Introduction
Endometriosis is a debilitating and chronic gynaecological illness defined by the existence of endometrial-like tissue beyond the uterus and exhibits similar hormonal responses (Ref. Reference Chapron1). The prevalence rate in the population cannot be known precisely, but about 10% of women in their reproductive years have endometriosis (Ref. Reference Shafrir2). Unfortunately, because an unequivocal clinical diagnosis is limited by individual differences, physician experience, the lack of specificity and non-invasive biomarkers, currently the delay in the diagnosis of endometriosis is inevitable (Ref. Reference Pascoal3). Endometriosis is related to a range of symptoms, the most common of which are subfertility and chronic pelvic pain (CPP) (Ref. Reference Zondervan, Becker and Missmer4), the latter must be taken seriously as it has a massive effect on the patient's daily life and works as well as bringing a substantial financial burden (Ref. Reference Lamvu5).
Although extensive and in-depth research put forward many hypotheses such as Sampson's implantation theory to explain its origin and pathogenesis, the exact aetiology remains elusive and controversial and the mechanism of pain associated with endometriosis is complex and still being investigated (Ref. Reference Wang, Nicholes and Shih6). Although the pathogenesis of endometriosis is not entirely appreciated, several risk factors including anatomical abnormality, endocrine, hereditary, oxidative stress, immunological factors and inflammation are all contributors to the initiation and progress of endometriosis (Ref. Reference Leone Roberti Maggiore7). The new ESHRE guidelines recommended hormonal therapy or surgery for the treatment of pain symptoms associated with endometriosis (Ref. Reference Becker8). However, they are not currently selected based on pain mechanisms (Ref. Reference Becker8).
Unfortunately, endometriosis tends to persist even after excising or ablating all visible lesions. Besides, the stage of endometriosis has a poor correlation between the degree of subjective pain and the location, amount, size and severity of endometriotic lesions (Ref. Reference Taylor, Kotlyar and Flores9). Endometriosis-related CPP may result from neo-neurogenesis, inflammatory pain, neuropathic pain, altered pain pathways, peripheral and central sensitisation and alterations in different brain regions (Refs Reference Zondervan, Becker and Missmer4, Reference Maddern10, Reference Liang11). Given that the current summary of pain mechanisms related to endometriosis remains sparse at the cellular and molecular levels, we attempt to explore its possible mechanisms in the peripheral and central nervous systems (CNS) and demonstrate brain changes in imaging and biochemistry thus providing a possible theoretical basis for future clinical treatment (Table 1; Figure 1).
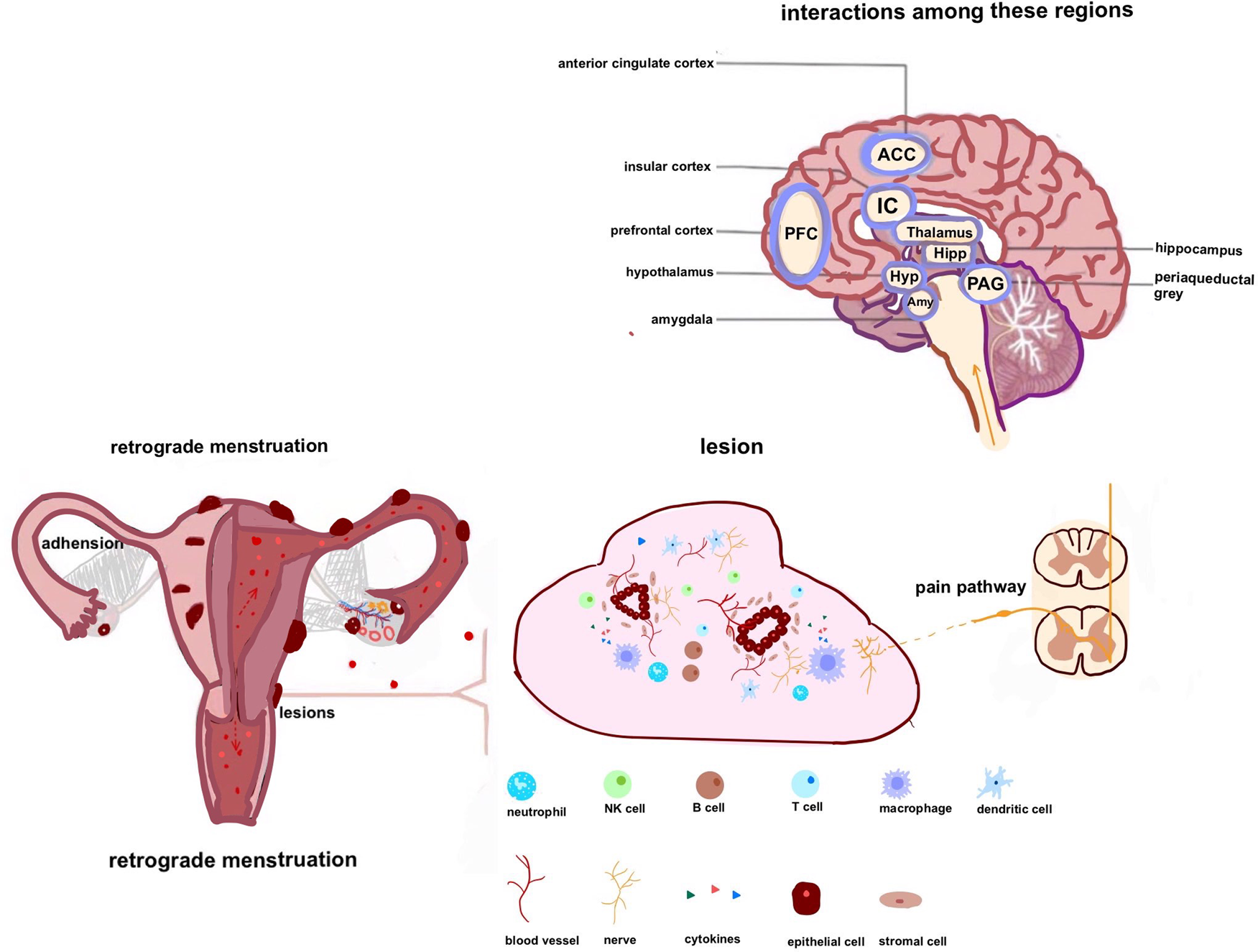
Fig. 1. Pain conduction pathway in endometriosis: endometrial debris can travel and infiltrate in several locations. In addition to stimulating neurogenesis and neovascularisation, it may release chemicals that attract diverse cells. The combination of these changes promotes microenvironmental changes around the lesion. Oxidative stress and inflammatory mediators can stimulate and sensitise pain-sensing nociceptors at primary afferent neurons in the area of the lesion. By modulating various ion channels, peripheral receptors result in increased sensitivity and hyperexcitability of nociceptor neurons (peripheral sensitisation). Inflammatory pain can progress into neuropathic pain as the disease state persists and progresses, causing neuronal damage. Pain processing may be altered by repetitive and persistent noxious stimulation, chronic inflammation and nerve injury, leading to central sensitisation. Besides, chronic pain is associated with alterations in the structure and function of the brain.
Table 1. Definitions of relevant terms in this review
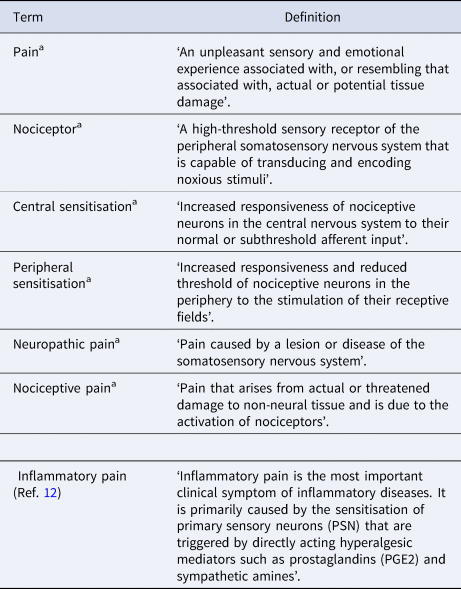
a Definitions of these terms are from the 2020 International Association for the Study of Pain (IASP).
Neurogenesis and endometriosis
Previous studies have revealed changes in the nervous system in endometriosis. In patients with endometriosis, nerve fibre density in the endometriotic lesion was 14 times higher than control (Ref. Reference Fassbender13). Deeply infiltrating endometriosis (DIE) showed elevated nerve density and expression of growth-associated proteins such as pan-neurotrophin receptors (NGFRp75) and nerve growth factor (NGF) (Ref. Reference Wang14). Even with minimal to mild endometriosis, the endometrium of individuals is predominantly innervated by substance P and calcitonin gene-related peptide (CGRP)-positive nerve fibres, cholinergic and adrenergic nerve fibres (Ref. Reference Bokor15). In endometrial biopsies performed on patients, researchers also identified more neuroendocrine cells than those from the control group (Ref. Reference Wang16). These small nerve fibres such as unmyelinated afferent C-fibres can transmit pain signals and modulate some functions of the autonomic nervous system (Ref. Reference Terkelsen17). The perineural invasion (PNI) (+) group, which had a higher nerve density, had higher visual analogue scale ratings for dysmenorrhoea, dyspareunia and chronic pelvic discomfort than the PNI (−) group (Ref. Reference Liang18). However, the nerve density and pain scores were only relevant as the causal link was not reported yet. In recent animal models, unlike previous findings (Ref. Reference McAllister, Dmitrieva and Berkley19), immunodeficient mice implanted with endometriotic lesions showed chronic pain behaviour but no significant difference in nerve density (Ref. Reference Tejada20). This also suggests that the mechanisms of pain associated with endometriosis are involved in an abnormality in the pain pathways with complicated pathogenesis.
The distribution of nerve fibres differs between normal human endometrium and endometriosis. Small nerve fibres were present in the functional layer of the endometrium in endometriosis patients, but similar innervation was not observed in healthy women (Ref. Reference Tokushige21). However, some researchers had countered that small nerve fibres are not unique to patients with endometriosis. It was not rigorous to use nerve fibres as a diagnosis (Ref. Reference Ellett22). It was suspected that the altered innervation of the functional endometrial layer may be caused by the together influence of other diseases rather than the effects of endometriosis alone (Ref. Reference May23). In addition, the distribution of nerve fibres within the endometriosis lesion was heterogeneous. In a cross-sectional study, by utilising a set of neuronal markers in samples from rectosigmoid endometriosis lesions, people detected larger nerve fibre densities at the edges of the nodules than at the centre (Ref. Reference Zomer24).
Endometriosis not only caused a change in the number and distribution of nerve fibres but also caused the nerve fibre types to change. Semaphorin, which was released by macrophages or activated fibroblasts in inflamed tissue in endometriosis patients, can induce degeneration of sympathetic nerves and necrosis, thus leading to the imbalance of the autonomic nervous system (Ref. Reference Scheerer25). In vitro experimental models, peritoneal fluid from patients with endometriosis can induce an increased amount of germinated sensory neurites in the dorsal root ganglion and the decrease of sympathetic ganglion neurites (Ref. Reference Barcena de Arellano and Mechsner26). An additional characteristic of endometriosis is that it is steroid-dependent with a complex hormonal imbalance (Ref. Reference Yilmaz and Bulun27). In preclinical studies, it was found that high 17β-oestradiol could alter the distribution and/or numbers of nerve fibres containing vesicular acetylocholine transporter (VAChT), nitric oxide synthase (nNOS) and vasoactive intestinal polypeptide (VIP) (Ref. Reference Jana28). This finding was supported by animal models. Both sympathetic and sensory nerve fibre markers were overexpressed at the site of induced endometriosis in mice. The increase in neurotrophic factors may be responsible for stimulating nerve development and boosting the growth of sensory nerves (Ref. Reference Ghersa29). Further research into the specific mechanisms, NGFs in rats' ovaries were found to affect the expression of neurotransmitter-related enzymes in noradrenergic and cholinergic systems, causing the corresponding neurotransmitter content to change and subsequent cascade response (Ref. Reference Benitez30).
Moreover, endometriosis lesions can also modify pain by affecting the activity of nerve fibres. People unearthed that patients with endometriosis had lower vagus nerve activity (Ref. Reference Hao31). Chronic pain often results from a depletion of the sympathetic nervous system (Ref. Reference Yeater32), and endometriosis also causes CPP. Although its mechanism is unclear, it encourages researchers to study the links between nerve change, inflammation and immune dysfunction (Refs Reference Scheerer25, Reference Ferrero33).
Nerves and neovessels
Experimental studies have shown that ectopic endometrial debris acquires neurovascular supplies through a series of complex mechanisms, and it then survives and develops (Ref. Reference Laschke and Menger34). In an attempt to treat pain, the patient with severe endometriosis received bevacizumab (Avastin®), a monoclonal antibody, resulting in the cessation of persistent dysmenorrhoea (Ref. Reference Bouquet de Joliniere35). In the process of angiogenesis, the vascular epithelial growth factor (VEGF) has a powerful biochemical influence including its well-known effects on neovascularisation and angiogenesis (Ref. Reference Apte, Chen and Ferrara36). The concentrations of macrophage migration inhibitory factor (MMIF), hypoxia-inducible factor-1a (HIF-1a) and VEGF were found to be higher in serum and endometrial tissue of endometriosis (EM) patients than in healthy women (Ref. Reference Zhang37). Among them, HIF may play a key role in maintaining the normal endometrial function, specifically in the expression of cellular and angiogenesis genes during progesterone withdrawal via the prostaglandin (PG) pathway during hypoxia (Ref. Reference Hsiao38). Stimulation of endometriotic cells or the significantly upregulated transforming growth factor-β (TGF-β) expressions in patients' peritoneal fluid can also upregulate VEGF levels by promoting the activation of a series of VEGF-related signalling molecules such as cyclooxygenase-2 (COX-2) (Ref. Reference Laschke and Menger34) or blocking the DNA-binding protein 2 pathway (Ref. Reference Young39). However, in a case-control study, there was no significant difference in VEGF mRNA expression between patients with endometriosis and controls (Ref. Reference Rashidi40). Based on clinical studies, it is possible that VEGF is particularly expressed in red endometriotic lesions and its expression correlates with the activity and the stage of endometriotic lesions (Ref. Reference Laschke and Menger34).
In fact, angiogenesis in endometriosis is a complex process in which multiple factors are involved (Ref. Reference Zhou41). Cells that have undergone drastic changes in the peritoneal fluid, such as macrophages, mast cells and other cells which are all involved in the regulation of angiogenesis (Ref. Reference Perricos42). Ectopic endometriotic lesions can also secrete chemokines that attract immune cells, whereas immune cells can secrete a variety of cytokines that influence the local microenvironment (Ref. Reference Zhou41). Within this microenvironment, the interleukin (IL) family is responsible for initiating inflammation as well as promoting angiogenesis by inducing the production of proangiogenic factors (Ref. Reference Fahey and Doyle43). However, angiogenesis and its indirect influence on pain remain controversial. Patients with higher vascularisation in DIE lesions did not score higher on patient pain questionnaires (Ref. Reference Raimondo44). But, the neovascularisation-based examination technique is effective for clearing hidden lesions and reducing postoperative pain recurrence (Ref. Reference Turco45).
Sensitisation and inflammatory/neuropathic pain
Chronic pain can be broadly divided into three categories: nociceptive pain (which inflammatory pain is part of), neuropathic pain and nociplastic pain (Ref. Reference Cohen, Vase and Hooten46). Inflammatory pain is caused by tissue injuries, which trigger inflammatory reactions (Ref. Reference Chang, Jiang and Chen47). Inflammation contributes to tissue pressure and dysfunction, and toxic substances around nociceptive receptors are responsible for neuropathic pain (Ref. Reference Lin48). The International Association for the Study of Pain (IASP) defines neuropathic pain as pain caused by a lesion or disease of the somatosensory system. People with neuropathic pain may have peripheral and/or central sensitisation (Ref. Reference Campbell and Meyer49). Clinical research of patients with endometriosis found that they suffer from complex pain, in which the neuropathic component also plays a role (Ref. Reference Coxon, Wiech and Vincent50). When a noxious stimulus acts on peripheral nociceptive neurons, they are activated to elicit action potentials. This nociceptive information follows sensory pathways into the CNS contributing to nociception (Refs Reference Levine, Fields and Basbaum51, Reference Tracey and Dickenson52). Peripheral inflammatory insult stimulates peripheral nociceptors. Over time, the reducing ligand-gated ion channel thresholds, altered adhesion receptor expression and permeability indicate that these nociceptors are more sensitive to slight environment changes and more likely to respond to painful stimuli (Refs Reference Maddern10, Reference Sadeghi53).
Among patients with endometriosis, researchers find inflammatory cellular infiltrates such as IL-10, COX-2, VEGF and pain mediator prostaglandin E2 (PGE2) (Ref. Reference Nanda54). Additionally, the level of pro-inflammatory factors such as TGF-β1, IL-15 and IL-7 are correlated with dysmenorrhoea severity in endometriotic implants (Ref. Reference Gueuvoghlanian-Silva55). Through the TGF-β–Smad signalling pathway, high levels of TGF-β1 in the peritoneal fluid contribute to peritoneal endometriosis (Ref. Reference Young39). It is not clear whether the association is the result of endometriosis alone, further research is needed (Ref. Reference Soni56). In other chronic inflammatory diseases, TGF-β1 injected into animal models inhibited CCL3/4 expression through the extracellular signal-regulated kinase (ERK) signalling pathway, reducing the inflammatory response and pain (Ref. Reference Zhang57). A pro-inflammatory cytokine, IL-1β, is overexpressed in endometriosis, which affects the neurotrophic factor, brain-derived neurotrophic factor (BDNF), through nuclear factor-κB (NF-κB) and Jun amino-terminal kinases (JNK) signalling pathways, exacerbating endometriosis-associated pain (Ref. Reference Yu58). Additionally, endometriosis-induced vaginal hyperalgesia was positively correlated with nerve-innervating cysts and PGEs in peritoneal fluid, because peripheral nociceptors' tansient receptor potential vanilloid 1 (TRPV1) thresholds may be affected by PGE2 (Ref. Reference Ma, Li and Xing59). Although the expression of COX-2 (the rate-limiting enzyme synthesised by PGE2) was increased in peritoneal fluid in patients with endometriosis and it fluctuated with the menstrual cycle (Ref. Reference Cho60). COX is implicated in neuroinflammation today, and excessive COX-2 expression can also contribute to neurodegeneration (Ref. Reference Mulet61). However, non-steroidal anti-inflammatory drugs, are weakly recommended in the current pain management guidelines (Ref. Reference Becker8).
It is well established that oxidative stress is implicated in the pathogenesis of endometriosis. When reactive oxygen species and antioxidant levels are imbalanced, they can produce inflammatory mediators and growth factors (Ref. Reference Scutiero62). Additionally, peritoneal protein oxidative stress markers were significantly associated with pelvic pain symptom scores in endometriosis (Ref. Reference Santulli63). Except for endometriosis lesions that secrete pain mediators, chemokines and cytokines, immune cells recruited by these substances also produce those abovementioned substances (Ref. Reference Bulun64).
A dysfunction of congenital or adaptive immune cells is a relevant mechanism for explaining endometriosis as well as its pain mechanisms (Ref. Reference Ma65). The variations in the proportion of dendritic cells in endometriosis were not only linked to the abnormal immune response and inflammatory environment, but also to the pain sensitisation and generation of pain symptoms (Ref. Reference Hey-Cunningham66). The stimulation of macrophages may result in the release of inflammatory mediators that attract circulating immune cells to inflammation sites, activate local immuneaccessory cells and affect peripheral nerve function (Ref. Reference Wu67). The innervated peripheral nerve transduces noxious stimuli created by these inflammatory mediators into action potentials that propagate from the nerve to the spinal cord (Refs Reference Malet and Brumovsky68, Reference Noh and Ismail69). Sensory neurons recruited macrophages in a hormone-dependent way that subsequently supported the growth of the lesion and promoted axonal sprouting of sensory neurons further invading the surrounding tissue (Ref. Reference Liang11). In addition, macrophage-derived insulin-like growth factor 1 (IGF-1) expression was increased in the mouse endometriosis model, and it may activate IGF-1R-mediated intracellular signalling pathways to promote pain hypersensitivity (Ref. Reference Forster70). The bidirectional interactions between mast cells and the nervous system have also attracted attention. When mast cells degranulate, histamine can trigger the release of neuropeptides (Ref. Reference Aich, Afrin and Gupta71), and peritoneal fluid from patients with endometriosis has higher levels of tryptase (a marker of degranulation) (Ref. Reference Borelli72). Reduced mast cell degranulation in endometriosis models has also been proven to reduce neurogenic inflammation and its resulting neurosensitisation (Ref. Reference Genovese73). C1q, mannose-binding lectin (MBL) and C1-INH concentrations are higher in the vicinity of endometrial lesions compared with the control group (Ref. Reference Sikora74). When C3 is blocked, it prevents the cascade of inflammatory signals in endometriosis (Ref. Reference Agostinis75) and also affects mast cell degranulation in mice with EM (Ref. Reference Agostinis76). Despite this, the method of modulating the immune system as an alternative therapy, such as using pentoxifylline, to treat endometriosis-associated pain remains ambiguous because there is insufficient evidence (Ref. Reference Grammatis, Georgiou and Becker77).
Hormonal influences on endometriosis-associated pain
Endometriosis is an oestrogen-dependent gynaecological disorder (Ref. Reference Gołąbek, Kowalska and Olejnik78). Studies have shown that oestrogen regulates visceral pain by increasing neuronal activity or modulating neuronal plasticity (Ref. Reference Sun79). The overexpression of steroidogenic factor-1, which participates in oestrogen biosynthesis, ultimately results in an overproduction of oestrogen (Ref. Reference Hu, Mamillapalli and Taylor80). The increase in local oestrogen biosynthesis could also be because of the up-regulation of aromatase expression induced by PGE2 (Ref. Reference Gonçalves81). Oestrogen can not only prolong induced hyperalgesia by regulating the autocrine mechanism activation at the plasma membrane but it is also related to neurogenesis and neurodegeneration by affecting proliferation or differentiation in neural stem/progenitor cells (Ref. Reference Ferrari, Araldi and Levine82). Researchers found in patients with endometriosis who received combined oral contraceptives that the amount of NGF and its receptors significantly decreased in the endometrium (Ref. Reference Latini83). The oestrogen and NGF signalling pathways interact, and the oestrogen receptor (ER) – a receptor can promote NGF-induced neurogenesis and differentiation (Ref. Reference Dzieran84). In peritoneal endometriotic lesions, slit guidance ligand 3, related to guiding axon growth, was regulated by ER agonists (Ref. Reference Greaves85). In addition, neuroimmune interactions modulated by oestrogen hormone signalling may contribute to the sensitisation of peripheral nerves (Ref. Reference Liang11). It is still controversial how oestrogen may regulate pain since activation of ER-β reduces nociceptive sensation in rat visceral pain models (Ref. Reference Cao86). A possible explanation is that oestrogen alters ERs in the spine, changing nociceptive sensation (Ref. Reference Zhang87). Experimental studies with endometriosis animals showed that inhibiting overactive protein kinase B (AKT) and ERK1/2 pathways reduced the pro-inflammatory microenvironment and aromatase P450 expression as well as E2 biosynthesis. It is a novel idea to suppress ER expression to prevent signal transduction in the future (Ref. Reference Arosh, Lee and Banu88).
Progesterone is one of the drugs used to treat endometriosis-related pain (Ref. Reference Becker8). A possible mechanism for its pain relief is Sig-1R, which is a mediator of pain and can bind progesterone. As a consequence of progesterone use, nociceptor excitability is decreased by reducing TRPV1 expression on their membrane (Ref. Reference Ortíz-Rentería89). Chronic pain in endometriosis is also associated with dysregulated hypothalamic–pituitary–adrenal (HPA) axis function (Ref. Reference van Aken90). Studies of chronic pain associated with endometriosis have linked cortisol delta and adrenocorticotropic hormone delta to the severity of menstrual pain in white women (Ref. Reference Ortiz91). It is important to note, however, that if cortisol levels are measured, the results may be reversed, depending on a variety of factors, including the location of the test and how stressed the crowd is (Ref. Reference van Aken90).
Other pain mechanisms
Endometriosis commonly causes anatomical distortions because of adhesions and fibrosis which can bring subfertility and persistent discomfort, as evidenced by considerable research (Ref. Reference van den Beukel92). Inside the fibrotic tissue of the lesion, new nerves were entrapped (Ref. Reference Anaf93), whereas there are also some rare conditions of endometriosis. Deep infiltrating endometriotic nodule entrapped the obturator nerve (Ref. Reference Kalkan and Daniilidis94). In a similar manner, sciatic endometriosis can also lead to sciatic nerve pain (Ref. Reference Kale95) and may be involved in central pain sensitisation via the fractalkine/CX3CR1/P38-MAPK (mitogen-activated protein kinases) signalling pathway (Ref. Reference Liu96). Compression of the sensory nerves that innervate the abdominal wall can also cause chronic pain, which may be one of the explanations for pain in abdominal wall endometriosis (Ref. Reference Kamboj, Hoversten and Oxentenko97). It should be noted that these are quite rare occurrences so is not broadly applicable to endometriosis-associated pain.
In addition to endometriosis, postoperative adhesion has also been shown to be a contributing factor to CPP in several studies (Ref. Reference van den Beukel92). But an absolute conclusion that adhesions cause pain cannot be drawn. Further research is needed to demonstrate the complex nature of adhesions in CPP (Ref. Reference Farag98). As most visceral organs seem particularly susceptible to mechanical distension, abnormal attachments may result in the increased stretch on internal organs causing persistent pelvic pain (Ref. Reference Grundy, Erickson and Brierley99). During the gradual enlargement of the cysts in patients with ovarian endometriosis, mechanical stimuli were detected by the nociceptors and mechanically sensitive cation channels opened, causing rapid depolarisation. The excited nociceptors carried information and sent noxious stimuli from the periphery to the spinal cord, resulting in pelvic deep discomfort and pain (Ref. Reference Basbaum100). In addition, the faster fibrosis around the ovary reduced the normal tissue space, resulting in iron accumulation and oxidative stress, promoting the development of endometriosis and forming a vicious circle (Ref. Reference Hayashi101).
Pain transmission in the spinal cord
The pain signal is then transmitted to the spinal cord dorsal horn and eventually to the CNS, where it is processed (Ref. Reference Maatuf, Geron and Priel102). Different groups of projection neurons and interneurons are involved in this process to maintain excitatory and inhibitory functions, respectively (Ref. Reference Finnerup, Kuner and Jensen103). Neurotransmitter glutamate is upregulated at the spinal level, resulting in enlargement of the pain field (Ref. Reference Hoffman104), while dysuria and/or defecation may also occur, thus explaining how coexistent conditions may occur with endometriosis (Ref. Reference Shafrir2). There was no surprise that patients with endometriosis experiencing CPP had myofascial dysfunction and sensitisation. When they adopt the relief posture, patients experience pelvic floor spasms caused by reactive contractions of the pelvic floor muscles (Ref. Reference Phan105). Because of the complexity of the mechanism of visceral pain, the actual cause of discomfort between two adjacent organs, such as the bladder and uterus, is frequently misinterpreted and misdiagnosed (Ref. Reference Lamvu5). Moreover, the interaction between autonomic innervation and visceral sensory neurons crossing the ganglion also contributes to neuroplasticity (Ref. Reference Gruber and Mechsner106).
Cell bodies of nociceptors, which innervate the viscera, reside in the dorsal root ganglion of the spinal cord (Ref. Reference Lopes, Denk and McMahon107). It is thought that inflammation-related cytokines, chemokines and other factors modulate ion channel activity in postsynaptic neurons by binding receptors or activating second messengers (Ref. Reference Hucho and Levine108). Heat-sensitive TRPV1 receptors found on capsaicin-sensitive peptide sensory neurons exhibit allosteric regulation under the influence of inflammation or neurotrophic factors (Ref. Reference Abbas109). The amount of upregulation of TRPV1 and TRPA1 receptors was closely correlated with dysmenorrhoea and dyschezia severity in DIE nodules in the rectum and sigmoid colon (Ref. Reference Bohonyi110). Oxidative stress is one of the pathophysiological factors causing endometriosis. Free oxygen radicals that penetrate through TPRV1 in DRGs can cause neuropathy and injury (Ref. Reference Fattori111). Because of the decreased activity of the mitochondrial enzyme ALDH2, reactive aldehydes in women with endometriosis do not degrade in time, resulting in the buildup of reactive aldehydes. The presence of reactive aldehydes activates the TPRA1 channel and modified pain signals (Ref. Reference McAllister112). Alterations in the expression of ectonucleotidases that may metabolise released adenosine triphosphate (ATP) in endometriosis contribute to the accumulation of ATP in the microenvironment (Ref. Reference Trapero113). It has been suggested that persistent stimulation of peripheral fibres may result in the release of inflammatory neurotransmitters and neuromodulators, including ATP, which influences glial cells (Ref. Reference Orr and Gensel114). Studies have shown that ATP activates the capsaicin-sensitive TRPV1 channel via P2Y receptors in DRG neurons (Ref. Reference Kwon115). In endometriosis, the expression of ligand-gated ion channel purinergic receptor P2X3 is increased, resulting in repeated neuronal sensitisation and persistent pain (Ref. Reference Trapero and Martín-Satué116). According to one explanation, activation of the transcription factor 3 (ATF3)/activator protein-1 pathway increases the expression of P2X3 in the dorsal root ganglion (DRG), resulting in endometrioid-related hyperalgesia (Ref. Reference Ding117). By selectively reducing or inhibiting P2X3 in the dorsal root ganglion, chronic pain was alleviated in rats, making it an effective method for modulating nociceptive signals and chronic pain (Ref. Reference Xiang118). The SCN11A(Nav1.9) was significantly higher in the peritoneum of women with CPP and endometriosis than in those with CPP alone (Refs Reference Greaves119, Reference Greaves120). In addition, acid-sensing ion channel activity was enhanced by PGE2 in DRG neurons, which resulted in acidosis-evoked pain, revealing a peripheral mechanism for PGE2 involvement in hyperalgesia (Ref. Reference Zhou121). Rodent models of endometriosis revealed abnormal activation of the NF-κB signalling in dorsal root neurons, resulting in the altered expression of ion channels such as TPRV1, TPRA1 and CGRP, causing chronic and spontaneous pain (Ref. Reference Fattori111). Despite studies exploring ion channels and endometriosis lesions, more research is needed to explain the relationship between its pain and ion channels or receptors in neurons (Ref. Reference Riemma122) (Figure 2).

Fig. 2. Afferent fibre excitability is influenced by changes in the density, distribution and expression of a variety of ion channels. For example, inflammatory mediators cause primary sensory neurons to have a lowered threshold resulting in depolarisation and increased responsiveness to stimulus.
Altered brain in endometriosis
There are connections between pain sensitisation and endometriosis in the CNS (Ref. Reference Zondervan, Becker and Missmer4). Neuroimaging and neurophysiological studies have found patients with chronic pain showed similar structural and functional changes in brain regions linked with pain cognition and emotional stability (Ref. Reference Maulitz123). Even worse, detrimental structural alterations in the brain structure can lead to pain maintenance commonly known as hyperalgesia (Ref. Reference Yang and Chang124). Secondary dysmenorrhoea is most often because of endometriosis. However between menstrual phases, several regions including caudate nucleus, hypothalamus and thalamus showed significant changes in grey matter (GM) volume in dysmenorrhoea subjects (Ref. Reference Tu125). Further, in women suffering from dysmenorrhoea, brain metabolism was different and the entorhinal cortex appears to be involved in the patient's increased response to painful stimuli (Ref. Reference Vincent126). However, it is not clear whether those patients with dysmenorrhoea do have endometriosis.
Women with CPP had reduced GM volume in the thalamus, whereas women experiencing endometriosis-associated CPP showed decreases in more brain regions, such as the thalamus, cingulate gyrus, putamen and insula (Ref. Reference As-Sanie127). Endometriosis may result in altered endogenous pain modulation since the insula is one of the key regions of the descending pain inhibitory system (Ref. Reference Matsuo128). The loss of neurons could be a logical explanation for a reduction in GM volume, but there is no evidence to support it (Ref. Reference Kang129). Functional magnetic resonance imaging (fMRI) showed that when patients with pain-activated brain areas, cortical connections were enhanced (Ref. Reference Ferdek130). Possibly because of the continued activation of the somatosensory pain system in patients with endometriosis, even during non-pain periods, the connectivity among the somatosensory cortex, dorsolateral prefrontal cortex, temporal cortex and orbitofrontal cortex was increased (Ref. Reference Ferdek130). Comparing endometriosis-associated CPP patients with pain-free controls, greater interconnectivity between the insula and the medial prefrontal cortex (mPFC) was identified (Ref. Reference As-Sanie131). The mPFC may display diverse roles in pain. In addition to its role in modulating pain, mPFC also could lead to chronification of pain through its corticosteroid projection (Ref. Reference Ong, Stohler and Herr132). Functioning connectivity between the mPFC and periaqueductal grey (PAG) was negatively related to glutamate (known as a major excitatory neurotransmitter in the brain) concentration (Ref. Reference Duncan133). Compared with women with pain-free endometriosis, the CPP group showed an elevated concentration of combined glutamine–glutamate in the anterior insular (Ref. Reference As-Sanie131). The descending inhibition pathway plays a key role in regulating central pain transmission via PAG (Ref. Reference Huang134). In the PAG, alterations in the expression of some receptors can affect the cross-talk between morphine receptor and N-methyl-d-aspartic acid receptor (NMDAR) thus affecting the analgesic signalling as well as individual pain response (Refs Reference Cortés-Montero135, Reference Torres-Reverón136). Some women with endometriosis suffer little or no pain despite the severity of disease, and this can be attributed to the larger volume of the PAG in their brain (Ref. Reference Brawn137).
When the abdomen of endometriosis macaques was activated below the standard pain threshold, abnormal activity of the thalamus and insular cortex was detected, indicating that these regions were responsible for the alteration of pain (Ref. Reference Yano138). After being administered with dienogest and morphine respectively, activation of these regions, observed by fMRI, was decreased (Ref. Reference Yano138). In general, exogenous opioids such as fentanyl are routinely used as strong analgesics, but because of the high risks of abrupt withdrawal and hyperalgesia, they are not recommended for chronic pain (Ref. Reference Colvin, Bull and Hales139). Prophylactic use of anti-NGF antibody mAb911 can, however, prevent the development of structural changes in the brain before they occur (Ref. Reference Buehlmann140). An endometriosis preclinical study has shown that COX-2 expression is elevated in spinal, thalamic and cortical areas, which are involved in processing pain in the CNS (Ref. Reference Greaves120). In turn, this results in excess PGE2 in the cerebrospinal fluid, where PGE2 has previously been found to function as a central pain sensitiser (Ref. Reference Umbrain141). Rats with EM pain sensitisation had fewer neurons in the thalamus and left olfactory tubercle, suggesting the thalamus might be involved in central sensitisation (Ref. Reference Zheng142). Some patients with endometriosis also have chronic pelvic pain syndrome (CPPS). Although CPPS patients also experienced changes in brain structures such as the thalamus, anterior cingulate cortex (ACC) and PFC (Ref. Reference Kuhn143).
The hippocampus has long been associated with cognitive functions but it is also involved in pain processing (Ref. Reference Wimmer and Büchel144). As shown by microarray analyses and confirmed by quantitative polymerase chain reaction, altered gene expression such as up-regulated Gpr88 and down-regulation of Nptx2 was found in several brain regions in endometriosis mice (Ref. Reference Li145). Connections between the hippocampus, thalamus, insula, striatum and cerebellum are increased in patients with endometriosis, and reductions in such connections when analgesic psychotherapy is used (Ref. Reference Beissner146). In the anterior segment of the hippocampus, the rAL region affects the HPA axis, which is associated with anxiety and pain (Ref. Reference Beissner146). However, studies have shown that the hypothalamic–pituitary–gonadal axis and HPA axis interact in women who suffer from dysmenorrhoea, so it is hard to pinpoint the exact mechanism (Ref. Reference Padda147). TRPV1 and NMDAR, modulating neuroplasticity and synaptic excitability (Ref. Reference Hansen148) were strongly expressed in the ACC, thalamus and hippocampus of rats with endometriosis-associated pain (Ref. Reference Zheng149). The formation of an immune oxidative environment within the hippocampus of endometriosis rats is one of the mechanisms leading to pain sensitisation, whereas the elevated oxidative state in the hippocampus was correlated with a lower level of BDNF (Refs Reference Xu150, Reference Cordaro151). Astrocytes and microglia are thought to be responsible for many inflammatory processes in affected brains, including secretion of inflammatory mediators and stimulation of the innate immune system (Ref. Reference Rostami152). In addition, mast cell degranulation was increased in the hippocampal tissues of endometriosis rats (Ref. Reference Cordaro151), whereas microglia and astrocytes are activated by mast cells, aggravating neuroinflammation (Ref. Reference Flores-Bonilla153) (Figure 3).
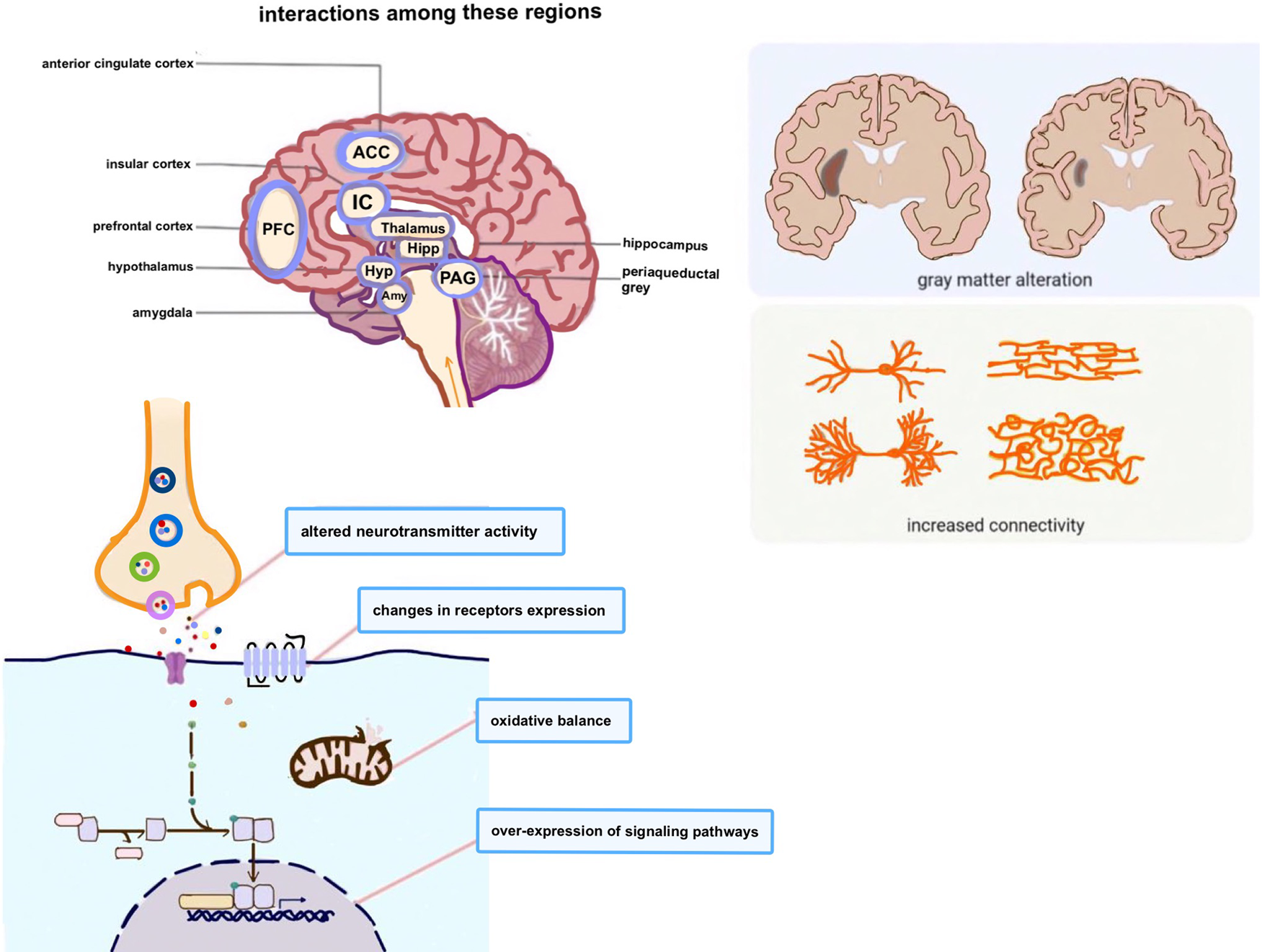
Fig. 3. Endometriosis-associated pain is linked to changes in brain structure and function, dysregulation of pain pathways and increased activity in brain regions. During the processing of pain information, multiple brain regions may interact with each other through several sophisticated mechanisms. Although complex and delicate interactions between distinct types of neurons in the specific regions have attracted high interest, the mechanisms underlying their mutual interactions are still not fully understood.
Conclusion
Pain in endometriosis is vital and complex, but few studies thoroughly outline the relationship between pain and the nervous system as well as brain metabolism. We summarise the most recent achievements in endometriosis-related pain in terms of neurogenesis, changes in pain transmission and peripheral and central sensitisation with corresponding changes in the cerebrum. As pain sensitisation in endometriosis is often overlooked in clinical practice, we hope that this review can inspire recognition of endometriosis-related pain and give more individualised treatment. In the future, with the wide utility of the multiparameter single-cell technique and the breakthroughs in neuroimaging, progress will be made in digging deeper into pain mechanisms in endometriosis, identifying new potential targets during the production and transmission of pain, developing novel pharmaceuticals precisely targeting the molecular mechanisms and enabling women to reduce the occurrence of chronic pain.
Search strategy and selection criteria
From October 2021 to June 2022, we searched databases on Medline, PubMed and Google using the key words ‘chronic pain’, ‘endometriosis’, ‘endometriosis and brain’, ‘pathogenesis’, ‘ion channels’, ‘angiogenesis’, ‘pain’, ‘nerve fibre’, ‘sensitisation’, ‘oestrogen’. There were no restrictions on article types, date of publication or language. For this review, we prioritised the most recent and definitive original articles, large randomised trials, meta-analyses and international guidelines.
Financial support
This work was supported by grants from the National Natural Science Foundation of China (No. 82071627).
Conflict of interest
The authors declare that they have no competing interests.






