Introduction
The term ‘cancer’ refers to a collection of more than 100 distinct illnesses, each defined by the uncontrolled and abnormal proliferation of cells. One of the most common diseases in the world is cancer. Tens of millions of individuals are diagnosed with cancer every year, and more than half of those people die from the disease (Ref. Reference Bray1). Cancer is the second most prevalent cause of mortality in many nations, following cardiovascular disorders. Cancer has already overtaken cardiovascular disease as the leading cause of death in many parts of the world, and this trend is expected to continue. As population ageing continues in many countries and older individuals are most vulnerable to cancer, cancer will continue to be a significant global health issue. When an individual was diagnosed with cancer only a few decades ago, their chances of survival were limited since there was little information available about the condition. Today, however, we are far more knowledgeable about it, including how to identify it sooner, treat it more successfully, and, most significantly, contribute to its prevention (Refs Reference Bray1, Reference Smith and Oeffinger2).
A large percentage of cancer fatalities are attributable, in part, to ineffective treatment techniques as a result of our poor understanding of cancer processes. Cancer models and treatments, on the other hand, are evolving and becoming more effective. Cancer cells in tumours have been perceived differently during the previous 20 years (Ref. Reference Hanahan and Weinberg3). In the beginning, cancer studies focused on cancer cells. However, more attention is being devoted to the tumour niche and tumour microenvironment, with a specific emphasis on non-cancer cells and intercellular communication. It is now understood that tumour development and cancer progression need communication between cancer and non-cancer cells (i.e. tumour stromal cells), which involves chemokines and cytokines, among other things (Refs Reference Mollica Poeta4, Reference Bhat5, Reference Nagarsheth, Wicha and Zou6).
Chemokines, which are considered chemotactic cytokines, are composed of more than 50 members. Based on the location of two N-terminal cysteines, these mediators are grouped into four subfamilies. CC chemokines (also called β-chemokines) are one of the subfamilies that consist of 27 chemokines. There have been 10 receptors (CC chemokine receptors (CCRs)) for these ligands until now (Ref. Reference Hughes and Nibbs7). As demonstrated by the correlation of their expression with the prognosis of patients with a certain type of tumour, chemokines and their receptors serve as essential components of the tumour microenvironment and cell interactions in the tumour niche. A chemokine can slow tumour growth by attracting tumour-infiltrating lymphocytes, which kill cancer cells, while also attracting tumour-promoting cells, such as tumour-associated macrophages and myeloid-derived suppressor cells (MDSCs), which collaborate with cancer cells in the tumour growth process (Refs Reference Shabgah8, Reference Navashenaq9, Reference Zamani10). Therefore, chemokines and their corresponding receptors might exert conflicting roles in cancer.
Many malignancies have a complicated chemokine network that affects immune cell infiltration, tumour cell proliferation, survival and migration, as well as angiogenesis. Chemokine receptors are expressed on immune cells, endothelial cells and tumour cells, and they may react to chemokine gradients. Chemokines are important determinants of the macrophage and lymphocyte infiltration in human malignancies, and they may also play a role in T-helper 2 cell polarisation (Ref. Reference Mollica Poeta4). Chemokine-receptor expression patterns in malignant cells vary depending on the cancer type. Cancer cell chemokine-receptor expression is linked to enhanced metastatic capability, according to studies using human cancer biopsy samples and mouse cancer models. Chemokine-receptor antagonists decrease macrophage infiltrates, cause tumour growth to stop or death and limit metastatic dissemination, according to preliminary laboratory evidence (Ref. Reference Bhat5). The cancer-chemokine network exposes similarities between the biology of inflammation and malignancy, similarities that help us better understand both diseases and point to novel therapy options (Ref. Reference Nagarsheth, Wicha and Zou6).
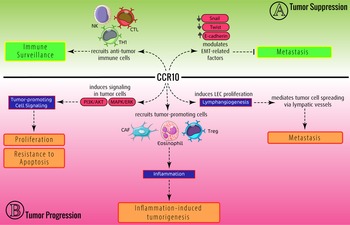
It has been shown that these chemokine ligands and receptors are dysregulated in various cancers. Therefore, it is concluded that this dysregulation might be involved in the development or suppression of tumours. Among CCRs, CCR10 is the latest identified CC chemokine receptor (Ref. Reference Marchese11). The proposed ligands for this receptor are CCL27 and CCL28 (Refs Reference Jarmin12, Reference Morales13, Reference Pan14, Reference Wang15). As discovered, the CCR10/CCL27–CCL28 axis plays several functions under pathophysiological conditions. The main role of the CCR10/CCL27–CCL28 axis is mediating the migration of several types of immune and non-immune cells. CCR10, via interaction with its ligands, develops innate lymphoid cells (ILCs) and Tγδ cells, leading to regulating unwanted inflammation and mediating homoeostasis. Moreover, this axis promotes inflammation via recruiting inflammatory immune cells under several conditions, resulting in inflammatory diseases (Fig. 1). Moreover, CCR10 and its ligands can be involved in cancers. Thus, in this study, we aimed to discuss the multifarious role of CCR10 and its ligands, CCL27/CCL28, in cancer development (Fig. 2).

Fig. 1. Role of CCR10 and its ligands (CCL27/CCL28) in non-malignant situations. (A) In homoeostatic roles, CCR10 recruits Tγδ cells and ILCs, leading to decreasing inflammation. Moreover, CCR10 is also involved in mucosal immunity and locomotor recovery after injury. (B) In pathological roles, CCR10 promotes inflammation by recruiting several inflammatory cells, including Th22 and mesenchymal progenitor cells (MPCs), leading to several autoimmune and inflammatory diseases such as rheumatoid arthritis (RA).

Fig. 2. Conflicting role of the CCR10/CCL27–CCL28 axis in the tumour. (A) In tumour-suppressive function, CCR10/CCL27–CCL28 inhibits metastasis through downregulating EMT-related genes. Moreover, CCR10/CCL27–CCL28 recruits anti-tumour immune cells to mediate immune surveillance, leading to tumour regression. (B) In tumour-promoting roles, CCR10/CCL27–CCL28 induces inflammation (via recruiting CAFs), tumour cell proliferation and lymphangiogenesis, resulting in tumour development.
CCR10, CCL27 and CCL28: characteristics, expression and primary functions
As has been mentioned, CCR10 is the latest member of the CCR family. The coding gene for CCR10 was first reported under the name G-protein coupled receptor 2 (GPR2) in 1994 (Ref. Reference Marchese11). It was not considered a chemokine receptor until 2000, when CCL27 (also called CTACK, ALP, ILC or ESkine) was established as a ligand for this receptor (Refs Reference Jarmin12, Reference Morales13). Soon after, CCL28 (also known as SCYA28, CCK1 or MEC), a chemokine predominantly expressed in mucosal areas, was recognised as another CCR10 ligand. CCL28 is more similar to CCL27 than any other chemokine (Refs Reference Pan14, Reference Wang15).
According to Bonini et al.'s study (1997), the human CCR10 gene has 72% homology with rat CCR10. Similar to other chemokine receptors and several G protein-coupled receptors, CCR10 has seven hydrophobic regions indicative of a seven-transmembrane helix-containing structure (Fig. 3). Moreover, CCR10 contains a potential N-linked glycosylation site, protein-kinase C phosphorylation site at the carboxyl terminus, and two casein kinase-2 phosphorylation sites. Despite other CCRs that have the DRYLAIVHA motif, the human CCR10 contains the DRYVAIARA motif directly following the third transmembrane domain (Ref. Reference Bonini16). Site-directed mutagenesis and crystallography studies propose that the extracellular domains of chemokine receptors are important interaction sites for their chemokine and peptide ligands. The main idea about chemokine and chemokine receptor interaction is that the extracellular loops (ECLs) of the receptor bind to the core domain of chemokines and help to position the ligand within the helical domains of the receptor (Ref. Reference Allen, Crown and Handel17). But specific research regarding the way of CCR10's interaction site with its ligands is required in future studies.

Fig. 3. Protein sequence and structure of CCR10. This receptor contains several extracellular (black sequences), transmembrane alpha helices (purple sequences) and intracellular (green sequences) domains. There is a disulphide bond between Cys113 and Cys191 (underlined cysteines). Although there is no specific research regarding the way of CCR10 interaction with the CCL27/CCL28 ligand, site-directed mutagenesis and crystallography studies propose that the extracellular domains of chemokine receptors are important interaction sites for their chemokine and peptide ligands. The main idea about chemokine and chemokine receptor interaction is that the ECLs of the receptor bind to the core domain of chemokines and help to position the ligand within the helical bundle of the receptor.
CCL28 (also called mucosa-associated epithelial chemokine (MEC)) is a chemokine that is required for proper mucosal immune activity (Ref. Reference Lazarus18). CCL28 gene in humans is encoded on 5p12, and according to Mickanin et al.'s investigation, CCL28's cDNA sequence predicts a 128-amino-acid protein with a 19-amino-acid signal sequence, yielding a mature protein of 109-amino-acids, making it one of the largest CC chemokines discovered to date. The presence of basic amino acids at the carboxy terminus of the mature protein is a noteworthy characteristic. The interaction of the released protein with heparin and heparin sulphate proteoglycans is most likely mediated by such clusters of basic residues. CCL28, similar to other chemokines, has various non-overlapping clusters of basic amino acids that agree with consensus heparin-binding motifs and 33 basic residues in the mature protein of 109 amino acids, suggesting that it is connected with the extracellular matrix. As a result, CCL28's predicted isoelectric point of 10.74 is abnormally high (Ref. Reference Mickanin, Bhatia and Labow19). Because of sharing 83 identical amino acids, mouse and human CCL28 are highly conserved. Moreover, at the nucleic acid level, mouse CCL28 is 76% identical to its human counterpart in the open reading frame (Ref. Reference Wang15).
CCL27 (also known as ESkine) is another chemokine that stimulates CCR10, and its effects are similar to those of CCL28 (Ref. Reference Homey20). CCL27 mature structure contains 88 residues with a 10 kDa molecular weight that activates the CCR10. CCL27 has a conventional chemokine monomeric structure; however its oligomeric structure is unclear. CCL27 and CCL28 are around 42% identical and form two overlapping disulphide bonds, according to their alignment. A third disulphide bond is formed by a pair of cysteine residues in CCL28. The hydrophobic residues Ile, Leu and Pro make up the majority of the N-terminal portions of CCL27 and CCL28. The Phe at position 1 (Phe1), which is replaced by an Ile in CCL28, and a second Pro at position 5 of CCL27, which lacks in CCL28, are two differences among others (Ref. Reference Jansma21). CCL27 exerts an intracellular function as well. In addition to the CCL27 protein, the CCL27 gene produces a nuclear protein called PESKY through alternative splicing, which is expressed in the testes, eyes and brain. This protein changes the expression of actin–cytoskeleton-associated genes, leading to migration and alteration in cell morphology (Ref. Reference Nibbs and Graham22). Other than PESKY, CCL27 consists of a nuclear location sequence which, upon interaction with CCR10, internalises into the cell nucleus and implements functions similar to PESKY (Ref. Reference Gortz23).
CCL27 promotes the migration of CD3+ and CD4+ lymphocytes. CCL28 and CCL27 cause B cells, especially immunoglobulin A (IgA+) plasma cells, and T cells to migrate via CCR10 (Ref. Reference Homey20). CCL27 is primarily produced by keratinocytes and has a skin-specific function, whereas CCL28 is secreted by mucosal tissues (Ref. Reference Lazarus18). As a result, these chemokines are critical in directing immune cells towards epithelial and mucosal tissues, where they engage in immune responses against pathogens (Refs Reference Lazarus18, Reference Homey20, Reference John24). CCL28 interaction with CCR3, another CCR28 receptor, promotes the development of allergies through the recruitment of eosinophils (Ref. Reference John24).
In terms of CCL27's interaction with CCR10, data reveal that CCL27 needs a phenylalanine at its N-terminal location in order to function properly, and that the C-terminal portion of CCL27, but not CCL28, seems to play a significant role in receptor activation. The sequences of CCL27 and CCL28, as well as the findings of numerous additional point mutations, demonstrate that receptor activation is not always dictated by the ligand's N-terminus sequence, but rather requires a far more sophisticated process. G protein-coupled receptors, which activate intracellular signalling pathways, and glycosaminoglycans, which are involved in cell surface localisation and transport, are two important interactions that chemokines have in vivo (Ref. Reference Jansma21). Despite the fact that chemokines have been proven to bind and activate their G protein-coupled receptors as monomers, many chemokines oligomerise when glycosaminoglycans are bound, and the capacity to oligomerise and bind glycosaminoglycans is essential for in vivo action. CCL27 has unusual oligomerisation behaviour, in which many equilibria involving low-affinity contacts between various surfaces seem to be active at the same time. In situations when CCL27 is monomeric by itself, however, contact with heparin increases oligomerisation. It was hypothesised that the oligomerisation state's plasticity allows CCL27 to adopt different oligomeric structures depending on the nature of the glycosaminoglycan-binding partner, providing a mechanism for increased glycosaminoglycan-binding and glycosaminoglycan-related functions diversity and specificity (Ref. Reference Jansma21).
Expression of CCR10/CCL27–CCL28 in cancer
Analysis of CCR10/CCL27–CCL28 expression in various cancers showed different patterns. In some cancers, their expression was increased, whereas some others showed a downregulated expression of this axis (Table 1). For example, CCL28 expression is downregulated in colon and ovarian cancers (Refs Reference Dimberg, Hugander and Wågsäter25, Reference Maghazachi, Sand and Al-Jaderi26). On the other hand, CCL27 expression is also decreased in melanoma, basal cell carcinoma (BCC) and colon carcinoma (Refs Reference Dimberg, Hugander and Wågsäter25, Reference Pivarcsi27). This indicates that these chemokines may have anti-cancer properties, at least during the early phases of tumour growth, which was confirmed by investigations of people with various kinds of breast cancer (Refs Reference Lin28, Reference Yang29). Therefore, treatments that enhance the production of these two chemokines have been shown to have anti-cancer properties (Refs Reference Gao30, Reference Degos31, Reference Gao32). In contrast, numerous studies have shown that expression of CCL27 and CCL28 was increased in lymphomas, bladder cancer, hepatocellular carcinoma (HCC) and multiple myeloma (Refs Reference Harasawa33, Reference Fujita34, Reference Notohamiprodjo35, Reference Hanamoto36, Reference Goteri37, Reference Hoeller38, Reference Masui39, Reference Zhong40, Reference Wu41, Reference Ren42, Reference Thangavadivel43). Although the underlying mechanisms regarding dysregulation of CCL27/CCL28 have not yet been defined in all experiments, some studies have indicated possible mechanisms which are discussed in the following.
Table 1. Role of CCR10 and its ligands in cancer development

a In this regard, CCL28 interacts with CCR3, but not with CCR10.
b Relevant receptor was not examined.
Cell signalling is one of the inducers or suppressors of gene expression. Epidermal growth factor receptor (EGFR), an activator of Ras signalling, was often found overexpressed or constitutively activated in squamous cell carcinoma (SCC) and BCC. Human EGF induced extracellular signal-regulated kinase (ERK) phosphorylation and reduced CCL27 expression in primary keratinocytes. Blockade of EGFR signalling or using a particular mitogen-activated protein kinase (MAPK) inhibitor (UO126) inhibited ERK phosphorylation and prevented the suppression of CCL27 expression in keratinocytes, indicating that ERK regulates CCL27 expression (Ref. Reference Pivarcsi27). Another possible mechanism relies on the expression of transcription factors such as hypoxia-inducible factor (HIF)-1α. Under hypoxic conditions, upregulation of HIF-1α promoted the expression of CCL28 in some cancer cells, including lung cancer and HCC (Refs Reference Liu and Wei52, Reference Huang53). In addition to HIF-1α, β-catenin signalling also induces transcription of CCL28 in gastric cancer cells, leading to tumour progression through the recruitment of Treg cells (Ref. Reference Ji55). Besides cell signalling and activation of a particular transcription factor, inflammatory cytokines also modulate chemokine expression. In this regard, tumour necrosis factor-alpha (TNF-α) has been reported to increase the expression of CCL28 in the tumour microenvironment (Ref. Reference Wu41).
Lysyl oxidase-like 2 (LOXL2) is a copper-dependent amine oxidase that belongs to the lysyl oxidase (LOX) family. Members of the LOX family catalyse the deamination of lysine residues in elastin and collagen to form covalent cross-links. LOXL2 upregulation has been observed in various tumours, including gastric, colon, breast, oesophageal and pancreatic carcinomas, which leads to tumour cell migration and invasion in vitro and in vivo, suggesting that LOXL2 contributes to tumour progression. In some cases, the enzymatic activity of LOXL2 is not required for its involvement in cancer progression. It has been shown that splice and truncated variants of LOXL2 (e.g. LOXL2Δ72 and LOXL2Δe13), which have impaired enzymatic domains, participate in tumour progression. The cDNA microarray analysis and real-time reverse transcription-polymerase chain reaction assay indicate that LOXL2Δ72 upregulated the expression of many genes involved in esophageal carcinoma (ESCC) cell migration, especially CCL28 (Ref. Reference Zou56). However, the underlying mechanism needs to be elucidated.
Role of CCR10/CCL27–CCL28 in immune surveillance and tumour regression
The immune system plays a critical role in the fight against malignancies. In mice with various immunodeficiencies, there is a high tumour incidence rate and a greater susceptibility to chemical carcinogen-induced cancers. Evidence suggests that tumour cells are able to evade immunological detection, which is called immune surveillance. Since the inception of the immune surveillance hypothesis, many advancements have been discovered (Ref. Reference Ribatti57). Regarding our subject, during the malignant transformation of keratinocytes, the homoeostatic and skin-associated chemokine CCL27 was shown to be gradually lost, indicating that tumour cells use this method to escape the immune system. Epidermis cancers may utilise various methods to avoid being attacked by the immune system. However, studies have shown that downregulation of CCL27 expression is a key immune escape strategy (Ref. Reference Pivarcsi27). CD4+ and CD8+ T lymphocytes were both involved in the antitumour activity induced by CCL27. These findings suggest that CCL27 plays a critical function in skin homoeostasis (Refs Reference Pivarcsi27, Reference Gao58). The importance of CCL27 expression in tumour and immune surveillance became apparent when the downregulation of this chemokine in endometrial cancer has led to tumour progression. In endometrial cancer, CCL27 downregulation resulted in impoverished natural killer (NK) cells in the tumour infiltrate, leading to tumour progression (Ref. Reference Degos31).
Other than immune surveillance, it has been shown that CCR10 might also be involved in the inhibition of tumour growth and metastasis. Administration of CCL28 to Oral squamous cell carcinoma (OSCC) cells (Ca9.22 and YD10B) inhibited RUNX3-prompted invasion and Epithelial-mesenchymal transition (EMT) in these cells through inducing retinoic acid response element (RARE)-related transcriptional activity and subsequently upregulating downstream RARβ expression by recruiting DNA methyltransferase and blocking histone deacetylase (HDAC1) enzymes (Ref. Reference Park49). Moreover, CCL28 binding to CCR10 enhanced expression of E-cadherin and downregulated EMT-related transcription factors Twist, Slug and/or Snail in OSCC cells. CCL28 not only inhibited OSCC invasion but also inhibited RANKL expression in OSCC cells, suppressing differentiation of osteoclastic precursors and thus inhibiting OSCC-induced bone destruction in vitro. To verify the latter effect of CCL28, it was shown that treatment of OSCC-xenografted murine models with CCL28 inhibits OSCC-dependent osteolysis via inhibiting osteoclast proliferation and inducing RARβ in vivo (Ref. Reference Park49).
CCR10/CCL27–CCL28 in cancer development
Besides the physiological and haemostatic functions of CCR10 and its ligands, it has been shown that these chemokines and receptor complexes act as mediators for tumour development.
Role of CCR10-related signal transduction in tumour growth and metastasis
Cell signalling and signal transduction play pivotal roles in cellular functions and survival. Among the different and various signalling pathways, mitogen-activated protein MAPK signalling has been linked to a variety of biological processes, including cell proliferation, survival, metabolic reprogramming and differentiation (Ref. Reference Guo59). CCL28-transfected breast cancer cells are found to have highly proliferative properties through inducing MAPK/ERK signalling pathways, which leads to significant cell proliferation. However, MAPK downstream proteins and mediators have not been characterised in this study (Ref. Reference Yang29). Despite unknown downstream genes for CCL28/CCR10 signalling in breast cancer, the results indicate that the interaction between CCL27 and CCR10 activates the ERK1/2 pathway, which ultimately enhances matrix metalloproteinase (MMP)-7 expression and promotes breast cancer cell invasion and migration. Thus, CCR10 may play a critical role in the invasion and migration of breast cancer cells (Ref. Reference Lin28). In glioma cells, the CCR10/CCL27 interaction triggered AKT signalling, leading to activation of PDGF and SRC genes and promoting cell proliferation and metastasis in vitro and in vivo (Ref. Reference Chen51). Furthermore, CCR10 ligation to CCL27 on melanoma cells initiates PI3K/AKT signalling, resulting in resistance to CTL- and Fas-induced apoptosis in these cells and immune evasion (Ref. Reference Murakami44).
Role of CCR10/CCL27–CCL28 in inflammation and immunomodulation
Inflammation is a set of events that involve the activation and infiltration of immune cells to initiate host defense against pathogens and plays an important role in tissue regeneration and repair. The role of inflammation and the immune system in cancer development has recently received a lot of attention. Administration of TNF-α to tetrachloromethane-induced and diethylnitrosamine-induced inflammatory HCC in murine models upregulated expression of CCL28 and CCR10 in the tumour microenvironment. Upregulation of CCR10 in HCC cells leads to activation of the PI3K/AKT signalling pathway, giving rise to much more tumour cell proliferation. These data indicate the effects of inflammation on tumour growth via inducing the CCR10/PI3K/AKT pathway (Ref. Reference Wu41).
In Hodgkin's lymphoma (HL), eosinophil accumulation (eosinophilia) is considered a pathological feature of this disease. The chemokine receptor CCR10 and its ligand CCL28 were discovered to be frequently expressed in HL-derived cell lines. CCR10 is known to be expressed preferentially by plasma cells, whereas CCL28 recruits eosinophils through CCR3 and plasma cells through CCR10 and CCR3. Therefore, CCL28-induced eosinophilia leads to inflammation in the tumour microenvironment. Recruited eosinophils express several receptors and ligands of the TNF superfamily, including CD40L, CD30L and CD95/FasL, resulting in the proliferation of lymphoma cells through providing antiapoptotic signals on the surface of HL cells (Refs Reference Pinto60, Reference Pinto61). Therefore, CCL28 promotes HL by inducing eosinophilia.
Lymphatic vessels are important in both physiological processes (e.g. fluid homoeostasis) and pathological conditions such as cancer. These vessels act as a key pathway for tumour cell migration and metastasis. As a result, the formation of new lymphatic vessels (lymphangiogenesis) and the expansion of the lymphatic system may contribute to the spread of tumour cells, indicating a bad prognosis for cancer (Ref. Reference Alitalo and Detmar62). The formation of lymphatic vessels is another consequence of inflammation in the tumour microenvironment. Macrophage-secreted TNF-α in the tumour microenvironment induces CCL27/CCL28 and CCR10 expression in the tumour cells and lymphatic endothelial cells (LECs), respectively, leading to migration of LECs towards a gradient of CCL27/CCL28 in the tumour microenvironment. Migrated LECs express vascular endothelial growth factor (VEGF)-D in response to TNF-α in the tumour microenvironment, resulting in lymphangiogenesis and possibly tumour cell migration through the formed lymphatic vessels (Ref. Reference Karnezis63).
Hypoxia promotes tumour development and raises the likelihood of metastasis, and it is associated with poor patient outcomes, which may be induced by a variety of physiological and biochemical changes in hypoxic tumours. As mentioned earlier, under hypoxic conditions, a transcription factor called hypoxia-inducible factor (HIF)-1α is induced, resulting in stimulation of transcription of various genes, including cytokines and chemokines. It has been shown that HIF-1α promotes the expression of CCL28 in some cancer cells, including lung cancer and HCC (Refs Reference Liu and Wei52, Reference Huang53). Hypoxia-induced CCL28 upregulation leads to recruitment and infiltration of CD4+ CD25+ Foxp3+ regulatory T cells (Tregs), which consequently give rise to tumour cell growth, invasion and angiogenesis and suppress immune surveillance in lung adenocarcinoma and HCC (Refs Reference Liu and Wei52, Reference Huang53).
Cancer-associated fibroblasts (CAFs) are activated myofibroblasts in the tumour microenvironment that promote tumourigenesis via secreting various mediators and altering the extracellular matrix. These cells can recruit from other sites or might be differentiated from other cell types, including mesenchymal stem cells or normal fibroblasts (Ref. Reference Arezoo64). Expression and production of CCL28 during chronic inflammation in the pancreatic ductal adenocarcinoma stroma and epithelia leads to attraction and chemotactic migration of CCR10-expressing stellate cells (pancreas-specific fibroblast cells) within the pancreatic tumour microenvironment. This recruitment of pancreatic stellate cells into the tumour microenvironment influences tumour malignancy and metastasis (Ref. Reference Roy65).
CCR10 and its ligands as tumour biomarkers
CCR10/CCL27
For many years, lymphocyte and leucocyte infiltration in the tumour has been considered a prognostic indicator (Ref. Reference Taghizadeh66). Recent research shows that clonally increased T cells in melanomas are not always linked with an efficient anti-tumour immune response and may represent distinct subsets with diverse roles. In reality, the tumoral lymphoid population contains both CD8+ cytotoxic lymphocytes and regulatory T cells with a CD4+/CD25high phenotype that suppresses the local antitumour response. According to a study, T lymphocyte density is negatively associated with tumour thickness in melanomas, independent of lymphocyte function or phenotype. This study indicated that CCR10 and its ligand CCL27 might be involved in aggressive behaviour and immunological evasion in melanomas, suggesting that CCR10 overexpression is a hallmark of malignant neoplastic cells. TNFα and interleukin-1beta (IL-1β) increase CCR10 expression in melanoma cells, and CCR10 expression correlates directly with tumour thickness and inversely with T lymphocytic density in melanomas. Moreover, CCR10 expression may be linked to melanoma aggressiveness and immune evasion (Ref. Reference Simonetti45).
Besides melanoma, CCR10 was also examined to distinguish other types of skin cancers and skin disorders. Cutaneous SCC, which comprises cells that arise from squamous cells in the epidermis, is one of the most common non-melanoma skin malignancies and is categorised as Clark level. Actinic keratosis (AK), bowenoid AK, and Bowen's disease, in which tumour cells are restricted to the epidermis, are believed to represent early stages of cutaneous SCC. BCC, another frequent skin cancer, consists of cells that mimic basal cells of the epidermis, but its origin remains to be identified. Seborrheic keratosis, a frequent benign skin malignancy, consists of a varied proliferation of prickles and basal cells. Examining CCR10 and CCL27 expressions under these conditions showed that CCR10 and CCL27 were highly expressed in SCC with more than Clark level III compared with BCC and Bowen's disease. In contrast, they were moderately expressed in SCC with less than Clark level II, bowenoid AK and AK. As a result, the expression of CCR10 and CCL27 may be stage-dependent. It has been hypothesised that CCR10 enhances neoplastic cell capacity to proliferate, infiltrate tissue, spread to lymph nodes and evade host immune responses in human melanoma (Ref. Reference Kai67). In contrast to this study, Pivarcsi et al. indicated that expression of CCL27 was decreased in SCC tissue samples. These discrepancies have been justified in this way that the populations of SCC-derived samples are different. Moreover, chronic inflammation is also a key risk factor for SCC development (Refs Reference Pivarcsi27, Reference Kai67). Furthermore, Martinez-Rodriguez et al. showed that increased CCL27 immunostaining in supratumoral epidermis is correlated with a better prognosis, a longer progression-free interval, and improved melanoma-specific survival. Therefore, it is concluded that intratumoral and extratumoral expression of CCL27 might change immune responses and prognosis (Ref. Reference Martinez-Rodriguez, Thompson and Monteagudo46).
Patients with non-muscle-invasive bladder cancer may benefit more from alternative therapies if the response to Bacillus Calmette–Guerin (BCG) can be predicted. A number of cytokine profiles have shown encouraging outcomes. However, practical application has been challenging. Using serum cytokines/chemokines collected before and during BCG treatment, prospective and longitudinal research was conducted to discover valid markers for BCG response. After screening, CCL27 was identified to be a top potential biomarker for predicting response to BCG therapy. Regulatory T cells were shown to be associated with serum CCL27 levels, suggesting that CCL27 may facilitate the recruitment of Tregs into the tumour microenvironment. Therefore, enhancement of Tregs because of increased levels of CCL27 might be a sign of poor prognosis after treatment with BCG immunotherapy (Ref. Reference Zhong40).
A frequent malignant tumour in south China and southeast Asia is nasopharyngeal carcinoma (NPC). NPC is caused by the Epstein–Barr virus (EBV), environmental, genetic and nutritional factors. Because EBV infection is linked to NPC, EBV-related indicators such as viral capsid antigen-specific IgA (VCA-IgA) have been extensively utilised in NPC screening. When used in initial screening for EBV antibody-positive NPC, VCA-IgA has excellent sensitivity but low specificity for NPC detection. Plasma CCL27 levels were significantly higher in the VCA-IgA+ healthy donors or the NPC patients compared with the VCA-IgA– normal subjects. Although there was no correlation between CCL27 levels and VCA-IgA titres or plasma EBV DNA content, further analysis revealed that CCL27 levels could discriminate between patients with early-stage NPC and healthy donors who were VCA-IgA positive. Together, CCL27 was effective in identifying VCA-IgA+ NPC patients (Ref. Reference Mao68).
Analysing CCL27 levels in cutaneous T cell lymphoma (CTCL) was also shown to be helpful in predicting tumour progression and regression. Serum CCL27 levels were elevated in CTCL subtypes (mycosis fungoides and Sezary syndrome) and correlated with other CTCL markers such as skin lesions, tumour burden index, serum soluble IL-2 receptor and CCL17. Moreover, CTCL patients who received treatment showed a significant reduction in CCL27 levels, indicating the value of the CCL27 examination in the prognosis of CTCL (Ref. Reference Masui39). Therefore, targeting CCL27 in CTCL might be a promising therapeutic approach (Ref. Reference Kagami69). Further investigations showed that interaction of CCL27 with CCR10 on circulating T cells from Sezary syndrome and mycosis fungoides leads to the homing of malignant T cells, contributing to the initiation of CTCL pathobiology (Refs Reference Fujita34, Reference Sokolowska-Wojdylo70).
CCR10/CCL28
The chemokine CCL28 is believed to function as a chemoattractant for leucocytes expressing CCR10 and/or CCR3. The CCL28 protein is expressed in both tumour and normal epithelial cells. Enzyme-linked immunosorbent assay revealed that CCL28 protein levels were substantially lower in colon cancers than in normal tissue but not in rectal tumours and normal tissue. Those with colon tumours showed substantially higher plasma CCL28 protein levels than patients with rectum tumours. These differences may indicate that distinct processes and signalling pathways enhance the production and release of CCL28 protein in the colon and rectum. More research on the CCL28 protein in colorectal cancer patients is required to determine its prognostic and therapeutic significance (Ref. Reference Dimberg, Hugander and Wågsäter25).
Classical Hodgkin's disease (HD) is distinguished by the presence of neoplastic Hodgkin and Reed-Sternberg (H-RS) cells amid extensive reactive cellular backgrounds (Ref. Reference Hanamoto36). In most cases H-RS cells are derived from the B-cell lineage, although their immunophenotypes are unique. As discussed, significant tissue eosinophilia is considered a characteristic histopathologic feature of HD (Refs Reference Pinto60, Reference Pinto61). The chemokine receptor CCR10 and its ligand CCL28 were discovered to be frequently expressed in HD-derived cell lines. CCR10 is known to be expressed preferentially by plasma cells, whereas CCL28 recruits eosinophils through CCR3 and plasma cells through CCR10 and CCR3. Expression of CCL28/CCR10 was found to be a prognostic marker for predicting eosinophilia in HD and poor prognosis in these patients (Ref. Reference Hanamoto36).
Analysing the expression of CCL28, CCR10, RARβ and CCR3 via immunohistochemistry in 117 human OSCC tissues showed that CCL28 expression was positively correlated with RARβ expression, which was associated with clinicopathological characteristics in OSCC patients. OSCC patients with higher expression of CCR10, CCL28 or RARβ had significantly better and prolonged survival times. These results suggested that OSCC patients with lower levels of CCR10, CCL28 or RARβ showed a higher possibility of bone invasion. As previously mentioned, CCL28 interaction with CCR10 inhibited OSCC-induced bone destruction by suppressing osteoclast differentiation and proliferation, which was correlated with survival analysis. Therefore, using CCL28/CCR10 expression might be helpful to predict OSCC invasion to the bone and survival of patients (Ref. Reference Park49).
A statistical regression model, which is called the risk prediction model, can correlate disease outcomes with various factors, especially gene expression patterns. Therefore, constructing a risk model using multiple genes might be promising for disease prediction (Ref. Reference Grant, Collins and Nashef71). In a study to identify an immune system-related prognostic signature for colorectal cancer, it was used a combined set of genes (8-IRG), including CCL28, NDRG1, SLC10A2, ESM1, TRDC, FGF2, UCN and UTS2. The results showed that the 8-IRG signature significantly correlated with tumour invasion, lymph node metastasis and tumour stage, reflecting the dysregulation of the immune system's mediators in the tumour microenvironment that can be considered a potential prognostic biomarker and therapeutic target in colorectal carcinoma (CRC) (Ref. Reference Wang72).
Moreover, finding protein–protein interaction (PPI) networks in which the physical interaction between different proteins is predicted has recently attracted much attention, leading to the discovery of putative protein targets of biomarker and therapeutic interest (Ref. Reference Titeca73). PPI network analysis in lung adenocarcinoma has indicated that mutated TP53 is a poor prognostic factor and its combination with EGFR mutation leads to a worse prognosis. In this study, PPI network analysis proposed that TP53/EGFR co-mutation accompanied by CCL28, GPC3, NPY and GPR37 might be novel prognostic markers that can also be used as therapeutic targets (Ref. Reference Wang74). Another study regarding PPI in CRC has shown that a prognostic gene signature comprising nine genes, including CCL28, TIMP1, GLDN, PCOLCE2, SLC4A4, NFE2L3, SCGB2A1, MMP1 and AXIN2, has good performance in the prediction of overall survival of CRC patients (Ref. Reference Chen75).
Examination of the response to therapies in cancer is an important issue that needs to be solved. Finding a suitable marker for analysing treatment efficiencies is highly encouraged. In an aggressive type of oesophageal adenocarcinoma, 11 potential genes were examined in which CCL28 and DKK-3 genes were considered potential predictors for response to adjuvant therapy. Moreover, CCL28 has been proposed as a therapeutic target that could potentially improve the treatment of oesophageal adenocarcinoma (Ref. Reference McLaren76). In another study regarding the prediction of drug resistance in cancer, it has been shown that analysing the expression of ABCC1/MDR1 accompanied by BCAR4, CCL28, SCGB2A2 and PIP in IPH-926 cells (human lobular breast cancer cells) might offer a new predicting tool for the evaluation of compounds to overcome drug resistance (Ref. Reference Krech77). As such, differential expression of CCL28, CXCL2, IL18, FDXR, HRAS and CHI3L1 may serve as a basis for predicting the effectiveness of 5-fluorouracil on breast cancer (Ref. Reference Tsao78). Furthermore, microarray analysis of multiple genes showed that examining the upregulation of genes, such as CCL28, CLIC5, PDCD4, RAB27A and TXNIP, can identify early transcriptome alterations during preoperative irradiation for rectal cancer, which may help us better understand tumour radioresistance (Ref. Reference Supiot79). These data indicate that a combination of examining CCL28 expression along with tumour-relative genes, which can be achieved by PIP network analysis, might provide a panel for predicting the prognosis and effectiveness of therapeutic approaches.
Therapeutic approaches
According to the above-mentioned involvement of CCR10/CCL27–CCL28 in cancer progression or regression, there is a growing body of evidence that designing therapeutic approaches based on this axis might be promising in the treatment of cancer (Table 2). As has been discussed, CCL27 is considered a T-cell-attracting chemokine. This chemokine attracts T cells and NK cells into the tumour microenvironment. It has been shown that adenoviral CCL27-infected tumour cells' injection into mice suppresses tumour growth through the infiltration of T cells and NK cells, suggesting the generation of specific immunity against tumour cells (Refs Reference Gao30, Reference Gao58). Therefore, it is suggested that CCL27 can be used as an adjuvant in immunotherapies to induce and infiltrate specific T cells against tumour cells (Ref. Reference Gao58). Furthermore, it has been mentioned that EGFR/Ras/ERK signalling in skin cancers leads to CCL27 downregulation, resulting in a disruption in skin homoeostasis and suppression of immune surveillance. Therefore, blocking this signalling pathway by tyrosine kinase inhibitors such as PD168393 or a specific MAPK inhibitor (UO126) might be helpful to treat skin cancer, which could give rise to CCL27 upregulation and recruitment of T cells (Ref. Reference Pivarcsi27). Other than tyrosine kinase inhibitors, there are some identified compounds that can induce CCR10 on anti-tumour immune cells. In this regard, it was shown that glatiramer acetate, dimethyl fumarate and monomethyl fumarate induce CCR10 expression on the NK cells, leading to migration of these cells towards tumour cells and enhancement of their cytotoxicity (Ref. Reference Maghazachi, Sand and Al-Jaderi26).
Table 2. Therapeutic approaches using CCR10/CCR27-CCL28 in cancer

The use of cytokines such as IL-2 to activate cancer patients' immune systems has long been a valuable treatment option, and cytokines are still a vital part of contemporary clinical cancer research (Ref. Reference Conlon, Miljkovic and Waldmann80). Because of the toxicity of IL-12 administration at high concentrations, it was suggested that a low dose combination of IL-12 with CCL27 increased significantly the infiltration of CD4+ and CD8+ T cells, perforin and interferon-γ, and showed milder pathological changes compared with IL-12 alone, indicating a promising approach in the treatment of ovarian cancer (Refs Reference Gao30, Reference Gao32). Therefore, the administration of CCL27 can be helpful in treating cancers in which this chemokine exerts anti-tumour functions.
All the mentioned therapeutic approaches regarding CCL27–CCL28/CCR10 in cancer were based on the anti-tumour function of this axis. As discussed, this axis also promotes tumour progression. Therefore, using approaches in which CCR10 and its ligands must be inhibited can also be promising in therapies. A proteasome inhibitor called bortezomib is currently being administered to myeloma patients. Around one-third of myeloma patients are initially resistant to bortezomib therapy, whereas most others acquire resistance over time with no clear prognostic indicators. It was found that CCL27, but not CCL28, is overexpressed in myeloma patients (i.e. expression more than 5000 pg/ml), causing resistance to bortezomib in myeloma cells. CCL27 protects and rescues myeloma cells from apoptosis induced by bortezomib. Moreover, this impact was shown to be highly correlated with stromal CCR10 expression and IL-10 production. It is concluded that blocking the CCR10/CCL27/IL-10-dependent myeloma crosstalk with stroma by siRNAs or antibodies might be a novel and tailored therapeutic target in early refractory myeloma patients (Ref. Reference Thangavadivel43).
It's been a decade since passive cancer immunotherapy began using monoclonal antibodies against particular tumour antigens. These antibodies are used to either inhibit or neutralise tumour-promoting factors (e.g. ligands, receptors and any antigen) or to mimic the functions of anti-tumour factors (Ref. Reference Lu81). Regarding the use of neutralising or blocking antibodies, it has been shown that administration of anti-CCR27 antibodies efficiently inhibited CCL27-expressing CCR10-B16 melanoma tumour development rather than CCL27– cells in mice, indicating the specificity of the treatment (Ref. Reference Murakami44). Other than CCL27, CCL28 is also involved in tumour development and is considered a Treg-infiltrating factor in liver, lung and colorectal cancers. In gastric cancer, it has been shown that using anti-CCL28 blocking antibodies abrogates Treg recruitment into the tumour microenvironment and even induces Th1 and CTL infiltration, leading to blockade of tumour growth (Ref. Reference Ji55).
Obstacles and limitations
The results show that chemokines play a complicated function in tumour development and cell proliferation. Because cancer type, stage of cancer and intratumour immune cell composition all influence treatment outcomes, considerable caution should be used while designing chemokine-based cancer therapeutics. Several strategies have been developed to augment chemokine-induced effects by increasing chemokine expression using various methods such as naked DNA plasmids, engineered tumour cells and transduced DCs. One of the major obstacles to the development of CCR10-based cancer treatment has been the lack of sufficient host response in the detection of immunogenic tumours. Only in the presence of APCs and lymphocytes, tumour cells can be recognised by the immune system. As a result, there has been a lot of interest in identifying the chemokine signals that facilitate immune-cell recruitment into tumours, with the goal of eventually investigating the potential for specific enhancement of antigen-presenting cells (APC) and effector T-cell infiltration as well as blocking the migration and function of Tregs and other immunosuppressor cells such as MDSCs and M2 macrophages. Several strategies have been developed to block chemokine-dependent responses by depleting chemokines and blocking their receptor signalling pathways. Chemokine redundancy is an immunology concept that refers to the phenomena of several cytokines exerting comparable activities and their capacity to do so. This is primarily owing to the fact that several chemokines share receptor subunits and intracellular cell signalling molecules/pathways. Since several chemokines exert similar effects, the blockade of CCR10 function might be compensated by functions of other chemokine complexes, leading to neutralising the treatment of cancer.
CCR10 is a multifunctional chemokine receptor that affects tumour development, angiogenesis and metastasis as well as immune cell infiltration. The balance between tumour-promoting and tumour-inhibiting variables determines how much CCR10 contributes to the overall outcome of tumour formation. CCR10 has been shown to be potentially bifunctional during tumour growth, with tumour-promoting and tumour-suppressive properties. As a result, additional research into the differences between CCR10's pro-tumour and anti-tumour actions is needed in order to produce more effective cancer treatments. Although the results of the mice tumour experiments are promising, there is still a long way to go in the use of CCR10-targeted medication to treat cancer in humans.
Concluding remarks and future perspectives
CCR10 is the latest identified CC chemokine receptor which can be ligated to two CC chemokine ligands, that is, CCL27 and CCL28. These chemokines are predominantly expressed and secreted in the epithelia, especially the skin (Ref. Reference Marchese11). Secretion of CCL27 and CCL28 leads to infiltration of CCR10-expressing cells such as T cells, immune cells and epithelia (Refs Reference Jarmin12, Reference Morales13, Reference Pan14, Reference Wang15). Other than the homoeostatic role of CCR10/CCL27–CCL28, this axis is also involved in the pathogenesis of diseases, especially cancer. Ligation of CCR10 to CCL27 or CCL28 triggers signal transductions into tumour cells such as PI3K/AKT and MAPK/ERK pathways, leading to tumour cell proliferation and growth (Refs Reference Pivarcsi27, Reference Guo59). Moreover, CCR10 ligation on inflammatory cells, including CAFs and eosinophils, promotes infiltration of these cells into the tumour microenvironment, leading to inflammation and tumour progression (Ref. Reference Roy65). Other than inflammatory cells, Tregs are also recruited into the tumour microenvironment, which inhibits anti-tumour immune responses. Additionally, CCL27 and CCL28 induce tumour cells to produce MMPs and EMT-dependent genes, including Twist, Slug and N-cadherin, to trigger metastasis. Furthermore, CCR10-expressing endothelial cells respond to CCL27 and CCL28 gradients of concentration, leading to angiogenesis and lymphangiogenesis (Ref. Reference Alitalo and Detmar62). Of note, the development of new blood or lymphatic vessels paves the way for tumour cells to invade and metastasise to distant organs (Ref. Reference Alitalo and Detmar62). In contrast, CCR10 also provides anti-tumour immune responses and is involved in immune surveillance depending on the tumour microenvironment. In this regard, it has been shown that ligation of CCR10 to CCL27 or CCL28 on lymphocytes and NK cells recruits these cells into the tumour microenvironment to fight against tumour cells. All things considered, the CCR10/CCL27–CCL28 axis acts as a double-edged sword in tumour development (Ref. Reference Degos31). The majority of the research on CCR10/CCL27–CCL28's role in cancer has been conducted in vitro, with just a small number of studies conducted in vivo, the majority of which have used animal models (Refs Reference Homey20, Reference Yang29, Reference Wu41, Reference Thangavadivel43, Reference Chen51). As a result, these findings from in vitro studies will be hard to translate into clinical studies. To make use of the findings in cancer therapy, diagnosis and prognosis, it is recommended that future research be more clinical in nature. Unlike CCL27, which merely binds to CCR10, CCL28 can interact with two receptors, CCR3 and CCR10. Therefore, determining the exact receptor for the function of CCL28 in cancer is highly recommended.
More importantly, the majority of studies looking into CCR10/CCL27–CCL28 as a biomarker have only examined its applicability and have not defined its action mechanism in that cancer, leading some to suggest that it would be more reliable if those studies also looked into the mechanism of CCR10/CCL27–CCL28 action in the suppression or promotion of cancer. Moreover, the mechanism of dysregulation of this axis has not yet been more elucidated. Non-coding RNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), are RNA molecules that do not have the ability to be translated into proteins such as mRNAs. These RNAs play crucial roles in gene regulation, gene translation, RNA splicing and DNA replication. It is strongly suggested that non-coding RNAs involved in regulating CCR10/CCL27–CCL28 in cancer be identified since they are important in gene regulation. Aside from that, several non-coding RNAs have been shown to be related to specific diseases, indicating that these RNAs should be explored on a time-sensitive basis. Therefore, analysing these RNAs in the regulatory axis of CCR10/CCL27–CCL28 might be promising in defining the mechanisms of CCR10/CCL27–CCL28's dysregulation. As discussed, chemokines and cytokines are part of a network connection. Using bioinformatics might accelerate finding these connections and decrease excess biological analysis (Ref. Reference Titeca73). Therefore, it is suggested that finding protein–protein and RNA–proteins interactions might broaden our knowledge regarding CCR10, CCL27 and CCL28 involvement in cancer.
Besides, the ability of the CCR10/CCL27–CCL28 axis to be targeted in therapeutic approaches is also discussed and some approaches have been explored. As mentioned, this axis acts as an infiltrating factor for antitumour immune cells. Therefore, using compounds that increase CCR10 on tumour-infiltrating NK or CTLs towards CCL27- or CCL28-expressing tumour cells may be beneficial. Moreover, CCL27 or CCL28 can be combined with other adjuvants such as cytokines in immunotherapies, suggesting that these combinations might introduce novel therapeutic protocols. Furthermore, CCR10 and its ligands might exacerbate tumour development. Designing molecules or drugs that block the signalling pathways or receptor–ligand interaction might also be a promising approach for treating cancers in which this axis plays a tumour-promoting role. On the whole, CCR10/CCL27–CCL28 appears to be a haemostatic axis whose role in cancer development should be studied further. Research in the future may open up new perceptions and expand our understanding of cancer biology, which would be beneficial.
Conflict of interest
The authors declare no conflicts of interest.