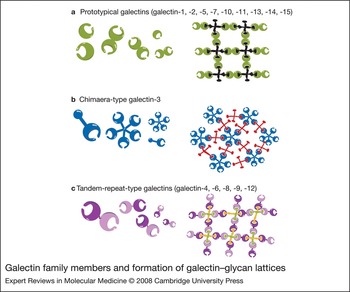
Galectin ancestors are present in very primitive organisms such as sponges and nematodes. As the animal kingdom evolved, the family expanded, presumably to meet the need for fulfilling more-complex tasks. To date, 15 members have been identified in mammals. Some have a wide tissue distribution, while others have higher tissue specificity; among the latter, each has a distinct tissue distribution pattern (Ref. Reference Cooper1). All galectins contain conserved carbohydrate-recognition domains (CRDs) of about 130 amino acids that are responsible for carbohydrate binding: prototypical galectins have one CRD (galectin-1, -2, -5, -7, -10, -11, -13, -14 and -15); the tandem-repeat-type galectins contain two homologous CRDs in a single polypeptide chain, separated by a linker of up to 70 amino acids (galectin-4, -6, -8, -9 and -12); and galectin-3 contains a nonlectin N-terminal region (about 120 amino acids) connected to a CRD (Fig. 1). A number of two-CRD galectins are known to exist in several isoforms, differing in the length of the linker sequences. Galectins were initially designated as S-type lectins, because some members require reducing conditions to maintain their activities (S stands for sulphydryl or thiol), but this is now a unique feature of only a small number of members (galectin-1 and -2) (Refs Reference Cooper1, Reference Cho and Cummings2).
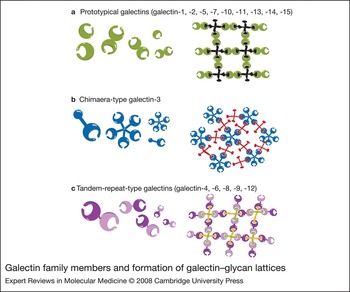
Figure 1. Galectin family members and formation of galectin–glycan lattices. Galectins can be subdivided into three groups: (a) prototypical galectins, containing one carbohydrate-recognition domain (CRD); (b) galectin-3, a chimaera-type galectin consisting of unusual tandem repeats of proline- and glycine-rich short stretches fused onto the CRD; and (c) tandem-repeat-type galectins, which contain two distinct CRDs in tandem, connected by a linker. Many galectins are either bivalent or multivalent with regard to their carbohydrate-binding activities: one-CRD galectins often exist as dimers; galectin-3 forms petamers upon binding to multivalent carbohydrates; and two-CRD galectins have two carbohydrate-binding sites. Thus, galectins can form lattices with multivalent glycoconjugates.
Sequence identity among most sequenced human galectin CRDs reported in the literature ranges from 20% to 50%. Notable exceptions include the C-terminal CRD of galectin-12, which is less than 20% identical to most other galectin CRDs; and galectin-10, -13 and -14, which have identities close to or greater than 50% to one another but below 20% to the CRDs of galectin-1, -2 and -12.
Each galectin has an individual carbohydrate-binding preference (Ref. Reference Hirabayashi3). Many galectins are either bivalent or multivalent with regard to their carbohydrate-binding activities: some one-CRD galectins exist as dimers; two-CRD galectins have two carbohydrate-binding sites and are thus at least bivalent; and galectin-3 forms pentamers upon binding to multivalent carbohydrates. Thus, galectins are capable of forming ordered arrays made of lectin and multivalent glycoconjugates (Refs Reference Fred Brewer4, Reference Sacchettini, Baum and Brewer5) (Fig. 1).
Galectin family members do not contain a classical signal sequence. Consistent with this feature, the proteins are localised primarily in the cytoplasm, as well as in the nucleus under certain conditions. However, they can be secreted and thus belong to the group of proteins that do not contain a signal sequence but can function outside cells (Ref. Reference Elola6).
The ability of recombinant galectins to exert various in vitro activities by engaging glycans on the cell surface as well as extracellular matrices has been extensively and convincingly documented (Refs Reference Elola6, Reference Ochieng, Furtak and Lukyanov7). Some galectins have been shown to bind to several different cell-surface antigens or receptors, in a carbohydrate-dependent manner. The picture that has emerged is that galectins do not have specific individual receptors, but that each can bind to a set of cell-surface or extracellular matrix glycoproteins containing suitable oligosaccharides. However, a large number of studies have demonstrated that galectins can also function inside cells and, interestingly, they may do so in a fashion that is independent of their carbohydrate-binding activities. Evidence is accumulating that galectins regulate signal transduction, by binding to intracellular ligands and participating in intracellular signalling pathways (reviewed in Ref. Reference Liu, Patterson and Wang8).
Galectin-1
The crystal structure of human galectin-1 consists of a six-stranded and a five-stranded β-sheet in an antiparallel arrangement (Ref. Reference Liao9). In solution, galectin-1 can form homodimers. The extracellular presence and function of galectin-1 is well established (Refs Reference Hsu and Liu10, Reference He and Baum11, Reference Rabinovich12, Reference Rubinstein13), even though the mechanism of its secretion remains to be determined. Recently, cell-surface glycans with affinity for galectin-1 have been shown to be important for the unconventional secretion of galectin-1, and intact carbohydrate-binding activity is essential for its secretion (Ref. Reference Seelenmeyer14). How interactions of galectin-1 with these glycans control its secretion remains to be elucidated. Evidence for intracellular functions also exist (Ref. Reference Liu, Patterson and Wang8), including pre-mRNA splicing activity (Ref. Reference Vyakarnam15).
Galectin-1 and the immune system
Galectin-1 is expressed by stromal cells surrounding pre-B cells, where it binds to the pre-B-cell receptor (BCR) and is involved in synapse formation between pre-B cells and stromal cells (Ref. Reference Gauthier16); through this interaction galectin-1 contributes to pre-BCR activation (Ref. Reference Rossi17). However, galectin-1 also negatively regulates B-cell proliferation and BCR-mediated signal transduction (Ref. Reference Yu, Siegel and Roeder18). Interestingly, galectin-1 binds to the B-cell-specific transcriptional coactivator OCA-B, and secretion of the lectin appears to be negatively regulated by this interaction (Ref. 18).
Galectin-1 has been shown to affect T-cell homeostasis by modulating cytokine production, proliferation and apoptosis. Perillo et al. (Ref. Reference Perillo19) found that galectin-1 induces apoptosis in subsets of thymocytes in vitro. The activity of galectin-1 in inducing apoptosis in activated T cells has subsequently been extensively studied by several research groups, and this topic has been comprehensively reviewed (Refs Reference Rabinovich20, Reference Hernandez and Baum21). Galectin-1 induces apoptosis through binding to selected cell-surface glycoproteins and triggering apoptosis signalling pathways. For example, in human T cell lines it triggers rapid translocation of endonuclease G from mitochondria to nuclei, in the absence of the cytochrome c release from mitochondria, nuclear translocation of apoptosis-inducing factor, and caspase activation that are commonly associated with apoptosis (Ref. Reference Hahn22). However, in another study galectin-1 was shown to sensitise human T cells to FAS (CD95)/caspase-8-mediated cell death through a mechanism involving an increase in the mitochondrial membrane potential, cytochrome c release, and participation of the ceramide pathway (Ref. Reference Matarrese23). Additional work further suggested that while galectin-1 induces surface phosphatidylserine exposure in activated T cells, rendering them positively stained by annexin V (a reagent commonly used for detecting apoptotic cells), it does not trigger a complete cell death pathway (Ref. Reference Stowell24). Thus, galectin-1 may trigger different endpoints along the apoptotic pathways in different target cells.
It has been shown that galectin-1 specifically suppresses T helper 1 (Th1)- and Th17-mediated immune responses, by inducing apoptosis in terminally differentiated T cells expressing specific carbohydrate ligands and/or by modulating cytokine production. Splenocytes from galectin-1-deficient (Lgals1 −/−) mice produce more interferon γ (IFN-γ; a Th1 cytokine) and interleukin 17 (IL-17; a Th17 cytokine) (Ref. Reference Toscano25), consistent with the higher sensitivity of Th1 and Th17 cells to apoptosis induced by galectin-1 (Ref. Reference Toscano25). Thus, increased galectin-1 expression or administration of galectin-1 is expected to cause the immune response to shift to the Th2 direction.
Importantly, galectin-1 also appears to mediate at least part of the immune inhibitory function of regulatory T cells. First, it is expressed by regulatory T cells, and its level further increases after the cells are activated. Second, the inhibitory effects of human and mouse CD4+CD25+ (regulatory) T cells are significantly reduced by blockade of galectin-1 (Ref. Reference Garin26). These results suggest another mechanism by which galectin-1 negatively regulates the adaptive immune response.
Galectin-1 also affects the physiology of monocytes and macrophages, as well as other antigen-presenting cells, through mechanisms that are independent of its ability to induce apoptosis. These include effects on antigen presentation and phagocytosis (Ref. Reference Barrionuevo27), and alternative activation of peritoneal macrophages (Ref. Reference Correa28). Furthermore, dendritic cells treated with galectin-1 have an enhanced migratory capacity through extracellular matrix (Ref. Reference Fulcher29). In addition, dendritic cells engineered to overexpress galectin-1 can stimulate naive T cells and induce apoptosis in activated T cells (Ref. Reference Perone30). Galectin-1 can affect other blood cells besides immune cells; for example, it can affect platelet activation and function (Ref. Reference Pacienza31).
The immunosuppressive activity of galectin-1 has been well demonstrated in vivo by using Lgals1 −/− mice. These mice exhibit more pronounced disease in an experimental autoimmune encephalomyelitis model (Ref. Reference Toscano25). In addition, they showed higher rates of fetal loss compared with wild-type mice in allogeneic matings, suggesting a role for galectin-1 in preventing fetal loss by mediating immune tolerance at the fetal–maternal interface (Ref. Reference Blois32). The immunosuppressive activity of galectin-1 has also been amply demonstrated by administration of recombinant galectin-1 or delivery of cells engineered to overexpress galectin-1 in several animal models of inflammatory and autoimmune diseases, including collagen-induced arthritis, concanavalin-A-induced hepatitis, experimental colitis, graft-versus-host disease, diabetes and experimental autoimmune uveitis (Refs Reference Levi, Tarrab-Hazdai and Teichberg33, Reference Offner34, Reference Rabinovich35, Reference Santucci36, Reference Santucci37, Reference Baum38, Reference Toscano39, Reference Perone40, Reference Bianco41). Whether the prevailing mechanisms underlying the immunosuppressive effects of galectin-1 involve the induction of T cell apoptosis, the modulation of cytokine production, or the generation of regulatory T cells (or the sum of these effects) remains to be investigated.
Galectin-1 and tumour development/progression
Upregulation of galectin-1 is well documented in different tumour types (Ref. Reference Camby42) and, in general, expression of this protein is associated with poor prognosis and acquisition of a metastatic phenotype (Ref. Reference Liu and Rabinovich43). Galectin-1 expressed by tumours favours tumour growth and influences tumour progression and metastasis by modulating various biological events, including cell migration, adhesion and angiogenesis (Ref. Reference Liu and Rabinovich43). Galectin-1 binds oncogenic H-RAS and promotes its anchorage to the plasma membrane, a process necessary for the role of this oncoprotein in neoplastic transformation (Ref. Reference Paz44); this results in sustained activation of RAF-1 and mitogen-activated protein kinase 1 (MAPK1), which contribute to tumour transformation (Ref. Reference Elad-Sfadia45). Other examples of the roles of galectin-1 in tumour biology include modulation of the in vivo migratory capacity of glioblastoma cells (Ref. Reference Camby46), and the sensitivity of tumour cells to chemotherapeutic agents (Ref. Reference Mathieu47).
Galectin-1 also plays a role in tumour cell evasion of the immune response. Galectin-1 contributes substantially to the immunosuppressive activity of melanoma cells, and this has been related to induction of apoptosis in tumour-directed cytolytic T cells by galectin-1 secreted by tumour cells and modulation of the Th1–Th2 cytokine balance (Ref. Reference Rubinstein48). In addition, recent studies revealed that Reed–Sternberg cells in classical Hodgkin lymphoma overexpress galectin-1, which favours a Th2 response and promotes the expansion of regulatory T cells (Refs Reference Gandhi49, Reference Juszczynski50), and thus may contribute to the immunosuppressive microenvironment in Hodgkin lymphoma.
Galectin-1 has an important role in tumour angiogenesis. Growth of tumours is diminished in Lgals1 −/− mice as a result of deficient angiogenesis (Ref. Reference Thijssen51). A further analysis revealed that galectin-1 expressed in endothelial cells is a target of the antiangiogenesis peptide anginex (Ref. Reference Thijssen51). Moreover, expression of galectin-1 by tumour-associated stromal cells in breast cancer correlates with increased tumour invasiveness (Ref. Reference Jung52) and galectin-1 expressed on endothelial cells in prostate tumour can inhibit T cell migration across endothelial cells (Ref. Reference He and Baum53). In addition, fluctuations in the levels of galectin-1 in the tumour microenvironment may influence attachment and detachment of cancer cells – a process that is essential for cancer progression (reviewed in Ref. Reference Elola6). Thus, galectin-1 expressed by tumour or stromal cells can favour tumour progression through modulation of several mechanisms, including cell migration, adhesion, angiogenesis and tumour immune escape.
Galectin-1 in the neural system and muscle development
Puche and Key (Ref. Reference Puche and Key54) showed that galectin-1 is expressed in primary sensory olfactory neurons, and immobilised recombinant galectin-l promotes neurite outgrowth from a mouse olfactory neuron cell line. This effect was neutralised by soluble galectin-1 and its inhibitor, lactose. They also showed that Lgals1 −/− mice had an aberrant topography of olfactory axons and a subset of primary sensory olfactory axons failed to project to their correct target sites in the caudal olfactory bulb in these mice (Ref. Reference Puche55). Recently, by using a proteomic approach, Sakaguchi et al. (Ref. Reference Sakaguchi56) identified galectin-1 as a factor that promotes proliferation of neural stem cells. This activity was confirmed by studying Lgals1 −/− mice and by demonstrating that exogenously added galectin-1 promotes proliferation of neural stem cells in the adult brain (Ref. Reference Sakaguchi56).
By contrast, Plachta et al. (Ref. Reference Plachta57) identified galectin-1 by proteomic analysis as a protein significantly upregulated in degenerating neurons, and showed that recombinant galectin-1 caused degeneration of neuronal processes. Furthermore, degeneration of neurons induced by a chemical was suppressed by galectin-1 inhibitors. In addition, elimination of nerve endings following axotomy was significantly delayed in Lgals1 −/− mice compared with wild-type mice.
Additional experiments are required to clarify why galectin-1 promotes growth of neural stem cells but causes degeneration of neuron processes. One possible explanation is that the active components responsible for these two processes are distinct. Horie et al. also found galectin-1 to be a soluble factor that enhances axonal regeneration (Ref. Reference Horie58) and determined that the active component is the oxidised form of galectin-1 (Ref. Reference Inagaki59), which, interestingly, does not have carbohydrate-binding activity. Galectin-1 is known to be oxidised and lose its carbohydrate-binding activity in the absence of reducing agents. Thus, the intriguing possibility exists that upon oxidation in the oxidising extracellular environment, this protein is transformed into another entity that functions distinctly from its reduced form. However, Sakaguchi et al. (Ref. Reference Sakaguchi56) did show that only the reduced form of galectin-1 promotes proliferation of neural stem cells.
During myogenesis, galectin-1 is expressed by proliferating myoblasts and secreted to the extracellular matrix, when these cells commence terminal differentiation and fuse into multinucleated fibres (Ref. Reference Cooper and Barondes60). Addition of galectin-1 to myogenic cells plated on an extracellular matrix protein inhibited myoblast spreading and fusion, suggesting that the protein regulates muscle cell interactions with the extracellular matrix that are critical to myogenic development (Ref. Reference Cooper, Massa and Barondes61). Additionally, exogenously added galectin-1 induced mesenchymal stem cells to differentiate into muscle and promoted regeneration of muscle (Ref. Reference Chan62). Furthermore, myoblasts derived from Lgals1 −/− mice showed reduced ability to fuse in vitro, and in these mice muscle fibre development at the neonatal stage is delayed and muscle fibre diameter at the adult stage is reduced (Ref. Reference Georgiadis63). These studies provide evidence that galectin-1 promotes muscle differentiation.
Galectin-2
X-ray crystallography of galectin-2 complexed with lactose revealed that the protein exists as twofold symmetric dimers containing two extended antiparallel β-sheets, in a β-sandwich arrangement (Refs Reference Lobsanov64, Reference Lobsanov65). Like galectin-1, galectin-2 can induce T-cell apoptosis (Ref. Reference Sturm66). In addition, the level of expression of galectin-2 is inversely related to the degree of colitis in a mouse model (Ref. Reference Paclik67), and treatment of mice with recombinant galectin-2 induced apoptosis in mucosal T cells and reduced colitis in this model.
Ozaki et al. (Ref. Reference Ozaki68) showed that smooth muscle cells and macrophages in human atherosclerotic lesions express both galectin-2 and the cytokine lymphotoxin α (LTA), and that galectin-2 binds to this cytokine. Furthermore, a single-nucleotide polymorphism (SNP) in the Lgals2 gene, which affects its transcription in vitro, was shown to be significantly associated with susceptibility to myocardial infarction. These findings suggest that galectin-2, at least in this Japanese population, can act as a link between the LTA cascade and the pathogenesis of myocardial infarction.
Galectin-3
Galectin-3 is the only member of the galectin family with an extended N-terminal region composed of tandem repeats of short amino acid segments (a total of approximately 120 amino acids) connected to a C-terminal CRD. Unexpectedly, chicken galectin-3 contains sequences indicative of a transmembrane domain (Ref. Reference Gorski69). The three-dimensional structure of galectin-3 CRD in complex with lactose or N-acetyl-lactosamine is composed of a five-stranded and a six-stranded β-sheet in a β-sandwich arrangement (Ref. Reference Seetharaman70), like galectin-1 and -2. Unlike galectin-1 and -2, this CRD does not exist as homodimers in the crystal. By nuclear magnetic resonance spectroscopy, galectin-3 was found to exist as monomers in solution and the N-terminal fragment has an unfolded, extended structure; however, in full-length galectin-3, the N-terminal domain residues 94 to 113 interact with the CRD (Ref. Reference Birdsall71).
Like other galectins, galectin-3 lacks a signal sequence required for secretion through the classical secretory pathway, but the protein is released into the extracellular space. Hughes' group has provided evidence suggesting that the rate-limiting step for galectin-3 secretion is its translocation to the cytosolic side of the plasma membrane (Ref. Reference Mehul and Hughes72). Galectin-3 can oligomerise in the presence of multivalent carbohydrate ligands and is capable of crosslinking glycans on the cell surface, thereby initiating transmembrane signalling events and affecting various cellular functions (reviewed in Refs Reference Ochieng, Furtak and Lukyanov7, Reference Liu and Rabinovich43, Reference Almkvist and Karlsson73). This self-association property is dependent on the N-terminal region of the protein. As this region is sensitive to proteases, such as collagenase and matrix metalloproteinases (MMPs) (Ref. Reference Ochieng74), the in vivo biological activities of galectin-3 are likely to be modulated by these enzymes.
Compared with other galectins, intracellular functions of galectin-3 have been more extensively documented (reviewed in Ref. Reference Liu, Patterson and Wang8). Like galectin-1, galectin-3 can induce pre-mRNA splicing (Ref. Reference Dagher, Wang and Patterson75). Several other intracellular functions have been reported for galectin-3 and, in some cases, intracellular proteins with which it interacts and that possibly mediate these functions have been identified. Of relevance to the intracellular functions of galectin-3, it is noteworthy that galectin-3 can be phosphorylated at the serine 6 (Ser6) and serine 12 residues (Ref. Reference Huflejt76) (see next section).
Galectin-3 in regulation of cellular responses
By using gene transfection and antisense approaches, galectin-3 has been found to have anti-apoptotic activity in a number of cell types against a diverse array of apoptotic stimuli. This topic has been extensively reviewed (Refs Reference Hsu and Liu10, Reference Hsu, Yang and Liu77). Mechanisms by which intracellular galectin-3 confers resistance to apoptosis remain to be fully elucidated, but existing information suggests that they may involve interaction with other regulators of apoptosis, including those operating in the mitochondria (Ref. Reference Hsu, Yang and Liu77). The anti-apoptotic activity of galectin-3 is apparently dependent on phosphorylation of Ser6 (Ref. Reference Yoshii78). The phosphorylation is also necessary for the export of the protein out of the nucleus when cells are exposed to apoptotic stimuli (Ref. Reference Takenaka79).
Galectin-3 can bind in a carbohydrate-dependent manner to extracellular matrix proteins, including the integrins α1β1 and αMβ1 (CD11b/18), and thus influences cell adhesion to extracellular matrices (Ref. Reference Hughes80). As mentioned above, galectin-3 can form pentamers upon binding to multivalent glycans. Conceptually, the multivalency of pentameric galectin-3 allows it to act as a bridge between cells and the extracellular matrix, and between cells, by binding simultaneously to glycans on two binding partners. Also, pentameric galectin-3 can form lattices with selected cell-surface glycans. This has been demonstrated for the T-cell receptor (TCR), epidermal growth factor receptor, and transforming growth factor β receptor. It was suggested that galectin-3-mediated lattice formation on the cell surface downregulates the activation of some receptors and attenuates endocytosis of others (Refs Reference Demetriou81, Reference Partridge82).
Finally, galectin-3 has been shown to regulate expression of certain genes, including cancer-related genes, such as those for cyclin D1, thyroid transcription factor 1 and mucin 2 (reviewed in Ref. Reference Nakahara and Raz83). It has also been shown to regulate c-Jun-N-terminal kinase 1 (JNK1) in mast cells (Ref. Reference Chen84). The underlying mechanisms for these effects are still unknown.
Galectin-3 in immune and inflammatory responses
Galectin-3 affects differentiation and growth of various immune cells: it induces apoptosis in T cells and neutrophils; and it activates several lymphoid and myeloid cells, including mast cells, neutrophils, monocytes and T cells, resulting in mediator release, superoxide anion production, and cytokine production (reviewed in Refs Reference Rabinovich20, Reference Liu85). Recombinant galectin-3 can also function like a chemokine in inducing migration of human monocytes and macrophages. Similar to chemokines, the activity is mediated through a pertussis toxin (PTX)-sensitive (G-protein-coupled) pathway and associated with a Ca2+ influx, and thus a specific chemokine receptor(s) may be involved (see references in Refs Reference Rabinovich20 and Reference Liu85). The receptors responsible for all of the above-described effects have not been established. The lectin may bind to several glycoproteins on the cell surface and may induce responses through different receptor(s) on different cell types.
Galectin-3 can also exert a suppressive effect on myeloid cells, as exemplified by inhibition of IL-5 production in human eosinophils (Ref. Reference Cortegano86). As mentioned above, galectin-3 can form lattices with the TCR complex, and through this mechanism serve as a negative regulator of TCR-initiated signal transduction. The fact that this lectin has promoting functions in some cells but suppressive activities in others may not be too surprising, since conceivably it can bind to either receptors that deliver positive signals or those that deliver negative signals.
Recombinant galectin-3 promotes adhesion of human neutrophils to laminin (Ref. Reference Kuwabara and Liu87) and to an endothelial cell line (Ref. Reference Sato88). In the case of the former, the lectin both serves as a bridge between the cell and the extracellular matrix and activates the cell through binding to cell-surface glycans, resulting in increased adhesive properties. By contrast, exogenously added galectin-3 attenuates interaction of thymocytes with thymic nurse cells in vitro (Ref. Reference Silva-Monteiro89), possibly through steric hindrance of the normal interactions between cell adhesion molecules that are involved in maintaining cell–cell or cell–matrix interactions.
Some evidence that endogenous galectin-3 possesses functions demonstrated by using recombinant galectin-3 has been provided. For example, adhesion of T cells to dendritic cells and macrophages can be inhibited by known galectin-3 inhibitors (Ref. Reference Swarte90). Additionally, endogenous galectin-3 was shown to participate in homotypic aggregation of monocytes induced by antibody against CD13 (Ref. Reference Mina-Osorio, Soto-Cruz and Ortega91), as this aggregation was inhibited by galectin-3 inhibitors.
Other activities of endogenous galectin-3 have been addressed by using cells from galectin-3-deficient (Lgals3 −/−) mice (reviewed in Ref. Reference Rabinovich20). Peritoneal macrophages from Lgals3 −/− mice are more sensitive to apoptosis induced by lipopolysaccharide plus IFN-γ, compared with those from wild-type mice. This is consistent with the anti-apoptotic functions of galectin-3. Comparisons between Lgals3 −/− and wild-type mice have revealed the function of galectin-3 in phagocytosis by macrophages mediated by the Fcγ receptor (Ref. Reference Sano92) and in mast cell responses induced by crosslinking of the cell-surface IgE receptor (Ref. Reference Chen84). The mechanisms by which endogenous galectin-3 regulates these activities still remain to be elucidated. In several cases, the responses in wild-type cells are not reduced by lactose, suggesting that the endogenous protein does not function by acting extracellularly as a lectin.
In vivo studies using Lgals3 −/− mice support the notion that galectin-3 promotes inflammatory responses (reviewed in Ref. Reference Rabinovich20). Reduced IgE-mediated responses of mast cells in vivo were observed in Lgals3 −/− mice. In addition, Lgals3 −/− mice exhibited attenuated infiltration of leukocytes relative to wild-type mice in a model of peritoneal inflammation. Lgals3 −/− mice developed lower lung eosinophilia compared with similarly treated wild-type mice following airway antigen challenge (Ref. Reference Zuberi93) in a mouse model of atopic asthma. These studies also revealed the promotion of Th2 polarisation by galectin-3. However, other studies showed reduced eosinophil infiltration following airway antigen challenge in rats and mice treated by intranasal delivery of cDNA encoding galectin-3 (Ref. Reference Lopez94). Thus, transgenic galectin-3 may not exactly reproduce the function of endogenous galectin-3, probably because it may not be expressed by the same cell types and may differ in the intra- versus extracellular mode of action.
Galectin-3 in tumour development/progression
The expression of galectin-3 is modulated in many different cancers (Ref. Reference Danguy, Camby and Kiss95). The role of galectin-3 in tumour growth, progression and metastasis has been extensively documented (reviewed in Ref. Reference Liu and Rabinovich43).
Roles in transformation, tumour growth and survival
There is evidence that galectin-3 expression is necessary for the initiation of the transformed phenotype of tumours and their subsequent growth (reviewed in Ref. Reference Liu and Rabinovich43), from studies involving inhibition of galectin-3 expression and ectopic expression of galectin-3 in cell lines. The mechanisms by which galectin-3 is involved in cell transformation and growth are not yet fully understood, but could be related to its ability to interact with oncogenic K-RAS and facilitate signal transduction from K-RAS to RAF and phosphoinositide 3-kinase (Ref. Reference Ashery96). Galectin-3 may also influence tumourigenesis through regulation of the cell cycle, as it has been shown to bind to β-catenin (a component of the WNT signalling pathway) and be involved in stimulating expression of cyclin D and c-MYC (Refs Reference Shimura100, Reference Shimura101). Its interaction with transcription factors may also be involved in these processes (Ref. Reference Paron99).
The most extensively studied function of galectin-3 that is relevant to tumour progression is inhibition of apoptosis (reviewed in Refs Reference Liu and Rabinovich43, Reference Nakahara, Oka and Raz97) in a range of tumour cell types exposed to diverse apoptotic stimuli (including chemotherapeutic agents). Very recently, evidence was provided that apoptosis induced by the tumour suppressor p53 involves repression of galectin-3 (Ref. Reference Cecchinelli98); thus, galectin-3 may be an effector molecule in the apoptosis-regulating pathway downstream of p53.
Role in metastasis
Recombinant galectin-3 has been shown to affect the motility of tumour cells and influence their invasiveness in vitro. However, both positive and negative effects have been reported (Refs Reference Le Marer and Hughes102, Reference Moisa103). With regard to endogenous galectin-3, transfectants of tumour cell lines overexpressing galectin-3 showed enhanced cell motility and in vitro invasiveness (Refs Reference Matarrese104, Reference O'Driscoll105).
Galectin-3 can also affect tumour metastasis by exerting its effect on the microenvironment of tumours. The presence of galectin-3 in the stroma has been shown to be a negative prognostic factor in breast cancer (Ref. Reference Moisa103). Galectin-3 has angiogenic activity in vitro, which may be related to its ability to induce migration of endothelial cells (Ref. Reference Nangia-Makker106). In addition, galectin-3 has been shown to play a critical role in activation of myofibroblasts in the liver and contribute to liver fibrosis induced by carbon tetrachloride (Ref. Reference Henderson107). Thus, this lectin may contribute to various other fibrotic processes.
Studies with animal models have provided evidence for the role of galectin-3 in tumour metastasis in vivo (reviewed in Ref. Reference Liu and Rabinovich43). For example, liver metastases of human adenocarcinoma xenotransplants in severe combined immunodeficient (SCID) mice can be inhibited by anti-galectin-3 antibody. Breast carcinoma cells overexpressing transgenic galectin-3 have higher metastatic potential. Furthermore, a C-terminal domain fragment of the protein (galectin-3C, which retains the carbohydrate-binding activity of galectin-3 but lacks the homo-oligomerisation property and so can inhibit the activities of galectin-3 by competing for the same glycans on the cell surface) inhibited tumour metastasis in an orthotopic nude mouse model of human breast cancer (Ref. Reference John108).
Galectin-3 in other pathological processes (atherosclerosis, diabetes, and wound healing)
Galectin-3 is expressed in foam cells and macrophages in atherosclerotic lesions (Ref. Reference Nachtigal109), and may participate in the development of atherosclerosis: compared with apolipoprotein (Apo)E-deficient mice, ApoE/galectin-3-double-knockout mice developed a significantly lower number of atherosclerotic lesions and atheromatous plaques at 36–44 weeks of age. This could be related to a pro-inflammatory role of galectin-3, as the double-knockout mice also exhibited a lower number of perivascular inflammatory infiltrates (Ref. Reference Nachtigal, Ghaffar and Mayer110).
Galectin-3 has been shown to bind to advanced glycosylation end products (AGEs) and is now considered as an AGE receptor (Ref. Reference Iacobini111); however, whether galectin-3 indeed serves as a cell-surface receptor and mediates the action of AGEs remains to be definitely established. Lgals3 −/− mice develop accelerated diabetic glomerulopathy compared with wild-type mice (Ref. Reference Pugliese112), but significantly less diabetes-mediated breakdown of the inner blood–retinal barrier (Ref. Reference Stitt113).
Galectin-3 may also play a role in wound re-epithelialisation. Corneal epithelial wound closure rates in Lgals3 −/− mice were significantly slower than in wild-type mice in various models of corneal wound healing, including corneas with excimer-laser ablations or alkali-burn wounds that were allowed to partially heal in vivo or in vitro (Ref. Reference Cao114). In addition, exogenous galectin-3 accelerated re-epithelialisation of wounded wild-type corneas but not Lgals3 −/−corneas. This suggests that simultaneous intra- and extracellular actions of galectin-3 may be required for wound healing. The mechanism by which galectin-3 regulates wound healing remains to be elucidated.
Galectin-4
Galectin-4, a two-CRD galectin, is predominantly expressed in the intestines and the stomach in rats. It has been identified as an adherens junction protein in pig oral epithelial cells, suggesting a role in the assembly of adherens junctions (Ref. Reference Chiu115). In intestinal epithelia, galectin-4 forms distinct soluble high molecular weight clusters with brush-border enzymes in the detergent-insoluble complexes known as lipid rafts (Ref. Reference Danielsen and van Deurs116).
The role of galectin-4 in lipid rafts has been studied by using RNA interference to deplete galectin-4 in a human colon adenocarcinoma cell line (Ref. Reference Delacour117). In galectin-4-depleted cells, protein markers of the apical membrane domain were trapped intracellularly, suggesting a defect in apical membrane protein sorting. Sulphatides with long-chain hydroxylated fatty acids, which are enriched in lipid rafts, were identified as high-affinity ligands for galectin-4. These findings suggest that by interacting with sulphatides to foster the clustering of lipid rafts, galectin-4 may play an important role in the apical delivery of proteins.
Galectin-4 has been shown to play a role in the pathogenesis of colitis in inflammatory bowel disease (Ref. Reference Hokama118). Galectin-4 stimulates CD4+ T cells from mice with colitis to produce IL-6, an inflammatory cytokine contributing to progression of colitis. Administration of a galectin-4 antibody to mice that have developed moderate intestinal inflammation suppresses development of the disease (Ref. Reference Hokama118).
An association between galectin-4 expression and breast cancer has also been noted. Normal breast tissues express very little, if any, galectin-4, but this galectin is induced in benign breast proliferative disease and is most highly expressed in ductal carcinoma in situ (Ref. Reference Huflejt and Leffler119).
Galectin-7
Galectin-7, a one-CRD galectin, is found mainly in stratified squamous epithelium and its expression correlates with keratinocyte differentiation. The crystal structure of human galectin-7 was determined in free form, as well as in complexes with galactose, galactosamine, lactose and N-acetyl-lactosamine (Ref. Reference Leonidas120). The structure revealed a fold with overall similarities to that of galectin-1 and -2, and to a greater extent that of galectin-10.
There is evidence that the galectin-7 gene is an early transcriptional target of the tumour suppressor protein p53 (Ref. Reference Polyak121). Bernerd et al. (Ref. Reference Bernerd, Sarasin and Magnaldo122) found that galectin-7 expression is upregulated rapidly after ultraviolet B irradiation of epidermal keratinocytes, parallel to p53 stabilisation and apoptosis induction. Furthermore, ectopic expression of galectin-7 causes significant cell death. These findings suggest that some of the pro-apoptotic effects of p53 on keratinocytes may be mediated by galectin-7. Subsequent studies by Kuwabara et al. (Ref. Reference Kuwabara123) demonstrated that galectin-7 promotes JNK activation and mitochondrial cytochrome c release.
Galectin-7 expression is markedly downregulated during the progression of malignancy in thyroid tumours (Ref. Reference Rorive124). Consistent with this, galectin-7-transfected human colon carcinoma DLD-1 cells grow more slowly than control transfectants under normal culture conditions, and form a lower number of colonies under anchorage-independent cell growth conditions. When inoculated subcutaneously into SCID mice, tumour formation from these cells is greatly reduced compared with control cells (Ref. Reference Ueda, Kuwabara and Liu125).
By contrast, there is evidence that galectin-7 is upregulated as mouse lymphoma cells progress toward a metastatic phenotype (Ref. Reference Moisan126). The development of thymic lymphoma is accelerated in mice inoculated with lymphoma cells overexpressing galectin-7. In addition, transfection of galectin-7 into lymphoma cells increases their metastatic behaviour, probably through upregulation of other genes, such as MMP-9 (Ref. Reference Demers, Magnaldo and St-Pierre127). Conversely, suppression of galectin-7 expression downregulates MMP-9 expression and decreases the dissemination and invasion of lymphoma cells to peripheral organs (Ref. Reference Demers128).
Finally, galectin-7 is also implicated in corneal wound healing – specifically in re-epithelialisation of wounds (Refs Reference Cao114, Reference Cao129) – probably through modulation of epithelial cell migration (Ref. Reference Cao130).
Galectin-8
Numerous galectin-8-related mRNA species have been found as a result of the presence of three unusual polyadenylation sites and alternative splicing of galectin-8 transcripts (Ref. Reference Bidon131). These mRNAs encode six galectin-8 isoforms: three with two CRDs, and three with only one CRD (Ref. Reference Bidon-Wagner and Le Pennec132).
Recombinant galectin-8 inhibits adhesion of human carcinoma 1299 cells to plates coated with integrin ligands and induces cell apoptosis (Ref. Reference Hadari133). Subsequent studies revealed that α3β1 integrin is a major galectin-8-binding protein. In accordance with its antiadhesive effects, transfection of galectin-8 into 1299 cells reduces colony formation (Ref. Reference Hadari133). Ligation of integrins by galectin-8 results in strong activation of integrin-mediated signalling cascades and leads to a distinctive cytoskeletal organisation and microspike formation (Ref. Reference Levy134). It was proposed that binding to galectin-8 modulates integrin interaction with the extracellular matrix, thereby regulating cell adhesion and cell survival (Ref. Reference Zick135). Galectin-8 also binds other integrins on various cell types – for example, integrin αM on neutrophils. The lectin enhances adhesive properties of neutrophils and induces superoxide production by these cells, possibly through binding to this integrin (Ref. Reference Nishi136).
Exogenously added galectin-8 suppresses the migration of human colon cancer cells (Ref. Reference Nagy137). Since galectin-8 expression is downregulated in colon cancer, it is conceivable that the cancer cells acquire increased migratory properties partly due to the reduced level of this lectin. Finally, there is evidence that galectin-8 can modulate the rate of internalisation of cell-surface receptors: when cells are bound to immobilised galectin-8, endocytosis of the insulin receptor is attenuated (Ref. Reference Boura-Halfon138). Furthermore, use of a cellular model of rheumatoid arthritis showed that galectin-8 can induce apoptosis in synovial fluid cells, possibly by interacting specifically with the CD44vRA isoform of CD44 (Ref. Reference Eshkar Sebban139). This suggests a possible mechanism by which this lectin affects the progression of this disease.
Galectin-9
The N-terminal CRD of galectin-9 forms homodimers both in the crystal form and in solution, and the three-dimensional structure is different from the canonical twofold symmetric dimers seen for galectin-1 and -2 (Ref. Reference Nagae140). Full-length galectin-9 forms stable dimers as well as multimers (Ref. Reference Miyanishi141). Three isoforms of galectin-9 differing in the lengths of their linker sequences have been identified (Refs Reference Spitzenberger, Graessler and Schroeder142, Reference Chabot143, Reference Sato144).
Similar to another two-CRD galectin, galectin-4, galectin-9 has also been found in lipid rafts (Ref. Reference Pioche-Durieu145). Interestingly, in renal epithelial cells, galectin-9 has been shown to be an integral plasma membrane protein with at least two transmembrane domains, functioning as a highly specific urate transporter (Refs Reference Leal-Pinto146, Reference Lipkowitz147, Reference Graessler148).
Human galectin-9 has been identified as a potent eosinophil chemoattractant produced by T cells (Refs Reference Matsumoto149, Reference Matsushita150). Further research suggests that the N- and C-terminal CRDs of galectin-9 interact with the same or closely related ligands on the eosinophil membrane (Ref. Reference Sato144).
Recombinant galectin-9 induces apoptosis in thymocytes, in a dose-dependent and lactose-inhibitable manner (Ref. Reference Wada151). Galectin-9 also induces death in Th1 cells, and not Th2 cells, through interaction with the Th1-specific cell-surface molecule TIM-3 (Refs Reference van de Weyer152, Reference Zhu153). An important role for galectin-9 in controlling the Th1 response in vivo has been confirmed by using the experimental autoimmune encephalomyelitis model (Ref. Reference Zhu153).
However, there is also evidence that galectin-9 favours the initiation of the adaptive immune response. Galectin-9 stimulates the maturation of dendritic cells (professional antigen-presenting cells) (Ref. Reference Dai154). Galectin-9-matured dendritic cells secrete IL-12 but not IL-10, and selectively elicit the production of Th1 cytokines by allogeneic CD4+ T cells. This effect may not be dependent on the lectin properties of this galectin, as a galectin-9 mutant lacking β-galactoside-binding activity still promotes maturation of dendritic cells. In addition, the effect of galectin-9 on dendritic cell maturation is only slightly inhibited by lactose. There is evidence that the MAPK p38 is an important mediator of galectin-9-induced dendritic cell maturation (Ref. Reference Dai154). The above findings that galectin-9 participates in initiation of an immune response by acting on dendritic cells are consistent with a recent report that the galectin-9 receptor TIM-3 is expressed on innate immune cells and promotes tissue inflammation (Ref. Reference Anderson155).
The percentage of galectin-9-positive cells in synovial tissue and the amount of galectin-9 in synovial fluid are significantly higher in patients with rheumatoid arthritis than in patients with osteoarthritis (Ref. Reference Seki156). Furthermore, a recombinant galectin-9 variant, which does not contain the linker peptide and is resistant to proteolysis, significantly induced apoptosis in fibroblast-like synoviocytes from rheumatoid arthritis patients and in cells in rheumatoid synovial tissue implanted into SCID mice. In addition, galectin-9 inhibits the development of Th17 cells and increases the frequency of T regulatory cells in experimental autoimmune arthritis (Ref. Reference Seki157). Thus, galectin-9 has the potential to be used as a therapeutic agent for treatment of rheumatoid arthritis, as well as other inflammatory diseases. In this regard, it is interesting that galectin-9 alters the course of nephrotoxic nephritis and glomerular hypertrophy in animal models (Refs Reference Tsuchiyama158, Reference Baba159).
In response to glucose stimulation, the glucose transporter GLUT-2, an N-glycosylated glucose transporter expressed in pancreatic β-cells, translocates to the plasma membrane and mediates the entry of glucose into β-cells. Interestingly, galectin-9 is associated with GLUT-2 on the β-cell surface (Ref. Reference Ohtsubo160). Ablation of the gene coding for a galactosyltransferase responsible for glycosylation of GLUT-2, which is essential for galectin-9 binding, reduces such association. This results in diminished cell surface GLUT-2 levels on the cell membrane upon glucose stimulation (Ref. Reference Ohtsubo160). Thus, galectin-9 may regulate glucose homeostasis, by facilitating the retention of GLUT-2 on the β-cell surface.
Galectin-9 is also expressed by a variety of tumour cells and may play an important role in tumour immunity by regulating the survival, proliferation and migration of both tumour cells themselves and immune cells in the tumour microenvironment (Ref. Reference Rabinovich20).
Galectin-10
Galectin-10 has a single CRD, sharing only six out of the eight residues directly involved in lactose binding in galectin-1, -2 and -7. It has affinity for mannose but not β-galactosides (Ref. Reference Swaminathan161). The three-dimensional structure of galectin-10 has been determined at 1.8 Å resolution in both free form (Ref. Reference Leonidas162) and a complex with mannose (Ref. Reference Swaminathan161). Despite its modest sequence homology to other galectins, the overall structural fold of galectin-10 is very similar to that of other galectins, especially galectin-7 (Ref. Reference Leonidas120). The molecule exhibits a ‘jellyroll’ structure, formed by two antiparallel β-sheets (Refs Reference Swaminathan161, Reference Leonidas162).
Previous studies suggested that galectin-10 was found only in eosinophils and basophils, but recent findings demonstrate that this lectin is also constitutively expressed in CD4+CD25+ regulatory T cells. Inhibition of endogenous galectin-10 in regulatory T cells by RNA interference restored their proliferative capacity and abrogated their suppressive activity on effector CD4+ T cells, indicating an essential role for galectin-10 in regulatory T cell function (Ref. Reference Kubach163).
Galectin-12
While the N-terminal domain of galectin-12 contains all the sequence elements predicted to form the two β-sheets found in other galectins, as well as conserved carbohydrate-interacting residues, its C-terminal domain shows considerable divergence from the consensus sequence. Nevertheless, the protein has lactose-binding activity, likely contributed by the N-terminal domain. The mRNA for galectin-12 contains features coding for proteins with growth-regulatory functions (Ref. Reference Yang164). These include a start codon in a sequence context that is suboptimal for translation initiation and AU-rich motifs in the 3′-untranslated region, which are known to confer instability to mRNA. Galectin-12 expression is upregulated in cells synchronised at the G1 phase or the G1–S boundary of the cell cycle. Consistent with this, ectopic expression of galectin-12 in cancer cells causes cell cycle arrest at the G1 phase and cell growth suppression (Ref. Reference Yang164).
Galectin-12 mRNA is over-represented in adipocytes, in which its expression is further upregulated by caloric restriction or treatment of obese animals with troglitazone, a ligand for the transcription factor PPARγ that improves insulin sensitivity (Ref. Reference Hotta165). Gene expression profiling of various mouse tissues revealed that galectin-12 and leptin are the only two genes that are specifically expressed in adipose tissue, with negligible expression in 14 other tissues examined (Ref. Reference Kouadjo166). Troglitazone-upregulated galectin-12 expression in adipose tissue correlates with adipocyte apoptosis, and overexpression of galectin-12 by transfection of COS-1 cells can induce apoptosis (Ref. Reference Hotta165).
Galectin-12 mRNA is markedly upregulated when preadipocytes differentiate into mature adipocytes (Ref. Reference Yang167). Downregulation of endogenous galectin-12 expression by RNA interference greatly suppressed adipocyte differentiation (Ref. Reference Yang167). Future studies may prove that galectin-12 plays an important role in the development of adipose tissue.
Galectin-15
Galectin-15 (ovgal11) cDNA was originally isolated from sheep stomach (abomasal) tissue infected with the nematode parasite Haemonchus contortus. Its expression is greatly upregulated in gastrointestinal tissue infected with helminth larvae, coincident with eosinophil infiltration and inflammation (Ref. Reference Dunphy168). Galectin-15 is also expressed in the uterus of sheep and forms crystals in the trophectoderm (Ref. Reference Gray169), and may regulate implantation and placentation by functioning as a heterophilic cell adhesion molecule between the conceptus trophectoderm and endometrial lumenal epithelium (Ref. Reference Farmer170).
Clinical implications/applications
Two decades of intensive studies since the cloning of the first galectin have generated a great deal of useful information regarding the structure, carbohydrate-binding specificity, and pattern of expression of galectins. In vitro and in vivo functional studies have also begun to yield significant insights into the roles of galectins in cultured cells and in the whole organism (summarised in Figure 2). Galectin research has spread into many fields, including immunology, cancer pathology, developmental biology, and neurobiology. The progress in the field has also revealed potential therapeutic applications; some of these are summarised below.

Figure 2. Selected biological functions assigned to different members of the galectin family. Galectins can promote a wide range of biological functions by clustering multiple multivalent glycoconjugates, thus triggering intracellular signaling. They can also bridge two cells of the same or different types, and bridge cells to extracellular matrix proteins. These glycan-binding proteins can also induce biological functions intracellularly, through protein–protein interactions. Selected biological functions mediated by individual members are summarised. IL-6, interleukin 6; Th, T helper; TIM-3, Th1-specific type 1 membrane protein; TCR, T-cell receptor.
Inflammatory diseases
The existing data suggest that recombinant galectin-1 could potentially be exploited as an effective therapeutic reagent for treating inflammatory and autoimmune diseases. These include rheumatoid arthritis, autoimmune hepatitis, autoimmune uveitis, diabetes, inflammatory bowel disease, graft-versus-host disease, and multiple sclerosis (reviewed in Ref. Reference Bianco41). Galectin-2 has a beneficial effect in colitis and thus may be used for treatment of inflammatory bowel disease (Ref. Reference Paclik67). In contrast, galectin-4 has a contributory role in colitis and neutralising antibody suppressed colitis in a mouse model (Ref. Reference Hokama118), and so targeting galectin-4 may be useful for treatment of inflammatory bowel disease. Galectin-9 has similar biological activities to galectin-1 with regard to induction of apoptosis in Th1 cells and there is evidence suggesting the utility of this lectin in treatment of inflammatory and autoimmune diseases, such as multiple sclerosis, nephritis and rheumatoid arthritis (Refs Reference Zhu153, Reference Seki156) (summarised in Table 1).
Table 1. Immunoregulatory and therapeutic effects of galectins in experimental models of autoimmunity and chronic inflammation

Abbreviations: Gal, galectin; IFN, interferon; IL, interleukin; MBP, myelin-basic protein; MOG, myelin oligodendrocyte glycoprotein; ND, not determined; OVA, ovalbumin; PLP, myelin proteolipid protein; siRNA, small interfering RNA; TNBS: 2,4,6-trinitrobenzene sulphonic acid.
The situation for galectin-3 is more complicated. In vitro studies using recombinant protein suggest that galectin-3 can either promote or suppress the response of inflammatory cells, depending on the cell types and experimental conditions (see section ‘Galectin-3 in immune and inflammatory responses’). Like galectin-1, -2 and -9, recombinant galectin-3 can induce apoptosis in T cells in vitro (Refs Reference Fukumori171, Reference Stillman172); thus, administration of galectin-3 might be expected to suppress the immune response under certain conditions. Indeed, plasmid coding for galectin-3 suppresses allergic airway inflammation in animal models (Ref. Reference Lopez94); however, importantly, galectin-3-deficient mice have reduced inflammatory responses, including allergic airway inflammatory response (Ref. Reference Zuberi93). Thus, it appears that endogenous galectin-3 promotes the allergic inflammatory response and its inhibitors may be useful for treatment for inflammatory conditions (summarised in Table 1).
Cancer
Given its known biological roles in tumour progression, galectin-1 has been postulated as an attractive target for anticancer therapies. In particular, there is evidence that galectin-1 produced by tumour contributes to tumour immune escape (Ref. Reference Rubinstein48); thus galectin-1 inhibitors could be used to counter this effect and enhance antitumour immunity. A vast amount of literature also suggests that galectin-3 plays an important role in tumour progression and metastasis, by functioning at a number of different points (see section ‘Galectin-3 in tumour development/progression’). Importantly, even though galectin-3 has been implicated in diverse biological pathways associated with tumour development and progression, in almost all cases, the effect of galectin-3 is cancer-promoting. Thus, galectin-3 inhibitors may be useful for treatment of various cancers by suppressing various pathways. Galectin-7, however, promotes apoptosis and suppresses growth in cancer cells (Refs Reference Kuwabara123, Reference Ueda, Kuwabara and Liu125). Thus, either gene therapy using galectin-7 cDNA or means to induce galectin-7 gene expression may be useful for treatment of selected cancers.
Neuronal regeneration
Galectin-1 induces neuronal degeneration and galectin-1-deficient mice exhibit reduced degeneration (Ref. Reference Plachta57). Thus, targeting this lectin may be useful for treating neuronal degeneration if suitable conditions are established. However, there are studies suggesting the promotion of neurite growth by galectin-1 (Ref. Reference Puche and Key54). The situation is confounded by the finding that oxidised galectin-1 promotes neurite growth (Ref. Reference Inagaki59). Regardless of this complication, if eventually endogenous galectin-1 is proven to induce neuronal degeneration, galectin-1 inhibitors can still be used, as it should be possible to generate ones that do not inhibit the oxidised form.
Other diseases
In view of the finding that galectin-3-deficient mice have delayed corneal wound healing and recombinant galectin-3 promotes the process (Ref. Reference Cao114), the use of recombinant galectin-3 or means to induce galectin-3 expression may be considered for treatment of chronic corneal wounds or other wounds. Recombinant galectin-7 may likewise be used for this purpose. Since galectin-3-deficient mice exhibit lower degrees of atherosclerosis (Ref. Reference Nachtigal, Ghaffar and Mayer110), galectin-3 may be a target for treatment of this disease. Finally, in view of the finding that galectin-12 is essential for adipocyte differentiation (Ref. Reference Yang167), inhibitors of this galectin may be exploited to reduce the amount of adipose tissues and considered for the treatment of obesity.
Research in progress and outstanding research questions
In view of their diversity in carbohydrate-binding specificity and activity, as well as temporal and spatial expression patterns, it is conceivable that different galectins are functionally distinct. There is little doubt that those members with significant presence outside the cell have extracellular functions such as cell–cell and cell–extracellular-matrix interactions, as well as binding to cell-surface ligands to initiate signal transduction. However, galectins have important functions inside the cell as well that may be related to their interaction with unglycosylated proteins, such as regulation of apoptosis. A summary of intracellular and extracellular functions of galectins is presented in Figure 3, using those relevant to tumour development as an example. Additional investigations are necessary to clarify further the dual intracellular and extracellular functions of galectins.
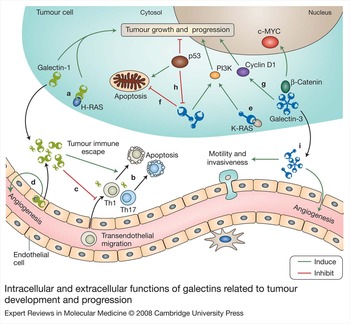
Figure 3. Intracellular and extracellular functions of galectins related to tumour development and progression. Galectins have both extracellular and intracellular functions. Several of these functions are relevant to tumour development/progression and some of them are depicted here. (a) Intracellularly, galectin-1 binds to oncogenic H-RAS and promotes its anchorage to the plasma membrane. (b) Extracellularly, galectin-1 contributes to immune evasion by inducing apoptosis in effector T cells or modulating the Th1–Th2–Th17 cytokine balance. (c) Galectin-1 also inhibits transendothelial migration of tumour-targeting T cells and (d) promotes angiogenesis. (e) Intracellularly, galectin-3 can mediate neoplastic transformation, by interacting with oncogenic K-RAS and promoting RAS-mediated signal transduction. It can also control tumour progression by (f) exerting antiapoptotic functions and (g) controlling the levels of regulators of cell cycle progression and cell proliferation, such as cyclin D1, c-MYC, and β-catenin. (h) Apoptosis induced by the tumour suppressor p53 involves suppression of galectin-3 expression. (i) Through extracellular mechanisms, galectin-3 promotes tumour cell migration as well as invasiveness and induces angiogenesis. Abbreviation: PI3K, phosphoinsositide 3-kinase; Th, T helper.
Identification of ligands that mediate the function of galectins is important for elucidating galectin actions. This is not a simple task because of the copious binding of galectins to irrelevant glycoproteins that are brought in close contact with galectins once cells are lysed. Several recent studies with cells deficient in MGAT5, an enzyme required for the production of high-affinity N-glycans for galectin-3, have generated some very interesting findings, implicating the interactions of this galectin with cell-surface glycans in the regulation of many cellular processes such as cell proliferation, differentiation and activation, as well as receptor endocytosis (Refs Reference Demetriou81, Reference Partridge82, Reference Lau173). However, the functional consequences of these interactions have not been demonstrated definitely and directly from the galectin side. Moreover, activities demonstrated by using recombinant proteins added to cells may not demonstrate the functions of endogenous galectins in that particular cell type.
The availability of galectin-knockout mice has been a great asset for researchers in this field. So far, several reports with knockout mouse models of galectin-1 and -3 have been published (see sections on these two proteins). Since some tissues simultaneously express several galectins, the possibility exists that there could be functional compensation by other galectin members in the knockout mice. However, distinct phenotypes in mice lacking either one of these two galectins support the functional uniqueness of different family members. Continued studies of these models, as well as mice deficient in other galectins and mice in which galectins are deleted conditionally and in a tissue-specific fashion, are important for a more complete understanding of functions of galectins.
As mentioned in the previous section, galectins have a great deal of therapeutic potential. However, before galectin-based therapeutic agents can be extrapolated to clinical settings, a more thorough understanding of the mechanisms involved in the functions of galectins is necessary. In this regard, several issues remain to be addressed, including: the extent of functional redundancy and specificity of action within the galectin family; the basis for the different functions exerted by the same galectin within different environmental contexts; the major function(s) of each galectin among the number of functions demonstrated; and whether the functions demonstrated for galectins in vitro are operative in vivo. In addition, it is critical to establish firmly whether the demonstrated functions of a given galectin are a result of its intracellular or extracellular effect, especially in the in vivo setting. This will determine whether the inhibitors used should target extracellular or intracellular space.
Acknowledgements and funding
We are grateful to the peer reviewers for critical and constructive assessment of our review. We also thank members of our laboratories for their contributions to the work cited in this review, and Diego Croci for his assistance in the preparation of the illustrations. We apologise to the authors of many relevant studies not cited because of space limitations. Work in authors' laboratories was supported by grants from the National Institutes of Health (RO1AI20958, RO1AI39620 and R21AR53116) to F.T.L. and by grants from The Cancer Research Institute Elaine R. Shepard Investigator, Agencia de Promoción Cientifica y Tecnologica (PICT 2006-603), and University of Buenos Aires and Fundación Sales to G.A.R. G.A.R. is a member of the research career of CONICET.