Introduction
Management of some tumour types has become an urgent dilemma. Over-diagnosis along with issues related to the prediction of patient survival are challenges still that exist in the context of cancer (ex. prostate cancer) (Ref. Reference Kim1). Circulating biomarkers can be assessed as a tool for early detection of cancer and reducing the rate of mortality (Ref. Reference Trinidad2). Liquid biopsy includes three conventional approaches including circulating tumour DNA (ctDNA), circulating tumour cells (CTCs) and extracellular vesicles (EVs) (Ref. Reference Sun3). Evaluation of CTCs and ctDNA provide useful knowledge about tumour evolution and the risk of tumour recurrence. Such approaches, however, have limited clinical utility due to high rates of false-positive outcomes and low rates of detection (Ref. Reference Liu4). However, multimodal analysis of ctDNA, CTCs and EVs is applicable using machine learning algorithm. Multimodal liquid biopsy can be used as an approach to improve the accuracy of liquid biopsy assays and to overcome low specificity and sensitivity of existing liquid biopsies (Ref. Reference Bu5).
EVs have emerged recently as cancer biomarkers (Ref. Reference Wang6). EVs contain genetic information of the original cell, and they can be used as blood biomarkers and in horizontal gene transfer for cancer therapy (Ref. Reference Balaj7). EVs secreted from tumour mediate cell-to-cell communication between tumour and its environment help to know more about cancer-related processes, such as progression and metastasis (Ref. Reference Probert8). The cargo carried by EVs is transferred from donor to recipient or acceptor cells. The EV-based cell-to-cell communication can be mediated through activation of receptors on surface of recipient cells. EVs can also affect the phenotype of acceptor cell/s through transferring membrane encapsulating cargoes (Ref. Reference Bonsergent9). Characterisation of individual EVs and their subpopulations will expand the current knowledge about communications among cells in physiological and pathological conditions (Ref. Reference Kim10). Generally, current approaches for EV purification demand time-consuming procedures. This along with high-cost antibodies and large sample volumes are predicaments for clinical utility of EVs in cancer diagnosis. In addition, there is no blood test specific for EV analysis in different types of cancers (Ref. Reference Liu11). In this review, we aimed to discuss about a possibility of using EVs as a diagnostic biomarker in solid tumours. Here, we have a focus over different types of methods for EV isolation and specification along with novel EV-related biomarkers for early cancer diagnosis.
Extracellular vesicles in cancer
Over the past decade (Ref. Reference Sun and Meckes12), tumour-derived EVs are regarded as biomarker reservoirs for cancer diagnosis (Refs Reference Sun3, Reference Liang13). EV are secreted abundantly from many cell types into circulation (Ref. Reference Liu4) and are able to easily cross biological barriers (Ref. Reference Najafi14). Human tumour microenvironment (TME) and plasma contain millions of diverse vesicles (Ref. Reference Whiteside15). EV cargo is transferred both locally and systemically and directs interactions within the TME in favour of tumour aggression and metastasis (Ref. Reference Hinger16). Malignant tumour cells release EVs that their uptake by less malignant cells will boost metastatic capacity in such cells (Ref. Reference Zomer17). EV release from cancer cells is considerably higher compared with non-cancerous cells (Ref. Reference Rontogianni18). The number of EVs in plasma of healthy individuals is about 109–1010/ml, but the concentration of such vesicles is increased to about 4- to 20-fold in disease conditions like cancer (Ref. Reference Lee19). Over 104 EVs can be released from a single tumour cell per day, which makes tumour-derived EVs highly frequent compared with other biomarkers within circulation (Ref. Reference Tian20). This is indicative of a need for developing sensitive methods for purification of EVs and their molecular profiling for clinical translation in cancer patients (Ref. Reference Liu11). Recipient cells release EV cargo in pH low condition, and exchange of such vesicles will impact their phenotype (Ref. Reference Bonsergent9).
EVs are assessed for deciphering the underlying inflammatory pathways occurring due to psychoneurological symptoms in prostate cancer patients (Ref. Reference Sass21). EVs harvested from pancreatic cancer patients are evaluated for protein cargo and comparting with that collected from healthy pancreatic epithelial cells. EVs from cancer cells were enriched in proteins responsible for vesicle generation and release along with oncogenic transformation of cells, whereas EVs from normal cells were enriched in proteins responsible for immune response. This indicates that tumour-derived EVs are able to initiate transformation of healthy cells into malignancy, and promote cancer spreading to other organs (Ref. Reference Servage22). Communication between prostate cancer cells with bone environment for further progression of cancer toward bone metastasis is mediated by EVs. EVs are, in fact, working as vehicles for delivery of RNAs that increase the risk of metastasis and enabling the growth of tumour cells within bone (Ref. Reference Probert8). EVs are also reported to be contributed to the metabolic events in breast cancer (Ref. Reference Chen23), and that such vesicles are reprogrammed (through targeting IL-3Rα) to blunt metastasis of triple-negative breast cancer (TNBC) (Ref. Reference Lopatina24). Purification of EVs from plasma in patients with ovarian, bladder and pancreatic cancer for surveying early cancer detection has shown the higher rate of early detection in pancreatic cancer compared with other cancer types (Ref. Reference Hinestrosa25). This is indicative of the cancer-type dependence for EV-based stratification of patients at early-stage cancer.
Classification and characterisation of extracellular vesicles
EVs are classified into three subgroups including exosomes, microvesicles (MVs) and apoptotic bodies (Fig. 1). They have differences in biogenesis, composition and functions (Ref. Reference Najafi14). A recommended nomenclature for EV identification is based on their size, which is due to a difficulty for definition of such particles based on their sub-cellular origin (Ref. Reference Ko26). MVs (microparticles or ectosomes (Ref. Reference Kowal27)) are large vesicles with a size ranging from 0.1 to over 1 μm (Ref. Reference Feng28). The size of apoptotic bodies is reached to about 1–5 μm (Ref. Reference Ko26). EVs are generally classified into small- and large-size subsets (Ref. Reference Crescitelli, Lässer and Lötvall29), but they typically have a size lower than 100 nm (Ref. Reference Feng28). Small EVs have a size lower than 200 nm (Ref. Reference Ko26). Large EVs derived from cancer cells with a size ranged from 1 to 10 μm are called oncosomes (Ref. Reference DeRita30). Exosomes constitute a major part of small EVs (Ref. Reference Kowal27). Large EVs are mainly conferred to the MVs (Ref. Reference Zeng31). Large and small EVs are released from glioma cells. As compared with non-cancer human umbilical vein endothelial cells (HUVECs), large EVs are secreted at considerably higher number from glioma cells, but both cell sources release similar number of small EVs (Ref. Reference Yekula32).
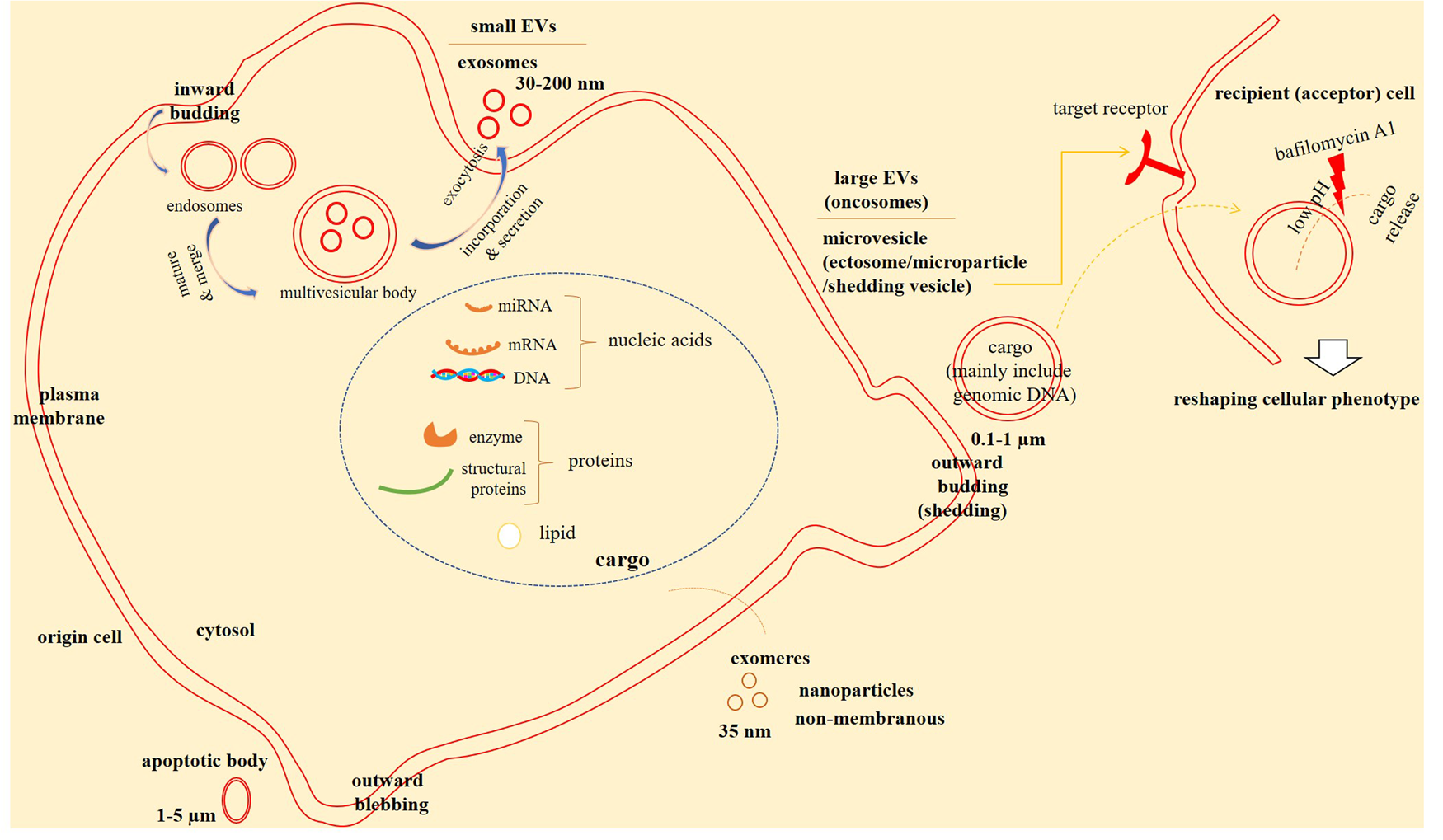
Fig. 1. Extracellular vesicles (EVs) in cell-to-cell communications. EVs are bilayer membrane structures that carry cargo including lipids, proteins and nucleic acids to affect target cells, acting as cell-to-cell communicators. Microvesicels (MVs), apoptotic bodies and exosomes are three subclasses of exosomes. Exosomes are generated from inward budding of plasma membrane in the form of endosomes. Endosomes are then become mature and merge to form mulivesicular bodies (MVBs) that are subsequently join the plasma membrane to release exosomes into the extracellular milieu. MVs are generated from outward budding of plasma membrane. Exomeres are non-membranous structures that are recently identified. Small EVs are mainly confer to the exosomes, while large EVs (also called oncosomes) are referred mainly to the MVs. EVs in recipient (or acceptor) cells release their cargo in pH low conditions and reshape the phenotype of such cells. Targeting EV cargo release in acceptor cells by agents, such as bafilomycin A1 can define therapy against cancer.
Large vesicles or oncosomes isolated from plasma of prostate cancer patients are enriched in tumour DNA, and the DNA is reflective of the genetic aberrations in the cell-of-origin and the genomic make-up of cancer. The fraction of this chromosomal DNA is more abundant compared to that in small EVs in which the same patients showed negligible DNA amount in small EVs (Ref. Reference Vagner33). KRAS mutation is common in pancreatic ductal adenocarcinoma (PDAC), and is considered as a critical driver of tumorigenesis, contributed to the initiation of cancer and progression toward metastasis (Ref. Reference Kamerkar34). Evaluation of mutant KRAS DNA in pancreatic cancer showed a considerably higher amount in small EVs at early stage, while at late-stage cancer such mutant DNA is similarly distributed among small and large EVs (Ref. Reference Hagey35). Thus, DNA distribution among various EV sizes is different between tumours. EVs isolated from metastatic melanoma patients and assessment of associated proteins in different subpopulations showed the general enrichment of large EVs and evolving several markers that are believed to be unique for small EVs, but there are markers specific for either one. For instance, both large and small low-density EVs are enriched in flottilin-1, while ADAM10 is enriched exclusively in small low-density EVs, and mitofilin is enriched in large EVs (Ref. Reference Crescitelli36).
Exosomes are distinct from MVs both structurally and functionally (Ref. Reference Kanada37). Exosomes are formed by inward budding of plasma membrane in the form of multivesicular bodies (MVBs) and their cargo is assembled within such endocytic membranes, whereas ectosomes are formed by outward budding of plasma membrane and their cargo is accumulated within plasma membrane (Refs Reference Meldolesi38, Reference Fan39) (Fig. 1). Recently, non-membranous nanoparticles with a size of about 35 nm called exomeres are identified (Ref. Reference Sandim and Monteiro40). The term EVP is used collectively for EVs and particles including exosomes and exomeres. EVPs are released at high concentrations (> 109) into peripheral blood, thereby providing adequate material for further analysis (Ref. Reference Hoshino41). Liquid biopsies can be provided for proteomic profiling of EVPs, which is important for early detection of cancers and monitoring therapy responses (Ref. Reference Whiteside15). Proteomic analysis of circulatory EVs in vitro can also be an effective tool for predicting their tissue-of-origin. In fact, proteomic profiling of EVs represents markers common among various cell types along with markers unique for a cell type. Diverse levels of the classical markers of small EVs, namely CD9, CD63 and CD81 are presented in different cell types (Ref. Reference Garcia-Martin42), but Grp78 and ARF6 are proteins related to apoptotic bodies and MVs, respectively (Ref. Reference Dao43). Proteomic profiling of EVs from tissue explants and plasma in cancer patients showed 90% sensitivity for VCAN, THBS2 and TNC in tissue explant EVPs, and the sensitivity for plasma EVPs was 95% (Ref. Reference Hoshino41). Immunoglobulins are the most frequent protein family packed within EVPs, and the specific types of EVP immunoglobulins can be used as a biomarker for distinguishing cancer patients from healthy individuals (Ref. Reference Pelissier Vatter44).
Virtues for the use of extracellular vesicles in cancer diagnosis
EV isolation can be a useful tool for cancer diagnosis and therapy. Evaluation of body fluids is a non-invasive way for cancer diagnosis (Ref. Reference Jang45), enabling detection of tumour risk in cancers hardly detected by routine methods. EV isolation from tumours like pancreatic cancer, which show cold immunity and represent poor prognosis (Ref. Reference Mortezaee46), can give us information about genetic changes and gene expression profile of this cancer type (Ref. Reference Jang45). EV signature is able to distinguish metastatic or non-metastatic breast cancer with high accuracy (~ 91%) (Ref. Reference Tian20). Isolation of EVs may provide important information for detection of diseases related to brain, such as cancers involving brain tissue. This is due to that EVs are able to cross blood-brain barrier (BBB) and transfer neurodegenerative and inflammatory molecules into the brain area (Ref. Reference Sass21). EVs can access tissues of whole body (Ref. Reference Wang47). Gut microbiome, for instance, is reflective of overall brain activity through the involvement of gut-brain axis. The microbiome affects the susceptibility of body to cancer, in which its altered activity can be contributed to the carcinogenic activity in an organ like brain. Generally, microorganisms cannot cross the BBB, but the EVs released from them have such capacity due to their nanosized diameter. Metagenomic analysis of microbial EVs collected from serum or brain tissue will provide a rich source of data for detection of brain tumour risk with high accuracy (Ref. Reference Yang48).
EVs are traceable within different biological fluids, such as saliva, urine, blood and breast milk, reflecting molecular fingerprint of the cell of origin. Such diverse sources infer a possibility of a molecular EV readout practically for all organs within the body (Ref. Reference Rontogianni18). Blood contains a huge number of non-vesicular materials, such as protein aggregates and free proteins (Ref. Reference Muraoka49), which requires appropriate techniques for effective isolation of EVs and identification of EV proteins. EVs can be traced within circulation at nearly early stages, and they are detectable throughout all disease courses (Ref. Reference Sun3). Thus, EVs can be obtained from blood samples collected for monitoring treatment responses. Conventional methods, by contrast, require several biopsies, which are not feasible due to demanding financial costs and put patients at higher risks (Ref. Reference Liu4). Another virtue for use of EVs in cancer diagnosis and therapy is their inherent stability (Ref. Reference Sun3). This is due to the protection made by EV lipid bilayer, which is able to protect the EV cargo from further degradation by external enzymes and proteases (Refs Reference Rontogianni18, Reference Chen50), thereby guarantying the integrity of cargos carried by them (Ref. Reference Sun3). The cargo includes RNAs along with proteins that serve as fingerprint of the cell of origin (Ref. Reference Shin51). This is indicative of the donor cell reflection of EV biochemical membrane composition, thereby granting the preferential tropism of exosomes toward their parented cell of origin (Ref. Reference Sancho-Albero52). The EV-based shuttle of miRNAs can be used as a prognostic and diagnostic biomarker in cancer patients. For instance, presence of miR-1246 in serum EVs from gallbladder cancer patients is indicative of cellular proliferation and invasion, and its combination with CA19-9 and CEA represents the higher diagnostic power in such patients. It is also reported that such miR type can be traced as an independent prognostic biomarker in such cancer (Ref. Reference Ueta53). Plasma profiling of EV-derived long RNAs is also reported to be of prognostic value in predicting treatment efficacy in breast cancer patients. Based on outcomes of one report, EV-derived long RNAs can be traced for distinguishing pathological responses in such cancer type (Ref. Reference Su54).
In-depth assessment of EV cargo yields higher specificity and sensitivity compared with whole plasma samples. This is due to that cancer-derived EVs are presumably enriched in molecules important for cancer diagnosis. These relevant molecules in such fraction are not detectable in plasma because of their mixing with highly frequent blood proteins (Ref. Reference Rontogianni18). In fact, EVs are enriched with contents that are selectively sorted from original cells. Furthermore, EVs are secreted actively from live cells rather than shedding from damaged or apoptotic cells (Ref. Reference Zhang55). EV cargo shows dynamic changes upon tumour progression (Ref. Reference Wendler, Stamp and Giamas56), which is indicative of the importance of EV evaluation for stage stratification.
Challenges for application of extracellular vesicles in cancer diagnosis
Like other agents, EV-based cancer therapy has its own issues, and current strategies are underway for solving such predicaments. First, isolation of purified EVs is a challenge in the area of EV-based therapy. EVs have rather small size, and contaminants are generally co-exist with these particles upon isolation (Ref. Reference Crescitelli, Lässer and Lötvall29). Second, very low concentration of exosomes in serum samples (<109 vesicles/ml) is another issue (Ref. Reference Jang45). Third, low levels of expressions for EV-related RNAs at early-stage cancer, detection of which at this stage is highly valuable (Ref. Reference Hu57). Fourth, insufficient sensitivity, restricted throughput, extended time for sample workup and no validate biomarker are other burdens in regard with the use of EVs in clinical setting (Ref. Reference Park58). Fifth, heterogeneity of EVs will make difficulties their isolation and detection of their specific subpopulations from other non-target EVs within circulation (Ref. Reference Wang6). Such heterogeneity is also referrable for some EV subtypes, such as exosomes in which two subpopulations of exosomes are identified: small (60–80 nm) and large (90–120 nm) exosomes (Ref. Reference Zhang59). Technical restrictions will be another issue, which hampers full separation of a specific subtype of EVs (Ref. Reference Zhang60).
Isolation of extracellular vesicles from tissue samples or plasma
Proteomic analysis of Extracellular vesicles and particles (EVP) harvested from human plasma and tissue samples gives us important information about the presence of tumour or even identifying tumour type. Based on results of a recent study, specificity of plasma and tissue EVP cargos for distinguishing tumour from normal tissues are 90 and 94%, respectively. Specific tumour-type proteins also exist within EVPs of plasma and tissue samples, which are useful biomarkers for detecting primary origin of tumour (Ref. Reference Hoshino41). Pan-cancer markers are EVP adhesion molecules and some metabolic enzymes. Examples of adhesion molecules are tenascin C, versican, CD36 and thrombospondin 2. A point important for consideration is the high similarity in proteomic profiling of tissue or cell-line derived EVPs between human and murine, while EVPs harvested from plasma are vastly different between the two, indicating low reliability of results from murine plasma EVPs when interpreted to human. Another point is that some proteins are exclusively detectable in plasma, not in tissue explants, so EVP proteins are seemingly reflective of cancer-related systemic changes (Ref. Reference Sandim and Monteiro40). Whiteside recently quoted on the idea and announced that EVPs with unique cancer-related proteomic profile are traceable only from plasma of cancer patients (Ref. Reference Whiteside15). EVs in plasma have a life-span of about 10 to 30 min, but they are constitutively released from cancer. It is impressive to note from a study by Lee et al.. who noticed a tumour suppressor activity for elevated plasm EVs after complete surgical removal of melanoma (Ref. Reference Lee19). This is indicative of a possible change in the cargo presented by EVs and an impact of a surrounding milieu on such variation in the activity of EVs post-therapy.
Programmed death-ligand 1 (PD-L1) is a checkpoint that its overexpression in the TME represent poor prognosis and confers immunosuppression (Refs Reference Mortezaee and Majidpoor61–Reference Majidpoor and Mortezaee64). In melanoma patients, circulatory exosomal PD-L1 is assessed in tissue biopsy and plasma samples. Exosomal PD-L1 is detected in plasma of all evaluated patients (n = 100), but only 67% of such cases were positive for PD-L1 in tumour biopsies. Comparisons are also made for soluble and exosomal PD-L1 within plasma, and there were considerably higher levels of this checkpoint in exosomes (64.26 pg/ml) compared with soluble PD-L1 (0.1 pg/ml). These are indicative that plasma detection of exosomal PD-L1 is more reliable than that detected in tumour biopsies, and quantification of PD-L1 is easier in exosomes compared to the soluble PD-L1 (Ref. Reference Cordonnier65). Serum assessment of exosomal PD-L1 is also applicable for monitoring metastasis in cancer patients, such as lung metastasis in osteosarcoma cases (Ref. Reference Wang66). A key and basic tip here is that tissue biopsy is invasive by nature, whereas plasma allows us to have a non-invasive method. Considering this along with what aforementioned, it could be asserted that plasma evaluation of EVs can be a desired route for EV-based analysis for tumour genomic map in cancer patients.
Urine analysis of extracellular vesicles for detection of malignancies of urogenital system
Urine is ideal for evaluation of EVs in cancers of urological system, such as prostate cancer because of its close proximity with such system (Ref. Reference Dhondt67). Prostasomes is an EV population detected in the prostatic and seminal fluid. Prostatic fluid is secreted into the urethra during urination, and its release can be stimulated using gentle prostate massage upon digital rectal examination (DRE), due to that the urine is called DRE urine. An increase in the amount of prostatic fluid in DRE urine is indicative of a rise in the rate of prostate-derived EVs (Ref. Reference Ramirez-Garrastacho68). Bottom-up OptiPrep density gradient (BU ODG) centrifugation for density-based urine fractionation is a useful tool for separation of highly-specific EVs from urine samples. In patients with prostate cancer, proteomic profiling of such vesicles can confirm the specificity of such cancer. BU ODG allows for gradient separation of three fractions of biomarkers: protein-enriched fraction at the bottom of ODG column (1.207–1.231 g/ml), Tamm-Horsfall Protein (THP)-enriched fraction (1.156–1.201 g/ml) overlaying the first and EV-enriched fractions on the top (1.087–1.109 g/ml) (Ref. Reference Dhondt67). Urinary EVs can be traced for miRNAs in cancer detection and medical checkup. Detection of such EVs can serve as biomarkers for detection of urologic (ex. prostate and bladder) as well as non-urologic (ex. liver, lung and pancreas) cancers (Ref. Reference Yasui69).
Detection of extracellular vesicles
EVs carry tetraspanins and other CD markers on their surface like what seen on surface of stem cells. Such markers can be investigated and stratified into common and specific markers. Specific markers can be used for purification of EVs from plasma for further evaluation of their content in cancer diagnosis and therapy.
Tetraspanins for extracellular vesicle detection
Tetraspanins specific to the endosomes, such as CD9, CD63 and CD81 are enriched in human EVs (Ref. Reference Jung70) and are considered as typical EV markers (Ref. Reference Ekström71). Expression of such tetraspanins along with the heat shock proteins (HSP70) can be served to distinguish EVs from other cytosolic vesicles (Ref. Reference Tucci72). CD9, CD63 and CD81 are all expressed in EVs derived from both prostate cancer stroma and the adjacent normal stroma with only low differences in their overall levels in EVs harvested from the two sources (Ref. Reference Shephard73). Anti-CD63 antibody can be used for capturing exosomes isolated by size-exclusion chromatography (SEC), as implemented for patients with head and neck cancer (Ref. Reference Theodoraki74). Magnetic beads and biochips can be conjugated with anti-CD9 antibody and used for capturing CD9+ EVs from culture medium or plasma (Refs Reference Vonlaufen75, Reference Zhang76). Based on outcomes of a recent report, an increase in the number of CD63+ CD81+ EVs in a tumour is interpreted as a better prognosis. High fraction of such EVs is accompanied by enriched pro-inflammatory phenotype of macrophages and increased cytolytic activity of immune system (Ref. Reference Fathi77).
Cancer-related CD markers
CDs 24, 29, 44 and 146 are cancer-related CD markers (Ref. Reference Ekström71). CD24 is a mucin-like antigen that is considered as an oncogene (Ref. Reference Jing78) and a marker of cancer stemness (Ref. Reference Altevogt79). CD24 is highly expressed in many types of solid cancers (Ref. Reference Jing78), and CD24+ EVs are detected in serum of patients with ovarian and breast cancers (Ref. Reference Altevogt79). CD29 is implicated in cancer metastatic diffusion and is highly expressed in exosomes collected from cancer patients (Ref. Reference Kang80). CD44+ exosomal transfer from highly metastatic ovarian cancer cells will induce a metastatic phenotype in low-metastatic cancer cells (Ref. Reference Shen81). CD44 is also described as a marker for detection of aggressive mesenchymal glioblastoma (GBM), which is identified at the surface of small EVs secreted from GBM cells. CD44 expression on small EVs from such cancer type is correlated with its mesenchymal phenotype and the invasive behaviour of cancer cells (Ref. Reference Lane82). CD146 is an inducer of epithelial-mesenchymal transition (EMT), and is correlated with poor prognosis, high tumour stage and TNBC (Ref. Reference Zeng83). Reduced expression of CD146 in EVs at one month after surgical resection is indicative of the importance of this marker for disease monitoring after surgery and therapy (Ref. Reference Bandini84). CD29 along with CD146 are also enriched in EVs of patients with Her2+ (vs. Her2−) cancer (Ref. Reference Ekström71). In addition, CD146 is a well-known biomarker of melanoma that is also expressed by EVs derived from this cancer type (Ref. Reference Porcelli85).
Extracellular vesicle contents for cancer detection
Melanoma signature using specific exosomal markers in human subjects is of prognostic and therapeutic value. This is comprised of HSP70, HSP90, TYRP2, VLA-4 and MET. Disease progression is predicted by exosomal co-expression of MET and TYRP2 (Ref. Reference Peinado86). Patients with malignant melanoma display P2X7 upregulation which is contributed to EV release and EV preferential miRNA selection, so it can be an appropriate prognostic marker and a therapeutic target in such patients (Ref. Reference Pegoraro87). PDCD6IP (ALIX) in small EVs is a protein that has the highest power for distinguishing patients with progressive melanoma from cases with no evidence of this disease. By contrast, upregulation of CNTN1 (contactin-1) occurs in small EVs only in cases with no evidence of melanoma following therapy (Ref. Reference Pietrowska88).
Exo-lncRNAs show considerable differences between breast cancer patients with healthy individuals. Among various exo-lncRNAs, VIM-AS1, ELDR and SNHG8 are found to be linked with disease progression (Ref. Reference Zhao89). Progressive colorectal cancer (CRC) cells release exosomal ADAM17 for their metastasis (Ref. Reference Sun90). Exosomal Cripto-1 can be assessed in serum of patients with perihilar cholangiocarcinoma, and it can be considered as a potential biomarker (Ref. Reference Hu91). Exosomal HOXD-AS1 is increased in serum of patients with prostatic cancer, and is regarded as a promising biomarker for diagnosis and treatment of the metastatic cancer. HOXD-AS1 is acting via modulation of the activity of miR-361-5p/FOXM1 axis, and is correlated with nodal and distant metastasis (Ref. Reference Jiang92). Post-DRE of exosomal lncRNA in urine is an effective way for early detection of prostatic cancer. Exploiting such way is superior to the routine prostate-specific antigen (PSA) testing, as it avoids unnecessary biopsies by 24.2% (Ref. Reference Li93). Epidermal growth factor receptor variant III (EGFRvIII) mRNA is identified in small and large EVs collected from glioma mice models (Ref. Reference Yekula32). In patients with GBM, MVs containing EGFRvIII are detected in serum of 7 per 25 cases (Ref. Reference Skog94). EGFRvIII containing EVs are transferred from glioma cells toward EGFRvIII– cancer cells, which is for induction of the activation of related genes and pathways, thereby potentiating anchorage-independent growth of tumour (Ref. Reference Al-Nedawi95). A point to consider is that markers suggested here are not all specific for each type of cancer. Cripto-1, for instance, is expressed at high levels in several number of human cancers (Ref. Reference Hu91). However, its evaluation can be an indicative of poor prognosis, and it can be assessed as a promising marker for early cancer detection. Thus, detection of Cripto-1 enables early management of cancer, thereby reducing the possibility of tumour metastasis. A summary of EV markers for detection of solid cancer types is presented in Figure 2.

Fig. 2. Extracellular vesicle (EV) contents for cancer diagnosis. EphA2 and alkaline phosphatase placental-like 2 (ALPPL2) and glypican-1 are early diagnostic markers used for diagnosis of pancreatic cancer. Evaluation of EphA2 is also useful for detection of non-small cell lung cancer (NSCLC) and colorectal cancer (CRC). EVs from right-sided colon cancer are enriched in LRG1 and SPARC. Expression of etraspanin-8 on EVs can be a predictor of future metastasis in NSCLC patients. FAK enrichment of small EVs is a marker to distinguish breast cancer from healthy subjects. Surface expression of developmental endothelial locus-1 protein (Del-1) on EVs is a marker for early detection of breast cancer. Exosomal HOXD-AS1 can be assessed as a biomarker of metastatic prostate cancer, and prostate-specific membrane antigen (PSMA) is a biomarker of prostate cancer and metastatic breast cancer. An increase in the serum level of epidermal growth factor receptor variant III (EGFRvIII) within small EVs is seen in glioblastoma (GBM) patients. MET, TYRP2 and P2X7 are contributed to the progression of melanoma. P2X7 overexpression determines selective EV content release for metastatic spread. Cripto-1 assessment in exosomes can be a potential biomarker for patients with perihilar cholangiocarcinoma.
Extracellular vesicle RNAs in solid cancer diagnosis
EVs mediate a dynamic and multi-directional communication between cells partly via delivering functional RNA (miRNA and mRNA) (Refs Reference Lai96, Reference Valadi97). Cancer-derived exosomes are contributed to the biogenesis of miRNAs independent on cell involvement. Precursor miRNAs can thus be processed by cancer exosomes into mature miRNAs, and that such exosomes mediate rapid mRNA silencing for reprogramming the transcriptome of target cells. Such peculiarity for tumour-derived exosomes will offer potentials for developing exosome-based biomarkers (Ref. Reference Melo98). miRNAs sorted to exosomes are seemingly modulated by the impact of cell activation on dynamic changes in transcriptome (Ref. Reference Squadrito99), and distinct classes of EV RNAs are exported selectively by cells, evidence of which is in CRC (Ref. Reference Hinger16). Detection of EV-miR-1246 in serum is of prognostic and diagnostic value in patients with gallbladder cancer. miR-1246 promotes proliferation and invasion of gallbladder cancer cells and is considered as an independent prognostic biomarker (Ref. Reference Ueta53). Exosomal miR-25/miR-203 is a biomarker for detection and monitoring treatment responses in patients with oesophageal squamous cell carcinoma (ESCC). Exosomal miR-25/miR-203 level is increased in patients with ESCC, and it shows reduction in its level after surgery. It is also linked with the lymph node metastasis in such cases (Ref. Reference Liu100). Compared with healthy subjects, urinary EV analysis from patients with benign prostatic hypertropia (BPH) and prostate cancer represents considerable upregulation of miR-21 and miR-346, while reduced levels of miR-23a and miR-122-5p were found in vesicles of such cases. Interestingly, levels of these miRNAs showed no considerable difference between patients with BPH and prostate cancer (Ref. Reference Dao43).
Methods for detection of extracellular vesicle-associated RNAs in cancer patients: EV-associated mRNAs and miRNAs are biomarkers for detection of cancer (Ref. Reference Hu57). Epigallocatechin-3-gallate (EGCG) is a polyphenolic biomolecule found in green tea, which is identified for its anti-oxidative activity and its anti-carcinogenic potential against a number of tumours (Ref. Reference Guo101). EGCG can also be used for isolation and detection of exosomal miRNAs in human plasma samples. EVs can be efficiently isolated via this easy-to-use method, effective for detecting EV miRNAs in cancer patients. Polyphenol has potent adhesive tendency to biological molecules, which allows exosome collection within biofluids, such as whole blood, serum, plasma and urine (Ref. Reference Jang45). Circulating EVs can be captured using nanoparticle biochips. Lipid-polymer hybrid nanoparticle (LPHN)-catalysed hairpin DNA circuit (CHDC) biochip is an effective tool for quantifying EV-associated RNAs. Signal amplification capacity allows the biochip to identify low levels of EV-mRNA in body fluids, such as serum, thereby enabling early detection of cancer. Low level of glypican-1 mRNA, for instance, can be detected using this biochip in serum EVs of patients with pancreatic cancer (Ref. Reference Hu57).
ExoNA-sensing chip is a microfluidic device for accurate control of fluid flow and an effective tool for cancer diagnosis. This device is equipped with three-dimensional nanostructured hydrogels for one-step detection of specific RNAs in intact exosomes. In breast cancer, for instance, such chip is equipped with a sensing hydrogel with probes enabling detection of the target exosomal mRNA (the breast cancer marker Erb-B2 receptor tyrosine kinase 2 [ERBB2] gene, along with a reference gene GAPDH). The hydrogel amplifies fluorescent signals at room temperature, and the encapsulation of probes with liposomes blocks generation of fluorescent signals from non-specific reactions. Precise analysis is also applicable via this device due to enabling the correction of value for variation in the number of exosomes among different individuals (Ref. Reference Lim102).
Protocols for isolation and detection of extracellular vesicles
A number of methods are available for EV isolation and detection in cancer diagnosis, a summary of which is represented in Tables 1 and 2.
Table 1. Common methods for extracellular vesicle (EV) isolation and purification in cancer

ELISA, enzyme-linked immunosorbent assay; UC, ultracentrifugation; and SEC, size-exclusion chromatography.
Table 2. Highly specific and novel methods for detection of extracellular vesicles (EVs) in cancer diagnosis

iddPCR, immune-droplet digital PCR; nPES, nanoplasmon-enhanced scattering; LPHN-CHDC, Lipid-polymer hybrid nanoparticle-catalysed hairpin DNA circuit; DPPIE, digital profiling of proteins on individual EV; and AFM-IR, atomic force microscope IR spectroscopy.
Labelling dyes
An ideal labelling method for visualisation of EVs requires to be specific for EVs, to has a desired half-life, to be stable and represent sufficient signal for preferential detection of nonoscaled EVs. Lipophilic dyes, such as PKH and DiI are commonly used for EV labelling (Ref. Reference Lai96). Pre-incubation of exosomes with the PKH green dye and then exposing them to target cells, such as cancer cells will enable examination of exosomal internalisation in cell/s of target (Ref. Reference Zhao103). PKH, however, is not specific for EVs due to labelling other lipid-containing components as well. Live-cell imaging techniques can be obtained using florescent labelling of EV membrane. EV labelling using palmitoylated fluorescent protein (PalmtdTomato) dye is more specific than PKH67 (Ref. Reference Lai96). PalmtdTomato is used for labelling membrane proteins on EVs (Ref. Reference Zaborowski104). PalmtdTomato labels predominantly the inner membrane of EVs, which will reduce potential disturbances in the composition of surface proteins in such vesicles (Ref. Reference Lai96).
Enzyme-linked immunosorbent assay for extracellular vesicle quantification
Enzyme-linked immunosorbent assay (ELISA) is a method for EV quantification in unpurified liquid samples. Conventional EV ELISA has several predicaments, such as long assay time, large sample consumptions and limited sensitivity (Ref. Reference Tan105). At least 50 μl undiluted plasma is required in a single replicate well of ELISA assay (Ref. Reference Liang13). Conventional ELISA and western blot are based mainly on bulk (rather than precise) measurement of individual EV heterogeneity. Bulk measurements mask the differences exist among individual EVs. ExoELISA assay is an advanced method developed in this area for single EV evaluation. ExoELISA assay is able to count single exosomes within plasma (limit of detection (LOD): 10 exosomes/μl). This method can be used for distinguishing breast cancer cases before/after surgery (Ref. Reference Zhang76).
Ultracentrifugation
Differential ultracentrifugation (UC) followed by density separation is an approach recently proposed for EV isolation (Ref. Reference Crescitelli, Lässer and Lötvall29). Generally, UC is a time-consuming method (Ref. Reference Tian20) in which Conventional differential UC is a heavy workload due to requiring consecutive centrifugation steps (Ref. Reference Correll106). Both small and large size EVs can be isolated using differential UC (Ref. Reference Crescitelli36). UC at 1 10 000 × g is exploited for collection of exosomes from CRC patients (Ref. Reference Dou107).
A fraction of urinary EVs is removed along with larger THP matrices during early low-speed centrifugation of urinary specimens, which introduces a bias. Recovery of such trapped EVs is applicable by depolymerisation using dithiothreitol, but it will result in the generation of smaller THP fibres that can be a source of contamination due to their co-sedimentation with EVs separated during high-speed centrifugation steps. A suggested approach for effective recovery of THP-entrapped urinary EVs and reducing contamination with proteins is the incorporation of alkaline wash and THP polymer reduction (Ref. Reference Correll106).
Size-exclusion chromatography
SEC is a simple and reproducible way for isolation of human EVs with high purity from blood samples (Ref. Reference Ueta53). Compared to the UC, SEC retain the integrity of small EVs and concomitantly reduces contamination with plasma proteins (Ref. Reference Vinik108). This is indicative of better recovery of urinary exosomal particles and proteins by SEC compared with UC (Ref. Reference Guan109). Besides, it is shown that EVs isolated by SEC are more functional compared to that for UC. This point is of value when EVs are applied for therapy (Ref. Reference Mol110). A disadvantage of SEC is the recovery of limited EV quantity (Ref. Reference Gámez-Valero111).
Precipitation
Differential UC, SEC and commercial precipitation methods, such as Exoquick are used commonly for separation of EVs from urine. Outcomes of a recent study, however, showed that such methods are sub-optimal for separation of highly-specific urine EVs (Ref. Reference Dhondt67). Precipitation and SEC are rapid methods for EV isolation compared to the UC. PRotein Organic Solvent PRecipitation (PROSPR) and polyethylene glycol (PEG) are examples of precipitating agents for EV isolation. A predicament for using PEG in EV isolation is the co-precipitation of proteins and contaminants, while SEC yields clean EVs (Ref. Reference Gámez-Valero111). Based on outcomes of one study, precipitation method for EV isolation also co-precipitated 9–15% of plasma proteins (Ref. Reference Karttunen112).
Immuno-PCR and immune-droplet digital PCR
Immuno-polymerase chain reaction (iPCR) is a powerful method for identification of EV proteins (Ref. Reference Sun and Meckes12). This immunoassay technique employs DNA for signal generation and utilises antigen–antibody interactions and amplification of PCR power. Immune-droplet digital PCR (iddPCR) is the latest utilisation of PCR technology. ddPCR can be integrated into iPCR for enabling protein detection with high sensitivity (Ref. Reference Zhou113). EV proteins are profiled using iddPCR amplification method, allowing multiplexed protein profiling of a single EV. EVs are labelled with antibody-DNA conjugates. Then, stochastic microfluidic EV is incorporated into droplets, and barcode signals are converted and amplified (using in situ PCR with florescent probes) for droplet imaging. The iddPCR enables ultrasensitive detection of rare proteins in a single EV. Microscopic resolution is not demanded for identification of single EV via such technique. This can be an effective tool for analysis of plasma EVs, enabling detection of molecular signature and monitoring therapy (Ref. Reference Ko114).
Nanotechnology for extracellular vesicle detection
Nanoplasmon-enhanced scattering assay
Nanoplasmon-enhanced scattering (nPES) assay is an inexpensive and ultrasensitive way for quantification of tumour-derived EVs. Gold nanoparticles including nanospheres and nanorods are used in this assay. Here, a sensor chip is considered for capturing EVs using specific antibodies and bondage of antibody-conjugated gold nanoparticles to EVs on this sensor chip. This will generate a local plasmon effect for increasing the specificity and sensitivity of detection for tumour-derived EVs. In this approach, quantification is applicable with low plasma sample (1 μl). As compared to the ELISA method, nPES requires lower sample volume, and it demands lower costs. nPES EphA2-EV assay can be used for distinguishing pancreatic cancer from pancreatitis. It can also be applicable for tumour staging and inspecting early responses to therapy (Ref. Reference Liang13). Nanoplasmonic exosome assay can be used for analysing exosomes secreted from ovarian cancer cells into ascites. Evaluation of CD24 and epithelial cell adhesion molecule (EpCAM) on ovarian cancer exosomes enables distinguishing them from benign cells (Ref. Reference Im115). CD24 and EpCAM are presented in exosomes of malignant ascites in patients with ovarian cancer (Ref. Reference Runz116).
Templated nanoplasmonics is a technology for multiparametric analysis of exosomes directly from clinical biofields. The same vesicles are undergoing simultaneous biomolecular and biophysical assessments. Here, Specific assessment of exosomal biomarkers is achieved through in situ assembly and growth of gold nanoshells on such vesicles. Multi-selective evaluation of various exosome markers, such as miRNAs and proteins are applicable through inspecting optical signals received from quenching fluorescent probes from the target-bound vesicles. This will distinguish such vesicles from non-vesicle particles or free molecular targets. This technology yields a rapid analysis in which only 15 min is required for multiplexed assessment with just 1 μl of plasma sample (Ref. Reference Wu117).
Nanopatterned microchips
Nanopatterned microchips are three-dimensional devices that enable analysis of the EV-related proteolytic enzymes through evaluation of their expression profile and proteolytic activity. Plasma samples can be obtained, and accurate classification of cancer patients is applicable by this approach. In vitro detection of cell invasion along with in vivo monitoring of tumour metastasis are applicable via this approach, which enables clinicians to be informed for taking personalised cancer therapy (Ref. Reference Zhang55).
Designing a microfluidic chip with three-dimensional nanopatterns enables detection of low exosomal levels in plasma and is important for early detection of cancer. In patients with ovarian cancer, such device can be exploited for detection of exosomal folate receptor alpha (FRα) protein, which can be a marker for early detection and monitoring progression of such cancer (Ref. Reference Zhang118). FRα is a receptor that is overexpressed in a number of solid cancers, such as TNBC, lung cancer and ovarian cancer, and upon bondage with folate it enables folate taken up by cells, which is a target for chemotherapy drugs, such as pemetrexed and methotrexate (Ref. Reference Scaranti119).
Magnetic nanoparticles
Lactoferrin conjugated 2,2-bis(methylol)propionic acid dendrimer-modified magnetic nanoparticles (LF-bis-MPA-MNPs)-based approach is a rapid and simple method for EV isolation. LF-bis-MPA-MNPs are chimeric nanocomposites. LF-bis-MPA-M can be used for isolation of EVs from several biological samples. In LF-bis-MPA-M method, a combination of biorecognition, electrostatic interaction and physical absorption are exploited for EV isolation, so it can be a high-yield method for isolation of EVs from urinary samples in both laboratory and clinics (Ref. Reference Dao43). Labelling MVs with magnetic nanoparticles and their detection by miniaturised nuclear magnetic resonance system yields a rapid and highly sensitive technique for direct profiling of circulating MVs. In a cancer like GBM, such system enables detection of specific MVs in cancer patients and allows comparison of the rate with that in non-cancer subjects. Such MVs can also be detected for predicting responses to therapy (Ref. Reference Shao120). Magnetic nanoparticles can be used for assessment of EV glycans. Many biomarkers used for clinical diagnostic purposes are glycosylated. EVs are harbouring a rich glycome, which is reflective of disease progression. Integrated magnetic analysis of glycans in EVs (iMAGE) is a technology designed for transduction of EV glycans into magnetic signals by polycore magnetic nanoparticles. Such signals are quantified through a sensor, and the result is a rapid (lower than 30 min) and sensitive (detection of about 104 EVs) assay (Ref. Reference Wang121).
ExoSCOPE
Real-time monitoring of targeted therapy provides important information about drug resistance in tumours and therapeutic responses (Ref. Reference Wang6). ExoSCOPE is a nanotechnology platform that can be applied for such purpose. ExoSCOPE is used for measurement of EV drug dynamics via which drug-target engagement is evaluated simultaneous with changes in protein expression profiles in a specific EV subtype. Assessment of EV drug dynamics is reflective of drug occupancy within target cells, thereby enabling real-time monitoring of treatment potency for a target drug (Ref. Reference Pan122).
Imaging systems for extracellular vesicle detection
Imaging flow cytometry (IFC) is proposed as a way for EV observation and quantification. Such technique requires low sample volume and reduced preparation time for specimens. IFC can be used for enhancing the sensitivity of particle detection for EV analysis (Ref. Reference Clark123).
Multiplexed reporters are optical reporters for labelling multiple population of EVs, enabling tracking and visualisation of EVs secreted from tumour, along with their uptake and exchange between various cell types both in vitro and in vivo. Here, membranes of EVs with different sizes are labelled with PalmGFP, PalmtdTomato, and PalmtdTomato encoding transcripts are tagged with a MS2 RNA-binding sequence for further visualisation and monitoring of both EV membrane and EV-packaged mRNA cargo. Visualisation of such EVs within tumour tissue is applicable using multiphoton intravital microscopy (Ref. Reference Lai96).
EV enrichment is occurring concurrent with a shift in metabolic systems toward biosynthesis, enabling in situ imaging of tumorigenic processes, including metastatic-related events in an unperturbed TME (Ref. Reference Tu124). Metabolic switching is considered as a tumour hallmark (Refs Reference Mortezaee125, Reference Mortezaee and Majidpoor126). Multicontrast nonlinear imaging can be utilised for visualising endogenous substances in breast cancer from both animal and human subjects. Fresh sections from human breast cancer can be readily imaged for detection of tumour-associated EVs. Nonlinear imaging can thus be a novel approach for cancer diagnosis and therapeutics (Ref. Reference Tu124).
Extracellular vesicle-based early detection of cancer
Early detection of cancer is the most focus of current studies. For a cancer like prostate, a localised cancer has an excellent prognosis with 5-year overall survival (OS) of 100%, but upon metastasis this rate is reduced to about 30% (Ref. Reference Najafi14). Metastasis is, in fact, the cause of almost all deaths from prostate cancer (Ref. Reference Majidpoor and Mortezaee127) and, in general, is contributed to more than 90% of deaths due to cancer (Ref. Reference Mortezaee128) . The 5-year OS rate for early-stage renal cancer and CRC is about 90%, but upon metastasis this rate is reduced to lower than 12% in the former and to lower than 10% in the latter (Ref. Reference Najafi14). These statistics are representative of the importance of early cancer detection and investigating novel biomarkers for such purpose in order to have effective cancer therapy. Cancer patients, particularly cases at early stages of disease, display low levels of biomarkers. Besides, detection of such low fractions may be impaired due to competition with non-specific peptides and proteins, which are abundant within blood. EVs due to being stable and able to keep their cargo contents, can be used for biomarker detection early in tumorigenesis (Ref. Reference Liang13).
EphA2 is overexpressed on EVs collected from pancreatic cancer cells, but it is absent on EVs derived from normal pancreatic tissue. EphA2 overexpression also occurs in early-stage non-small cell lung cancer (NSCLC) and CRC, which is indicative of the essence of evaluation of this biomarker for early cancer detection (Ref. Reference Liang13). Alkaline phosphatase placental-like 2 (ALPPL2) is a membrane protein and a potential biomarker for diagnosis of PDAC at early stages. ALPPL2 is available in EVs released from pancreatic cancer (Ref. Reference Shin51). Glypican-1+ circulatory exosomes is another biomarker for early detection of pancreatic cancer. Glypican-1+ exosomes identify KRAS mutations, which has 100% correlation with the mutation of this gene in tumours. KRAS is a gene abundantly mutated in patients with pancreatic cancer (Ref. Reference Melo129). Alternating current electrokinetics (ACE) platform for EV purification from plasma is able to detect pancreatic cancer at stage 1 of the disease in 95.5% of cases. This rate is higher compared with the respective 74.4 and 43.8% detection rate in ovarian and bladder cancer patients (Ref. Reference Hinestrosa25).
Breast cancer is a leader of death in women and despite advances in the field, identifying novel choices for early detection of such cancer is a crucial challenge (Ref. Reference Esposito130). Diagnosis of breast cancer at early stages allows for immediate surgery without the need for prior radiation or chemotherapy, which represents an optimistic prognosis. Carcino-embryonic antigen (CEA) and carbohydrate antigen 15-3 (CA15-3) are biomarkers that are currently assessed for screening breast cancer and monitoring treatment responses. However, the specificity and sensitivity of such blood markers are low, which asks for surveying novel efficient diagnostic methods for early detection of breast cancer (Ref. Reference Su54). The sensitivity of CA15-3, which is the most frequently used marker for patients with metastatic breast cancer is about 60–70%. Developmental endothelial locus-1 protein (Del-1) is a surface protein on circulating EVs that its concentration is considerably higher in breast cancer patients, and its rate is returned to normal after surgical resection of tumour. The sensitivity and specificity of Del-1 for detection of breast cancer are 84–99% and 74–86%, respectively (Ref. Reference Moon131). Extracellular vesicle long RNA (exLR) d-signature score is higher in breast cancer compared with a benign disease. The d-signature can be exploited for detection of early-stage breast cancer from the control and benign groups with area under the curve (AUC) of 0.94 and 0.88, respectively, which is indicative of the potential of exLR d-signature for detection of early-stage breast cancer. exLRs, such as exMSMO1 can also be assessed in plasma for predicting responses to the neoadjuvant therapy. Silencing MSMO1 considerably augmented sensitivity of breast cancer cell lines to chemotherapy (Ref. Reference Su54).
Thermophoretic analysis of extracellular vesicle surface proteins for early detection of prostate cancer
Each year, more than a million of needle biopsies from prostate are performed in the USA mainly due to fluctuations or elevation of PSA, which prone patients to the increased risk of infectious complications (Ref. Reference McKiernan132). In addition, not all prostate cancer cases show elevated level of PSA in which 30% of men reach a false negative rate with PSA lower that 4 ng/ml (Ref. Reference Shephard73). It is also not applicable to link a specific rate of PSA level in patients with metastatic prostate cancer (Ref. Reference Rode133). Besides, tracing for PSA as a cancer screening marker can cause overdiagnosis and overtreatment. Thus, optimal replacement for PSA testing is imperative for strengthening detection rate and reducing unnecessary biopsies (Ref. Reference Li93). Tracing surface proteins from EVs using thermophoretic profiling is a promising way for early cancer diagnosis and classification (Ref. Reference Liu11). A virtue for the use of thermophoretic aptasensor is no need for EV pre-isolation. Instead, thermophoretic enrichment and aptamer-bound EVs are used for producing an amplified fluorescent signal, the intensity of which is indicative of expression of serum EV surface proteins (Ref. Reference Tian20). Sensitivity and specificity of this method for detection of stage 1 cancers are 95 and 100%, respectively. In regard with prostate cancer, such approach can be exploited efficiently for detection of PSAs and discriminating prostate cancer from benign prostate enlargement. Thermophoretic profiling is inexpensive and fast way and requires low serum volumes (lower than 1 μl) (Ref. Reference Liu11). Thermophoretic profiling is also applicable for discrimination of metastatic from non-metastatic breast cancer and for monitoring treatment responses of the metastatic cancer (Ref. Reference Tian20).
Extracellular vesicles for detection of some solid cancer types
Extracellular vesicle proteomic assay for detection of breast cancer subtypes
In regard with breast cancer, proteome profiling of EVs released from diverse cell lines can give us important information about different molecular subtypes. Different subtypes of breast cancer have specific molecular pathways and diverse biological processes. Different kinases and proteins are evolved in EVs, so evaluation of EVs can be exploited as a non-invasive tool for discriminating between various breast cancer subtypes. It was found EV enrichment essentially with factors contributed to metastasis in patients with TNBC. These factors were proteins related to angiogenesis, cell migration and extracellular matrix (ECM) organisation. Cellular proliferation, by contrast, is a hallmark of HER2 breast cancer subtype, which was elicited in EVs harvested from this tumour subtype. Proteins enriched in HER2-EVs act in cell-to-cell adhesion and are related to the ERBB2 signalling (Ref. Reference Rontogianni18).
Extracellular proteomic assay for detection of colorectal cancer
CRC is a tumour that shows high rates of prevalence and mortality (Ref. Reference Najafi14). EV protein profile is different in patients with right-sided compared with left-sided colon cancer. Right-sided colon cancer is more prone to develop metastasis. Leucine-rich alpha-2-glycoprotein 1 (LRG1) and secreted protein acidic and rich in cysteine (SPARC) are upregulated in EVs collected from right-sided colon cancer, thus they can be considered as markers of tumour sidedness (Ref. Reference Zhong134).
EV Click Chips for diagnosis of hepatocellular carcinoma
EV Click Chips is an EV purification system for detection of hepatocellular carcinoma (HCC). In this system, four approaches are used for EV purification: EV capturing, multi-marker cocktails of antibodies, nano-structured substrates and micro-fluidic chaotic mixers. Plasma samples are collected, and HCC specific mRNA markers are quantified using reverse transcriptase PCR system (Ref. Reference Sun3).
Evaluation of extracellular vesicle heterogeneity
EVs released from different individual cells display considerable variations in their phenotypes and quantity. Such high heterogeneity may also occur in tumour-derived EVs (Ref. Reference Zhang76). In fact, tumours have heterogenous nature due to genetic variations among individual cells, and that such heterogeneity is also reflective in EVs (Ref. Reference Zomer17). Based on interpretations of a quite recent systematic review, the heterogeneity in EV samples can be explained partially by lack of appropriate methods for proper dissection of EV composition. It requires attention that the biological composition of EV membranes is very heterogenous, so differences are seen among individual cells (Ref. Reference Gudbergsson135). Information collected from EV-to-EV heterogeneity can be helpful for developing new methods for cancer diagnosis. Localised fluorescent signals generated from rolling circle amplification (RCA) which is a useful tool for assessing individual EV heterogeneity. The whole method is called digital profiling of proteins on individual EV (DPPIE). Here, an anti-CD9 engineered biochip and multiple DNA aptamers are used for EV capture from plasma and specific label of surface membrane proteins on EVs, followed by RCA for generation of localised fluorescent signals, which can be observed with a confocal microscopy. DPPIE has an ultrasensitive EV analysis capacity enabling profiling of multiple proteins on a single EV. DPPIE can be used for distinguishing the EV in patients with lung squamous cell carcinoma from that in lung adenocarcinoma cases (Ref. Reference Zhang76). Atomic force microscope IR spectroscopy (AFM-IR) is a useful tool for identifying the structure and composition of EVs, so it can be applied in cancer diagnosis and therapy. This approach only requires a basic understanding of chemistry and spectroscopy, and data are completed at about 24 h. AFM-IR is able to detect proteins, nucleic acids and lipids within a single EV from a population of vesicles and to compare different population of EVs. This technique will enable comparing individual EVs both qualitatively and quantitatively. AFM-IR has a high resolution (<20 nm), which allows for interrogating variations in structure and composition in a single EV. Assessment of such variations is also important for tracing changes occur upon encapsulation of EVs with drug molecules used for drug carriers in cancer therapy. A point to consider is that AFM-IR is semiquantitative due to the heterogeneity of vesicles (Ref. Reference Kim10).
Conclusions and future perspectives
Discovering highly accurate and non-invasive biomarkers in cancer diagnosis is the main focus of the current area of cancer research. Over the past decade, several molecular signatures are developed, but they have no overlaps and are failed for validation in independent cohorts of patients (Ref. Reference Kim1). EVs are under continuous evaluation for being used as prognostic and diagnostic biomarkers. EVs provide a window toward genetic state of individual cancers (Ref. Reference Balaj7). They show heterogeneity in tumours and conventional methods are based mainly on bulk measurements (Ref. Reference Zhang76). PalmtdTomato is an effective way for labelling EV membrane proteins, as compared to the PKH67. In regard with ELISA, ExoELISA is preferred over conventional methods due to enabling single exosome count. Compared to the ELISA, nPES needs lower sample volume, and it demands lower costs. Isolation of small and large size EVs is applicable using differential UC. As compared to the UC, more functional EVs can be isolated using SEC. Precipitation and SEC are rapid methods for EV isolation compared to the UC. SEC yields clean EVs, while contaminants exist in precipitation methods. Rare single EV proteins can be detected using iddPCR. LF-bis-MPA-M is a high-yield method and a rapid and simple way for EV isolation from urine. AFM-IR allows for interrogating variations in structure and composition of a single EV, but it is semiquantitative. A number of works are being exploited for finding EV markers specific for a tumour type and enabling cancer detection at early stages. Thermophoretic profiling is a fast and an inexpensive way, which requires low serum volumes and rendering high sensitivity and specificity for early cancer detection without a need for EV pre-isolation. Research is underway for evolving more specific markers in order to distinguish different subtypes of EVs and to overcome challenges related to the complexity of biofluids for sample preparation, as well as resolving technical variations for further analysis of samples (Ref. Reference Geeurickx136). High-throughput EV profiling is now applicable using stimuli-mediated systems. Here, stimuli-responsive copolymers are installed onto phospholipid bilayer of exosomes, which enables exclusive enrichment and purification of exosomes, and non-vesicle particles are not co-exist in samples. Development of such systems will definitely boost research regarding the EVs in basic and clinics, and can be a substitute for conventional methods, such as precipitation and UC in terms of purity, isolation yield and retained bioactivity (Ref. Reference Liu137). Another advance in the field is the use of reference biological materials, such as recombinant EV (rEV), which has biophysical and biochemical traits of sample EVs. rEVs are stable and can be tailored to express a protein of interest, such as cancer cell-specific membrane protein, and they can be served as an internal control for data normalisation and evaluation of pre-analytical variables (Refs Reference Geeurickx136, Reference Geeurickx138). In the future, we will be notifying of more advances in the field through developing more systems of isolation with high reliability in isolation and purification of EVs and marker-specific stratification of such vesicles for cancer diagnosis at the earliest stages. Due to the growing number of cancers diagnosed in the world and the chronic inflammation remained due to the SARS-CoV-2 induced disease pandemic event the need for reliable non-invasive biomarkers will be more sensible.
Acknowledgements
This work is approved by Ethical Committee of Kurdistan University of Medical Sciences (The ethical code: IR.MUK.REC.1401.114).
Author's contributions
Collection and revision of information, KM, J.M and F.F; Conceptualisation, F.F and KM; writing, original draft preparation, review and editing, JM, and KM. Authors have read and agreed to publish of the manuscript.
Financial support
This research received no external funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This work is approved by Ethical Committee of Kurdistan University of Medical Sciences.
Consent for publication
The authors of the paper have been read and approve and consent the final version for publication.
Availability of data and material
Not applicable.






