1. Introduction
The Upper Cretaceous – lower Palaeocene Scollard Formation in the area of Dry Island Buffalo Jump Provincial Park in central Alberta has long been known to produce fish material, starting with the amiid Cyclurus fragosa (Jordan, Reference Jordan1927) being named based on over 60 isolated elements collected by CM Sternberg. The Pisces Point locality, sampling the Scollard Formation within the park, has been known for its fish material since one of the authors (AGN) visited in 1989 with the locality’s discoverer, local naturalist Tim Showalter. This site was named for the teleost specimens found in the fine-grained mudstones that are associated with the sandstones that bear amiid remains. Collecting at Pisces Point in the 2000s and 2010s led to the recovery of diverse teleosts. These include two species of osteoglossomorphs (one of which, Wilsonichthys aridinsulensis, has been formally described; Murray et al. Reference Murray, Newbrey, Neuman and Brinkman2016), an ostariophysan (Brinkman et al. Reference Brinkman, Divay, Newbrey and Neuman2018), an esocid (Brinkman et al. Reference Brinkman, Murray, Newbrey and Neuman2016) and specimens of two different acanthomorph. One of the latter was a poorly preserved partial specimen; the other, better preserved in part and counterpart, is the subject of this study.
Acanthomorph material from Pisces Point was first signalled at a conference a number of years ago (Newbrey et al. Reference Newbrey, Wilson, Neuman, Murray and Brinkman2010). That acanthomorph was represented by a single nearly complete, articulated fish, about 45 mm in total length, which was considered to be aged 5 years, based on growth lines in a vertebral centrum. Newbrey et al. (Reference Newbrey, Wilson, Neuman, Murray and Brinkman2010) identified it as a paracanthopterygian based on the presence of a full neural spine on the second preural centrum and two epurals in the caudal skeleton. They further associated it with Percopsiformes as it shared with that order the presence of six branchiostegal rays and an anterodorsal excavated margin on the opercle; they could not place it within any previously named family, however, because it revealed a mosaic of characteristics indicating affinities with different families. Unfortunately, that acanthomorph specimen is now missing and, despite many efforts, it has not been seen since 2014. However, another specimen has been recovered from Pisces Point, which likely represents the same taxon and is here described as the oldest-known freshwater articulated paracanthopterygian material.
2. Geological setting
The Pisces Point locality in Dry Island Buffalo Jump Provincial Park samples the lower Scollard Formation, with the sediments being about 1–2 m above the base of the formation. The Pisces Point fish-bearing sediments are located just above the Kneehills Tuff, a distinctive marker bed now considered to be in the Battle Formation, dated as 66.5– 67.0 Ma based on potassium argon dating (K–Ar; Eberth, Reference Eberth, Currie and Padian1997). The Battle Formation and the Scollard Formation are part of the Edmonton Group; the exposed part of this group ranges from 72–73 Ma (radiometric dating) to an estimated 63.5 Ma (Eberth, Reference Eberth, Currie and Padian1997) in the area near Pisces Point. The Scollard Formation is well known for its dinosaur and mammal remains, and is dated as late Maastrichtian through early Palaeocene in age. The Cretaceous–Palaeogene (K-Pg) boundary is found within the formation, and separates it into a lower non-coaly interval and an upper coaly interval (Eberth, Reference Eberth, Currie and Padian1997); the upper interval includes the No. 14 (Ardley) coal seam. The Pisces Point locality is located in the lower part of the formation, so is late Maastrichtian in age.
The Scollard Formation sediments were deposited in freshwater environments (Gibson, Reference Gibson1977), with the lower part of the formation characterized as an alluvial succession with palaeochannel fills, levee and splay deposits, and flood deposits (Eberth, Reference Eberth, Currie and Padian1997). The Pisces Point locality itself samples a palaeochannel with alternating sandstone and mudstone layers, interpreted as resulting from periodic alternation between active channel and stagnant waters in the channel (pers. comm., Dave Eberth and Dennis Braman, 2012). The locality may have been an ox-bow cut off from the main channel with lower water flow, perhaps seasonal, and stagnant ponds (pers. comm., Dave Eberth and Dennis Braman, 2012). The articulated fish remains are found in both the sandstone and mudstone layers, but the teleost material is more common in the mudstone layers.
3. Materials and methods
3.a. Specimens
The articulated fossil acanthomorph specimen described here is housed in the Royal Tyrrell Museum of Palaeontology (TMP), under catalogue number TMP 2013.046.1421 (part and counterpart of the percopsiform). The specimen was prepared by Allan Lindoe using fine needles under a dissecting microscope. Photographs were taken under polarized light using a Nikon DXM 1200C digital camera mounted on a Zeiss Discovery V8 stereo microscope, as well as under natural light using an Olympus OM-D E-M10 camera with macro lens. Isolated acanthomorph elements that are here identified as percopsiform based on comparison with the articulated specimen are from the collections of the TMP, the University of Alberta Laboratory for Vertebrate Palaeontology (UALVP) and the Royal Saskatchewan Museum (RSM).
3.b. Phylogenetic analysis
The relationships among the Paracanthopterygii are under study by a number of other researchers (e.g. the laboratory of T Grande) who are using both molecular and morphological techniques; we therefore focus here more narrowly on the relationships among the extant and extinct Percopsiformes, using only morphological characters that can be scored for the fossils. A number of morphological characters have been used in various analyses that include percopsiform fishes, although these studies were mostly focused on interrelationships among the lineages within Paracanthopterygii, and did not explicitly test intrarelationships among the percopsiform constituent subgroups (which is our purpose here). The characters we use are from Rosen & Patterson (Reference Rosen and Patterson1969), Patterson & Rosen (Reference Patterson, Rosen and Cohen1989), Murray (unpub. MSc thesis, University of Alberta, 1994), Wilson & Murray (Reference Wilson, Murray, Arratia and Viohl1996), Murray & Wilson (Reference Murray, Wilson, Arratia and Schultze1999), Grande et al. (Reference Grande, Borden, Smith, Arratia, Schultze and Wilson2013, Reference Grande, Borden, Wilson and Scarpitta2018) and Davesne et al. (Reference Davesne, Gallut, Barrie, Janvier, Lecointre and Otero2016). The final list of characters we used, with a discussion of each, is provided in online Supplementary Appendix 1 (available at http://journals.cambridge.org/geo). We excluded characters that are irrelevant to percopsiform intrarelationships or that cannot be scored for the fossil taxa. Some characters were slightly rewritten without comment to better clarify the features, but characters that were more greatly modified from other analyses, by combining two character states into one for example, are noted as ‘modified’ in the list. Multiple authors may have noted features in certain taxa, but the references given in online Supplementary Appendix 1 for each character are those that explicitly used the character to indicate relationships. We added a few additional characters that we noticed during the course of this project.
We chose a variety of taxa as outgroups. The extant genus Polymixia is often considered the sister group to the Acanthomorpha including Paracanthopterygii (e.g. Johnson & Patterson, Reference Johnson and Patterson1993) or just to the Paracanthopterygii (e.g. Grande et al. Reference Grande, Borden, Smith, Arratia, Schultze and Wilson2013); we included Polymixia lowei and also two fossil Polymixiiformes (Cumbaaichthys oxyrhynchus and Boreiohydrias dayi). Within the Paracanthopterygii, Sphenocephaliformes has been variously found as the basal-most lineage (e.g. Murray, unpub. MSc thesis, University of Alberta, 1994) or placed in a polytomy with Percopsiformes and all the other paracanthopteryigans (e.g. Grande et al. Reference Grande, Borden, Smith, Arratia, Schultze and Wilson2013). We included Xenyllion zonensis and X. stewarti, the North American members of the order, along with Sphenocephalus (based on the literature and photographs of specimens) to represent the Sphenocephaliformes. Although Stylephorus, the only genus of the Stylephoriformes, may be a key member of the Paracanthopterygii (e.g. Grande et al. Reference Grande, Borden, Smith, Arratia, Schultze and Wilson2013; Davesne et al. Reference Davesne, Gallut, Barrie, Janvier, Lecointre and Otero2016), we did not have access to any specimens of this highly derived fish, and so did not include it in our analysis of percopsiforms. The two other paracanthopterygian lineages (following Grande et al. Reference Grande, Borden, Smith, Arratia, Schultze and Wilson2013), the Gadiformes and Zeiformes, are represented in our analysis by Lota lota and Microgadus proximus (both Gadiformes) and Zeus faber (Zeiformes).
We included as many fossil members of the Percopsiformes as we could, and personally examined available specimens for features. Remaining data were taken from the literature, as noted in online Supplementary Appendix 2. The data matrix is given in online Supplementary Appendix 3.
The phylogenetic analyses were run in PAUP version 4.0 (Swofford, Reference Swofford2002) using parsimony analysis in a branch and bound search. All characters were run unordered and unweighted. The analyses used tree bisection reconnection (TBR) with furthest addition sequence. Tree length was calculated in PAUP, with additional tree statistics calculated in Mesquite (Maddison & Maddison, Reference Maddison and Maddison2018). The first analysis was run with no constraints. An additional analysis was run using a constraint to keep Amblyopsis, Aphredoderus and Trichophanes as part of a monophyletic Percopsiformes, as traditionally constituted.
3.c. Anatomical abbreviations used in figures
aa, anguloarticular; ach, anterior ceratohyal; brst, branchiostegal ray; cc, compound centrum; cl, cleithrum; den, dentary; dfin, dorsal fin; ect, ectopterygoid; ep1–2, epurals 1–2; fr, remains of frontal; hsp, haemal spine; hy1–6, hypurals 1–6; hyo, hyomandibula; l, left; lac, lacrimal; meth, mesethmoid; mx, maxilla; npu2, npu3, neural spine of second or third preural centrum; nsp, neural spine; op, opercle; pal, palatine; pcl, postcleithrum; phy, parhypural; pmx, premaxilla; pop, preopercle; psph, parasphenoid; pt, pterygiophore; ptt, posttemporal; pu2, pu3, second or third preural centrum; q, quadrate; r, right; ra, retroarticular; sn, supraneural; sop, subopercle; sym, symplectic; u2, second ural centrum; un1, un2, first or second uroneural.
3.d. Institutional abbreviations
RSM, Royal Saskatchewan Museum, Regina, Saskatchewan, Canada; TMP, Royal Tyrrell Museum of Palaeontology, Drumheller, Alberta, Canada; UAMZ, University of Alberta Museum of Zoology, Edmonton, Alberta, Canada; UALVP, University of Alberta Laboratory for Vertebrate Palaeontology, Edmonton, Alberta, Canada; UCMP, University of California Museum of Paleontology, Berkeley, California, United States of America.
4. Systematic palaeontology
The new species is placed in the Paracanthopterygii based on having a caudal skeleton with a full neural spine on the second preural centrum and two epurals, and 16 branched principal rays. It has a single supraneural dorsal to the neural spines, as is found in both the Percopsiformes and Sphenocephaliformes. The fossil lacks the distinctive opercle and preopercle of the latter and is instead placed in the Percopsiformes, with which it additionally shares the absence of a postmaxillary process and a distinctive dorsal process on the opercle. It also shares with the Percopsidae (Percopsis Agassiz, Reference Agassiz1849; Erismatopterus Cope, Reference Cope1870; Amphiplaga Cope, Reference Cope1877; Libotonius Wilson, Reference Wilson1977; Mcconichthys Grande, Reference Grande1988; Lateopisciculus Murray & Wilson, Reference Murray and Wilson1996; Massamorichthys Murray, Reference Murray1996) a distinctive form of the dentary which has a large ventral excavation.
Subdivision TELEOSTEI Müller, Reference Müller1845
Sept ACANTHOMORPHA Rosen, Reference Rosen, Greenwood, Miles and Patterson1973 (sensu Johnson & Patterson, Reference Johnson and Patterson1993)
Superorder PARACANTHOPTERYGII Greenwood, Rosen, Weitzman & Myers, Reference Greenwood, Rosen, Weitzman and Myers1966
Order PERCOPSIFORMES Berg, Reference Berg1940
FAMILY incertae sedis
Lindoeichthys n. gen.
Type species. Lindoeichthys albertensis n. sp.
Derivation of name. The generic name is in honour of Allan Lindoe, University of Alberta, who, over the course of a 50-year career, has prepared this and many other fossil fish.
Diagnosis. As for species.
Lindoeichthys albertensis n. sp.
Holotype. TMP 2013.046.1421, an almost complete fish, preserved in part and counterpart (Fig. 1), missing the tip of the tail and the dorsal skull roof. The part consists of the head, body and part of the caudal fin skeleton, whereas the counterpart does not preserve any elements of the head but has the caudal skeleton and partial caudal fin rays.

Fig. 1. Photograph of the part and counterpart of the holotype of Lindoeichthys albertensis n. gen. et sp., TMP 2013.046.1421. Scale bar: 1 cm.
Type locality and horizon. The Pisces Point locality, Dry Island Buffalo Jump Provincial Park, Alberta Canada, sampling the Upper Cretaceous (Maastrichtian) Scollard Formation.
Derivation of name. The specific epithet is for the province of Alberta, from where the fossil was recovered.
Diagnosis. Percopsiform fish (used here in the classic sense to include the families Percopsidae, Mcconichthyidae, Aphredoderidae and Amblyopsidae, as well as the genus Libotonius) differing from all other members of the order by the presence of bifurcating serrations around the angle of the preopercle. Further differs from the other genera by having much shorter jaws relative to head length than in the two species of Libotonius; by having fewer anal-fin spines (three) than Lateopisciculus (four) but having more than Libotonius, Erismatopterus (two) and Percopsis (one or two); and by having fewer vertebrae (34 or 35) than Mcconichthys (38) and Massamorichthys (43–45), and more than Amphiplaga (31–32), Erismatopterus (29–30), Lateopisciculus (31), Aphredoderus (30) and all amblyopsids except Forbesichthys (27–31; counts from Woods & Inger, Reference Woods and Inger1957; Cooper & Kuehne, Reference Cooper and Kuehne1974). Also differs from amblyopsids and aphredoderids by lacking lateromaxillae.
5. Description
The matrix surrounding the specimen includes relatively large grains of sand, which did not allow some elements to be preserved. The following description is based on both the part and counterpart; some elements and measurements are restored or combined from examination of both pieces.
5.a. General body form
This is a slender fish with a large, well ossified head (Fig. 1). The standard length (SL) of the holotype is 70.9 mm; the caudal fin rays are not fully preserved so a total length cannot be determined, but the fin appears to be forked. The head length (HL) is 21.8 mm, and head depth (HD) is 20.3 mm. The body is not fully preserved so a depth cannot be measured, but it appears the body would have been about as deep as the head.
The dorsal fin is situated in the middle of the body, only slightly closer to the tail (distance between posterior insertion of dorsal fin and end of hypural plates is 21.1 mm) than it is to the head (distance between anterior origin of dorsal fin and tip of premaxilla estimated from part and counterpart at 32.1 mm). The much smaller anal fin lies under the posterior part of the dorsal fin with a preanal distance of 46.2 mm. The pectoral fins are positioned low on the body and the pelvic fins insert just behind them.
5.b. Cranial elements
The skull roof is not well preserved. Fragments of bone belonging to the frontal and parietal are present, but no details can be discerned (Fig. 2). The parasphenoid is visible in the posterior half of the large orbit. The basioccipital is identified posterodorsal to the opercle, in a position that would have been medial to the opercle in life.

Fig. 2. Photograph and interpretive drawing of the head of the holotype of Lindoeichthys albertensis n. gen. et sp., TMP 2013.046.1421. The specimen was coated in ammonium chloride and several images were focus-stacked in PhotoShop. Arrow indicates deep ventral embayment of dentary. Scale bar: 1 cm.
5.b.1. Jaws
The jaws are fairly well preserved, with the posterior part of the left maxilla present, as well as parts of both right and left premaxillae and dentaries, and the left anguloarticular (Fig. 2). The maxilla expands into a broad rounded posterior end; the anterior end is not preserved. The premaxillae have low ascending processes, and a postmaxillary process is just evident on the left premaxilla, mostly covered by the maxilla. Small tooth bases are preserved on both premaxillae on the exposed surface of the alveolar process. Although the maxilla is not complete, it is likely that it would have been longer than the premaxilla. There are no supramaxillae. The dentaries narrow at the symphysis but are of similar height for the length of the bone. The left dentary is preserved in ventrolateral view and clearly shows the deep embayment or excavation below the toothed surface that is the wide open sensory canal characteristic of percopsiforms (personal observation; Fig. 2). The anguloarticular has a long, narrow anterior portion, with a relatively low dorsal projection. There is a large retroarticular at the posteroventral corner.
5.b.2. Suspensorium and branchial bones
The hyomandibula, quadrate, symplectic, ectopterygoid and endopterygoid of the suspensorium are all at least partially preserved (Fig. 2). The hyomandibula is tall, with a broad, single head. The ventral portion is covered by the preopercle, but the large rounded condyle for articulation with the opercle is visible. Anteriorly, there is a short, wide flange on the hyomandibula. The anterior tip of the symplectic is preserved in articulation with the quadrate. The quadrate is preserved with the large condyle visible and the rest of the bone partially obscured as it angles into the matrix. The ectopterygoid lies along the anterior edge of the quadrate; it is a small bone, with a distinct angle in the middle. There is no sign of teeth and no dorsal process on the ectopterygoid. Above the ectopterygoid, the broad plate of the endopterygoid is partially visible. It appears to be edentate.
Remains of the anterior ceratohyal are visible; there is no evidence of a beryciform foramen. There are six acinaciform branchiostegal rays with percopsoid projections present on at least the anterior four. It cannot be determined which rays are associated with the anterior or posterior ceratohyals.
5.b.3. Circumorbital bones
The lacrimal is the only element of the circumorbital series that is at least partially preserved. The limits of the bone are difficult to determine, but it is a large bone, with a strong dorsal process. There is no evidence of spines ornamenting the ventral edge, and no evidence of canal pores or a flange on the lateral surface.
5.b.4. Opercular series
The opercle is partially preserved, but is displaced posteroventrally from the presumed life position. There is a strong horizontal ridge that extends posteriorly to form a small spine. A similar strong ridge forms the anterior edge of the bone; it would likely have formed a ventral spine but this is displaced underneath the preopercle and so not visible. The dorsal portion of the opercle above the horizontal ridge is not fully preserved; it is excavated anteriorly above the articular facet for the hyomandibula, but appears complete posteriorly, with no excavation or recurved process. The subopercle is large, filling the space ventral and posterior to the opercle. An interopercle is not distinguishable.
The preopercle is the best preserved bone in the series. The vertical and horizontal limbs form a 90° angle, with the horizontal limb being about 70% as long as the vertical limb. There is an open flange on both limbs that would have covered the sensory canal. The posterior edge of the vertical limb is ornamented with numerous small spines, which continue around the posteroventral corner of the bone.
5.c. Post-cranial elements
5.c.1. Vertebral column
There are 32 preural vertebral centra clearly preserved, with a gap behind the basioccipital which includes bony remains that indicate another centrum might have been present. In addition, the compound centrum (fused first preural plus first ural centra) and the second ural centrum (u2) are present, for an estimated total count of 34 or 35 centra. Ribs are present starting anteriorly on the second clearly preserved centrum (which should be the third centrum of the vertebral column) and there are 11 pairs of ribs in total. Whether the anterior ribs are borne on parapophyses cannot be determined. There are 23 centra (including the compound centrum and u2) behind the insertion of the first anal pterygiophore, which are considered to be the caudal centra although the presence of a haemal arch on all of them cannot be confirmed. The centra are longer than high anteriorly, but become progressively shorter and deeper towards the tail.
Anterior neural arches are preserved on the counterpart. There is a small, irregularly shaped supraneural above the first clearly preserved neural spine (Fig. 3); however, there may be an additional neural spine anterior to this one, so the first dorsal pterygiophore inserts behind either the third or fourth neural spine. The anterior dorsal-fin pterygiophores interdigitate with the neural spines such that there is only ever a single pterygiophore between two neural spines.
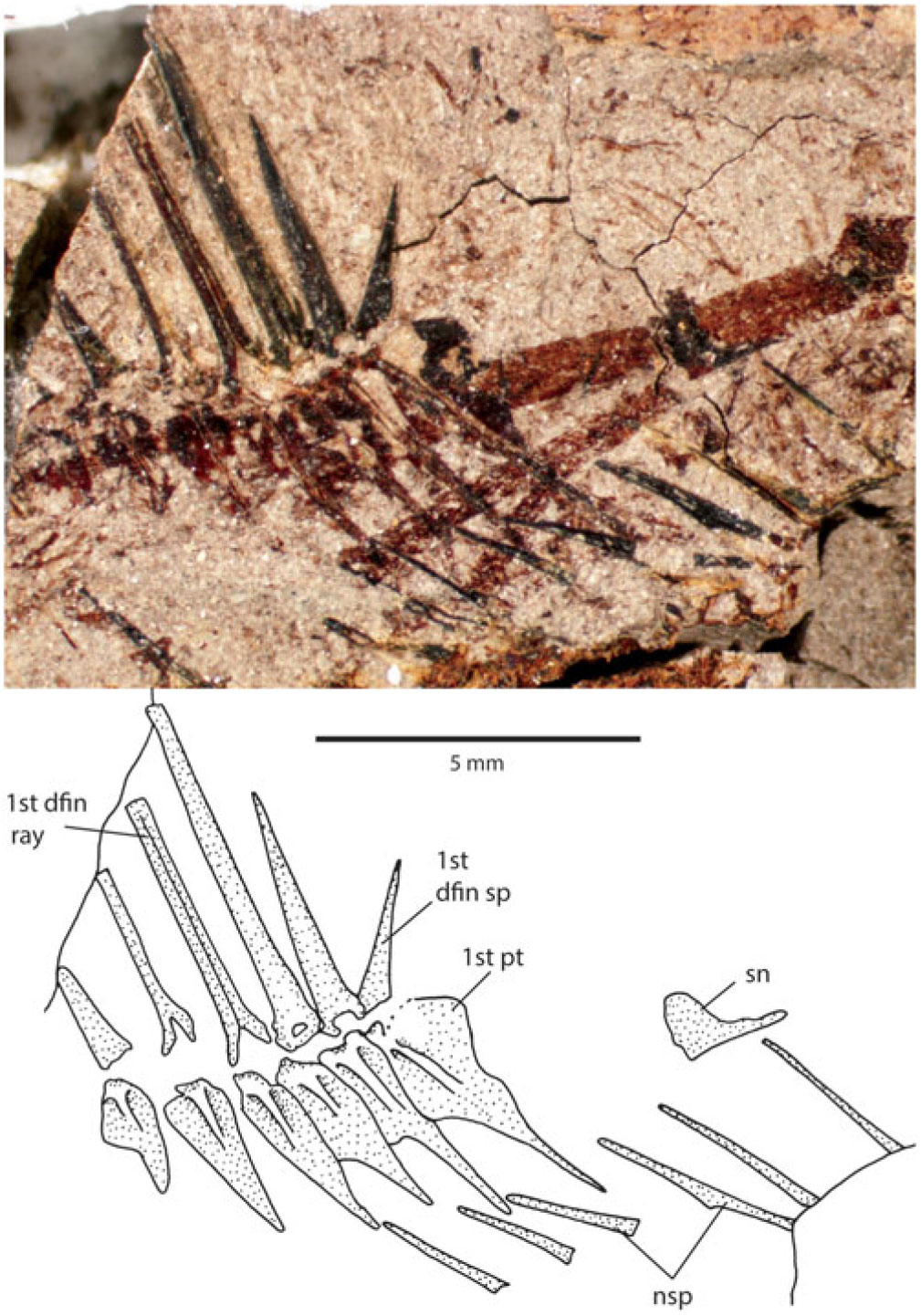
Fig. 3. Photograph and interpretive drawing of the anterior neural spines and supraneural bone of holotype of Lindoeichthys albertensis n. gen. et sp., TMP 2013.046.1421. Scale bar: 5 mm.
5.c.2. Median and paired fins and girdles
The dorsal fin is situated mid-back; it has three spines and 14 branched rays (the last two counted separately). The dorsal fin base is 16.6 mm long. The anal fin also appears to have three spines, and 11 anal-fin rays. A crack in the fossil runs through the anal fin, but the fin base is estimated at 10.7 mm long. There are nine vertebral centra between the insertion of the first dorsal and first anal pterygiophores. The first anal pterygiophore is much larger than the rest and reaches almost to the vertebral column, and so forms a haemaxanal complex.
There are about 18 pectoral-fin rays; they reach posteriorly past the pelvic girdle and anterior portion of the pelvic-fin rays. There are eight rays in the pelvic fin. The pelvic-fin rays are quite long, measuring about 8.6 mm (allowing for a crack running through the fin), but do not reach all the way to the origin of the anal fin.
5.c.3. Caudal fin and skeleton
The caudal skeleton is quite well preserved on the counterpart of the holotype (Fig. 4), although there is a crack in the specimen that extends between the second and third preural centra. This crack also runs through the haemal spine on the third preural centrum such that the tip of this spine is preserved on one side of the crack, and the rest of the spine is preserved on the other.

Fig. 4. Photograph and interpretive drawing of the tail of holotype of Lindoeichthys albertensis n. gen. et sp., TMP 2013.046.1421, coated in ammonium chloride. Arrows indicate first (unbranched) principal rays of upper and lower lobe of the caudal fin. Scale bar: 0.5 cm.
There is a compound centrum (presumed to be the first preural plus first ural centra) bearing the parhypural and first two hypurals. All three of these bones are broad and abut one another along their lengths. The parhypural has a proximal flange on the anterior edge. There are four upper hypurals, for a total of six hypurals in the fin skeleton. The upper hypurals also abut one another along their lengths, and become progressively narrower dorsally. There are two uroneurals; the first is shorter than the second which reaches distally to the posterior end of the hypurals. Two narrow, long epurals are positioned close to the uroneurals, leaving a gap between the anteriormost epural and the posteriormost neural spine. The neural spine, associated with the second preural centrum (pu2), is long, thin and reaches distally almost as far as the epurals. A second neural spine is located just anterior to this, but the proximal end is missing such that it cannot be determined if it is also associated with pu2 or is the spine of the third preural centrum. There are eight branched principal rays in both the ventral and dorsal lobes of the fin, for a caudal-fin formula of I,8,8,I.
5.c.4. Scales
No complete scales are preserved on the holotype. However, a few small ctenii are preserved on the ventral flank in front of the anal fin, indicating the presence of at least some ctenoid scales. Whether these are actually spinoid or ctenoid (sensu Roberts, Reference Roberts1993) cannot be determined.
6. Remarks
The new taxon is placed in the Paracanthopterygii based on the presence of a full neural spine on the second preural centrum and two epurals in the caudal skeleton, two features that define the group. The presence of six branchiostegal rays indicate the fish can be further included in Percopsiformes.
7. Results of phylogenetic analysis
The branch-and-bound analysis with no constraints resulted in a single shortest tree (Fig. 5a) of length 122 steps with a consistency index of 0.516 and retention index of 0.667 (calculated in Mesquite). In this analysis, the new species from the Pisces Point locality, Lindoeichthys albertensis, is recovered as a member of Percopsiformes, in a clade with the Percopsidae plus Mcconichthys. We did not include any features to unite the two species of Libotonius, but if the two are forced to be sister groups, the tree length only increases by one step. We accept that these two species belong to the same genus and are members of the Percopsidae as originally described (Wilson, Reference Wilson1977, Reference Wilson1979).

Fig. 5. Results of phylogenetic analyses. (a) Single-most parsimonious tree resulting from the first analysis with no constraints (tree length, 122 steps). (b) 50% majority rule consensus tree of 30 shortest trees of tree length 126 steps, when the Percopsiformes is constrained to be monophyletic only with the inclusion of Amblyopsis, Aphredoderus and Trichophanes. Daggers indicate extinct taxa. Alternate clades in (b) that could be identified as Percopsidae are indicated with dashed lines.
Mcconichthys is recovered positioned basal to the traditional members of the Percopsidae, so that it either can be considered as the sister group to Percopsidae (in its original family Mcconichthyidae) or it could be included in the Percopsidae. Trichophanes, Aphredoderus and Amblyopsis form successive sister groups to the representatives of Zeiformes and Gadiformes, all within the Paracanthopterygii. If Trichophanes and Aphredoderus are forced to be sister groups, as previously considered, the tree length increases by three steps.
Sphenocephalus and the two species of Xenyllion form a clade, here considered to be the family Sphenocephalidae, which is coextensive with Sphenocephaliformes. Boreiohydrias and Cumbaaichthys join Polymixia in the Polymixiiformes, which is where they were placed when originally described (Murray & Cumbaa, Reference Murray and Cumbaa2013; Murray, Reference Murray2016).
In our second analysis, the Percopsiformes was constrained to be monophyletic with the inclusion of Amblyopsis, Trichophanes and Aphredoderus, as it is traditionally conceived. This analysis resulted in 30 shortest trees of length 126 steps. The strict consensus of the 30 trees was very poorly resolved, but the 50% majority rule consensus tree (Fig. 5b) has numerous clades found in 90–100% of the trees; it has a consistency index of 0.500 and retention index of 0.644 (calculated in Mesquite). In this tree based on the constrained analysis, Lindoeichthys is placed as the sister group to Percopsis. Polymixiiformes and Sphenocephaliformes are recovered as clades, although the arrangement of the polymixiiform genera differs from the unconstrained analysis by having Boreiohydrias sister to Polymixia instead of Cumbaaichthys. The relationships among Zeus, Lota and Microgadus are the same in both analyses. Although Amblyopsis, Aphredoderus and Trichophanes are forced to be within a monophyletic Percopsiformes, these three genera still do not form a clade as expected based on traditionally conceived relationships.
8. Discussion
8.a. Phylogenetic analysis
Most of the characters included in the analysis exhibit homoplasy, and in many cases a rearrangement of the taxa does not increase the tree length by more than one or two steps. Clearly, the phylogenetic result cannot be considered robust. However, a few interpretations can be made.
The traditional conception of the order Percopsiformes includes Amblyopsidae and Aphredoderidae, and this traditional group has been recovered in recent molecular analyses (e.g. Dillman et al. Reference Dillman, Bergstrom, Noltie, Holtsford and Mayden2011; Grande et al. Reference Grande, Borden, Smith, Arratia, Schultze and Wilson2013) and at least one morphological study (Davesne et al. Reference Davesne, Gallut, Barrie, Janvier, Lecointre and Otero2016, although amblyopsids were not included in the analysis); however, none of the genera included here to represent amblyopsids (Amblyopsis) and aphredoderids (Aphredoderus and Trichophanes) were recovered as part of the Percopsiformes in our unconstrained analysis. When we constrained Percopsiformes to include those taxa, the three genera were recovered in a basal position in the order in our analysis. The morphological characters we used do not support the membership of aphredoderids and amblyopsids within Percopsiformes.
Murray & Wilson (Reference Murray, Wilson, Arratia and Schultze1999) also removed Aphredoderus from the Percopsiformes; however, that was somewhat controversial and has not been supported by subsequent studies, primarily because they left Amblyopsis with the Percopsiformes. Amblyopsis and Aphredoderus share a jugular vent and presence of lateromaxillae (see Armbruster et al. Reference Armbruster, Niemiller and Hart2016), which intuitively seem to be strong characters to unite the two. In our current results, these two genera are not recovered as sister groups in the unconstrained analysis, but the tree length increases by only a single step if the genera are forced together. In the constrained analysis, Amblyopsis and Aphredoderus are recovered as sister groups to the exclusion of Trichophanes.
The clade comprising the uncontested percopsids (Percopsis, Amphiplaga, Erismatopterus, Lateopisciculus and Massamorichthys), along with Mcconichthys, Libotonius and Lindoeichthys, was recovered in both of our analyses and these taxa are united by having the portion of opercle dorsal to the horizontal ridge constricted to the middle portion of the bone (character 15, state 2), a feature also shared with Trichophanes and Aphredoderus, and a single postcleithrum with an enlarged dorsal plate (character 26, state 1), a feature shared with Trichophanes.
Lindoeichthys is placed as the basal-most percopsiform in our unconstrained analysis, with Mcconichthys being the next most-basal taxon in the order. Our interpretation of the second centrum not being foreshortened in Mcconichthys removes one of the three characters that Grande (Reference Grande1988) used to unite Mcconichthys with ‘higher’ paracanthopterygians (the group including gadiforms). The second character Grande (Reference Grande1988) used, first neural spine in close articulation with supraoccipital crest, is now interpreted as a convergence; the third character, absence of parapophyses on at least the first three and up to seven vertebrae with ribs inserting in ventrolateral cavities in the centra, was not included in our analysis as many of the fossils were impossible to code for it.
Percopsidae excluding Mcconichthys but including Libotonius are united by having a dorsal process on the maxilla, which may or may not be present in Lindoeichthys albertensis, and a wide open sensory canal in the dentary which is present in Lindoeichthysbut not Mcconichthys. Switching the positions of Lindoeichthys and Mcconichthys on the single tree resulting from the unconstrained analysis only increases the tree length by a single step and, in the constrained analysis, Lindoeichthys is well within the Percopsidae as the sister group to Percopsis. Based on the two analyses, we are confident that Lindoeichthys is a member of the Percopsidae.
8.b. Temporal range of Percopsiformes
The relationships found in the unconstrained phylogenetic analysis fit well with the geological age of the taxa. Lindoeichthys, from the Maastrichtian Age, is recovered as the basal-most percopsiform, followed by Mcconichthys from early Palaeocene time. The Percopsidae had made its appearance by middle Palaeocene time, with Lateopisciculus and Massamorichthys, which we recovered as sister taxa, close to the Eocene Amphiplaga. However, the extant Percopsis is nested between the Eocene Libotonius and Eocene Erismatopterus, Amphiplaga and the two middle Palaeocene taxa. In the constrained analysis, the placement of Lindoeichthys as the sister to Percopsis is not congruent with the ages of the fossil taxa, with the Cretaceous Lindoeichthys sister to the extant Percopsis and the early Palaeogene genera more basal than both.
In addition to the articulated specimens, freshwater acanthomorphs are represented in the Upper Cretaceous strata of the Western Interior of North America by isolated elements from vertebrate microfossil localities. These elements include dentaries that have previously been referred to the Percopsiformes (Brinkman et al. Reference Brinkman, Newbrey, Neuman, Eaton, Titus and Lowen2013, Reference Brinkman, Newbrey, Neuman, Wilson, Clemens, Horner and Hartman2014). In a sequence of localities from southern Utah that extend from the Cenomanian to the late Campanian in age, acanthomorphs first occur in the Coniacian Age (Brinkman et al. Reference Brinkman, Newbrey, Neuman, Eaton, Titus and Lowen2013). Their remains become increasingly abundant in younger assemblages, and in three localities from the upper portion of the upper Maastrichtian Hell Creek Formation, an average of 50% of all teleost precaudal centra are from acanthomorph fishes (Brinkman et al. Reference Brinkman, Newbrey, Neuman, Wilson, Clemens, Horner and Hartman2014, table 3). On the basis of dentaries and other tooth-bearing elements, three different acanthomorphs could be identified: the enigmatic Platacodon, Priscacara sp. and percopsiforms. Percopsiforms are represented by dentaries that share distinctive features with extant members of the group. These features include the presence of a wide open sensory canal bordered dorsally by a thickened ridge and bridged anteriorly by a bar of bone that crosses the canal, bordering a pore at the anterior end of the dentary. Further, the tooth-bearing surface is a broad pad covered by numerous tiny teeth showing no variation in size across the surface of the jaw and showing no linear arrangement of the teeth. Comparison of the isolated dentaries with the dentary of Lindoeichthyssupports their percopsiform identification (Fig. 6). These dentaries therefore provide additional evidence of the diversity and distribution of the group in the western interior of North America. In the series of Upper Cretaceous Utah vertebrate microfossil localities, acanthomorph dentaries first occur in the upper Campanian Kaiparowits Formation (Brinkman et al. Reference Brinkman, Newbrey, Neuman, Eaton, Titus and Lowen2013). Their presence in contemporaneous beds in Alberta is documented by dentaries from the Belly River Group (Fig. 6b), which were referred to as acanthomorph indet. by Neuman & Brinkman (Reference Neuman, Brinkman, Currie and Kopplelhus2005, fig. 9.7E–H). Four distinct dentary morphotypes are present, indicating that a high level of diversity had been reached by this time. Percopsiform dentaries are present in younger formations, including the Hell Creek Formation (Brinkman et al. Reference Brinkman, Newbrey, Neuman, Wilson, Clemens, Horner and Hartman2014), Lance Formation (Fig. 6c), and the lower Palaeocene Ravenscrag Formation (Fig. 6d).

Fig. 6. Isolated percopsiform dentaries from vertebrate microfossil localities compared with the dentary of Lindoeichthys albertensis n. gen. et sp.: (a) Lindoeichthys albertensis n. gen. et sp.; (b) the late Campanian Belly River Group of Alberta, TMP 2019.060.13; (c) the late Maastrichtian Lance Formation of Wyoming, UALVP 59912; and (d) the early Palaeocene Ravenscrag Formation RSM P1594-59. Dentaries are shown in occlusal, internal and external views. The photograph of UALVP 59912 has been reversed for comparison. Scale bar: 2 mm.
The Percopsiformes were present in the fresh waters of North America during Late Cretaceous time, with articulated specimens documenting their presence during late Maastrichtian time and isolated dentaries first occurring during late Campanian time. By the Palaeocene Epoch, they had diversified into three lineages (Lindoeichthys, Mcconichthys and Percopsidae).
9. Conclusions
Lindoeichthys albertensis is the oldest-known articulated freshwater acanthomorph from North America. This fossil represents the earliest-known percopsiform fish, and documents that paracanthopterygians invaded North American fresh waters soon after the retreat of the marine Western Interior Seaway, which was home to paracanthopterygians such as Xenyllion. The articulated percopsiform material allows us to confidently identify isolated elements, particularly dentaries, as belonging to one or more species of percopsiform. These isolated elements provide evidence that acanthomorphs had colonized freshwater environments by the Coniacian, with percopsiforms definitely represented in the Campanian strata in North America.
Prior to the material described here, the oldest freshwater acanthomorph reported is Spinocaudichthys oumtkoutensis from the Cenomanian Stage of Morocco. Spinocaudichthys could not be included in any acanthomorph group and was left incertae sedis, with the suggestion it might be a paracanthopterygian (Filleul & Dutheil, Reference Filleul and Dutheil2001). The holotype is a small, elongate fish with a poorly preserved head (Filleul & Dutheil, Reference Filleul and Dutheil2001). A second specimen of Spinocaudichthys, but perhaps representing a different species, also lacks a well-preserved head (Davesne et al. Reference Davesne, Gueriau, Dutheil and Betrand2018). The preservation of the material makes its affinities difficult to assess, but it clearly exhibits acanthomorph features and is therefore the oldest known freshwater acanthomorph. Furthermore, Davesne et al. (Reference Davesne, Gueriau, Dutheil and Betrand2018) noted the caudal skeleton of Spinocaudichthys, with the presence of a full neural spine on the second preural centrum and two epurals, indicates affinities of this genus with paracanthopterygians excluding Polymixiiformes. The freshwater habitats of Spinocaudichthys and Lindoeichthys indicate two separate incursions of paracanthopterygian fishes into fresh waters during Late Cretaceous time.
The earliest acanthomorphs are known from marine deposits of Albian–Cenomanian age from North America (e.g. Murray, Reference Murray2016). With Spinocaudichthys in Cenomanian deposits of Morocco, it seems in the eastern Tethys acanthomorphs invaded fresh waters soon after the original evolution of the group. In North America, there is a lag between the earliest marine forms (e.g. Murray, Reference Murray2016) and the first freshwater acanthomorphs reported here. The phylogenetic relationships of the new freshwater fish, indicating it is a basal form of Percopsiformes, are consistent with the age of this fossil; the more basal marine paracanthopterygians (Xenyllion spp.) inhabited the Western Interior Seaway a few million years prior to the evolution of their freshwater relative.
Acknowledgements
We thank Allan Lindoe, University of Alberta for expert preparation of the fossil material the technicians and volunteers of the Royal Tyrrell Museum for assisting in fieldwork, especially A. Fotheringham, R. Sanchez, R. Russell and P. Ralrick. Financial support for fieldwork was provided by the Royal Tyrrell Museum Cooperating Society. We are particularly grateful to the editor and reviewers D. Davesne and M. Wilson for their very helpful comments that allowed us to improve the manuscript. The material reported here was collected under permit from Alberta Parks and Recreation (permits 09-006 (DBB); permits 10-086, 11-083, 12-064 and 13-128 (MGN)]. Financial support to AMM is provided by Natural Sciences and Engineering Research Council of Canada Discovery (grant no. 327448).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756819001328.








