1 and 2 had no statistical correlation, regardless of which taxa were included (Fig. 7). No statistical correlation was recovered from Diplocynodon and, with only two specimens, this analysis could not be conducted for Allognathosuchus independently of the rest of the data. Asiatosuchus showed strong correlation with relative warp 6 (Fig. 7) and weak correlation with relative warp 10 (online supplementary Table S4). Relative warp 6 appears to summarize only subtle differences in landmarks of the relative anterior extents and contacts between the prefrontal, nasal, frontal and lacrimal bones (Fig. 7). Trending with time, the frontal landmarks appear to reach slightly more anteriorly while the prefrontal landmarks do not reach as anteriorly in MP 13 Asiatosuchus. This trend is statistically significant, but is admittedly limited to the seven samples available. Boverisuchus only had statistical correlation with relative warp 27, which only summarized 0.79% of taxon variation (online supplementary Table S5).
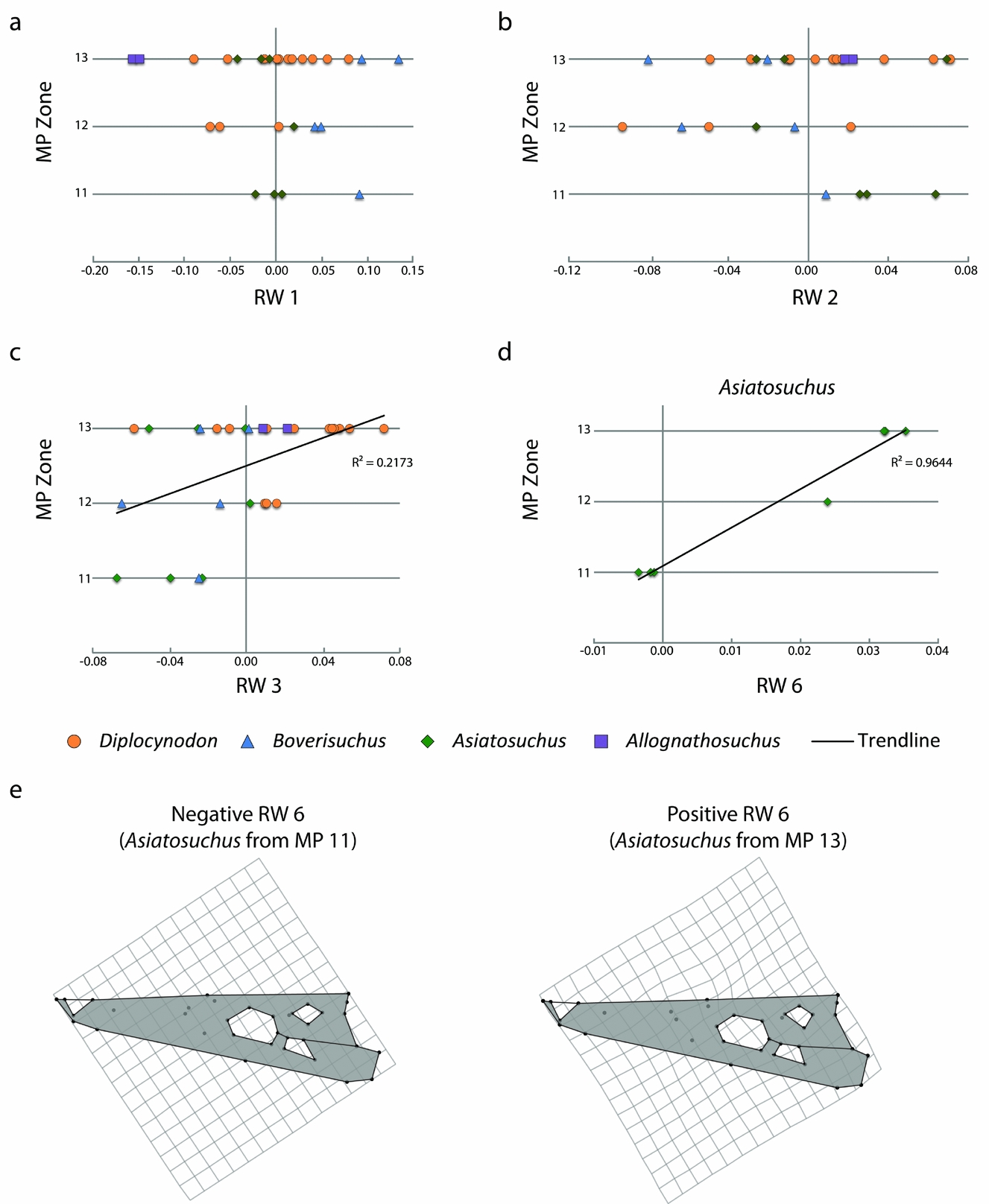
Figure 7. Geiseltal crocodylian morphospace through the preserved mammal Palaeogene zones, MP 11–13, spanning the ‘Geiseltalian’ European land mammal age. These are limited to individuals with complete skulls and do not represent all genera occurring within each zone. (a) XY scatter plot, showing RW 1 of the Geiseltal dataset from MP 11 to 13, which showed no statistically correlated trend. (b) XY scatter plot, showing RW 2 of the Geiseltal dataset from MP 11 to 13, which showed no statistically correlated trend. (c) XY scatter plot, showing RW 3 of the Geiseltal dataset from MP 11 to 13, which showed only a weak statistical correlation; see online supplementary Table S2. (d) Strongest statistical correlation with time within the Geiseltal dataset, RW 6 v. MP zone in Asiatosuchus; see online supplementary Table S4. (e) Deformation grids showing negative to positive relationships of landmarks along the RW 6 axis, as generated from the consensus of the full Geiseltal dataset.
As only reasonably complete skulls were included, these results are only representative of the specimens preserved well enough for the study. Each taxon, except Allognathosuchus, has representative fossils in each stratigraphic zone. The lack of Diplocynodon with complete skulls in MP 11 does not mean that Diplocynodon was not present at this time. The MP 11 zone only contained three Asiatosuchus and one Boverisuchus skull. More skulls were preserved within the MP 12 zone: three Diplocynodon, one Asiatosuchus and two Boverisuchus. The MP 13 zone contained the most specimens that could be used for this study (n = 18) with three Asiatosuchus, two Allognathosuchus, two Boverisuchus and eleven Diplocynodon.
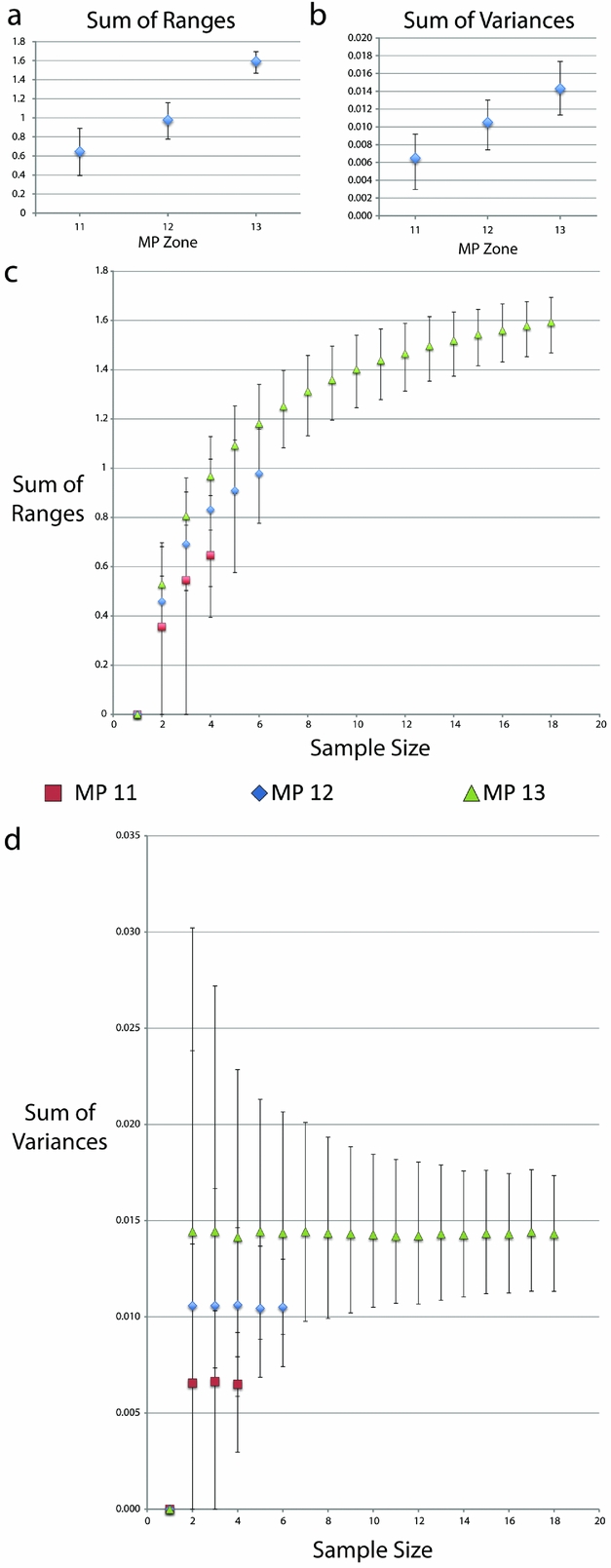
Both range and variance metrics steadily increase with time across the MP zones (Table 3; Fig. 8). There is a statistically signficant difference between the sum of ranges during MP 13 compared to both MP 11 and 12, as well as the sum of variances between MP 11 and MP 13 (Table 3; Fig. 8). However, when the data were resampled by rarefaction, statistical significance is lost when sampling was limited to the smallest common sample size (for both four and six individuals), as indicated by overlapping 95% confidence intervals (Fig. 8). It should however be noted that greater variation would be expected during MP 13 regardless of sample size due to Allognathosuchus only having been present during this particular MP zone.
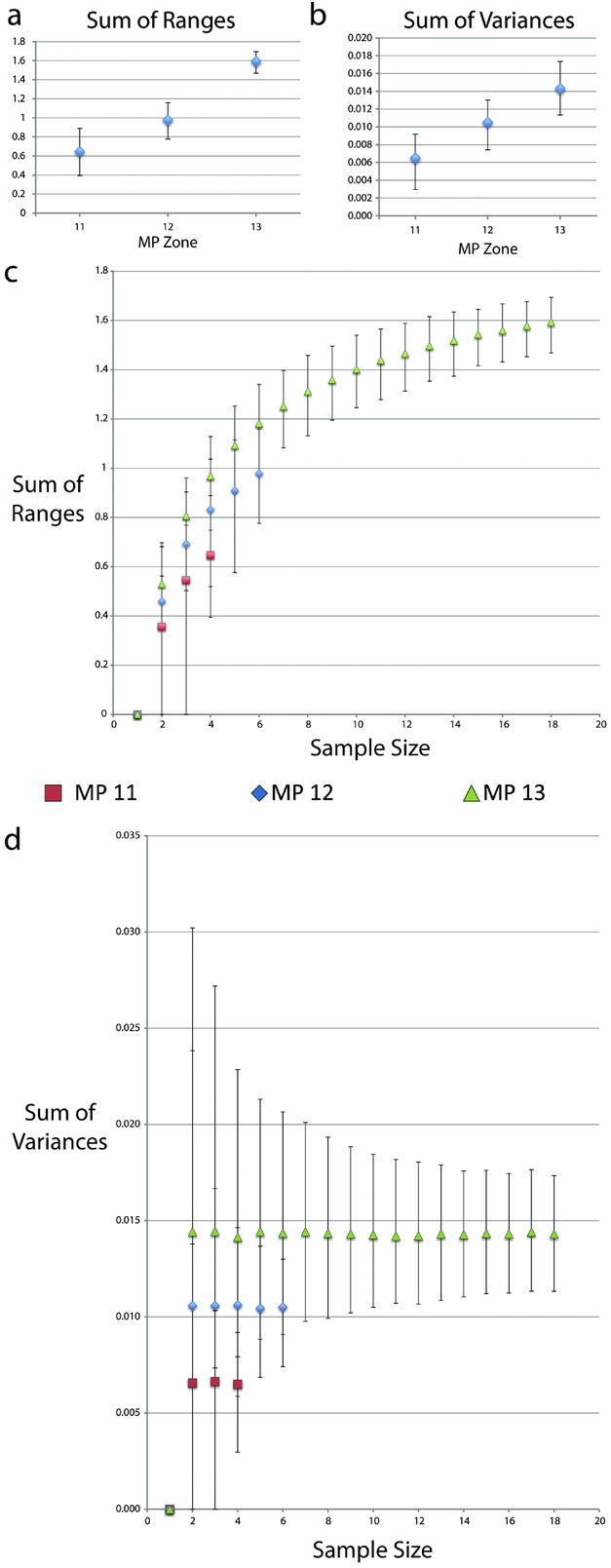
Figure 8. Disparity metrics with 95% confidence intervals from the Geiseltal geometric morphometric analysis encompassing all 27 relative warps axes. Data were generated using Rare 1.2 (Wills, Reference Wills1998). Key in middle refers to XY scatter plots of both (c) and (d). (a) Sum of ranges data from whole samples at each MP zone. (b) Sum of variances data from whole samples at each MP zone. (c) Sum of ranges data resampled using bootstrapping methods, creating rarefaction curves. (d) Sum of variances data resampled using bootstrapping methods, creating rarefaction curves.
In the interests of presenting alternative views, we split the landmark file into three seperate tps files and reran the relative warps analysis for each of the MP zones independently. Since these analyses were run using only the members present during each of the MP zones, the average skull shape was reset for each, meaning deviation from the centre is different for each zone. This analysis is not meant to identify trends with age, but to look at each zone as an independent collection of specimens. The MP 11 zone shows clear separation between the one specimen of Boverisuchus and the three Asiatosuchus in relative warps 1 and 2 (Fig. 9). The MP 12 specimens all plotted separately, but the one Asiatosuchus plotted very close to a Diplocynodon specimen. Not surprisingly, the same overlap between Asiatosuchus and Diplocynodon persisted in MP 13. During MP 13, Boverisuchus and Allognathosuchus again have very distinct separation in morphospace.

Figure 9. Geiseltal crocodylian morphospace separated by mammal Palaeogene zones, MP 11–13. Each plot represents a separate analysis using MP zone-specific subsets of the original landmark dataset. In each plot, the horizontal axis is relative warp 1; the vertical axis is relative warp 2.
3.b. Comparison to extant crocodylians
The relative warps analysis including the Geiseltal specimens and modern crocodylians resulted in 66 axes. The first two axes (RW 1 and RW 2) summarize 19.66% of the total variation within the combined analysis, which summarize more variation than any other axis (online supplementary Fig. S2; available at http://journals.cambridge.org/geo). We tested the combined analysis for correspondence between Kendall's shape space and Euclidean coordination by regressing Procrustes distance against Euclidean distance in tpsSmall 1.28 (F. J. Rohlf, unpub. program, 2013: http://life.bio.sunysb.edu/morph/soft-tps.html). The resulting high correlation (0.999996) indicates strong agreement between the two projections.
The primary axis of variation, RW 1, again involves mostly differences in landmarks reflecting brevirostrine versus longirostrine skulls (Fig. 10). The relationship of landmarks in this respect is the same as that found using only the Geiseltal specimens. The only notable difference is that negative RW 1 in the combined dataset also has a constriction of the landmarks of the supratemporal fenestra, resulting in a more bony skull table. Positive RW 1 landmarks share all the same traits found in the Geiseltal-only dataset, with a much stronger emphasis on premaxillary landmarks being relatively further from orbital landmarks, resulting in a longer snout in proportion to the rest of the head. This more extreme elongated skull shape is due to the inclusion of the longirostrine gharial (Gavialis gangeticus) and false gharial (Tomistoma schlegeliii). The positive RW 1 landmarks of the supratemporal fenestrae are also expanded, resulting in a less bony skull table. The second axis, RW 2, also mostly summarizes variation in width, but the direction of width is reversed. The other features describing differences in landmarks of RW 2 in Geiseltal are not comparable with the combined dataset. Instead, negative RW 2 is characterized by: (1) lateral margin landmarks that are further from the midline, resulting in a robust, wider snout; (2) landmarks that are more widely set between the quadratic condyle and quadrate-quadratojugal suture; and (3) more anterior extent of the jugal-quadratojugal suture along the infratemporal fenestra, resulting in a slightly different angle of the fenestra in dorsal view. Positive RW 2 is characterized by: (1) more close placement of landmarks from the lateral margin to the midline, particularly at the premaxilla, resulting in a more pointed, narrow skull shape; (2) slight difference in the quadratic condyle landmark, resulting in a slightly narrower condyle; and (3) more posterior position of the landmarks, representing the jugal-quadratojugal suture along the infratemporal fenestra, resulting in a more angled fenestra in dorsal view.
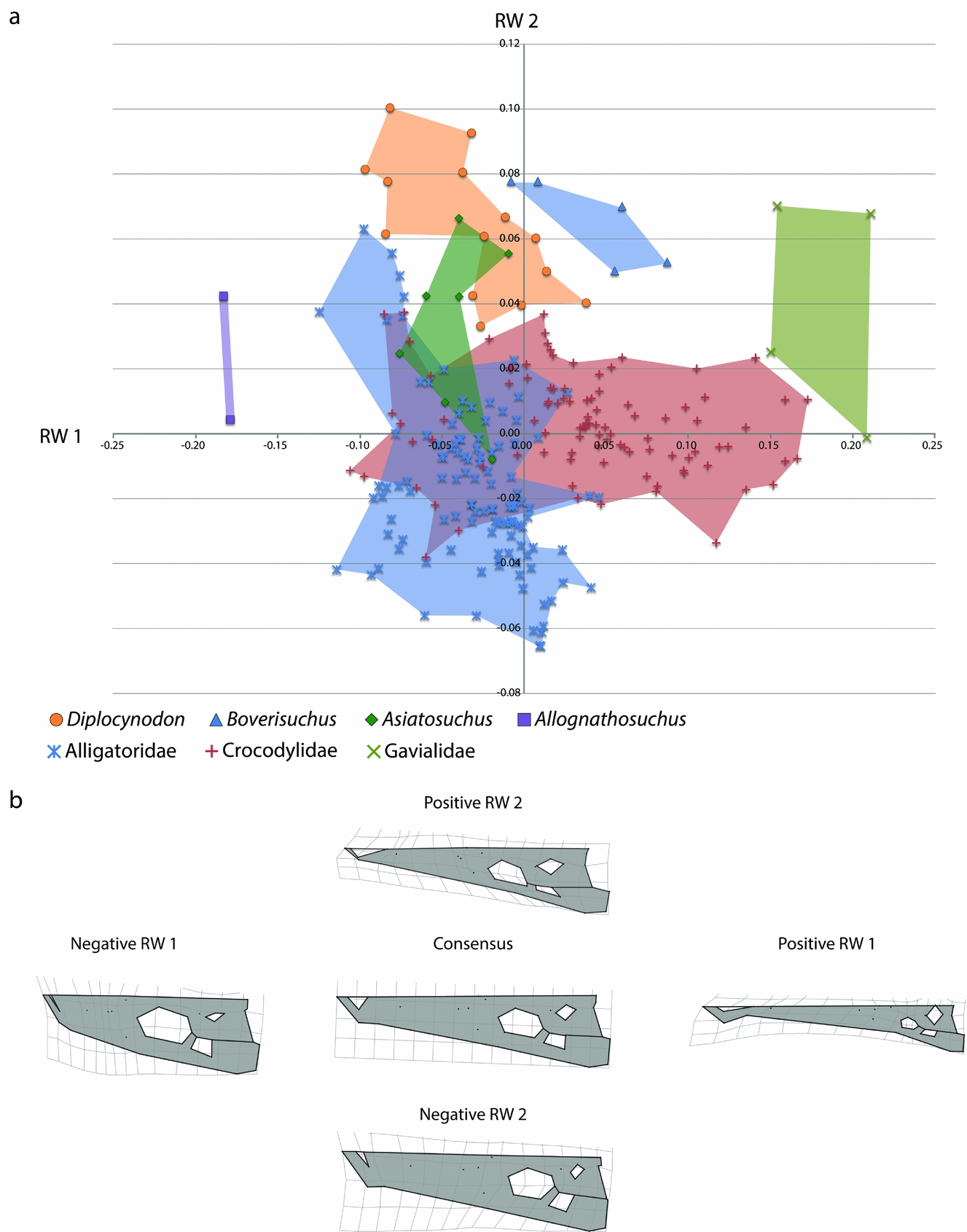
Figure 10. (a) Morphospace of Geiseltal crocodylian skulls (n = 28) and extant crocodylian skulls (n = 218) utilizing geometric morphometric analysis. The horizontal axis is relative warp 1; the vertical axis is relative warp 2. (b) Deformation grids representing landmark placement centred on the consensus configuration and deviating in positive and negative directions for relative warps 1 and 2.
The RW 1 v. RW 2 morphospace plot, including both Geiseltal crocodylians and extant crocodylians, shows separation of Boverisuchus, Allognathosuchus and Diplocynodon from all living species of Crocodylia (Fig. 10). The only overlap with extant taxa was Asiatosuchus, which overlapped with members of both Alligatoridae and Crocodylidae. The overlap between Asiatosuchus and Diplocynodon is still present, although this overlap does not coincide with any extant crocodylian in the dataset. Diplocynodon are characterized by positive RW 2, with more specimens verging on negative RW 1. The range of Diplocynodon in RW 1 is very similar to that of extant Alligatoridae. There was only minimal overlap in RW 2 with extant Crocodylidae, but much more overlap with some members of living Alligatoridae. When these two factors are combined, however, Diplocynodon appears as a distinct group from the extant members. Boverisuchus is characterized by only positive RW 2 and all but one specimen had positive RW 1. In terms of RW 2, Boverisuchus overlaps with Diplocynodon and some of Asiatosuchus, but is more distinct in terms of its more positive RW 1 values. The extant population shows that modern alligatorids vary mostly in terms of RW 2, while crocodylids vary most in terms of RW 1 landmark positions. The RW 1 variation of Crocodylidae is likely due to the inclusion of the false gharial, Tomistoma schlegelii, but is also contributed to by the slender-snouted crocodile, Mecistops cataphractus.
4. Discussion
4.a. Correlations between size, taphonomy and stratigraphic age
Due to the manageable number of axes from the Geiseltal component of the analysis, we ran statistical correlation along all 27 relative warps axes. The correlation between centroid size and relative warps axes was strongest in Asiatosuchus, accounting for a total of 13.97% of taxon variance (online supplementary Table S4; available at http://journals.cambridge.org/geo). This indicates a small but noteworthy allometric effect on skull shape. The fact that one of these axes (RW 6) also has statistically significant correlation with stratigraphic age (Fig. 7) is likely related. The other relative warps that correlated with size had no correlation with stratigraphic age in Asiatosuchus (online supplementary Table S4), indicating non-directional change in these allometric patterns through time.
Taphonomic compression affected the fossils of Geiseltal differently. Only Diplocynodon and Boverisuchus had representative specimens of both preservation states (notable compression or little to no compression). Both of these taxa are intermediate in size while Asiatosuchus, the largest taxon, had only uncompressed specimens (n = 7) and Allognathosuchus, the smallest taxon, had only compressed specimens (n = 2). This differential preservation is most likely caused by the difference in size, supported by the strong correlation between the centroid size and taphonomy within the full Geiseltal dataset (p-value = 0.0020; online supplementary Table S6). However, taphonomy appears to have had at most a minor effect on the geometric morphometric data. None of the first five relative warps axes (accounting for a total of 42% of variation) had a statistically significant correlation with taphonomy (online supplementary Table S2). Within the full Geiseltal dataset, correlation was recovered with only RW 6 and 7 (accounting for a total of 10.85% of variation). Even though RW 6 had statistically significant correlation with taphonomy in the full Geiseltal dataset, there was no such correlation within the taxon with the greatest sampling, Diplocynodon (online supplementary Table S3). This may indicate a more taxonomic bias to taphonomy in RW 6 than strictly shape altering, particularly considering Allognathosuchus were compressed and Asiatosuchus were not. Probably as a result of the taxonomic bias of taphonomy, centroid size had a statistical correlation with taphonomy but only when the entire dataset was considered. No such correlation existed within Diplocynodon or Boverisuchus (online supplementary Table S6). Correlation between taphonomy and shape was statistically significant within Diplocynodon for only relative warps 7 and 10 (accounting for a total of 8.51% of taxon variance; online supplementary Table S3). No statistical correlation was found between taphonomy and the relative warps of Boverisuchus (online supplementary Table S5). Most of the discussion of these data is focused on the first and second axes, which summarize more of the variation than any of the other axes and lack a significant correlation with taphonomy, both as a whole and within each taxon (online supplementary Tables S2–S6).
Although Boverisuchus, Asiatosuchus and Diplocynodon are all found at each MP zone (11–13), reasonably complete skulls were not recovered at each level. The graphics of Figures 7 and 9 should therefore be interpreted with some reservation. The general lack of statistically significant correlation between relative warp axes and stratigraphic age show only minor directional change through time (Fig. 7). The strongest correlation between age and relative warps (RW 6 of Asiatosuchus; online supplementary Table S4) is the only clear example of directional change within the skull structure of Geiseltal crocodylians, towards subtle differences in the relative anterior extent of the frontal and prefrontal landmarks. By looking at each MP zone independently (Fig. 9) we can see how each system existed on its own, even though it does not identify statistical trends through time.
4.b. Reconstructing prey preference in Geiseltal crocodylians
4.b.1. Total body length of Geiseltal crocodylians
Datasets have been generated in the past from extant crocodylians in order to estimate total body length in fossil taxa using the living crocodylian species Crocodylus porosus, Crocodylus moreletii, Gavialis gangeticus and Alligator mississippiensis (Woodward, White & Linda, Reference Woodward, White and Linda1995; Sereno et al. Reference Sereno, Larsson, Sidor and Gado2001; Farlow et al. Reference Farlow, Hurlburt, Elsey, Britton and Langston2005; Platt et al. Reference Platt, Rainwater, Thorbjarnarson, Finger, Anderson and McMurry2009; Young et al. Reference Young, Bell and Brusatte2011 b). Given the presence of both crocodyloids and alligatoroids at Geiseltal, it seemed appropriate for this study to use multiple methods for total length estimation. We have provided the dorsal skull length measurements of each of the Geiseltal fossil specimens in Table 1.
There is a large difference in skull morphology between the Geiseltal crocodylians and G. gangeticus and, as a result, we decided not to use these data. Femoral dimensions from A. mississippiensis were published by Farlow et al. (Reference Farlow, Hurlburt, Elsey, Britton and Langston2005) in order to calculate total body length but, given the lack of preserved femora with most Geiseltal crocodylians, this would also be impractical. This method was tested with Diplocynodon, Asiatosuchus and Boverisuchus from Geiseltal, and every specimen but one resulted in over-prediction of total body length (Farlow et al. Reference Farlow, Hurlburt, Elsey, Britton and Langston2005). Regressions for determining total body length from cranial length of modern A. mississippiensis were presented by Woodward, White & Linda (Reference Woodward, White and Linda1995) and converted to a cranial length: total length ratio by Young et al. (Reference Young, Bell and Brusatte2011 b). As Allognathosuchus and Diplocynodon are both alligatoroids (Brochu, Reference Brochu2004; J. Martin, unpub. Ph.D. thesis, Univ. Lyon, 2009; Martin, Reference Martin2010; Martin & Gross, Reference Martin and Gross2011; Delfino & Smith, Reference Delfino and Smith2012; Martin et al. Reference Martin, Amiot, Lécuyer and Benton2014), we have applied the calculation methods derived from A. mississippiensis (Woodward, White & Linda, Reference Woodward, White and Linda1995; Young et al. Reference Young, Bell and Brusatte2011 b; Table 1). As Asiatosuchus belongs to crocodyloidea (Vasse, Reference Vasse1992; Delfino & Smith, Reference Delfino and Smith2009; Brochu, Reference Brochu2013) and is more similar in size and morphology to C. porosus than to C. moreletii, we chose to use the data derived from C. porosus (Sereno et al. Reference Sereno, Larsson, Sidor and Gado2001). These total body length estimates are also provided in Table 1.
As Boverisuchus is neither a crocodyloid nor an alligatoroid (Brochu Reference Brochu2013), the method of total body length calculation was more problematic. Only one complete skeleton of Boverisuchus is known (GMH Leo X-8001-1938). Unfortunately, the skull of this specimen is preserved in ventral view only, and a dorsal skull length can only be approximated. Best estimation of the dorsal skull length from the ventral surface is 36.5 cm, and we measured 2.06 m for the total length of the skeleton. This measurement is nearly identical to that recorded by Farlow et al. (Reference Farlow, Hurlburt, Elsey, Britton and Langston2005). This creates a cranial length to total length ratio of 0.17727, higher than reported for C. porosus or A. mississippiensis (Woodward, White & Linda, Reference Woodward, White and Linda1995; Sereno et al. Reference Sereno, Larsson, Sidor and Gado2001; Young et al. Reference Young, Bell and Brusatte2011 b). These are instead more similar to low values of G. gangeticus, but still much lower than for metriorhynchids (Sereno et al. Reference Sereno, Larsson, Sidor and Gado2001; Young et al. Reference Young, Bell and Brusatte2011 b). Farlow et al. (Reference Farlow, Hurlburt, Elsey, Britton and Langston2005) attempted to use femoral data from A. mississippiensis to predict the size of this same Boverisuchus specimen from Geiseltal. The calculation they generated from the specimen's femur was much higher than the measured total length of the specimen (Farlow et al. Reference Farlow, Hurlburt, Elsey, Britton and Langston2005). The researchers suggested that an unusually large femur length: total length ratio could have caused this problem (Farlow et al. Reference Farlow, Hurlburt, Elsey, Britton and Langston2005). The study also returned higher-than-measured estimates calculated from cranial lengths derived from Alligator and C. porosus (Farlow et al. Reference Farlow, Hurlburt, Elsey, Britton and Langston2005). In the absence of a larger dataset for a more closely related taxon, we elected to use this single ratio to estimate body sizes of the other fossil Boverisuchus of Geiseltal (Table 1). The average from the Boverisuchus specimens of this study (1.41 m) is smaller than the estimates from either A. mississippiensis (1.56 m) or C. porosus (1.72 m).
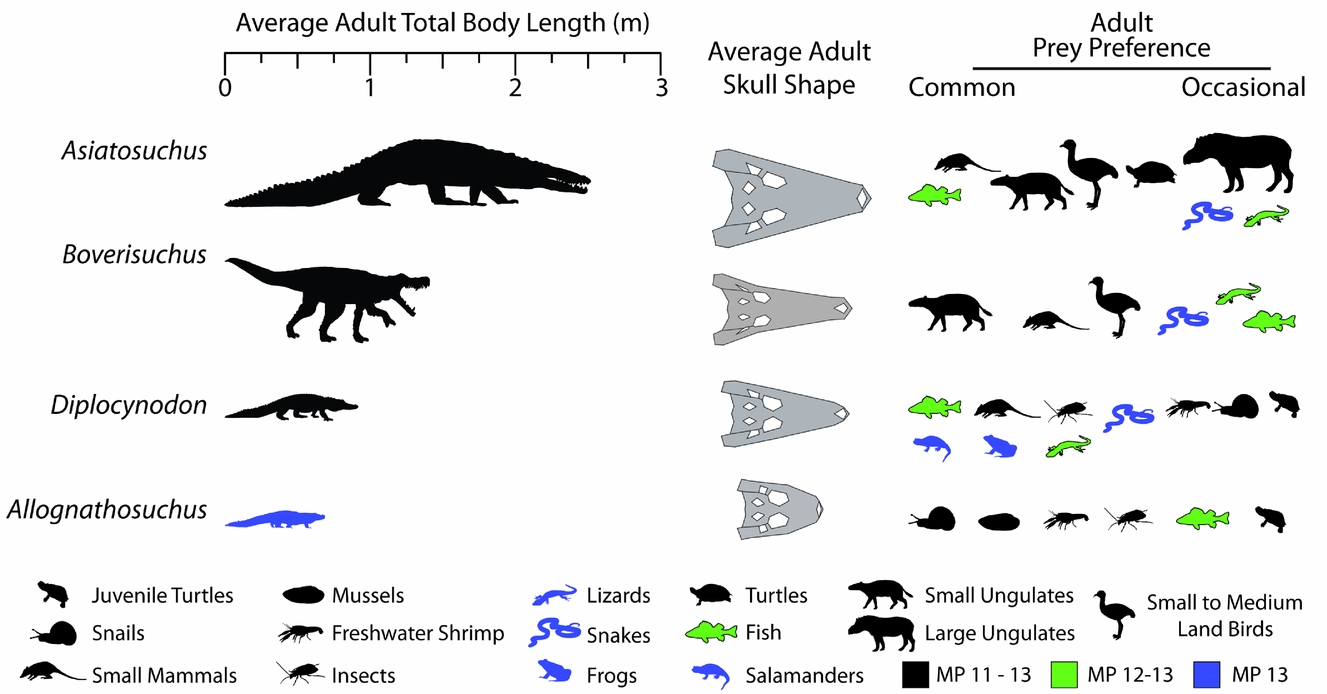
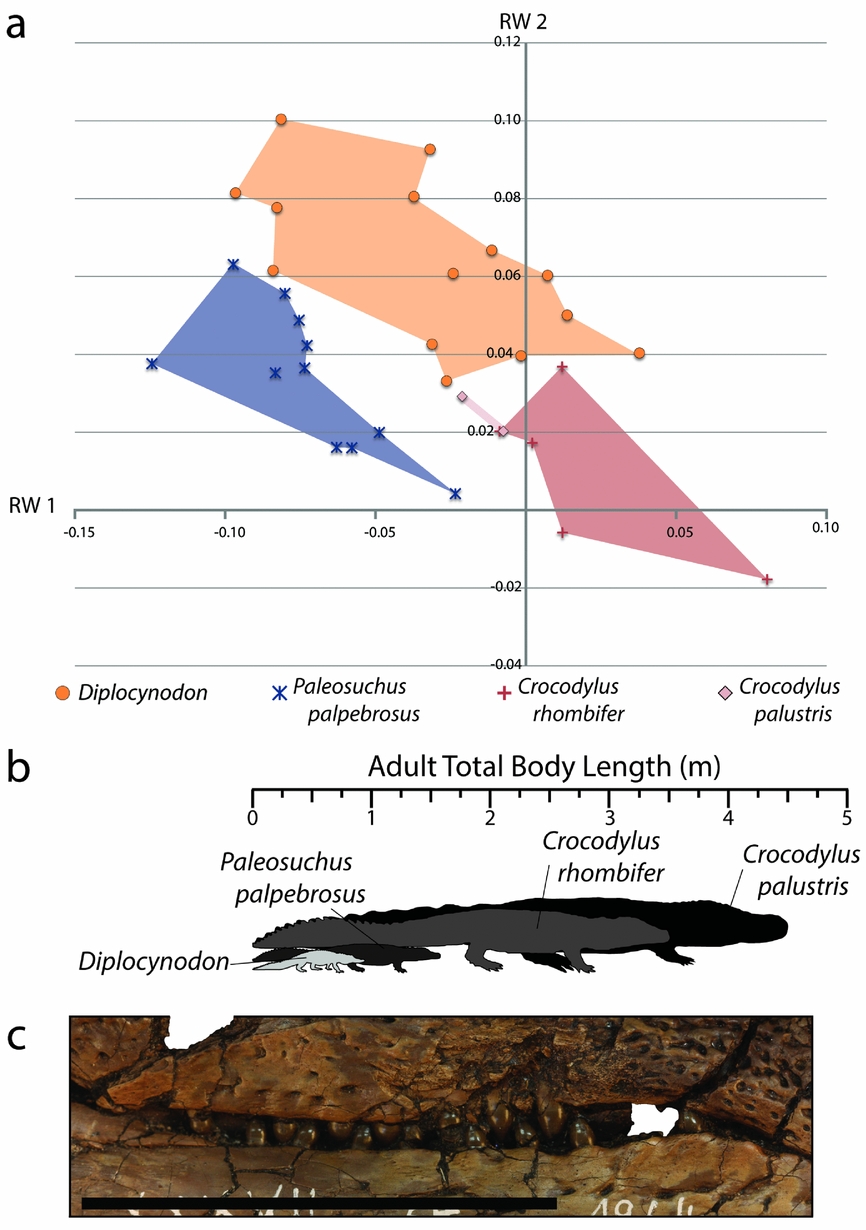
The averages of these values are presented in Figure 11, showing the relative sizes of the Geiseltal crocodylians. Asiatosuchus represents the largest Geiseltal crocodylian by far with an average length of 2.52 m, and is the largest predator from the fossil site, being much larger even than the mammalian carnivores. Boverisuchus was still quite large at an average adult length of 1.41 m. Diplocynodon averaged around 0.91 m, being consistently smaller than Asiatosuchus and Boverisuchus. The smallest are the Allognathosuchus at only 0.71 m average adult length. These small crocodylians are adults despite their small stature. At least three other individuals are known from Geiseltal that also represent small adults, although their skulls were not complete enough to be included in this study.
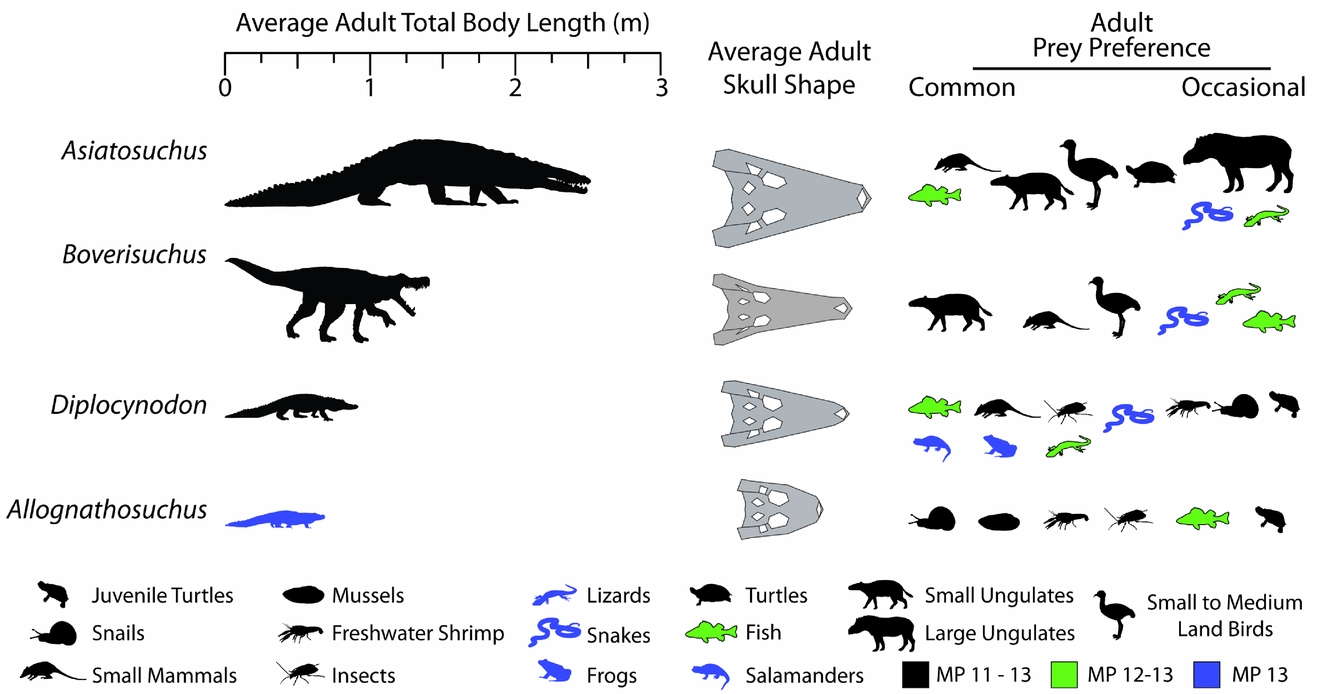
Figure 11. Reconstructed total body length estimated from fossil measurements (Table 1) and known relationships between head and body length in crocodylians (see Discussion 4.b.1 for details). Average adult skull shapes were formed from consensus plots of morphospace exclusive to the taxon. Reconstructed diet determined from similarity to extant morphospace and known fossil taxonomic occurrences at the Geiseltal-Fossillagerstätte, as well as other information; see Discussion 4.b.2–5 for details. MP stands for mammal Palaeogene zone.
4.b.2. Estimating potential prey preference of Allognathosuchus
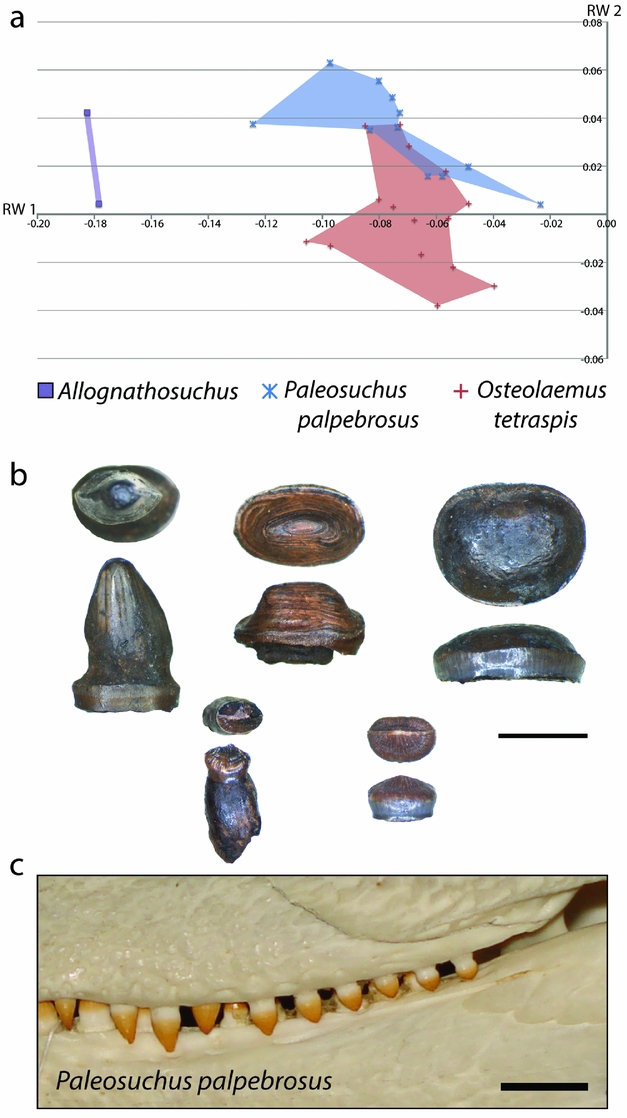
From comparison of skull shape and body size data for each Geiseltal taxon to the extant dataset, we can estimate the likely diet of these extinct crocodylians. The closest similarities within the extant geometric morphometric dataset to Allognathosuchus were the dwarf caiman, Paleosuchus palpebrosus and the dwarf crocodile, Osteolaemus tetraspis (Fig. 12). The size of these living crocodylians rarely exceeds 2 m (Eaton, Reference Eaton, Manolis and Stevenson2010; Magnusson & Campos, Reference Magnusson, Campos, Manolis and Stevenson2010), making them the smallest species of living crocodylians. Body size estimates for Allognathosuchus are even smaller, less than 1 m (Table 1).
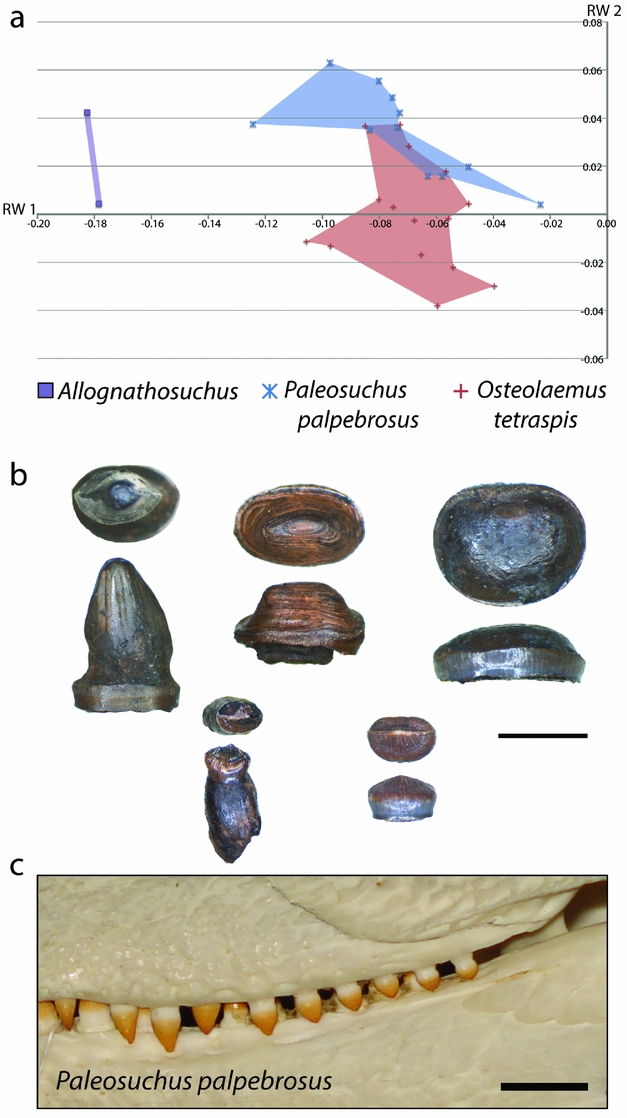
Figure 12. (a) XY scatter plot of relative warps 1 and 2 from the combined analysis showing only Allognathosuchus and extant species with closest similarity. (b) Examples of dentition from Allognathosuchus in worn condition (top three specimens) and unworn (bottom two specimens); each tooth is shown in occlusal and lateral view; GMH III-10173-1949; scale bar 5 mm. (c) Comparable dentition in Paleosuchus palpebrosus in left lateral view (ICN 1818); scale bar 1 cm.
In addition to similar landmark configuration, the dwarf crocodile and dwarf caiman both have heterodont dentition with sharpened teeth anteriorly and molariform teeth posteriorly (Frey & Monninger, Reference Frey and Monninger2010; Fig. 12). The posterior dentition of Allognathosuchus is remarkably molariform, being very flat without an apical point, and would likely have served well for a durophagous diet (Fig. 12). Furthermore, these teeth are often heavily worn (Fig. 12), showing strong similarity to teeth of fossil and modern crocodylians previously interpreted as crushing hard food items including molluscs, crustaceans and turtles (Ösi & Barrett, Reference Ösi and Barrett2011). The dwarf crocodile and dwarf caiman commonly eat insects, snails, crabs, shrimp and fish (Magnusson, Vieira da Silva & Lima, Reference Magnusson, Vieira da Silva and Lima1987; Pauwels et al. Reference Pauwels, Barr, Sanchez and Burger2007). Based on the diet, dentition and shape similarity of Paleosuchus and Osteolaemus, and the small size of Allognathosuchus, it was likely restricted to similar small, armored prey. Possible prey items that lived at the same MP 13 zone of Geiseltal included crustaceans (Beurlen, Reference Beurlen1938), bivalves (Krumbiegel, Reference Krumbiegel1990) and gastropods (Krumbiegel, Reference Krumbiegel1963). Also available were insects (Weidlich, Reference Weidlich1987) and fish (Voigt, Reference Voigt1934; Gaudant, Reference Gaudant1988), which likely rounded out the diet based on the modern diet of both Paleosuchus and Osteolaemus (Magnusson, Vieira da Silva & Lima, Reference Magnusson, Vieira da Silva and Lima1987; Pauwels et al. Reference Pauwels, Barr, Sanchez and Burger2007). Turtles were suggested as a probable prey item of Allognathosuchus (Carpenter & Lindsey, Reference Carpenter and Lindsey1980) due to their very blunt teeth and stout jaw. Turtles were present at this time at Geiseltal (Hummel, Reference Hummel1935) but, due to the small size of Allognathosuchus, adult turtles were likely too large. However, juvenile turtles may have been an occasional prey item.
It should be noted that, being an alligatoroid (Brochu, Reference Brochu2004), Allognathosuchus would be predicted to be more similar to living forms due to the stronger phylogenetic effect on rostral morphology found in a 3D study of modern crocodylian skulls (Piras et al. Reference Piras, Luciano Teresi, Buscalioni and Cubo2009, Reference Piras, Buscalioni, Teresi, Raia, Sansalone, Kotsakis and Cubo2014). However, the more severely negative RW 1 traits of Allognathosuchus, combined with its highly flattened rear teeth, appear to represent adaptation towards a strongly durophagous diet to a greater degree than other Geiseltal genera.
4.b.3. Estimating potential prey preference of Boverisuchus
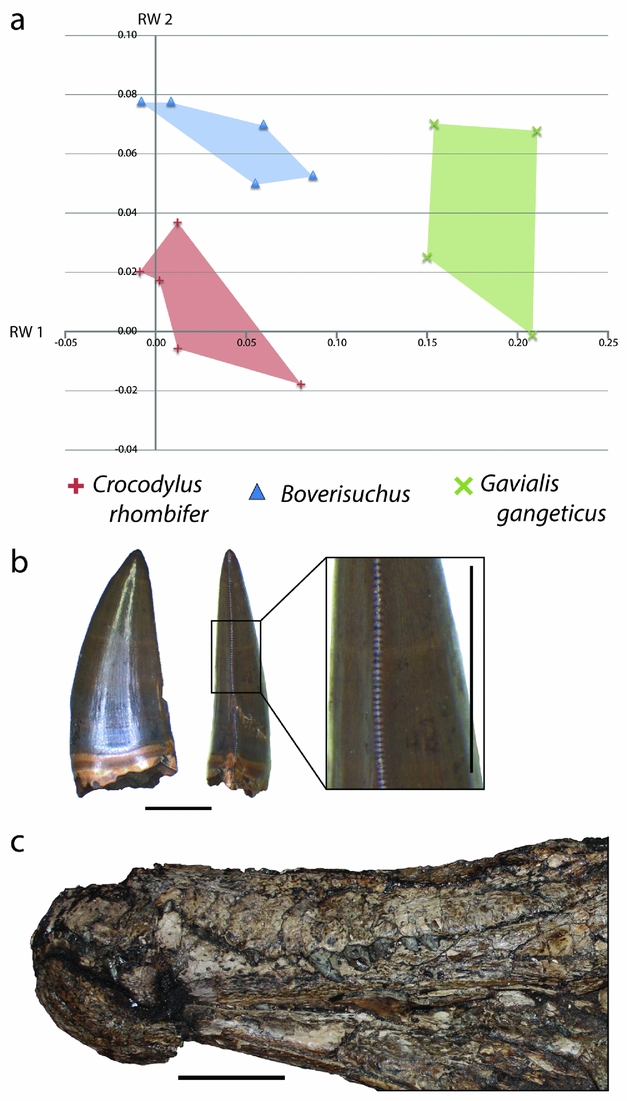
Within the combined Geiseltal and extant RW 1 v. RW 2 morphospace plots, Boverisuchus occupied a wholly different morphospace from all modern crocodylians and the other members of the Geiseltal fauna (Fig. 10). The positive but low RW 1 and positive RW 2 placement of Boverisuchus landmarks indicated a narrow, triangular snout. This shape was similar in terms of RW 2 to certain specimens of Diplocynodon. The landmark configurations of Boverisuchus specimens did not reflect nearly as extreme values of RW 1 as Gavialis skulls (Fig. 13) but were still longirostrine, and the general triangular shape of the skull is consistent in some ways with Gavialis. Furthermore, the shape analysis placed Boverisuchus from Geiseltal nearest to one member of the Cuban Crocodile (Crocodylus rhombifer; Fig. 13). The Cuban Crocodile has been noted for having a more terrestrial behaviour, having been seen far from water sources and even jumping on land for semi-arboreal hutias (Morgan & Albury, Reference Morgan and Albury2013). The Indian Gharial (Gavialis gangeticus) is however strictly fluvial in habitat (Stevenson & Whitaker, Reference Stevenson, Whitaker, Manolis and Stevenson2010). The dietary preferences of these two taxa reflect their preference of habitat. The Cuban Crocodile has a diet consisting mostly of small terrestrial mammals (Soberón, Ramos & Barr, Reference Soberón, Ramos and Barr2001), while the gharial consumes almost exclusively fish (Stevenson & Whitaker, Reference Stevenson, Whitaker, Manolis and Stevenson2010). RW 1 values of Boverisuchus are more consistent with C. rhombifer, while RW 2 values are more consistent with Gavialis. Boverisuchus landmarks with respect to these two relative warps axes do not match either taxon, indicating a potentially different purpose but with at least some similarity to both.
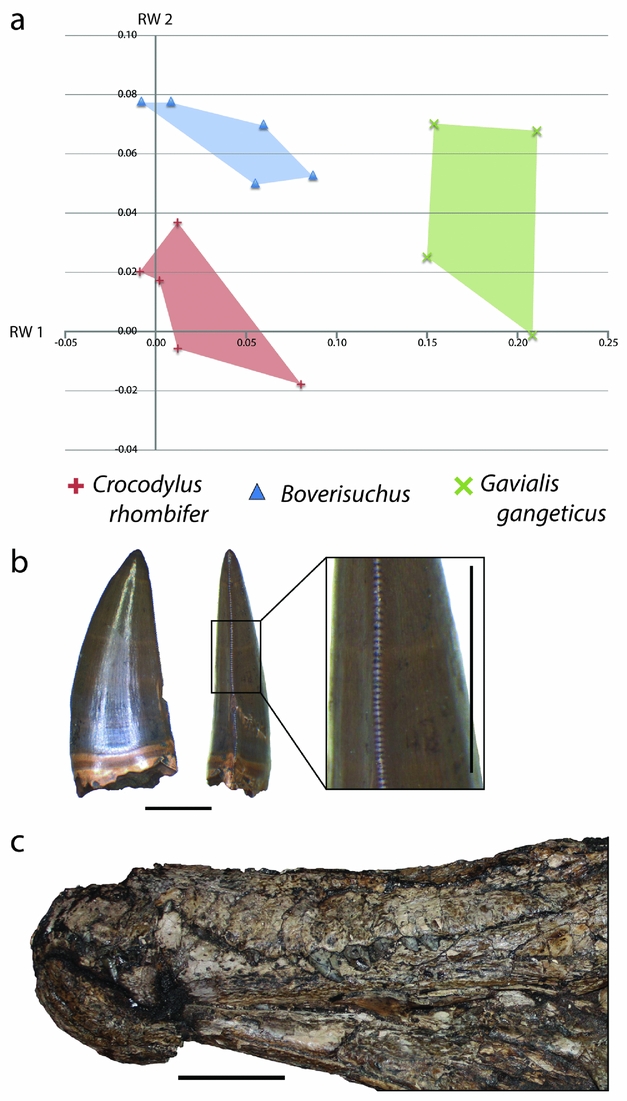
Figure 13. (a) XY scatter plot of relative warps 1 and 2 from the combined analysis showing only Boverisuchus and extant species with closest similarity. (b) Example of a serrated tooth from Boverisuchus in lateral and posterior views; GMH VI-1500-1952; both scale bars 5 mm. (c) Lateral view of exposed dentition of Boverisuchus; GMH Leo X-8001-1938; scale bar 10 cm.
To interpret the potential diet for Boverisuchus, it helps to also incorporate other known features of its morphology. For example the teeth have true serrations (Fig. 13), an unusual trait not seen in modern crocodylians and only seen in a small number of fossil crocodyliforms. Serrated teeth are typically seen in either fully terrestrial predators, such as theropod dinosaurs and Komodo dragons (D'Amore & Blumenschine, Reference D'Amore and Blumenschine2009), or in fully marine predators such as some sharks (Frazzetta, Reference Frazzetta1988). Truly serrated teeth (excluding false-ziphodonty, sensu Young et al. Reference Young, de Andrade, Brusatte, Sakamoto and Liston2013) are known to have evolved independently in multiple lineages of Crocodyliformes, typically from taxa interpreted as being either terrestrial (e.g. sebecids, baurusuchids or mekosuchines; Montefeltro, Larsson & Langer, Reference Montefeltro, Larsson and Langer2011; Sobbe, Price & Knezour, Reference Sobbe, Price and Knezour2013; Kellner, Pinheiro & Campos, Reference Kellner, Pinheiro and Campos2014) or marine (e.g. certain metriorhynchids; Young et al. Reference Young, de Andrade, Brusatte, Sakamoto and Liston2013). Given that the Geiseltal site is quite far from a marine habitat, even during middle Eocene time, the terrestrial preference seems more likely. In each case, serrations are useful for cutting prey that cannot be swallowed whole. This enables land predators to consume prey that is much larger than their jaws. For terrestrial predators that do not chew their food, this is necessary for defleshing the prey when force alone is not sufficient (D'Amore & Blumenschine, Reference D'Amore and Blumenschine2009). The teeth of Boverisuchus bow ventrally with elongated upper teeth exposed when the jaws are shut (Fig. 13), which is much more similar to the morphology of C. rhombifer than to Gavialis. Furthermore, Boverisuchus has longer limbs in relation to its body (Farlow et al. Reference Farlow, Hurlburt, Elsey, Britton and Langston2005) as well as hoof-like phalanges (Brochu, Reference Brochu2013), both of which have been interpreted as reflecting a more terrestrial habitat. Combining the evidence for different skull shape, tooth morphology, limb length and phalangeal morphology, Boverisuchus appears to have been adapted for a very different method of prey acquisition from living crocodylians as well as from other Geiseltal crocodylians. Given the skull shape similarity, greater dentition similarity and connections to terrestrial (not fluvial) habitat (Soberón, Ramos & Barr, Reference Soberón, Ramos and Barr2001), the Cuban Crocodile seems to be a better analogue than the Gharial for Boverisuchus.
There are some clues as to crocodylian predation of terrestrial prey within the middle Eocene fossil records of Germany. The Geiseltal fossil site is well-known for horses that were comparatively small in size, for example, Propalaeotherium (Hellmund & Koehn, Reference Hellmund and Koehn2000; Hellmund, Reference Hellmund2013 a). A small publication made during the ongoing excavations stated: ‘Krokodilzähne in Kieferknochen von Paläohippiden deuten darauf hin, daß diese von Krokodilen angefallen und gebissen wurden’ (original quote in German by Krumbiegel, Reference Krumbiegel1959, p. 119), which translates as ‘Crocodile teeth in the jawbones of primitive horses [formerly called palaeohippids] suggest that these were attacked and bitten by crocodiles’.
No crocodile teeth preserved within a horse jaw have yet been found within the Geiseltal Collection; however, they may have become disassociated during the last 56 years. An observation from the German fossil site Eckfeld (MP 13) states, regarding a fossil primate jaw, that ‘bite marks in the front of the mandible and the [dis]solution of tooth enamel point in the case of the Periconodon specimen to crocodile digestion’ (Franzen, Reference Franzen2004). Crocodylian predation of non-aquatic prey such as small horses and primates would be consistent with Boverisuchus as a terrestrial predator, although it does not mean that these animals were necessarily attacked by Boverisuchus specifically.
Crocodylus rhombifer is known to eat small mammals such as hutia (Soberón, Ramos & Barr, Reference Soberón, Ramos and Barr2001). Similarly sized mammals were also present at Geiseltal, including insectivorans, marsupials and primates (Hellmund, Reference Hellmund2007). These animals may have provided an appropriate prey source for Boverisuchus. Other prey of similar size at Geiseltal could have included occasional large snakes and/or lizards (Hellmund, Reference Hellmund2007). Given the similarity in RW 2 between Boverisuchus and Gavialis, fish may also have been at least a component of its prey preference. It should be noted that snakes, lizards and fish grow far more common at Geiseltal up to MP 13 and have yet to be recovered from MP 11 (Hellmund, Reference Hellmund2007; Table 1). Although it would represent a different kind of prey, Geiseltal fossils of terrestrial non-volant birds such as Palaeotis have been recovered that could have fallen prey to a terrestrial predator even at adult size (Houde & Haubold, Reference Houde and Haubold1987). Additionally, juveniles of the larger bird Gastornis may also have been a source of prey (Fischer, Reference Fischer1962; Hellmund, Reference Hellmund2013 b). The only mammalian cursorial carnivore of comparable size from Geiseltal was the creodont Oxyaenoides bicupsidens, but this taxon was only present during MP 11 (Morlo, Reference Morlo1999; Hellmund, Reference Hellmund2007; Table 1); competition from other large-bodied land predators would therefore have been low to non-existent at Geiseltal.
4.b.4. Estimating potential prey preference of Diplocynodon
The Diplocynodon of Geiseltal placed in RW 1 v. RW 2 morphospace not occupied by any modern crocodylian, although it did overlap with some specimens of Asiatosuchus. As an alligatoroid (Delfino & Smith, Reference Delfino and Smith2012) this is somewhat surprising, given the known phylogenetic effect on morphological disparity in Alligatoroidea (Piras et al. Reference Piras, Luciano Teresi, Buscalioni and Cubo2009). However, Diplocynodon of Geiseltal plotted close to another alligatoroid, the dwarf caiman (Paleosuchus palpebrosus), as well as two crocodyloids, the Indian Mugger (Crocodylus palustris) and the Cuban Crocodile (Crocodylus rhombifer). Skull shape in these crocodylians seems to be characterized by positive RW 1 and RW 2 values near the consensus (Fig. 14). The Diplocynodon specimens closest to P. palpebrosus had more pointed snouts, with positive RW 2 and negative RW 1 traits. In turn, the Diplocynodon closest to C. rhombifer and C. palustris have less pointed snouts with low positive RW 2 and near-consensus RW 1 traits.
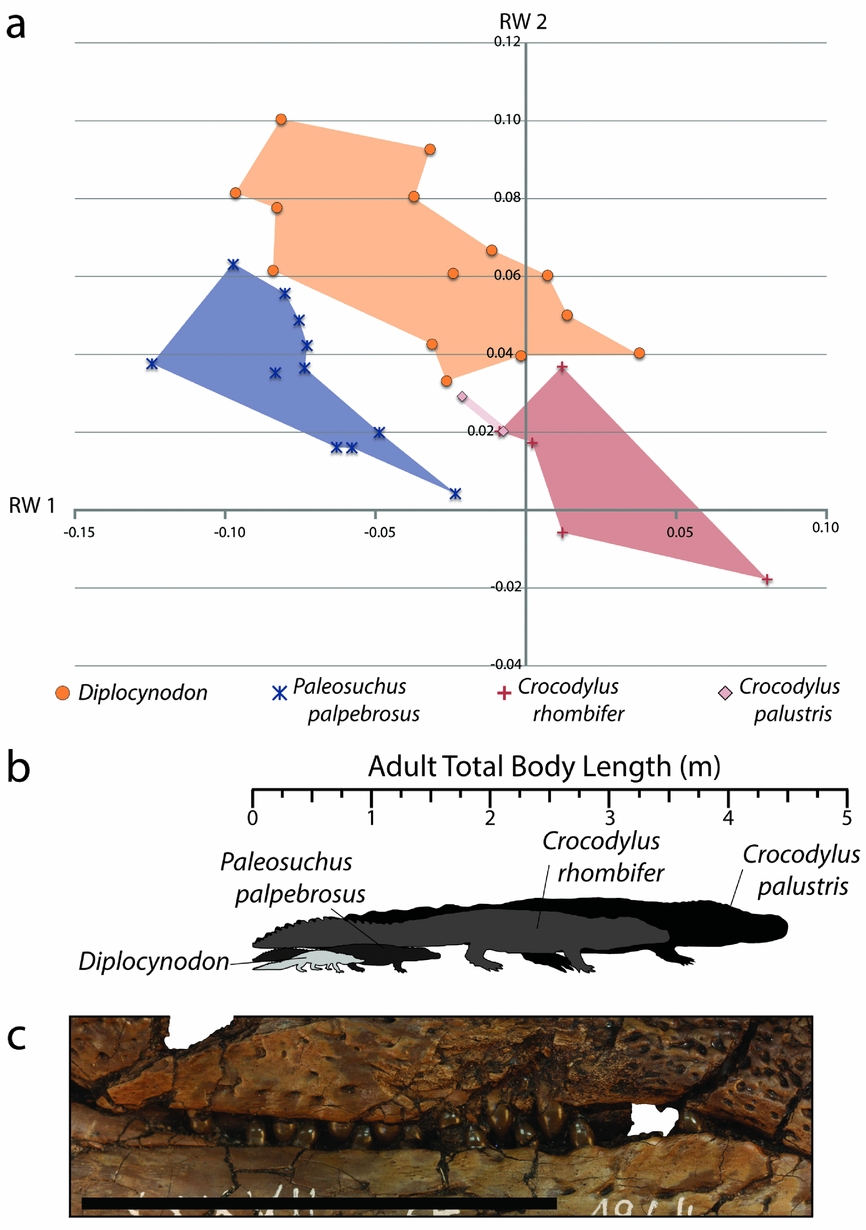
Figure 14. (a) XY scatter plot of relative warps 1 and 2 from the combined analysis showing only Diplocynodon and extant species with closest similarity. (b) Size comparison between Diplocynodon and the three extant taxa identified in the combined geometric morphometric analysis as having similar RW 1 v. RW 2 morphospace above; total body length values for extant taxa are: 1.6 m for Paleosuchus palpebrosus (Medem, Reference Medem1981), 3.5 m for Crocodylus rhombifer (Varona, Reference Varona1966) and 4.5 m for Crocodylus palustris (Chang et al. Reference Chang, Gaschal, Qadri and Shaikh2012); Diplocynodon estimate from this study. (c) Right lateral view of rear dentition of Diplocynodon from Geiseltal; GMH XXXVII-54-1964; scale bar 5 cm; anterior is to the right.
The diets of these three extant crocodylians are rather different from each other. The dwarf caiman regularly consumes insects, crustaceans, molluscs and fish (Magnusson, Vieira da Silva & Lima, Reference Magnusson, Vieira da Silva and Lima1987). The Indian Mugger, quite a bit larger than Diplocynodon, regularly consumes insects (as juveniles), crustaceans, fish, frogs, reptiles, small mammals and occasional large mammals as adults (De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). As stated, the Cuban Crocodile is known for a diet consisting mostly of small terrestrial mammals (Soberón, Ramos & Barr, Reference Soberón, Ramos and Barr2001).
Considering the shape similarity in RW 1 v. RW 2 morphospace with these three crocodylians and their widely variable dietary information, it appears Diplocynodon was a small generalist. Of the three extant forms, the size of adult Diplocynodon is most similar to P. palpebrosus (Fig. 14). Comparing the diet of the dwarf caiman to prey available at Geiseltal, Diplocynodon would likely have eaten from a variety of small prey, including those with armor. Studies of modern crocodylians found that bite force scales with body size in all living species (as measured on land; Erickson et al. Reference Erickson, Gignac, Steppan, Lappin, Vliet, Brueggen, Inouye, Kledzik and Webb2012, Reference Erickson, Gignac, Lappin, Vliet, Brueggen and Webb2014), so this difference of size likely translates to a difference in potential prey, both in terms of prey hardness and prey size. The small stature of Diplocynodon likely precluded large prey that similarly shaped C. palustris can consume, meaning that prey such as ungulates, adult land birds and adult turtles would not be likely food sources. The dentition of Diplocynodon (Fig. 14) is not nearly as flat or bulbous as Allognathosuchus (Fig. 12), but more similar to anterior teeth of P. palpebrosus (Fig. 12) than to the round, peg-like teeth of C. palustris or the more compressed teeth of C. rhombifer.
However, given the RW 1 v. RW 2 morphospace similarity to both C. palustris and C. rhombifer, Diplocynodon may have regularly consumed small mammals (Soberón, Ramos & Barr, Reference Soberón, Ramos and Barr2001; De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). The lack of features associated with terrestriality in Diplocynodon indicates that, like most crocodylians, Diplocynodon was adapted to an aquatic habitat as in extant P. palpebrosus and C. palustris (Magnusson, Vieira da Silva & Lima, Reference Magnusson, Vieira da Silva and Lima1987; De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). Given their presence in the diet of P. palpebrosus and young C. palustris, small prey such as insects, fish, frogs and reptiles were a likely part of the diet of Diplocynodon. Fossils of insects, fish, frogs, salamanders, lizards and snakes have been recovered from the same levels as Diplocynodon at Geiseltal (Fig. 11). However, fish, amphibians, snakes and lizards are much more common during MP 13 at Geiseltal and are unknown so far from MP 11 (Hellmund, Reference Hellmund2007; Table 1).
4.b.5. Estimating potential prey preference of Asiatosuchus
In the combined morphospace of RW 1 v. RW 2, Asiatosuchus specimens were characterized by negative RW 1 and mostly positive RW 2 traits (Fig. 10). Perhaps as a result of the high morphological disparity of crocodyloids (Piras et al. Reference Piras, Luciano Teresi, Buscalioni and Cubo2009), the morphospace of Asiatosuchus overlapped with three different genera of extant crocodylians (Fig. 15): Osteolaemus tetraspis (Dwarf Crocodile); Paleosuchus palpebrosus (Dwarf Caiman); and Caiman (C. crocodilus and C. yacare). The Asiatosuchus morphospace is also very close to that of Crocodylus palustris and Crocodylus novaeguineae (Fig. 15). These six species in four genera have fairly similar skull landmark configurations, with the specimens closest to Asiatosuchus being characterized by positive RW 1 traits but near the consensus. The Asiatosuchus with lower RW 2 traits were most similar to the caimans (Caiman and Paleosuchus) and the two true crocodiles (C. palustris and C. novaguinae). The Asiatosuchus with the highest RW 1 were nearest Osteolaemus, with similarly robust snouts.

Figure 15. (a) XY scatter plot of relative warps 1 and 2 from the combined analysis showing only Asiatosuchus and extant species with closest similarity. (b) Left lower dentition of Asiatosuchus in left lateral view; GMH XIV-4757b-1956; scale bar 1 cm. (c) Enlarged view of Asiatosuchus tooth in anterior and labial views; GMH XIV 2401 1954; scale bar 1 cm. (d) Comparison of Asiatosuchus skull from Geiseltal (GMH XIV 4757a-1956) and modern specimen of Crocodylus palustris (ZMB 37193, Zoological Collection of the Museum für Naturkunde, Berlin); scale bar 5 cm. (e) Internal view of a costal section of the carapace of the freshwater turtle Geochelone eocaenica (GMH XXXVI-416-1963) with crocodylian bite mark preserved, including surrounding impact scar; scale bar 5 cm. (f) Enlarged view of the bite mark of the same specimen in (e); scale bar 1 cm. bm – bite mark; d6 – sixth dentary tooth; d11 – eleventh dentary tooth; d15 – fifteenth dentary tooth.
The skull shape of these extant crocodylians is somewhat varied as in Asiatosuchus, and their diet is also fairly diverse. As mentioned above, the dwarf crocodiles and dwarf caimans commonly eat insects, snails, crabs, shrimp and fish (Magnusson, Vieira da Silva & Lima, Reference Magnusson, Vieira da Silva and Lima1987; Pauwels et al. Reference Pauwels, Barr, Sanchez and Burger2007); however, their adult size is much smaller than that of Asiatosuchus. The spectacled caiman, Caiman crocodilus, typically consumes much of the same food, but with a greater portion of its diet coming from fish as individuals approach adulthood (Magnusson, Vieira da Silva & Lima, Reference Magnusson, Vieira da Silva and Lima1987). Adult Crocodylus palustris, the Indian Mugger, tend to eat crustaceans, fish, reptiles, small mammals and occasional large mammals (De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). Unfortunately, there is little to nothing published regarding the natural diet of the New Guinea crocodile, Crocodylus novaeguineae. Asiatosuchus does not appear to have any of the terrestrial adaptations described above for Boverisuchus.
Of the six most similar skull shapes within the extant dataset the specimens of Asiatosuchus seem most similar to C. palustris (Fig. 15), a large-bodied generalist ambush predator (De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). The similarity between the taxa is in terms of the body size, an overall flattened skull, a robust and wide snout and rounded, peg-like dentition in the rear of the jaw (Fig. 15). The only difference in diet between C. palustris and the others described above is a lack of insect consumption as adults (De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). For the diet of Asiatosuchus, small prey items such as insects, snails and crustaceans only appear to have been a regular part of the diet at young ages when body size was most similar to adults of P. palpebrosus and O. tetraspis (rarely exceeding 2 m; Eaton, Reference Eaton, Manolis and Stevenson2010; Magnusson & Campos, Reference Magnusson, Campos, Manolis and Stevenson2010). However, without well-preserved juveniles of Asiatosuchus, it is unknown if skull shape at a young age was also similar to these crocodylians.
Given that there was some landmark similarity to Caiman in RW 1 v. RW 2 morphospace, the fact that Caiman gain a greater portion of their diet from fish as they grow larger (Magnusson, Vieira da Silva & Lima, Reference Magnusson, Vieira da Silva and Lima1987) may indicate that fish played a significant role in the diet of Asiatosuchus. Fish is also a regularly consumed item in C. palustris (De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). Again, it should be noted that fish are unknown from MP 11 at Geiseltal and are only common during MP 13 (Hellmund, Reference Hellmund2007; Table 1). A single record of a lepisosteid is known from MP 12, but numerous fish have been recovered from MP 13 (Gaudant, Reference Gaudant1988; Gaudant & Haubold, Reference Gaudant and Haubold1995). In earlier stratigraphic ages, fish and other aquatic animals were either not fossilized or the crocodylians relied more on their other prey preferences. The consumption of reptiles by C. palustris (De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011) may indicate that occasional large snakes and lizards were also consumed by Asiatosuchus, but again these are more common during MP 13 and unknown for MP 11 (Hellmund, Reference Hellmund2007; Table 1). The large size of Asiatosuchus and its similarity in RW 1 v. RW 2 morphospace to C. palustris suggests larger prey was viable in adulthood. By comparing this sort of prey of the Indian Mugger (deer, sambars, buffaloes; De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011) to that found at Geiseltal, the most likely candidates are large and small ungulates such as Lophiodon and Propalaeotherium, present during all three MP zones discussed. Also present at Geiseltal are condylarths and several other groups of small mammals that represent sizes similar to prey of these extant crocodylians (Hellmund, Reference Hellmund2007).
Based on studies of living crocodylians (Erickson et al. Reference Erickson, Gignac, Steppan, Lappin, Vliet, Brueggen, Inouye, Kledzik and Webb2012, Reference Erickson, Gignac, Lappin, Vliet, Brueggen and Webb2014), the larger size of Asiatosuchus (Table 1) would likely mean a proportionally greater bite force than Diplocynodon. As mentioned above, this has been used in C. palustris for consuming large ungulates (De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). Furthermore, the similarly sized and shaped C. palustris have been documented eating multiple kinds of testudines (De Silva et al. Reference De Silva, Amarasinghe, de Silva and Karunarathna2011). A fossilized predation attempt on the middle Eocene turtle Neochelys of Spain has been described previously as having been caused by Asiatosuchus (Jiménez-Fuentes, Reference Jiménez-Fuentes2003). A partial carapace of the adult freshwater turtle Geochelone eocaenica (GMH XXXVI-416-1963) was recovered from Geiseltal (MP 13) with a clear crocodylian tooth puncture mark, consistent with teeth of Asiatosuchus (Fig. 15). The blunt, round teeth and robust jaws of Asiatosuchus likely assisted in managing armored prey. Given the similarity of shape between Asiatosuchus and Diplocynodon, but notable difference in size (Fig. 6), these taxa seem to have similar differentiation as sympatric Crocodylus porosus and C. johnstoni (Webb, Manolis & Sack, Reference Webb, Manolis and Sack1983). Interestingly, a study by Martin (J. Martin, unpub. Ph.D. thesis, Univ. Lyon, 2009) also found similarity in geometric morphometric morphospace between Asiatosuchus (n = 4) and Diplocynodon (n = 7), although these were from multiple sites and geological ages.
5. Concluding remarks
The fossil crocodylians of the Geiseltal fauna represent a situation of high diversity and provide a good framework for studying partitioning of prey preference within related taxa. As crocodylians are typically thought of as ‘opportunistic feeders’, there will be overlap among certain occasional prey items (Fig. 11). It is however clear from the present analysis that, within a community of opportunistic feeders, there are still adaptations and specializations that likely enabled these taxa to cohabitate. Also, the time when all four crocodylian genera are found together (MP 13) is also when far more aquatic prey species are recovered as fossils at Geiseltal. Considering these fauna are also present from MP 11 at Messel (Morlo et al. Reference Morlo, Schaal, Mayr and Seiffert2004) and MP 13 at Eckfeld (Neuffer et al. Reference Neuffer, Gruber, Lutz and Frankenhäuser1996), this interaction evidently persisted in central Germany for the entire Geiseltalian period (MP 11–13) but may have reached its peak at Geiseltal during MP 13. Studies have previously linked crocodylian diversity to changes in climatic conditions, primarily temperature (Markwick Reference Markwick1998 a, b; Martin, Reference Martin2010; Martin et al. Reference Martin, Amiot, Lécuyer and Benton2014; Mannion et al. Reference Mannion, Benson, Carrano, Tennant, Judd and Butler2015). This high crocodylian diversity occurs during the middle Eocene period characterized by warm temperatures (Mosbrugger, Utescher & Dilcher, Reference Mosbrugger, Utescher and Dilcher2005; Tütken Reference Tütken2014), which likely played a large part in the ability of these ectotherms to diversify.
Acknowledgments
Primary funding for this work was contributed by the German Federal Cultural Foundation (Kulturstiftung des Bundes). Funding for travel to form the extant dataset came from the National Science Foundation Grant DEB-0733725 and DSGC 640179, funds from the Florida Museum of Natural History (FLMNH), the Smithsonian Tropical Research Institute Paleobiology Fund, the Geological Society of America Graduate Student Research Grant, the R. Jerry Britt, Jr. Paleobiology Award, the Gary S. Morgan Student Research Award and the R.W. Moriarty Science Seminar Series of the Carnegie Museum of Natural History (CMNH). Thanks to J. I. Bloch, P. D. Polly and J. J. Head for advice and assistance with forming the extant geometric morphometric dataset. Fossil preparation was conducted by M. Stache of the GMH. Thanks to Dr Monika Hellmund for assistance with high-magnification photographs. Thanks to Dr M. Wills for the use of the programming code for Rare used in this study. Assistance with specimens and access to extant herpetological collections was provided by D. Kizirian and R. Pascocello (AMNH), K. Krysko (FLMNH), S. Rogers (CMNH) and F. Tillack (ZMB). The authors would also like to thank the reviewers and editors who helped to improve the quality of this manuscript. Cordial thanks to G. Krumbiegel, Halle (Saale), a nestor of the study of the Geiseltal-Fossillagerstätte, especially during excavations from the middle 1950s to the early 1990s, for personal communications with the authors regarding ongoing Geiseltal research.
Declaration of interest
None.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0016756815001041.