1. Introduction
1.a. Previous work and purpose
The Tertiary vertebrate record of México is scattered across the country. Spanning the Eocene–Pliocene interval (i.e. Early Wasatchian – Early Blancan North American Land Mammal Ages or NALMAs), it includes among others remains of marsupials, creodonts, perissodactyls, probocideans, carnivores, rodents and lagomorphs (Montellano-Ballesteros & Jiménez-Hidalgo, Reference Montellano-Ballesteros, Jiménez-Hidalgo, Vega, Nyborg, Perilliat, Montellano-Ballesteros, Cevallos-Ferriz and Quiroz-Barroso2006). In particular, the Eocene localities and faunas of Lomas las Tetas de Cabra (Wasatchian, Baja California; Novacek et al. Reference Novacek, Ferrusquía-Villafranca, Flynn, Wyss and Norell1991), Marfil (Bridgerian–Uintan, Guanajuato; Fries, Hibbard & Dunkle, Reference Fries, Hibbard and Dunkle1955; Black & Stephens, Reference Black and Stephens1973; Ferrusquía-Villafranca, Reference Ferrusquía-Villafranca, Black and Dawson1989), Rancho Gaitan (Chadronian, Chihuahua; Ferrusquía-Villafranca, Reference Ferrusquía-Villafranca1969; Ferrusquía-Villafranca, Galindo-Hernández & Barrios-Rivera, Reference Ferrusquía-Villafranca, Galindo-Hernández, Barrios-Rivera, Arroyo-Cabrales and Polaco1997; Ferrusquía-Villafranca et al. Reference Ferrusquía-Villafranca, Jiménez-Hidalgo, Ortiz-Mendieta, Bravo-Cuevas, Montellano-Ballesteros and Arroyo-Cabrales2002) and Yolomécatl (Uintan, Oaxaca; Jiménez-Hidalgo et al. Reference Jiménez-Hidalgo, Smith, Guerrero-Arenas and Alvarado-Ortega2015; Ferrusquía-Villafranca et al. Reference Ferrusquía-Villafranca, Ruíz-González, Torres-Hernández, Anderson, Urrutia-Fucugauchi, Martínez-Hernández and García-Villegas2016) stand out for their biodiversity. It should be noted that the latter is the southernmost Eocene fauna of North America, and includes mammal species belonging to at least five Orders: Carnivora, Rodentia, Condylarthra, Artiodactyla and Perissodactyla (Jiménez-Hidalgo et al. Reference Jiménez-Hidalgo, Smith, Guerrero-Arenas and Alvarado-Ortega2015; Ferrusquía-Villafranca, unpublished data). Among the Artiodactyla species found, Leptomeryx sp. (Leptomerycidae) was previously known from the Chadronian Rancho Gaitan local fauna (Ferrusquía-Villafranca, Reference Ferrusquía-Villafranca1969; Ferrusquía-Villafranca, Galindo-Hernández & Barrios-Rivera, Reference Ferrusquía-Villafranca, Galindo-Hernández, Barrios-Rivera, Arroyo-Cabrales and Polaco1997). This taxon is a small (rabbit-sized), primitive, hornless brachydont/mesodont ruminant, that lived during Middle–Late Eocene (Late Uintan) to Early Miocene time (Early Hemingfordian) (Damuth, Reference Damuth, Damuth and MacFadden1990; Webb, Reference Webb, Janis, Scott and Jacobs1998). Palaeoecologically, Leptomeryx is considered to be a forest-dwelling mammal that thrived on tree leaves and fruits (Clark, Beerbower & Kietze, Reference Clark, Beerbower and Kietze1967; Retallack, Reference Retallack1983; Wall & Collins, Reference Wall and Collins1998).
The Leptomeryx sp. of this study was found in Yolomécatl, Oaxaca, some 1480 km south of its known former location in Mexico (Rancho Gaitan, near Ojinaga, Chihuahua), at c. 12° latitude, crossing the Tropic of Cancer. We decided to use the carbon and oxygen isotopic relationships recorded in the tooth enamel of an Oaxacan specimen referred to this taxon in order to infer its diet and habitat, compare them with those inferred from Leptomeryx species from temperate North America, and decide whether or not the latitudinal difference influenced the diet and habitat of Leptomeryx in southern (tropical) North America.
1.b. Study area
The study area includes c. 90 km2 of rugged terrain within the Mixteca Region, NW Oaxaca State, Sierra Madre del Sur Morphotectonic Province, SE Mexico, between latitudes 17° 25′ and 17° 36′ N and longitudes 97° 29′ and 97° 36′ W (Fig. 1). The Cenozoic sequence unconformably overlies carbonate rock units of Late Jurassic – Late Cretaceous age. The area also includes the Mixteco/Oaxaca Terrane boundary, namely the Tamazulapam fault (Nieto-Samaniego et al. Reference Nieto-Samaniego, Alaniz-Alvarez, Silva-Romo, Equiza-Castro and Mendoza-Rosales2006; Morán-Zenteno, Cerca & Keppie, Reference Morán-Zenteno, Cerca and Keppie2007).

Figure 1. Location and geology of the study area.
The Tertiary sequence (Ferrusquía-Villafranca et al. Reference Ferrusquía-Villafranca, Ruíz-González, Torres-Hernández, Anderson, Urrutia-Fucugauchi, Martínez-Hernández and García-Villegas2016) consists of five lithostratigraphic units: two volcanic and one shallow intrusive of Eocene–Oligocene age, as well as two epiclastic and subordinately pyroclastic units of Eocene – early Late Oligocene age. Finally, Quaternary deposits and soils unconformably overlie the preceding units (Fig. 1). The structural record chiefly includes folds in the Mesozoic units and faults in the Tertiary units. Palaeontologically, the most interesting unit is the Yolomécatl Formation, an c. 650 m thick, vertebrate-bearing, red clastic lacustrine/fluvial succession that fills the namesake triangular graben, which is genetically related to the Tamazulapam fault dynamics. Felsic tuff sheets interbed this succession; one yielded an 39Ar–40Ar age of 40.7 Ma (Ferrusquía-Villafranca et al. Reference Ferrusquía-Villafranca, Ruíz-González, Torres-Hernández, Anderson, Urrutia-Fucugauchi, Martínez-Hernández and García-Villegas2016), which dates this unit and its fauna as of late Middle Eocene age (i.e. Late Uintan NALMA).
1.c. Stable isotopes
Three main approaches are used for inferring the diet and habitat for Pleistocene and earlier extinct mammals: biological actualism, morphofunctional analyses and biochemical carbon/oxygen markers (Andrews & Hixson, Reference Andrews and Hixson2014). Carbon is incorporated into plants through photosynthesis in three pathways: C3, C4 and CAM (O'Leary, Reference O'Leary1988).
The C3 photosynthetic pathway occurs in trees and shrubs and some temperate grasses, with carbon isotopic values ranging between –34 ‰ and –22 ‰ (van der Merwe & Medina, Reference van der Merwe and Medina1989, Reference van der Merwe and Medina1991; Cerling et al. Reference Cerling, Harris, MacFadden, Leakey, Quade, Eisenmann and Ehleringer1997; Koch, Reference Koch1998). On the other hand, the C4 photosynthetic pathway has δ13C values between –14 ‰ and –10 ‰, and is usually found in grasses as well as trees and shrubs from warm regions (Smith & Epstein, Reference Smith and Epstein1971; Cerling, Reference Cerling, Sage and Monson1999; Medrano & Flexas, Reference Medrano, Flexas, Azcón-Bieto and Talón2000). The third photosynthetic pathway, CAM (crassulacean acid metabolism), is found in succulent plants such as cacti, bromeliads or agaves, with δ13C values between –35 ‰ and –12 ‰ (Gröcker, Reference Gröcker1997; Andrade et al. Reference Andrade, de la Barrera, Reyes-García, Ricalde, Vargas-Soto and Cervera2007).
Herbivores eat plants, incorporating the carbon from those plants into their tissues and structures such as dental enamel. The isotopic values are correlated with those of the plants, but vary in carbon isotopic composition by as much as a 14.1 ‰ increment (Cerling & Harris, Reference Cerling and Harris1999). Based on that variation, modern animals that eat C4 plants will have δ13C values between –2 ‰ and 2 ‰. Carbon isotopic values between –9 ‰ and –19 ‰ will be found in herbivores eating C3 plants, while those eating both types of plants will have δ13C values between –2 ‰ and –9 ‰ (MacFadden & Cerling, Reference MacFadden and Cerling1996). However, given that C4 plants became dominant by Hemphillian time (c. 8 Ma ago), this classification is not readily applied to older, pre-Hemphillian mammal taxa. Zanazzi & Kohn (Reference Zanazzi and Kohn2008) have therefore proposed that δ13C values of –15 ‰ to –21 ‰ indicate the presence of mesic, closed-canopy forest; –13 ‰ to –8 ‰ woodlands; and –8 ‰ xeric grasslands.
On the other hand, oxygen is incorporated into animals by inhalation, from water in food and mainly by ingested water. Such oxygen is in equilibrium with what is lost through CO2 exhalation, faeces, urine and sweat. Other factors such as physiology, climate and habitat can modify such balance (Sánchez, Reference Sánchez, Alcorno, Redondo and Toledo2005). The ingested oxygen mostly comes from the ingested water that is present from rain water, in turn affected by latitude, longitude and rain quantity, but mainly temperature (Dansgaard, Reference Dansgaard1964; Castillo, Morales & Ramos, Reference Castillo, Morales and Ramos1985). Oxygen isotopic composition (18O/16O) is frequently used for palaeoclimatic and palaeoecological studies (Bocherens et al. Reference Bocherens, Koch, Mariotti, Geraads and Jeager1996; Kohn, Reference Kohn1996; Sponheirmer & Lee-Thorp, Reference Sponheirmer and Lee-Thorp1999; Schoeninger, Kohn & Valley, Reference Schoeninger, Kohn, Valley, Ambrose and Katzemberg2000).
2. Materials and methods: sample extraction and preparation
A bulk sample (belonging to the Colección Nacional de Paleontología, Instituto de Geología, Universidad Nacional Autónoma de México) was taken from isolated chick teeth and processed in the Stable Isotope Laboratory at the Instituto de Geología, UNAM, by the method proposed by Koch, Tuross & Fogel (Reference Koch, Tuross and Fogel1997). First, 20 mg of enamel was ground and sieved (125 μm mesh) to obtain a fine and uniform powder. Then 10 mL of hydrogen peroxide at 30 % was added to eliminate the organic matter. After 2 h, the samples were centrifuged and the hydrogen peroxide decanted and washed again three times with water type I (grade HPLC 18.2 MΩ).
After the washing, 5 mL of a buffer solution, Ca(CH3CO2)2-CH3COOH 1.0 M, pH 4.75, was added and the mixture was allowed to rest for 9 h. The buffer solution was decanted and the samples were washed another three times with water type I. Finally, to eliminate any remaining water, ethanol was added and the solution was left for 20 h in an oven at 90 °C. Isotopic ratios were determined with a Finnigan MAT 253 mass spectrometer with a dual inlet system, and auxiliary Gas Bench equipment with a GC Pal autosampler with a temperature-controlled aluminium plate adjoined to the mass spectrometer (Révész & Landwehr, Reference Révész and Landwehr2002). Results were reported as δ18OVPDB and δ13CVPDB, normalized using NBS-19, NBS-18 and LSVEC to the Vienna Pee Dee Belemnite (VPDB) scale in accordance with the corrections described by Coplen (Reference Coplen1988), Werner & Brand (Reference Werner and Brand2001) and Coplen et al. (Reference Coplen, Brand, Gehre, Gröning, Meijer Harro, Toman and Verkouteren2006). For this technique, the standard deviation was 0.2 ‰ for oxygen and carbon.
Finally, we compared the isotopic values of carbon and oxygen with those obtained by Zanazzi & Kohn (Reference Zanazzi and Kohn2008) of Leptomeryx speciosus from the Late Eocene (Chadronian) White River Group and L. evansi from the Orellan part of this group (Table 1).
Table 1. Carbon and oxygen isotopic values of Leptomeryx sp. from the Yolomécatl Formation, L. speciosus and L. evansi from the White River Group. The δ18OVSMOW values of specimens from White River Group were transformed to δ18OVPDB using Faure's (Reference Faure1977) equation: δ18OVPDB= (0.97002 × δ18OVSMOW) −29.98. δ18O values are expressed in VPDB ‰. White River Group values were taken from Zanazzi & Kohn (Reference Zanazzi and Kohn2008).

3. Results
The δ13C value of the Leptomeryx sp. specimen from Yolomécatl is –12.5 ‰ and that of δ18O is –4.1 ‰. The carbon isotopic value falls within the range reported by Zanazzi & Kohn (Reference Zanazzi and Kohn2008) for Leptomeryx speciosus and L. evansis from the Chadronian and Orellanian portions of the White River Group. In the case of δ18O value, this is similar to that of Leptomeryx speciosus but different from that shown by L. evansi (Fig. 2a–d).
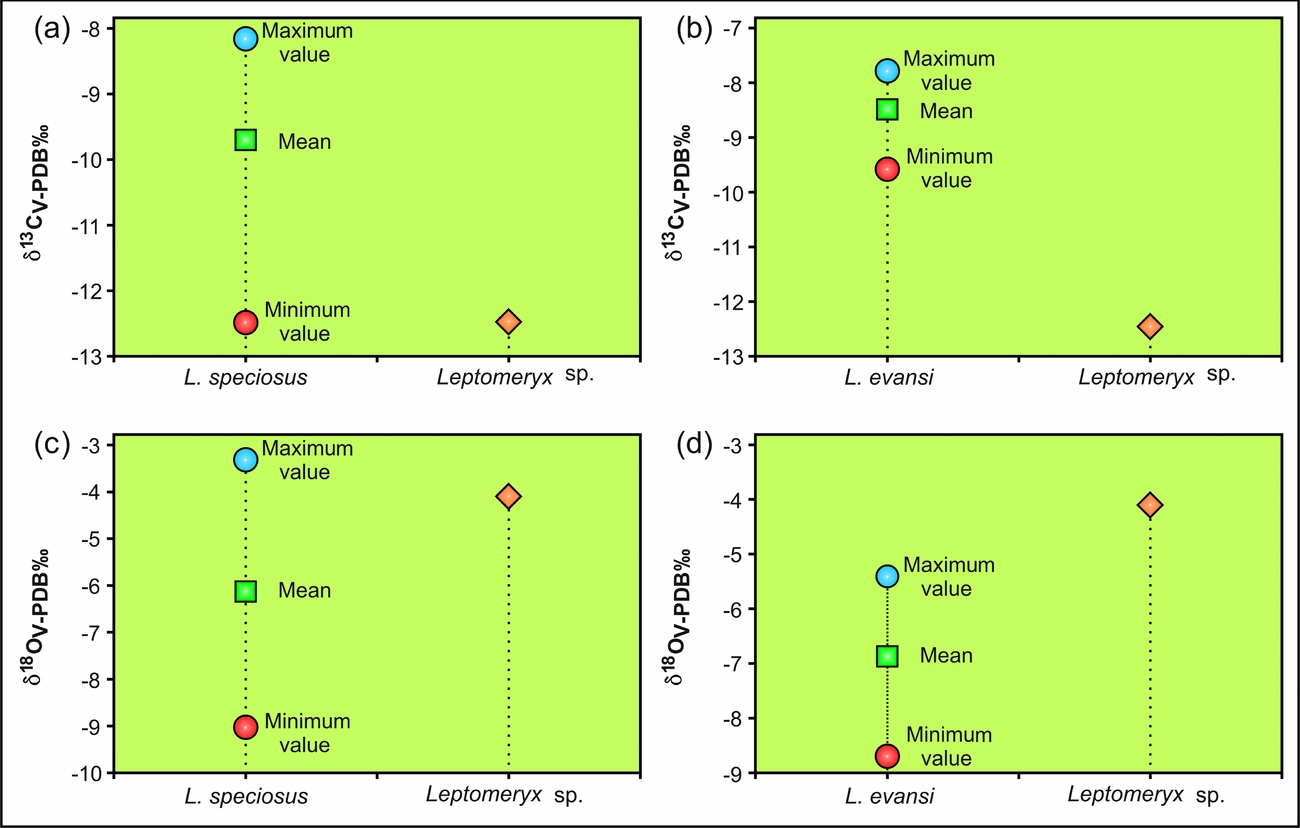
Figure 2. Comparison of (a, b) carbon and (c, d) oxygen isotopic values of Leptomeryx specious and L. evansi from the White River Group and Leptomeryx sp. from the Yolomécatl Formation.
4. Discussion
4.a. Diet
The carbon value of Leptomeryx sp. indicates that this individual fed only on C3 plants; Wall & Collins (Reference Wall and Collins1998) and Webb (Reference Webb, Janis, Scott and Jacobs1998) had pointed out that Leptomeryx was a small herbivore that fed on leaves and fruit, which are C3 plants (Medrano & Flexas, Reference Medrano, Flexas, Azcón-Bieto and Talón2000). Zanazzi & Kohn (Reference Zanazzi and Kohn2008) mentioned that the Late Eocene (Chadronian) Leptomeryx speciosus from the White River Group fed on C3 plants as observed in the Yolomécatl specimen. The Early Oligocene (Orellan) Leptomeryx evansi from the same group had a mixed C3/C4 diet however, which indicates that C4 plants were ingested or, alternatively, that the C3 plants on which L. evansi fed were water-stressed (due to a scarcity/lack of water), an environmental condition that altered its δ13C values; this is confirmed by microwear studies (see Zanazzi & Kohn, Reference Zanazzi and Kohn2008; Mathis & MacFadden, Reference Mathis and MacFadden2010; Shackelton, Reference Shackelton2016). Further, Zanazzi & Kohn (Reference Zanazzi and Kohn2008) indicated that the δ18O values of Leptomeryx are consistent with these mammals having had an incompletely developed anterior intestine fermentation system, or that they depended on water to accomplish anterior intestine fermentation, and that they possibly fed at night, when humidity is greater, behaving just as the extant Indonesian Tragulus javanicus (mouse deer).
4.b. Habitat
The oxygen isotopic value of the Yolomécatl individual is similar to that of Leptomeryx speciosus (Chadronian, White River Group) and different from L. evansis (Orellan, same group), as shown in Table 1. However, the δ18O values of L. evansi fall within the range of L. speciosus values (see Table 1).
Likewise, the isotopic results obtained from the Yolomécatl Leptomeryx sp. indicate that it was a forest or forest/savanna ecotone dweller, as was the Chadronian L. speciosus from the White River Group, and clearly different that the Orellan L. evansi from the same group which preferred open, somewhat xeric vegetation areas (Zanazzi & Kohn, Reference Zanazzi and Kohn2008; Lukens, Reference Lukens2013).
On the other hand, Webb (Reference Webb, Janis, Scott and Jacobs1998) indicated that the Late Eocene Leptomeryx species lived in open forests. The palynologic record obtained from the Yolomécatl Formation discloses the presence of arboreal and herbaceous taxa in NW Oaxaca at that time, lending credence to this assertion (see online Supplementary Table S1, available at http://journals.cambridge.org/geo). In addition, the record of Amynodontopsis sp., Merycoidodon sp., Miohippus sp., Perchoerus probus, Poebrotherium sp. and Trigonias sp. from the same formation (Jiménez-Hidalgo et al. Reference Jiménez-Hidalgo, Smith, Guerrero-Arenas and Alvarado-Ortega2015; Ferrusquía-Villafranca, unpublished data), which were dwellers of forests, open forests or savannas (Zanazzi & Kohn, Reference Zanazzi and Kohn2008; Bottrell, Reference Bottrell2009; Boardman, Reference Boardman2013; Boardman & Secord, Reference Boardman and Secord2013; Evans & Janis, Reference Evans and Janis2014), also strengthens this theory.
5. Conclusions
The results of this study lead us to conclude that the Leptomeryx sp. from the Late Eocene (Uintan) Yolomécatl Formation fed only on C3 plants and lived in a forest or in a forest/savanna ecotone. This scenario is consistent with the palynologic record of this formation, which indicates the presence of arboreal and herbaceous vegetation cover, and with the presence of mammal taxa known to lived in an environment similar to that of Leptomeryx sp. Both records disclose a tropical environment in northwestern Oaxaca during Late Eocene time.
Acknowledgements
We are indebted to the Instituto de Geología, Universidad Nacional Autónoma de México (UNAM) for its support, and to the Dirección General de Asuntos del Personal Académico de la UNAM (DGAPA) for their financial assistance through grants PAPIIT IN110614 and IA10407 to develop this project. The analysis was carried out in the Laboratorio de Isótopos Estables and Laboratorio Universitario de Geoquímica Isotópica (LUGIS) of Institutos de Geología and Geofísica, UNAM by our colleagues Edith Cienfuegos Alvarado and Francisco J. Otero Trujano, to whom we are grateful. We also thank two anonymous reviewers for their suggested changes that contributed to the improvement of this publication.
Declaration of interests
None
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756817000747.





