Introduction
The discovery of a second vascular system in the body goes back to the ancient Greeks, most importantly Herophilos (335–280 BCE) and Erasistratus (310–250 BCE), who described a vascular system resembling what we now call the lymphatic system. The lymphatic network was updated in more detail almost four centuries ago (in the mid-17th century) by Gasparo Aselli as ‘lacteal vessels’ and was grounded on his observations of a dog's abdomen (Ref. Reference Loukas1). Since then, and until two or three decades ago, the lymphatic vasculature had received much less attention than blood vessels, mostly because of the difficulty of distinguishing between blood and lymph vessels. With the discovery of several cellular markers which are exclusively, or at least predominantly, expressed by lymphatic endothelial cells (LECs), our knowledge of lymphology has increased remarkably. These tools have enabled the study of the molecular mechanisms of lymphangiogenesis, the growth and formation of new lymphatic vessels (LVs) that have appeared to be an important phenomenon in many pathological conditions in various organs. In this paper, we review the current state of knowledge of the lymphatic system, with a focus on the role of lymphangiogenesis in renal diseases. Furthermore, we explore the possible mechanistic link between renal lymphangiogenesis and other pathological processes such as inflammation and fibrosis. Finally, we discuss the potential value of renal lymphangiogenesis as a new target for therapeutic intervention in renal diseases.
Lymphatic vessels
Anatomy and physiology
Unlike blood capillaries, lymphatic capillaries consist of overlapping oak leaf-shaped endothelial cells (ECs), with incomplete or no basement membrane. Lymph capillaries lack smooth muscle cells or pericyte coverage, but are equipped with thin fibrillar structures called anchoring filaments, which permit expansion and prevent collapse. By connecting the abluminal surface of LECs to the surrounding interstitial matrix, anchoring filaments enable these cells to respond to changes in interstitial fluid pressure, as in oedema, when high interstitial pressure tends to collapse LVs (Ref. Reference Wiig and Swartz2). The precollecting segment does exhibit some coverage (Ref. Reference Florey3); however, collecting vessels are fully equipped with perivascular SMCs (Ref. Reference Reddy and Staub4). To ensure their unidirectional fluid movement, LVs possess two kinds of valves to prevent lymph backflow: overlapping endothelial junctions in the initial lymphatics serve as a primary valve (also called a microvalve) which allows fluid to flow from the interstitial space into the lumen of the LVs while considerably reducing the leakage back into the interstitium, and the traditional intraluminal valve serves as a secondary valve (Ref. Reference Trzewik5).
With their special structural and functional properties, LVs are central to a wide variety of actions in the body. They play a central role in the maintenance of tissue fluid homoeostasis, immune surveillance and trafficking, and fatty acid absorption. LVs drain excess fluid entering the interstitial space from the blood capillaries, and take up extravasated cells and macromolecules and transport them unidirectionally to the draining lymph node, thoracic duct and into the inferior vena cava. LVs thus complete one of the most important physiological functions, by returning interstitial tissue fluid back to the blood circulation. LVs are not simple ducts, since LECs actively participate in a variety of vital biological processes such as reorganisation and remodelling of the extracellular matrix (ECM), modulation of capillary morphogenesis, cellular migration and more importantly, immune response and peripheral tolerance (Ref. Reference Wiig and Swartz2).
Visualisation and phenotyping of LECs
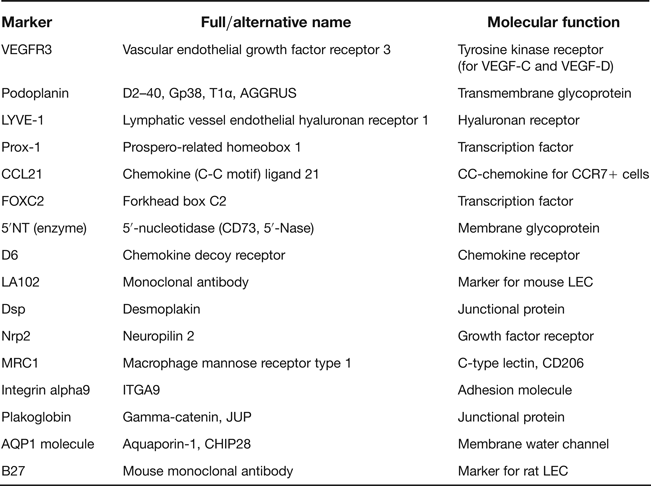
Until recently, our understanding of the lymphatic vascular system was much more limited than of its counterpart blood vasculature. The main reason was a lack of reliable tools to differentiate between LECs and blood endothelial cells (BECs). With the discovery of several markers predominantly expressed on LECs, knowledge in this field has substantially improved in recent years. In this respect, although several markers have been identified to date, the extent of their expression and reliability in all pathological conditions has long been a matter of debate. The consensus, however, is that there still is no perfect and ideal marker available that works reliably in all disease conditions in different organs. Several markers have been proposed in the literature (Table 1); however, the most extensively tested and studied markers yielding satisfactory results are LV endothelial hyaluronan receptor 1 (LYVE-1), vascular endothelial growth factor receptor 3 (VEGFR-3), prosperorelated homeobox transcription factor 1 (Prox1) and mucin-type transmembrane glycoprotein podoplanin. Care should be taken when using these markers, as many other cells and structures can show positivity. Therefore, multiple markers need to be used for confirmation.
Table 1 Summary of markers for lymphatic vessels

LECs at developmental stages
The heterogeneity of LECs at different developmental life stages is a complicated matter and the origins of LVs have been controversial. To date, two major hypotheses have been put forward to explain the origins of LVs: one proposes the budding of primary lymph sacs ‘centrifugally’ by endothelial budding and lymphangiogenesis; the other involves a role for mesenchymal cells. The first centrifugal sprouting hypothesis was proposed by Florence Sabin, based on her observation of LVs sprouting from the jugular veins of pig foetus (Ref. Reference Sabin6). This hypothesis is widely supported, principally by mouse molecular genetics (Ref. Reference Srinivasan7). Several years later, a second alternative model was proposed by Huntington and McClure. This hypothesis proposes that the initial lymph sacs develop in the mesenchyme while, in contrast to the previous model, their origins are independent of the veins (Ref. Reference Huntington and McClure8). However, it has been shown that LVs start growing from distinct ECs which express Prox-1 in the anterior cardinal vein (Ref. Reference Wigle and Oliver9), and that the establishment of blood circulation, which happens in humans at around the sixth to seventh week of embryonic development, is a prerequisite for LV development. Later, this Prox-1-expressing EC population migrates and establishes the primary lymph sac and then, by remodelling, forms the LV system (Ref. Reference van der Putte10). The reader is referred to recent excellent reviews (Refs Reference Schulte-Merker, Sabine and Petrova11, Reference Yang and Oliver12, Reference Koltowska13) for further details on lymphatic development.
LVs in kidney
Before the discovery of specific markers, observations by light or electron microscopy and dye injection studies had revealed no major differences in the distribution of the renal lymphatic system in mammals (Refs Reference Albertine and O'Morchoe14, Reference Cuttino15, Reference Peirce16, Reference Eliska17, Reference Kriz18). Later studies taking advantage of more specific markers such as podoplanin, LYVE-1, Prox-1 and VEGFR-3 confirmed the previous findings on the distribution of renal lymphatics (Refs Reference Lee19, Reference Tanabe20, Reference Matsui21, Reference Voss22). D2–40, a monoclonal antibody which recognises human podoplanin, validated the pattern of human renal LVs that had been uncovered by previous investigations (Refs Reference Sakamoto23, Reference Ishikawa24, Reference Bonsib25). This antibody is a promising marker for the study of human LVs and is in use in daily clinical practice.
Using these novel markers, recent studies have provided in-depth information on the development of renal LVs in the embryonic and adult rodent kidney. Although the extra-renal lymphatic network in mice is well developed at embryonic day (E) 12, the first intrarenal LV is observed at E13 (Ref. Reference Lee19). In contrast, no LVs can be found in developing rat kidney until E20, at which point the first LVs are observed in the renal hilus and interstitium around the pelvis (Ref. Reference Tanabe20). There are no LVs at that point around the arcuate arteries and in the cortical area. However, one day after birth, several small LVs can be observed around the branches of arcuate veins and arteries in the corticomedullary region; since no LVs can be found at this time on the cortex, these LVs appear to be unconnected to the cortical area. The first small LVs in the cortex of rat kidneys are observed around the interlobular arteries at days 10 to 20 after birth (Ref. Reference Tanabe20). LVs accompany intrarenal arteries and veins up to the interlobular branch in the cortex in adult rat kidneys, and occasionally some LVs can be observed near glomeruli. Although there can be several LVs in the renal pelvis, virtually no LVs have been reported in the parenchymal tissue in the cortex and medulla (Refs Reference Tanabe20, Reference Matsui21).
Two lymphatic systems have been observed in human kidneys. One is the intrarenal LVs, which show the same pattern of distribution as in rat kidneys. They follow the intrarenal distribution of blood vessels up to the level of interlobular arteries and veins in the cortex. LVs in the cortical area are occasionally observed in the tubulointerstitial space and around glomeruli, whereas LVs are absent or very rarely found in the outer or inner medulla. LVs are also found in the adipose tissue of the hilum, around the blood vessels beneath the interstitium of the pelvic transitional epithelium, and also in the vicinity of the hilar and sinus arteries and veins (Refs Reference Ishikawa24, Reference Bonsib25). The second capsular lymphatic system is found in addition to these blood vasculature-accompanied intrarenal LVs (Refs Reference Holmes, O'Morchoe and O'Morchoe26, Reference Bell27, Reference Clark and Cuttino28). As the number of LVs decrease in the outer cortex, these capsular LVs mostly drain superficial cortical lymph and run parallel to the renal capsule, eventually connecting to LVs in the renal sinus in the hilum. In healthy kidneys, these capsular LVs drain little fluid, but if there is a urinary tract obstruction (UUO), these LVs become the main route of lymph drainage (Ref. Reference Holmes, O'Morchoe and O'Morchoe26). The specific roles of this dual lymphatic network in renal health and disease remain unclear and warrant future investigation. A schematic representation of the anatomy of renal lymphatics is presented in Figure 1.

Figure 1 Anatomy of renal lymphatics. Renal lymph vessel after entering the kidney follows the same hierarchical distribution as of renal veins and arteries, up to the level of interlobular arteries. In normal kidney, there is almost no lymphatic vessel (LV) in cortical interstitium and few may observe in close proximity to glomeruli. In the medullary part, LVs are absent or very rarely observe. Kidney capsule has also some LVs. (Modified with permission from ref. 75)
Renal lymphangiogenesis
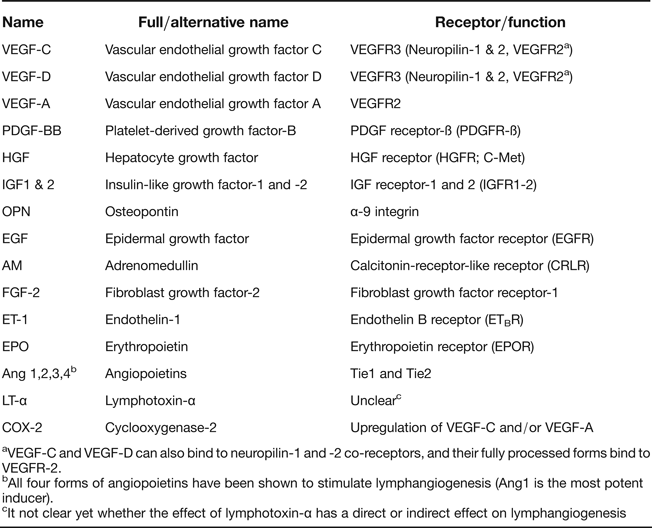
Lymphangiogenesis in adults occurs in physiological processes and in a number of pathological conditions. Although there are several similarities between these processes, pathological lymphangiogenesis requires some additional factors (Ref. Reference Hirakawa29). Physiological lymphangiogenesis occurs in embryogenesis, during the development of the corpus luteum and in wound healing in adults (Refs Reference Otsuki, Magari and Sugimoto30, Reference Martínez-Corral31). To date, various growth factors and mediators have been shown to promote lymphangiogenesis in different organs, and their number continues to increase (Table 2, Refs Reference Zheng, Aspelund and Alitalo32, Reference Marino33, Reference Lee34). The best-known factors are vascular endothelial growth factor-C (VEGF-C) and VEGF-D. VEGF-C signals primarily through its receptor, VEGFR-3, play a crucial role in the proliferation, migration and survival of LECs. VEGF-C has a vital role in LV development and VEGF-C deficiency results in embryonic death in mice (Ref. Reference Karkkainen35). VEGF-D is another major inducer of lymphangiogenesis, also signalling via VEGFR3, but its ability to promote lymphangiogenesis is less important than that of VEGF-C. Both VEGF-C and -D are also able to induce angiogenesis (Refs Reference Benest36, Reference Rissanen37). In the next sections, we will discuss recent findings surrounding lymphangiogenesis and its implications in kidney diseases.
Table 2 Summary of lymphangiogenic growth factors and mediators

aVEGF-C and VEGF-D can also bind to neuropilin-1 and -2 co-receptors, and their fully processed forms bind to VEGFR-2.
bAll four forms of angiopoietins have been shown to stimulate lymphangiogenesis (Ang1 is the most potent inducer).
cIt not clear yet whether the effect of lymphotoxin-α has a direct or indirect effect on lymphangiogenesis
Inflammation and inflammatory cells
Lymphangiogenesis has been reported to occur at sites of tissue inflammation, following immunisation and bacterial infection. It is also involved in many inflammatory conditions such as psoriasis, chronic airway inflammation, Crohn's disease, inflammatory bowel disease, rheumatoid arthritis, transplant rejection and tumour metastasis (Ref. Reference Alitalo38). An increasing body of data supports those hypotheses proposing the existence of mutual interaction between inflammation and lymphangiogenesis. LVs form the exit route for inflammatory cells, cytokines, chemokines, inflammatory exudate fluid and macromolecules from tissues to lymph nodes, where the immune response arises. LECs efficiently react to microenvironmental alterations. In this sense, the inflammatory process can affect LVs, while LVs also play a crucial role in promoting inflammatory responses. A wealth of evidence from many investigations has shown that LVs are not just passive conduits but active players in many biological responses such as immunoregulation, which is necessary for immune surveillance and inflammation (Ref. Reference Card, Yu and Swartz39).
Chemokines
Secondary lymphoid tissue chemokine (SLC/CCL21), which is constitutively expressed by LECs in small LVs under a steady state, is induced and secreted in large amounts in an inflammatory microenvironment. CCL21 has been shown to be a key regulator of the translymphatic migration of dendritic cells (DC) and other leucocytes in both homoeostasis and in inflammation (Ref. Reference Johnson and Jackson40). CCL19 is also secreted by LECs, but in very small amounts compared with CCL21 (Ref. Reference Kriehuber41). Like BECs, LECs express macrophage inflammatory protein (MIP-3ß/CCL20) and monocyte chemotactic protein (MCP-1/CCL2) upon stimulation with several inflammatory cytokines such as tumour necrosis factor α. LECs also express several other chemokines and a large number of chemoattractants such as CCL5 (RANTES), CXCL12 (SDF-1) and CX3CL1 (Fractalkine), which together with CCL21 promote leucocyte transmigration through LVs (Refs Reference Randolph, Angeli and Swartz42, Reference Haessler43). In the precollector segment, LECs express low podoplanin and significantly upregulated CCL27, whereas in the initial capillaries, LECs – which also express high level of podoplanin – secrete CCL21. It has been suggested that precollector segments with low podoplanin most probably act in trafficking pathogenic CCR10+ T lymphocytes in inflammation, highlighting the potentially distinct functions of LV segments in normal tissue homoeostasis and inflammation (Ref. Reference Wick44). Seminal work by Kerjaschki et al. (Ref. Reference Kerjaschki45) has shown that LECs express CCL21 in renal biopsies from rejected kidney transplants. They observed that the transmembrane sialomucin-like glycoprotein podoplanin is bound to CCL21 on the basolateral membrane of LECs in nodular infiltrates, unlike in the luminal expression of podoplanin in normal tissue. This result confirms the active role of podoplanin in CCL21 binding, and the recruitment of CCR7+ inflammatory cells, assigning a unique role in the organisation of nodular infiltrates in the renal transplants.
Receptors
LECs upregulate intercellular adhesion molecule 1 (ICAM-1), junctional adhesion molecule 1 (JAM1), vascular cell adhesion molecule (VCAM-1) and E-selectin in response to inflammatory stimuli. These adhesion molecules help leucocyte transmigration by promoting leucocyte–endothelial adhesion (Ref. Reference Johnson46). Another receptor which has been shown to be expressed by LECs in renal allografts is CXCR7 (Ref. Reference Neusser47). CXCR7, a receptor for ligands CXCL11 or CXCL12, is involved in leucocyte trafficking by LVs in allografts. CXCR7 is expressed by LECs in allograft tissues, whereas its expression is restricted to smooth muscles and some peritubular ECs in the healthy control kidney. CXCR7 expression is increased in renal allograft rejection both in LVs and blood vessels, and can play a role in the recruitment and activation of inflammatory cells in renal allografts. It is deemed that CXCR7 might be involved in adhesion of cells to LVs and peritubular capillaries in the kidney. However, the exact role of this upregulation in renal vasculature is not yet understood clearly (Ref. Reference Mancardi48). Future targeting interventions are warranted to further clarify the biological function and importance of this CXCR7 upregulation in renal allograft injury.
Duffy antigen/receptors for chemokines (DARC) and protein D6 are also expressed by LECs and are able to bind with high affinity to different inflammatory chemokines (Ref. Reference Gardner49). D6 is thought to act as a chemokine scavenger; while in contrast, DARC presents chemokines (Ref. Reference Pruenster50). Although both are integral to inflammatory reactions, their importance and exact interplay with LECs in kidney inflammation and the resolution of inflammation is not investigated yet. Sphingosine-1-phosphate receptor 1 (S1P1), a receptor for sphingosine-1-phosphate (S1P), is expressed on LECs (Refs Reference Akiyama51, Reference Pham52). S1P promotes LEC migration and tube formation in vitro as well as lymphangiogenesis in vivo. S1P1 is also involved in lymphangiogenesis (Refs Reference Aoyagi53, Reference Yoon54). FTY720, a microbe-derived immunosuppressive that acts as an agonist for the S1P receptors, inhibited lymphangiogenesis in experimental islets allografts and significantly prolonged graft survival (Ref. Reference Yin55). The role of S1P has been investigated in various renal diseases (Refs Reference Kim56, Reference Long and Price57, Reference Kramer58). Modulation of S1P1 receptor on renal lymphatics might serve as a new strategy to prevent renal lymphangiogenesis.
Leucocytes
The important role of leucocytes in lymphangiogenesis has been extensively investigated (Ref. Reference Loffredo59). Several subsets of leucocytes are able to produce mediators and growth factors at the site of inflammation that can promote lymphangiogenesis, such as mast cells, macrophages, neutrophils, DCs, B cells, T cells and inflammatory fibroblasts (Refs Reference Angeli60, Reference Conrady61, Reference Kataru62, Reference Tan63). Macrophages play a prominent role in these subsets. Macrophages participate in lymphangiogenesis in two distinct ways: by producing paracrine lymphangiogenic stimuli and mediators, or by directly trans-differentiating into LECs (Ref. Reference Ran and Montgomery64). However, the exact role and the significance of the contribution attributed to macrophages in postnatal lymphangiogenesis are still under investigation (Ref. Reference Hall65). We and other groups have shown that macrophages can be actively involved in renal lymphangiogenesis in several diseases (Refs Reference Matsui21, Reference Sakamoto23, Reference Adair66, Reference Lee67, Reference Poosti68, Reference Suzuki69). However, studies of several kidney injury models have shown that CD68+ macrophages are not the main source of lymphangiogenic mediators, at least in producing VEGF-C. In a mouse UUO model, macrophage depletion by clodronate liposomes markedly abolished renal lymphangiogenesis (Ref. Reference Lee67). In the same disease model in rats, Suzuki et al. (Ref. Reference Suzuki69) showed that unlike macrophages, tubular epithelial cells are the major contributor to renal lymphangiogenesis, by secreting large amounts of VEGF-C. In a time-course study of adriamycin-induced nephropathy, we found that renal lymphangiogenesis significantly increases at week 12, while there is no macrophage influx up to that time. Even after prominent influx of ED1-positive cells at later time points, <5% of these cells showed VEGF-C expression by double immunofluorescent staining (Ref. Reference Yazdani70). This implies that the participation of macrophages in lymphangiogenesis is highly context-dependent, at least in the kidney. Besides, the precise role of different subtype of macrophages such as M1 (classically activated, pro-inflammatory) and M2 (alternatively activated, anti-inflammatory) in inducing lymphangiogenesis is not well characterised. Specifically targeting macrophages, and its subtypes, in the various renal disease models with high lymphangiogenesis will help understand the role of macrophages in renal lymphangiogenesis and is currently a focus of our study.
Nodular infiltrates are shaped by the accumulation of T cells, B cells, DCs and probably macrophages. These structures resemble tertiary lymphoid organs (TLOs), are commonly formed during chronic inflammatory conditions and have been shown to be constantly associated with LVs in the kidney (Refs Reference Kerjaschki45, Reference Heller71). Recently, we observed strain-dependent lymphocytic infiltration and TLO formation in the kidneys of aged mice (Ref. Reference Huang72). Candidate genes that can be linked to ageing-associated TLO formation have been identified. To date, it remains unclear what the functional importance of these structures could be in renal diseases. They seem to be a part of a local immune system (Ref. Reference Carragher, Rangel-Moreno and Randall73), although whether TLOs and secondary lymphoid organs possess the same functional properties has not yet been clarified (Ref. Reference Aloisi and Pujol-Borrell74).
In summary, inflammatory cells and their mediators can induce renal lymphangiogenesis in kidney diseases accompanied by tubulointerstitial inflammation. This expansion of LV network in cortical region of the kidney seems to be a positive response of microenvironmental milieu in order to increase the washout of overloaded interstitial fluid in both inflammatory and oedematous injuries in renal diseases (Ref. Reference Seeger, Bonani and Segerer75). However, it is not known yet to what extent these newly formed LVs are truly functional in fluid, inflammatory cells and macromolecular transport from the kidney. Using in vivo imaging techniques or injecting dyes into renal capsule will help to understand the significance of this lymphatic expansion in removing interstitial fluids in different kinds of kidney injury models. Moreover, using knock-out animal models for different inflammatory cells, or drugs specifically targeting/depleting leucocytes will be informative to unravel the role of leucocyte subsets in renal lymphangiogenesis.
Fibrosis
Fibrosis has a close connection to inflammatory cells and mediators, as clearly stated in a recent comprehensive review ‘no inflammation, no fibrosis’ (Ref. Reference Wick76). Therefore, it is highly likely that lymphangiogenesis in the kidney or other organs are associated with fibrosis. Several studies of distinct organs and disease conditions, and renal diseases, have reported that new LV formation occurs in diseases accompanied by fibrotic conditions (Refs Reference Matsui21, Reference Sakamoto23, Reference Lee67, Reference Suzuki69, Reference Zampell77). However, the causal relationship between lymphangiogenesis and fibrosis, evidenced by collagen deposition and scar formation, has not been completely elucidated. Lymphangiogenesis is proposed to be involved in the pathogenesis of idiopathic pulmonary fibrosis (Ref. Reference El-Chemaly78). Recently, ectopic secretion of PDGF-B by LECs, which signals through its PDGFR-ß receptor on mural cells, was shown to recruit aberrant mural cells on LVs. This attachment of mural cells to LVs hampers their drainage capacity, and this defect in the lung's LVs has been reported to be a critical step in progressing to pulmonary fibrosis. This process occurs in the early stage of pulmonary fibrosis development, and by blocking the PDGF-B/PDGFR-ß signalling, the authors were able to prevent recruitment of mural cells to LVs, to restore lymphatic drainage and to ameliorate pulmonary fibrosis (Ref. Reference Meinecke79). This elegant study revealed a crucial role for LVs in the pathogenesis of fibrotic diseases, although it is not yet clear whether the same process holds true for other fibrotic conditions, such as renal fibrosis. Sakamoto et al. (Ref. Reference Sakamoto23) evaluated biopsies from several different chronic renal diseases for LVs by immunohistochemistry. They observed significant increases in LV density (LVD) in tubulointerstitial lesions, which correlate with the degree of tissue interstitial fibrosis. CCR7-positive fibrocytes migrate to the kidney in response to CCL21 chemokine and contribute to the pathogenesis of renal fibrosis. Blocking this CCL21/CCR7 pathway has shown to reduce kidney fibrosis (Ref. Reference Sakai80). As LECs have been shown to actively express and secrete CCL21 chemokine in the kidney (Ref. Reference Kerjaschki45), it is likely that blocking LEC expression of CCL21 could prevent renal fibrosis. We found that prominent lymphangiogenesis in proteinuric kidneys occurs before the influx of macrophages, deposition of collagens and interstitial fibrosis (Ref. Reference Huang72). It appears that lymphangiogenesis would occurs before clear signs of fibrosis, at least in our rat proteinuric model, and this is more in line with a profibrotic response.
TGF-β showed both inhibitory and stimulatory effect on lymphangiogenesis (Refs. Reference Oka81, Reference Kinashi82, Reference James, Nalbandian and Mukouyama83), and LECs are also able to secrete TGF-β (Refs. Reference Podgrabinska84, Reference Malhotra85). Whether LECs in renal disease conditions can secrete TGF-β and play a direct role in fibrogenesis needs further investigations. Zhao et al. (Ref. Reference Zhao86) recently proposed that VEGF-C directly induces fibrogenesis. It is therefore very intriguing to examine VEGFR-3 decoy receptor strategies to catch away and block VEGF-C in some renal diseases with, for example, VEGF-C secretion by activated tubular epithelial cells. By this intervention both fibrosis and lymphangiogenesis should be prevented at the same time.
Figure 2 shows how activated renal lymphatic endothelium contributes to inflammation and fibrosis, and therefore could substantially contribute to tubulointerstitial remodelling in kidney diseases.

Figure 2 The connection between renal lymphangiogenesis, inflammation and fibrosis in the progression of tubulointerstitial remodelling in kidney diseases. In many renal diseases, the initial insults via different mechanisms are able to activate tubular epithelial cells. Upon activation, injured tubular cells start making and secreting a variety of chemokines and meditators which have huge impacts on several key processes in kidney diseases, such as renal inflammation and fibrosis. The reciprocal connection between inflammation and fibrosis are very well studied and known. Both infiltrated inflammatory cells, (myo)fibroblasts and their mediators are shown to promote indirectly renal lymphangiogenesis and remodelling. However, these effects are mutual. In addition to indirect effects, activated tubular epithelial cells (mostly of proximal tubules) secrete specific lymphangiogenic growth factors such as VEGF-C and VEGF-D; therefore directly stimulate the formation of new lymphatic vessel in tubulointerstitial area. Lymphatic endothelial cells under this inflammatory microenvironment also secrete several chemokines as among which CCL21, which can attract CCR7-expressing inflammatory cells and fibrocytes into the perilymphovascular space, and in this way exacerbate further inflammatory and fibrotic reactions.
Transplantation
The exact role of LVs and lymphangiogenesis in organ transplantation remains under debate. Lymphangiogenesis is suggested to be beneficial, for example, by draining inflammatory cells and cytokines, but could also be detrimental, by transporting antigen-presenting cells to lymph nodes and initiating immune responses. Inhibiting lymphangiogenesis in rodent corneal transplantation models was reported to be beneficial by promoting allotransplant survival and appears to be a promising therapeutic tool in enhancing corneal transplant outcomes (Refs Reference Ling87, Reference Tang88, Reference Dietrich89, Reference Uehara90, Reference Zheng, Lin and Ling91). Corneal lymphangiogenesis appears to have negative effects on human cornea graft survival, and has been suggested as a sign of poor prognosis in corneal transplant rejection (Ref. Reference Zheng, Lin and Ling91). Lymphangiogenesis has also been shown to be a common phenomenon in solid organ transplantation, at least in the lungs, kidneys and heart (Refs Reference Geissler92, Reference Dashkevich93, Reference Stuht94). Specific blocking of lymphangiogenesis in experimental heart and islet transplantation models enhances graft survival (Refs Reference Yin55, Reference Nykanen95). Nykänen et al. (Ref. Reference Nykanen95) convincingly demonstrated in experimental mouse cardiac allografts that targeting LVs with anti-VEGFR-3 antibody decreases the number of LECs that express CCL21, infiltrating CD4 + T cells and arteriosclerosis, suggesting a novel intervention to regulate alloimmune activation in solid organ transplantation. Adair et al. (Ref. Reference Adair66) showed that almost all rejected kidney transplantation samples had increased LV numbers as a common phenomenon. Biopsies from human renal allografts showed active lymphangiogenesis, with an approximately 50-fold increase in LV numbers being associated with nodular mononuclear infiltrates (Ref. Reference Kerjaschki45). LECs have been reported to express CCL21 chemokine and attract CCR7+ cells, such as lymphocyte and DCs, providing evidence of an active role for these LECs in organising nodular infiltrates around LVs in the kidney (Ref. Reference Kerjaschki45). This pioneer study highlighted the newly formed LVs and perilymphovascular infiltrates as important origins for alloimmune responses, which have a central role in renal transplant rejection. We, among others, also observed renal lymphangiogenesis in experimental renal transplantation rat models (Refs Reference Vass96, Reference Rienstra97, Reference Palin, Savikko and Koskinen98). Inhibition of the mammalian target of rapamycin (mTOR) blocked lymphangiogenesis, and this anti-lymphangiogenic effect can most probably explain prolonged lymphoedaema in renal transplanted patients treated with rapamycin (Ref. Reference Huber99). In line with these findings, the beneficial effect of sirolimus, another mTOR inhibitor, in ameliorating experimental chronic kidney allograft injury has been related largely to its anti-lymphangiogenic properties (Ref. Reference Palin, Savikko and Koskinen98). In addition to sirolimus-related lymphedema, Ersoy. and Koca: (Ref. Reference Ersoy and Koca100) recently reported everolimus-related lymphedaema in renal transplantation, which indicates the anti-lymphangiogenic activity of everolimus. The therapeutic value of these mTOR inhibitors in blocking lymphangiogenesis remains to be elucidated in some conditions where new lymph vessel formation is detrimental. Blocking lymphangiogenesis seems to be a double-edged sword intervention after organ transplantation. It might be beneficial by interrupting the route of antigen-presenting cells (APCs) to reach lymph node and preventing raising of alloimmune response or detrimental by hampering the clearance of inflammatory cells, interstitial fluids and macromolecules. This area needs further experimental and clinical studies in order to prove the pros and cons of therapeutic values of targeting renal lymphatic and lymphangiogenesis in the course of kidney transplantation.
Renal microenviromental homoeostasis and oedema
LVs have a central role in maintaining fluid transport in the body, and disruption of this pivotal function leads to accumulation of tissue fluid and oedema formation. Interstitial fluid pressure increases in conditions where lymphatic drainage is insufficient, such as in lymphoedaema, causing tissue swelling, increased hydraulic conductivity and remodelling of interstitial matrix (Ref. Reference Mendez101). Renal interstitial oedema and swelling are common as a consequence of the tubulointerstitial injuries in chronic kidney diseases associated with proteinuria (Ref. Reference Rodriguez-Iturbe, Herrera-Acosta and Johnson102). The establishment of renal lymphatic circulation appears to be crucial to renal homoeostasis and function.
Experimental disruption of lymphatic circulation in rat kidneys causes protein-rich fluid retention in renal interstitium, fibrosis, renal cell apoptosis and severe proteinuria, and collectively leads to marked chronic kidney failure (Refs Reference Zhang103, Reference Zhang104). On the other hand, lymphangiogenic therapies have shown promising results in restoring lymphatic function and drainage, thereby ameliorating tissue oedema (Ref. Reference Choi105). Provoking lymphangiogenesis by viral vector overexpression of VEGF-C or exogenous administration of recombinant VEGF-C profoundly decreased interstitial fluid accumulation in experimental lymphoedaema models (Refs Reference Szuba106, Reference Cheung107). Of remarkable interest is a recent study by Planas-Paz et al. (Ref. Reference Planas-Paz108) which convincingly showed that increased interstitial fluid pressure can mechanically prompt lymphangiogenesis in embryos. They demonstrated that mechanical stretching of LECs induces their proliferation in embryonic life, leading to increased LV's size to improve their ability to drain fluid. This mechanism could be important for oedematous disorders in adult life.
Fascinating discoveries from Titze's lab (Refs Reference Machnik109, Reference Machnik110, Reference Wiig111) on the subject of nonosmotical storage of sodium in the subcutaneous interstitium involving immune cells and LVs, usher in a new era in the sodium homoeostasis and hypertension field. This unique sodium storage subsequently induces lymphangiogenesis in the skin via the macrophage/VEGF-C signalling pathway, thereby creating buffering phenomena to halt hypertension in the salt-overload state. This lymphangiogenic response to high-salt diet appears to be specific to the skin and is not observed in the kidney. Interestingly, we found that plasma VEGF-C is modulated by salt intake, at least in proteinuric patients with chronic kidney disease, with higher VEGF-C levels being observed during high salt intake. We also reported increased VEGF-C level in proteinuric patients compared with healthy control individuals for both sodium intakes (Ref. Reference Slagman112). Circulating VEGF-C is also higher in preeclamptic women compared with normal pregnant women (Ref. Reference Lely113). Although the response of VEGF-C to altered sodium intake in preeclamptic women remains unclear, this does suggest that increase in the lymphangiogenic state also occurs in preeclampsia, as a parallel to our observation of CKD proteinuric patients (Ref. Reference Toering, Navis and Lely114). These data emphasise that blood pressure regulation is not only a kidney affair and actually is more complex, highlighting the role of LVs and immune cells in hypertensive disorders. How these advances can be transferred to daily clinical practice for preventive or curative therapy is the issue of current research area in this field.
Proteinuria
Many renal diseases are associated with proteinuria. Since proteinuria is independently associated with the decline in renal function, anti-proteinuric treatment comprises a major cornerstone in clinical nephrology. Nevertheless, annihilation of proteinuria is very difficult, and most patients slowly progress towards renal failure. This indicates the need for additional treatment modalities: not only in trying to reduce proteinuria even further, but also to reduce the harmful effects of proteinuria downstream. Overexposure to filtered plasma proteins injures and activates tubular epithelia cells, and upon activation they start secreting several kinds of chemokines and mediators (Ref. Reference Mono115). We recently showed in a time-course study of experimental rat proteinuric nephropathy that proteinuria can trigger renal lymph vessel formation (Ref. Reference Yazdani70). Secretion of VEGF-C by activated proximal tubular epithelial cells secondary to proteinuria was suggested to be the main source for promoting proteinuria-driven renal lymphangiogenesis. We observed in this model that early proteinuria cannot evoke renal lymphangiogenesis up to six weeks. However, prolonged and chronic proteinuria by activation of the tubular epithelial cells can trigger massive renal lymphangiogenesis at week 12. In addition, anti-proteinuric intervention prevented this renal lymphangiogenesis. We found that renal lymphangiogenesis occurs in line with tubular activation (evidenced by osteopontin expression), profibrotic reactions (interstitial α-SMA); however, before the influx of ED1-positive macrophages, and before deposition of collagen I and III. This suggests that renal lymphangiogenesis is an early response, at least in our model, and starts prior to the development of fibrosis and inflammation. As already mentioned, LECs also express CCL21, which can attract CCR7+ fibrocytes and immune cells and are therefore able to promote renal fibrosis. Although we did not investigate whether LECs express this chemokine at the early phase (6 weeks), blocking lymphangiogenesis appears to be a promising intervention to prevent proteinuria-mediated tubulointerstitial inflammation and fibrosis, and is currently under investigation. Another observation was concomitant tubular expression of osteopontin which, after VEGF-C, is a potent lymphangiogenic factor, at least in the melanoma cell line (Ref. Reference Liersch116). To date, the role of osteopontin in renal lymphangiogenesis remains unknown.
Diabetic nephropathy
Hyperglycaemia has been linked to LV remodelling and lymphangiogenesis in various organs (Refs Reference Moriguchi117, Reference Haemmerle118, Reference Kivela119, Reference Yin120). Sakamoto et al. (Ref. Reference Sakamoto23) reported that lymphangiogenesis and VEGF-C expression was significantly higher in human biopsies with type 2 diabetic nephropathy than in nondiabetic renal diseases. Notably, there was no correlation between renal lymphangiogenesis, the extent of fibrosis and inflammation in these biopsies, which could suggest that diabetic conditions have a direct impact on the growth of renal lymphatics. Similarly, oedematous renal tissues with nephropathy and renal lymphangiogenesis have been observed in diabetic mouse kidneys (Ref. Reference Uchiyama121). However, the effect of glucose and advanced glycation end-products on VEGF-C production and other lymphangiogenic mediators which orchestrate renal lymphangiogenesis in diabetic nephropathy and its impact on renal pathophysiology has not yet been fully explored.
Renal cancer
The potent impact of lymphatic invasion and lymphangiogenesis in cancer development and metastasis has been extensively investigated, and has been suggested to be associated with poor prognosis in several human malignant tumours (Ref. Reference Karaman and Detmar122). However, the significance of lymphangiogenesis, compared with angiogenesis, is unclear in renal cancers, even in the most common kidney tumour, renal cell carcinoma. Despite the fact that the VEGF-C/D/VEGFR-3-axis has been reported to be important in lymphatic tumour spread (Ref. Reference Lin123), others (Ref. Reference Bierer124) found no correlation for this axis with clinicopathological parameters. Using the D2–40 antibody and applying an immunohistochemistry approach, one study (Ref. Reference Horiguchi125) reported that intratumoural LVs and lymphatic invasion are associated with renal cell carcinoma tumour aggressiveness, and that patients with intratumoural LVs have poor prognosis. Two other studies reported that intratumoural LVs rather than peritumoural LVs correlate with prognostic values such as distant metastasis, and can be used as indicators of high risk of renal cell carcinoma metastasis (Refs Reference Ozardili126, Reference Debinski127). However, other studies aiming to evaluate lymphangiogenesis in renal cell carcinoma were largely unable to find intratumoural LVs and no correlation between these findings (intratumoural and peritumoural LVs) and prognostic clinical parameters was found (Refs Reference Bonsib25, Reference Baldewijns128, Reference Iwata129). LV involvement within the sinus and renal pelvis have also been proposed to be sources for tumour cell spread via LVs and lymph node metastasis in clear cell renal cell carcinoma (Ref. Reference Ishikawa130). Recently, in a large single institution analysis of renal cell carcinoma patients, capillary-lymphatic invasion was suggested to be independently associated with renal cell carcinoma death and metastasis (Ref. Reference Eisenberg131). Although this needs to be validated by further studies, Kroeger et al. (Ref. Reference Kroeger132) reported for a small cohort that clear cell renal cell carcinoma with lymphatic spread can be characterised by unique molecular, clinicopathological and genetic changes, such as high epithelial VEGFR-2 protein expression and low chromosome 3p loss at the genetic level. Other studies have revealed no induction of VEGF-C in renal cell carcinoma (Ref. Reference Voss22) and a very limited ongoing lymphangiogenesis (Ref. Reference Iwata129). Collectively, the conflicting data on lymphangiogenesis in renal cell carcinoma does not yet allow any clear conclusion on its potential roles in tumour growth and metastasis and warrant future investigations.
Summary
LVs and lymphangiogenesis are actively involved in many pathological conditions. In various diseases, LEC activation and lymphangiogenesis is the response of LVs to disturbances in microenvironmental homoeostasis in order to maintain the physiological conditions. One of the main functions of LVs is to enforce the balance between interstitial fluid transport and maintaining tissue homoeostasis. Therefore, it is conceivable that any injury that has a negative effect on LV function, leads to tissue fluid accumulation and interstitial oedema. In almost all chronic kidney diseases, especially those associated with proteinuria, interstitial swelling and oedema is a common finding. This swelling of the renal interstitial space is known to induce remodelling of the interstitial matrix and, as a consequence, renal fibrosis. As CKD is a multifactorial disease with complex pathophysiology, renal oedema can be considered as a one of the active factors in promoting CKD. The potential significance of LVs can be evaluated by modulating its development in experimental models of CKD. Potential strategies, including increasing LV number and/or function, have been proposed as a therapeutic option in patients suffering from lymphedaema. These interventions may have the same promising effects in kidney diseases associated with renal oedema. In many cases, an increase in LV number might be detrimental by exacerbating the inflammatory milieu and thereby actually promoting the disease condition. There is a well-established reciprocal interaction between inflammation and lymphangiogenesis. Inflammatory cells and mediators are the most potent lymphangiogenic inducers, and the activated LECs in this inflammatory milieu secrete several chemokines which attract inflammatory cells around those LVs which are further upholding that inflammatory microenvironment. Recent evidence showed that modulating lymphangiogenesis could serve as a therapeutic strategy in inflammatory diseases. Many renal diseases are associated with inflammatory reactions; therefore targeting LVs might be useful in reducing the progression of renal inflammation. Several fibrotic renal diseases are closely associated with renal lymphangiogenesis. Although not investigated specifically in renal diseases yet, a direct causal link between lymphangiogenesis and fibrosis in other organs has also been proposed. LECs are able to secrete various mediators and chemokines which not only promote the inflammatory process, but are also directly involved in the initiation and progression of fibrosis. Inhibiting renal lymphangiogenesis, and as a result, blocking the expression of several mediators by LECs might be considered as a therapeutic rationale in preventing the progression of renal fibrosis. Proteinuria triggers renal lymphangiogenesis. Currently, the significance of this proteinuria-induced renal lymphangiogenesis is not known. Since proteinuria is associated with renal oedema; LV formation may be a positive response in order to promote removal of accumulated excess interstitial fluids from the kidney. In addition, renal lymphangiogenesis is one of the interstitial remodelling processes downstream of proteinuria. The direct blocking of lymphangiogenesis may not be an effective approach, as continuous activation of tubular epithelial cells by ultrafiltrated plasma proteins plays the major role in the expression of lymphangiogenic growth factors such as VEGF-C. Stimulation of renal lymphangiogenesis might be useful as an add-on to other drugs that directly target activated tubular cells or downstream secreted mediators. Lymphangiogenesis in the context of transplantation may be a double-edged sword. Lymphangiogenesis can be beneficial in order to remove the inflammatory cells and mediators as well as excess fluid from the transplanted organ and in this way preventing oedema and further tissue injury. On the other hand, lymphangiogenesis can be detrimental as they facilitate transport of APCs as well as chemokines, cytokines, and inflammatory mediators to the draining lymph nodes and initiating alloimmuno reactions thereby promoting graft rejection. The usefulness of blocking pre- and post-transplant lymphangiogenesis has been shown in experimental models of organ transplantation. However, this has not been described renal transplantation yet and needs further investigation before it can be considered as a clinical approach in renal transplant medicine.
Lymphangiogenesis is mostly investigated in the cancer field of research as one of the main routes for tumour metastasis. Several kinds of renal cancers are also shown to be associated with pre and/or intratumoural lymphangiogenesis. Inhibiting lymphangiogenesis might also be a therapeutic option to target malignancies of the kidney. Extensive investigations that are currently being performed in both the experimental and clinical cancer field will definitely provide us with more knowledge about the role and therapeutic values of anti-lymphangiogenic therapy in preventing tumour growth and metastasis, and may have the same implication in kidney tumours.
Perspective
The active role of LVs and lymphangiogenesis in many pathological conditions is well-established today. Recently, the anti-VEGFR3 antibody for blocking tumour lymphangiogenesis entered phase 1 clinical trials. However, the beneficial or detrimental effects of lymphangiogenesis would appear to be very context-dependent and organ-specific. As such, both promoting lymphangiogenesis – for example, by using VEGF-C – and inhibiting it could be served as therapeutic modalities. The available knowledge brings the renal lymphatic network forward as an intriguing and promising structure in modulating interstitial homoeostasis, inflammation and fibrosis in kidney diseases. Specific targeting of lymphangiogenesis in renal disease models would appear to be crucial to unravelling the causal relation between lymphangiogenesis and fibrosis, and could be a novel therapeutic strategy to prevent renal fibrosis, progression to End Stage Renal Disease and renal function loss. Improved understanding of the detailed mechanisms involved in renal lymphatic functions and remodelling are needed and require future research to provide possible therapeutic interventions for curative or preventive approaches in renal diseases.
Financial support
Saleh Yazdani is financially supported by the Graduate School of University Medical Centre Groningen.
Conflicts of interest
None.
Acknowledgement
The authors would like to thank Professor Mohamed R. Daha for his critical comments and reading the manuscript.