1. Introduction
In most living reptiles, teeth are continuously shed and replaced to ensure a regular bite and a lifelong capability to engulf prey (Richman & Handrigan, Reference Richman and Handrigan2011). However, the process of tooth development in many fossil reptilian groups has only recently become a topic of study (Erickson, Reference Erickson1996 a; Zaher & Rieppel, Reference Zaher and Rieppel1999; Rieppel & Kearney, Reference Rieppel and Kearney2005; Caldwell, Reference Caldwell2007; Chinsamy, Tunoğlu & Thomas, Reference Chinsamy, Tunoğlu and Thomas2010, Reference Chinsamy, Tunoğlu and Thomas2012). Hence, in order to gain a deeper understanding of the odontogenesis in Mosasauridae, a speciose family of secondarily aquatic lizards that became the dominant marine tetrapods in the Late Cretaceous about 98–66 million years ago, we examined periodic dentinal markings in a small number of isolated marginal tooth-crowns collected from the marine Cretaceous of southern Sweden.
Examination of incremental growth lines is an effective method of assessing dentine formation rates and tooth development times in both extant and extinct vertebrates (e.g. Erickson, Reference Erickson1996 a and references therein). Incremental markings form through periodic alterations in collagen fibre trajectories and/or variations in the mineralization process (Kawasaki, Tanaka & Ishikawa, Reference Kawasaki, Tanaka and Ishikawa1980; Bhaskar, Reference Bhaskar1991; Erickson, Reference Erickson1996 b; Nanci, Reference Nanci2008), thereby providing a continuous and often permanent record of the odontogenesis (Erickson, Reference Erickson1996 a). Such increments also contribute to our understanding of those mechanisms that underlie morphological change (Dean, Reference Dean, Teaford, Smith and Ferguson2000), and are thus of importance in the scientific fields of developmental and evolutionary biology.
Despite being illustrated already in the mid-1800s by Richard Owen (Reference Owen1841, Reference Owen1845; see Erickson, Reference Erickson1996 a), the importance of dentinal laminations was not recognized until the works of Andresen (Reference Andresen1898) and von Ebner (Reference Ebner and Scheff1902, Reference Ebner1906). These latter authors described concentric striations in the teeth of humans and other primates that are now being referred to as incremental lines of von Ebner and Andresen, respectively (e.g. Dean & Scandrett, Reference Dean and Scandrett1996). Long-period Andresen lines are spaced between 4 and 14 days apart owing to endogenous biorhythms (Kawasaki, Tanaka & Ishikawa, Reference Kawasaki, Tanaka and Ishikawa1980; Dean & Scandrett, Reference Dean and Scandrett1996; Dean, Reference Dean, Teaford, Smith and Ferguson2000), and usually contain short-period lines of von Ebner, where the distance between each lamination represents the daily secretion of dentine (Bhaskar, Reference Bhaskar1991; Dean, Reference Dean and Moggi-Cecchi1995, Reference Dean1998; Dean & Scandrett, Reference Dean and Scandrett1996; Hillson, Reference Hillson2005).
Using periodic chemical (i.e. fluorochrome) labelling, Erickson (Reference Erickson1996 b) was able to determine the rate and duration of dentine secretion in the modern crocodylians Alligator mississippiensis and Caiman crocodilus. Moreover, by counting and measuring the increments laid down between the fluorochrome injections, he (Erickson, Reference Erickson1996b ) demonstrated that these laminations form on a daily basis (as they do in most amniotes; e.g. Erickson, Reference Erickson1996a , Reference Erickson b ; Kamiya et al. Reference Kamiya, Yoshida, Kusuhashi and Matsuoka2006; Nanci, Reference Nanci2008; Kierdorf et al. Reference Kierdorf, Kierdorf, Witzel, Intoh and Dobney2009). Subsequently, Erickson (Reference Erickson1996 a) broadened his investigation to also include Mesozoic crocodylians and nonavian dinosaurs. Comparable markings were found in the fossilized teeth of both groups, to suggest homology given their close phylogenetic relationship (Erickson, Reference Erickson1996a ). More recently, Chinsamy, Tunoğlu & Thomas (Reference Chinsamy, Tunoğlu and Thomas2010, Reference Chinsamy, Tunoğlu and Thomas2012) demonstrated the presence of periodic dentinal increments in a tooth-crown of the derived mosasaur Mosasaurus hoffmanni, and building on Erickson's (Reference Erickson1996a , Reference Erickson b ) model, they estimated that it took 511 days to deposit the dentine at the level of sectioning.
In the present contribution, concentric laminations observed in histological sections of three isolated mosasaur teeth (assigned to Dollosaurus sp., cf. Platecarpus and Tylosaurus ivoensis, respectively) from the Kristianstad Basin, southernmost Sweden, are compared to morphologically similar markings in a tooth-crown of the marine crocodylian Aigialosuchus sp. collected from the same area. We begin with a description of various microstructural features that have previously been used to determine crocodylian and mosasaur dentine secretion rates (see Erickson Reference Erickson1996a , Reference Erickson b ; Chinsamy, Tunoğlu & Thomas, Reference Chinsamy, Tunoğlu and Thomas2010, Reference Chinsamy, Tunoğlu and Thomas2012). We then estimate the time it took to develop the dentine at the level of sectioning. Finally, we conclude with a brief discussion on tooth development times and replacement rates in mosasaurs.
2. Geological setting
The Cretaceous deposits of the Kristianstad Basin sensu Erlström & Gabrielson (Reference Erlström and Gabrielson1992) are bordered to the southwest by the Linderödsåsen and Nävlingeåsen horst ridges, whereas the northern demarcation is more diffuse with several outliers of marine strata of Campanian–Maastrichtian age (Fig. 1). The Cretaceous sediments comprise primarily shallow marine calcarenites, calcisilitites and calcareous sandstones, although terrigenous material in conglomerates and boulder beds indicates that nearby land served as important source areas (Christensen, Reference Christensen1975). For more comprehensive descriptions of the geology, development and depositional environments of the Kristianstad Basin, see Christensen (Reference Christensen1975), Bergström & Sundquist (Reference Bergström, Sundquist, Kornfält, Bergström, Carserud, Henkel and Sundquist1978), Lidmar-Bergström (Reference Lidmar-Bergström1982) and Erlström & Gabrielson (Reference Erlström and Gabrielson1986, Reference Erlström and Gabrielson1992).

Figure 1. Geological map of southernmost Sweden showing the location of the Kristianstad Basin in the northeastern corner of the province of Skåne and the localities from which the analyzed teeth were collected (modified from Lindgren & Siverson, Reference Lindgren and Siverson2002, fig. 1).
With the exception of a single Aigialosuchus sp. tooth (LO 11621 t) from the lowermost Campanian (Gonioteuthis granulataquadrata belemnite Zone, Ullstorp site; see Erlström & Gabrielson, Reference Erlström and Gabrielson1986 and Siverson, Reference Siverson1993 for locality information), all fossils examined herein were collected from strata representing a narrow stratigraphic interval (i.e. the informal Belemnellocamax mammillatus zone) corresponding to the highest belemnite zone in the lower part of the European two-fold division of the Campanian Stage (see Christensen, Reference Christensen1975). Three localities exposing sediments of latest early Campanian age (Åsen, Ivö Klack and Ugnsmunnarna; Fig. 1) produced the teeth described below. For locality information, see Lindgren & Siverson (Reference Lindgren and Siverson2002), Lindgren et al. (Reference Lindgren, Currie, Siverson, Rees, Cederström and Lindgren2007) and Eriksson et al. (Reference Eriksson, Lindgren, Chin and Månsby2011, fig. 2).
3. Methodology
The material selected for histological analysis comprises four isolated, presumably shed marginal tooth-crowns (LO 11621 t – LO 11624 t) representing three genera of mosasaurs (Dollosaurus, cf. Platecarpus and Tylosaurus) and one genus of crocodylian (Aigialosuchus). Comparisons with more complete dentitions in mosasaur and crocodylian skeletons from North America and west-central Europe (see Lindgren & Siverson, Reference Lindgren and Siverson2002, Reference Lindgren and Siverson2004, Reference Lindgren and Siverson2005; Lindgren, Reference Lindgren2005 a, b) suggest that all teeth originate from the mid-section of the dental ramus in sub-adult to adult individuals.
Prior to sampling, all tooth-crowns were measured (Table 1), photographed (the crowns were covered with ammonium chloride prior to being photographed) and moulded. Thereafter, the teeth were vacuum-embedded in a clear polyester resin, and millimetre-thick transverse sections were produced using a slow-speed diamond saw (the precise location of each section is listed in Table 1). Additionally, a partial longitudinal section was produced from LO 11621 t (Aigialosuchus sp.) because previous studies (e.g. Erickson, Reference Erickson1996 b) have indicated that longitudinal sections may provide additional histological details. The thin-sections were mounted on petrographic slides using polyester resin, ground to optical translucency (i.e. to about 50–100 μm thickness), polished on a felt pad with aluminium oxide powder, and imaged (at × 5–40 magnification) using a Nikon DS-Fi1 camera attached to a binocular microscope. Histological features were documented with particular focus on incremental growth line distribution, spacing and counts. Notes were made also on other potential endogenous markings, as well as on enamel thickness, state of the osteocyte lacunocanalicular system, and possible taphonomic/diagenetic artefacts. In accordance with the published methodology of, for example, Dean (Reference Dean1998), Erickson (Reference Erickson1996 a, b) and Nanci (Reference Nanci2008), the different types of incremental laminations were identified based on their widths and morphology. In each section examined, the most complete sequences of short- and (if present) long-period lines were measured using an image analysis software (NIS-Elements BR 3.10) from digital micrographs, and the average distance between the individual lines was calculated. These numbers were then extrapolated to include the entire thickness of the dentine (measured using the multipoint circle tool in NIS-Elements BR 3.10) in order to obtain values of the total dentine accumulation at the level of sectioning.
Table 1. Measurements and section locations of sampled tooth-crowns

Depository acronym. LO – Department of Geology, Lund University, Lund, Sweden.
4. Histological analyses
The dental morphology of the mosasaur and crocodylian taxa dealt with in this work has been described in detail by, for example, Persson (Reference Persson1959, Reference Persson1963), Lindgren (Reference Lindgren2005 a, b) and Lindgren & Siverson (Reference Lindgren and Siverson2002, Reference Lindgren and Siverson2004, Reference Lindgren and Siverson2005); here we provide additional microstructural data obtained from recent histological analyses. Basic measurements, such as tooth-crown height, width and length (as preserved), are presented in Table 1.
4.a. LO 11622 t (Dollosaurus sp.)
Two transverse histological sections were made from LO 11622 t (Fig. 2); one near the apex and one at approximately mid-crown height. The apical section preserves closely spaced dentinal tubules that radiate from the margin of the pulp cavity and outward. Numerous odontocyte lacunae are also visible, and these are oriented with their long axis parallel to the tubules. A small number of laminations run parallel to the pulp circumference. The lines are faint, irregularly spaced and too few to allow a confident quantitative analysis. Numerous taphonomically induced micro-cracks are also present, and these have served as loci for bacterial or fungal activity (cf. Underwood, Mitchell & Veltkamp, Reference Underwood, Mitchell and Veltkamp1999), resulting in the partial loss of endogenous microstructures.

Figure 2. Dollosaurus sp., LO 11622 t, isolated marginal tooth-crown. (a) Specimen photographed in labial view prior to histological analysis. Level of sectioning is marked with a horizontal bar. (b) Transverse mid-crown section of LO 11622 t (labial side faces downwards). (c) Demarcated area in (b) showing periodic incremental markings here interpreted as lines of von Ebner. Scale bars represent 10 mm (a), 5 mm (b) and 500 μm (c).
The transverse section taken at mid-crown height is similar to the apical one in that it contains multiple radially oriented dentinal tubules and elongated odontocyte lacunae (Fig. 2b). However, those areas affected by microorganisms are considerably smaller, and consequently the dentine is rich in preserved increments (some of which are accentuated by secondarily deposited minerals). These laminations run perpendicularly to the tubules and are most pronounced in the peri-pulpal part of the dentine, where 105 consecutive lines with a size range of 8–23 μm and an average width of 12.2 μm were documented (Fig. 2c). By extrapolation (mean thickness of dentine = 5194 μm), the sectioned part of the tooth-crown holds a total of 426 periodic dentinal markings.
4.b. LO 11623 t (cf. Platecarpus)
Two transverse histological sections were made from LO 11623 t (Fig. 3): one close to the apex and one at approximately mid-crown height. In the apical section, the enamel displays one distinct and one faint lamination. No increments were observed in the dentine; however, closely spaced dentinal tubules could be seen radiating from the vicinity of the pulp cavity and outward. The dentine is also rich in dark odontocyte lacunae with stubby canaliculi. As in LO 11622 t, the lacunae are elongate and oriented with their long axis parallel to the tubules. Diverging channels, joined in discrete networks and probably made by bone-boring bacteria and/or fungal hyphae, surround a number of taphonomically induced micro-cracks.

Figure 3. cf. Platecarpus, LO 11623 t, isolated marginal tooth-crown. (a) LO 11623 t in labial view prior to histological analysis. Level of sectioning is marked with a horizontal bar. (b) Transverse mid-crown section of LO 11623 t (labial side faces downwards). (c) Demarcated area in (b) showing long-period incremental markings (here interpreted as Andresen lines – black arrowheads), radially oriented dentinal tubules, possible calcospherites (arrow), and elongate osteocyte lacunae. (d) Demarcated area in (c) showing a second set of finer concentric laminations here interpreted as lines of von Ebner (better preserved striae are marked with white arrowheads). Scale bars represent 10 mm (a), 5 mm (b), 1 mm (c) and 100 μm (d).
The enamel of the mid-crown section holds three weak and incomplete increments consisting of alternating dark and light bands. The section is rich in both endogenous microstructures and diagenetic artefacts, such as micro-fractures filled with brownish matter. Towards the circumference the dentine is largely deteriorated by microorganisms (Fig. 3b, c). Micro-borings are also present in the region of the pulp cavity, although some original structures, including darkly stained mineral globules (possibly calcospherites) remain (Fig. 3c, white arrow). Radially oriented dentinal tubules and odontocyte lacunae are dispersed throughout the dentine (Fig. 3b, c, d). The blackish lacunae are elliptical, and remains of the canalicular system occur as thread-like extensions from the surfaces of the secondarily filled cavities (Fig. 3d). Two sets of incremental growth lines were measured: one set of ten consecutive long-period lines, characterized by alternating opaque and transparent bands, with a size range of 102–201 μm and an average width of 141 μm (Fig. 3c), and one set of 128 short-period lines with a size range of 6–19 μm and an average width of 11.3 μm (Fig. 3d). By extrapolation, at the level of sectioning the tooth holds a total of 342 short-period lines with one long-period line for every twelfth or thirteenth short-period line (mean thickness of dentine = 3860 μm).
4.c. LO 11624 t (Tylosaurus ivoensis)
Two transverse histological sections were made from LO 11624 t (Fig. 4): one close to the apex and one at approximately mid-crown height. In the apical section, the enamel contains four incomplete increments. Evidence of bacterial or fungal activity is apparent towards the periphery of the dentine, thus limiting the amount of observable primary features in this part of the section. Odontocyte lacunae and dentinal tubules appear scattered throughout the dentine, the latter of these microstructures being intersected by a continuous set of 77 incremental laminations ranging in size from 10 to 26 μm and with an average width of 15.7 μm. Given that the thickness of the dentine is 6441 μm, there are 410 daily increments at the level of sectioning. Additionally, a few coarser markings occur among the short-period lines, roughly 100–200 μm apart.
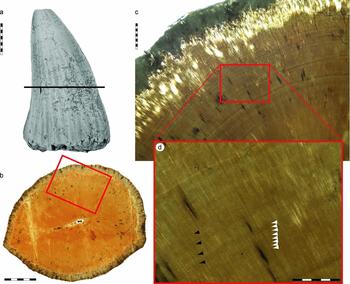
Figure 4. Tylosaurus ivoensis, LO 11624 t, isolated marginal tooth-crown. (a) Specimen photographed in labial view prior to histological analysis. Level of sectioning is marked with a horizontal bar. (b) Transverse mid-crown section of LO 11624 t (labial side faces downwards). (c) Demarcated area in (b) showing long-period incremental markings (here interpreted as Andresen lines), radially oriented dentinal tubules and elongate osteocyte lacunae. (d) Demarcated area in (c) showing presumed Andresen lines (black arrowheads) and a second set of finer concentric laminations here interpreted as lines of von Ebner (better preserved striae are marked with white arrowheads). Scale bars represent 10 mm (a), 5 mm (b), 1 mm (c) and 500 μm (d).
In the mid-crown section, only one incremental line can be traced over some distance in the enamel. Although the perimeter of the dentine is remodelled by bone-boring bacteria and/or fungal hyphae, its inner parts are well preserved with abundant dentinal tubules and elongate odontocyte lacunae. One set comprising 16 consecutive long-period lines, with a size range of 122–275 μm and an average width of 219 μm, is present in the peri-pulpal dentine (Fig. 4d, black arrowheads). Additionally, there is one set of 82 short-period lines with a size range of 9–34 μm and an average width of 19.4 μm (Fig. 4d, white arrowheads). Thus, at the level of sectioning the tooth-crown holds a total of 487 short-period lines (mean thickness of dentine = 9440 μm) with one long-period line for every eleventh or twelfth short-period line.
4.d. LO 11621 t (Aigialosuchus sp.)
Three histological sections were made from LO 11621 t (Fig. 5): one transverse section close to the apex, one transverse section at approximately mid-crown height, and one longitudinal section near the base of the crown. In the apical section, the enamel holds three incomplete increments preserved as alternating opaque and transparent laminae. Most primary features of the dentine are obscured by an interconnected lattice of thread-like channels that was probably made by bone-boring bacteria and/or fungal hyphae, although densely spaced, radially oriented dentinal tubules and a few faint, concentric laminations are visible in some areas.

Figure 5. Aigialosuchus sp., LO 11621 t, isolated tooth-crown. (a) LO 11621 t as it appeared (in labial view) prior to histological analysis. Level of sectioning is marked with a horizontal bar. (b) Transverse mid-crown section of LO 11621 t (labial side faces downwards). (c) Demarcated area in (b) showing periodic incremental markings (arrowheads) here interpreted as lines of von Ebner. Scale bars represent 5 mm (a, b) and 500 μm (c).
The section taken at mid-crown height is also partially affected by microorganisms, particularly in the peripheral parts of the dentine (Fig. 5b). Close to the pulp cavity, fine dentinal tubules extend radially from the crown centre and outward (Fig. 5c). These tubules are intersected perpendicularly by a number of concentric and gently undulating incremental growth lines (consisting of alternating dark and light bands) of somewhat varying widths (6–19 μm) and thickness (Fig. 5c, arrowheads). A strong band at about 1200 μm from the centre of the pulp cavity is surrounded by a micro-crack, about 1 μm wide. This crack indicates a weakening zone in the dentine, which may have been exploited by bacteria and/or fungi (Fig. 5c). Proximal to the pulp cavity, increments are regular and well preserved, whereas distally, the laminations are more irregular and diffuse. Fifty-one consecutive short-period growth lines were measured, rendering an average width of 12.7 μm. By extrapolation (mean thickness of dentine = 3287 μm), the tooth-crown contains a total of 259 dentinal increments over the sectioned surface. The longitudinal section did not yield any additional information.
5. Discussion
Lines of von Ebner form through a circadian rhythm involving alternating accumulation and mineralization of dentine (Nanci, Reference Nanci2008), and the daily increments seen in extant crocodylian teeth are presumably deposited in a similar manner (Erickson, Reference Erickson1996 b). The short-period markings observed in Aigialosuchus sp. have a morphology and size range consistent with the periodic increments documented by Erickson (Reference Erickson1996 b), and homologous increments are also found in Dollosaurus sp., cf. Platecarpus and Tylosaurus ivoensis, thus corroborating the presence of von Ebner's lines in Mesozoic crocodylian and mosasaur dentine (cf. Erickson, Reference Erickson1996a ; Chinsamy, Tunoğlu & Thomas, Reference Chinsamy, Tunoğlu and Thomas2010, Reference Chinsamy, Tunoğlu and Thomas2012).
Daily dentine formation rates (and incremental line widths) in modern amniotes are between 1 and 30 μm regardless of the size of the tooth (and animal), something that led Erickson (Reference Erickson1996 a) to conclude that there are important structural or physiological constraints that limit the amount of dentine that can form on a daily basis. The consequences of these restraints are interesting, particularly when considering animals that attain gigantic proportions. During the evolution of obligatory marine mosasaurs, body size increased and so did tooth size (Russell, Reference Russell1967; Lingham-Soliar, Reference Lingham-Soliar1995). However, the time required to develop the teeth seemingly increased as well, as indicated by the growth line counts in T. ivoensis (410 and 487 days at the apex and mid-crown section, respectively) and Mosasaurus hoffmanni (511 days; see Chinsamy, Tunoğlu & Thomas, Reference Chinsamy, Tunoğlu and Thomas2010, Reference Chinsamy, Tunoğlu and Thomas2012) – two enormous species with massive marginal tooth-crowns – compared to cf. Platecarpus (342 days at mid-crown height) (the moderately robust crown of Dollosaurus sp. takes an intermediate position with 426 daily increments at mid-crown height). Although both T. ivoensis and M. hoffmanni undoubtedly were capable of eating almost anything that they encountered, including sea turtles and other mosasaurs (e.g. Massare, Reference Massare1987 and references therein), the risk of long-term incapacity to overwhelm prey following breakage or other severe dental injuries may have been high (assuming that tooth replacement rates declined in conjunction with prolonged tooth development times, as they do in extant animals; cf. Kline & Cullum, Reference Kline and Cullum1984; Erickson, Reference Erickson1996 a). Nonetheless, in similarity with the giant tyrannosaurid dinosaurs (see Erickson, Reference Erickson1996 a), this evolutionary trade-off presumably was compensated for by allometric modifications of the teeth, including, for example, the development of more robust crowns and broader tooth bases (Massare, Reference Massare1987; Lingham-Soliar, Reference Lingham-Soliar1995; Lindgren & Siverson, Reference Lindgren and Siverson2002). Not only did these adaptations enable the capture of larger prey items, but they also increased the resistance of the teeth to mechanical damage, thereby extending their functional longevity (Lingham-Soliar, Reference Lingham-Soliar1995).
In addition to lines of von Ebner, coarser and more widely spaced incremental markings were observed in LO 11623 t (cf. Platecarpus) and LO 11624 t (T. ivoensis) (Figs. 3, 4). These long-period lines contain between 11 and 13 short-period lines each, to suggest formation periodicities similar to those of Andresen lines in extant animals (Dean & Scandrett, Reference Dean and Scandrett1996). However, unlike typical Andresen lines, which are spaced on average between 15 and 30 μm apart (Dean & Scandrett, Reference Dean and Scandrett1996), the long-period lines documented in this study have a mean width of 141 μm (cf. Platecarpus) and 219 μm (T. ivoensis), respectively. Assuming depository rhythms similar to those of Andresen lines, this would imply that the amount of dentine secreted at any given time was between 4 and 15 times higher in mosasaurs relative to modern mammals (cf. Dean & Scandrett, Reference Dean and Scandrett1996). Given that mosasaurs, as opposed to most mammalian taxa, continuously shed and replaced their teeth, significantly higher tooth formation rates are expected (and in line with calculated tooth formation times in other reptilian groups, including Aigialosuchus, other crocodylians, and nonavian dinosaurs; Erickson, Reference Erickson1996 a, b; Sereno et al. Reference Sereno, Wilson, Witmer, Whitlock, Maga, Ide and Rowe2007; D'Emic et al. Reference D'Emic, Whitlock, Smith, Wilson and Fisher2009). Nonetheless, in a study devoted to Bauer's (Reference Bauer1898) collection of ichthyosaur tooth sections, Scheyer & Moser (Reference Scheyer and Moser2011) pointed out that similarly spaced incremental lines are variably assigned to as von Ebner and Andresen lines by different authors (see also Dean, Reference Dean1998, Reference Dean, Teaford, Smith and Ferguson2000). Moreover, they (Scheyer & Moser, Reference Scheyer and Moser2011) documented two sets of incremental markings in ichthyosaur dentine: a first set comprising very fine laminations situated about 2–3 μm apart, and a second set of coarser markings where spaces varied around 15–20 μm. Scheyer & Moser (Reference Scheyer and Moser2011) interpreted the thin, short-period lines as those of von Ebner based on their comparable thickness to the daily increments in human dentine, whereas the coarser, long-period lines were referred to as Andresen lines. If this interpretation is correct, then the short-period lines recorded in mosasaur and nonavian dinosaur dentine would represent Andresen lines, which in turn would result in even shorter tooth development times than those estimated by us and others (cf. Erickson, Reference Erickson1996 a; Sereno et al. Reference Sereno, Wilson, Witmer, Whitlock, Maga, Ide and Rowe2007; D'Emic et al. Reference D'Emic, Whitlock, Smith, Wilson and Fisher2009; Chinsamy, Tunoğlu & Thomas, Reference Chinsamy, Tunoğlu and Thomas2010, Reference Chinsamy, Tunoğlu and Thomas2012). However, increments with a periodicity of less than a day (infradian markings) have been identified in some animals (see Dean & Scandrett, Reference Dean and Scandrett1996), and it should also be pointed out that Maxwell, Caldwell & Lamoureux (Reference Maxwell, Caldwell and Lamoureux2012) failed to find any regular incremental banding pattern in their sample of sectioned ichthyosaur teeth. Even though we were unable to confirm the presence of laminations spaced less than about 6 μm apart, we cannot rule out the possibility of three different sets of incremental markings in mosasaur dentine because of potential preservational artefacts, such as diagenetic colouration or secondary mineralization, which might obliterate the original ultrastructure of the dentine.
In order to fully understand the process of tooth development and replacement in mosasaurs, more comprehensive studies based on larger sample sizes are required. However, because the incremental growth lines observed in mosasaur marginal teeth are homologous to those in the dentine of extant animals, it is likely that the dentinal deposition rhythms we record herein represent primitive features that can be found in all tooth-bearing vertebrates.
Acknowledgements
We gratefully acknowledge logistical support for the project provided by the Department of Geology, Lund University. We thank Jan Rees, Peter Cederström and Filip Lindgren for collecting some of the specimens examined in this study and for putting their private collections at our disposal. This research was supported by grants from the Swedish Research Council, the Crafoord Foundation and the Royal Swedish Academy of Sciences.








