Introduction
Ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one) is a synthetic seleno-organic compound, exhibiting glutathione peroxidase (GPx)-like activity (Ref. Reference Parnham and Sies1), and electrophilic and potential antioxidant properties (Ref. Reference Sakurai2). Its general mechanism of action is reaction with essential thiol groups in proteins (Refs Reference Noguchi3, Reference Yang, Shen and Ong4). The well-known thiol-containing compounds cysteine (Cys/CySS), glutathione (GSH, GSH/GSSG) and proteins thioredoxin (Trx, Trx-(SH)2/Trx-SS), and thioredoxin reductase (TrxR) (Refs Reference Pereira5, Reference Oliveira and Laurindo6), are all regulated by ebselen (Ref. Reference Parnham and Sies1). Cysteine is one of the least abundant amino acids in proteins and due to its unique nucleophilic thiol group, is able to participate in a broad range of chemical modifications. The nucleophilic thiol group on cysteine is a well-known biological target of ebselen (Ref. Reference Niemeyer7). GSH is a tripeptide that contains glutamate (Glu), glycine (Gly) and cysteine (Cys). This small peptide is the most abundant intracellular antioxidant (Ref. Reference Hansen and Harris8). It is mainly found in the form of reduced GSH (GSH) and oxidised GSH (GSSG), with the vast majority reduced during normal conditions (Ref. Reference Lv9). It also has auxiliary functions in metabolism and cellular signalling (Ref. Reference Arteel and Sies10). As the most abundant store of cellular thiols and the major component maintaining the vital redox equilibrium, intracellular GSH is crucial. Ebselen can bidirectionally regulate GSH metabolism, most commonly removing intracellular GSH by binding its thiols (Refs Reference Yang, Shen and Ong4, Reference Yang, Shen and Ong11).
Trx is a 12 kDa redox protein, containing five β-strands surrounded by four α helices, a structure known as the Trx fold that is found throughout the oxidoreductase superfamily (Ref. Reference Ghareeb and Metanis12). Mammalian TrxRs can reduce both prokaryotic and mammalian Trx proteins, while Escherichia coli TrxR has a narrow substrate specificity and catalyses the reduction of only bacterial Trx (Ref. Reference Nordberg and Arnér13). Three isoforms of Trx have been identified in mammalian cells: cytosolic Trx (Trx1), mitochondrial Trx (Trx2) and a Trx variant with increased expression in spermatozoa (SpTrx/Trx3) (Ref. Reference Xie14). TrxRs are selenium-dependent dimeric flavoproteins 112–130 kDa. The family includes the cytosolic TrxR1 and the mitochondrial TrxR2 (Ref. Reference Li15). These reductases are essential in maintaining redox balance in the cytoplasm and mitochondria, respectively (Ref. Reference Marshall16). Both Trx and TrxR are potential therapeutic targets of ebselen. Recent findings show that through inhibiting Trx or TrxR, ebselen increases the production of reactive oxygen species (ROS) in prokaryotic cells (Ref. Reference Ren17), making TrxR a potential therapeutic target (Refs Reference May18, Reference Gustafsson19). Thus, the thiol reactivity of ebselen makes it a multi-target molecule that can affect many biological processes.
Ebselen also plays an unprecedented role in regulating cell signal transduction. Ebselen can directly or indirectly regulate the activities of target proteins and exert pharmacological effects on many biological processes. These include anti-inflammatory and (Refs Reference Xu20–Reference Sharma22) pro-apoptotic (Refs Reference Hilchie23, Reference Yoshizumi24) activities and effects on oxidative stress (Ref. Reference Kim25) and cell differentiation (Ref. Reference Li26). Ebselen operates in different signalling pathways through various modes of action. It reduces inflammation by affecting the expression of proteins, such as c-Jun N-terminal kinase (Jnk) (Ref. Reference Bi21), interleukin-2 (IL-2) (Ref. Reference Thabet and Moustafa27) and IL-8 (Ref. Reference Xu20). Ebselen can positively regulate Jnk (Ref. Reference Yoshizumi24), cytochrome C (Ref. Reference Boireau28), nuclear factor erythroid 2-related factor 2 (Nrf2) (Ref. Reference Kim25), and negatively regulates caspase 3 (Ref. Reference Laird29), B cell lymphoma protein-2 (Bcl-2) (Ref. Reference Wei30) and tumour necrosis factor-α (TNF-α) (Ref. Reference Park31). Alterations in the expression of these proteins affect apoptosis and oxidative stress. Ebselen influences cell differentiation by regulating smooth-muscle actin (α-SMA) (Ref. Reference Park32), phosphoinositide 3-kinase (PI3K), etc. (Ref. Reference Li26). Understanding the effects of ebselen on thiol-containing targets will facilitate further studies and the discovery of new pathways that can be used for the development of novel pharmacological strategies.
Based on extensive research, several potential clinical applications of ebselen in the treatment of different diseases have been reported in cell lines studies (Refs Reference Xu20, Reference Bi21, Reference Thabet and Moustafa27, Reference Shi33), animal experiments (Refs Reference Boireau28, Reference Oostwoud34, Reference Garland35, Reference Lynch36, Reference Soyman37) and clinical trials (Refs Reference Beckman38, Reference Sharpley39, Reference Singh40, Reference Kil41). In mammalian cells, ebselen works as a GPx-like compound and peroxiredoxin mimic through Trx and TrxR to scavenge hydrogen peroxide and peroxynitrite (Ref. Reference Ren17). Recent studies using cell lines have shown that ebselen can be used in the fight against atherosclerosis and chronic kidney disease (Refs Reference Yoshizumi42, Reference Vera43) as well as cancers, including breast cancer (Ref. Reference Thabet and Moustafa27) and C6 glioma (Ref. Reference Shi33). Also active in prokaryotic cells, ebselen is a competitive inhibitor of bacterial TrxR and ebselen treatment elevates ROS levels (Ref. Reference Ren17). Hence, ebselen could help combat infectious organisms. It has inhibitory effects on Gram-positive bacteria (Refs Reference Thangamani, Younis and Seleem44, Reference Thangamani, Younis and Seleem45), Gram-negative bacteria (Refs Reference Chen and Yang46, Reference Zou47), and eukaryotic pathogens such as fungi and parasites (Refs Reference Thangamani48, Reference Felli Kubica49, Reference Eltahan50). Even for viral infections (Ref. Reference Thenin-Houssier51), particularly COVID-19, ebselen has been shown to have outstanding inhibitory activity. Ebselen's neuroprotective action and ability to counter oxidative stress have been demonstrated in animal experiments using brain disease models, including Alzheimer's disease (AD) (Refs Reference Martini52, Reference Klann53), depressive disorder (Ref. Reference Antoniadou54), brain ischaemia/stroke (Refs Reference Sui55, Reference Imai56), cystitis (Ref. Reference Wang57) and peritonitis (Ref. Reference Zou47). Clinical trials have confirmed the efficacy of ebselen in acute ischaemic stroke (Ref. Reference Yamaguchi58) and acute middle cerebral artery occlusion (Ref. Reference Ogawa59). Including a 320-enrolled-patient-trail revealed that patients who started ebselen within 24 h of stroke onset has a significant improvement than those who started treatment after 24 h, and a 99-recruited-patient-trail determined a corresponding significant reduction in the volume of cerebral infarct of patients who started treatment within 6 h of onset. Meanwhile, following ongoing clinical trials showed that ebselen is also a promising compound in treating diabetes mellitus (ClinicalTrial.gov NCT00762671), Meniere's disease (ClinicalTrial.gov NCT03325790, NCT02603081), bipolar disorder (ClinicalTrial.gov NCT03013400), hearing loss (ClinicalTrial.gov NCT01452607), ototoxicity (ClinicalTrial.gov NCT02819856), temporary auditory threshold shift (ClinicalTrial.gov NCT01444846) and other conditions. Thus far, a large number of studies have demonstrated that ebselen has multiple pharmacological effects. In short, though ebselen has not yet been approved as a drug for any specific disease, its efficacy and tolerance in humans has shown that ebselen can be a promising therapeutic compound.
The aim of this review is to summarise our current understanding of the multiple molecular targets of ebselen and the biological processes and its effect on diseases, in order to help the future exploration of novel targets and develop new pharmacological uses for this compound (Fig. 1).

Fig. 1. Bioactivity and chemical structure of ebselen (2-phenyl-1,2-benzisoselenazol-3(2H)-one).
Targeting thiol-containing compounds
Ebselen acts mainly by targeting intracellular thiol-containing macromolecules, particularly cysteine, GSH and thiol proteins (e.g., Trx and TrxR) which are listed in Table 1.
Table 1. List of thiol compounds affected by ebselen

Targeting cysteine
Cysteine is one of the most inherently reactive amino acids, and has a variety of important biochemical functions such as catalysis and redox regulation (Ref. Reference Wang60). The unique chemical properties of the thiol group in cysteine are involved in different biological transformations and functional sites, such as disulphide (S–S) bond (Refs Reference Poole61, Reference Backus62). As a common target for covalent inhibitors, the highly nucleophilic cysteine thiol provides an ideal anchor for electrophilic molecules (Ref. Reference Maurais and Weerapana63). Under various conditions, ebselen can react with a large number of cysteine thiols (Refs Reference Sakurai2, Reference Azad64, Reference Terentis65). It inhibits the activation of superoxide dismutase-1 (SOD1), it is known to cause amyotrophic lateral sclerosis (ALS); New Delhi metallo-b-lactamase (NDM-1), an enzyme resistant to a wide range of β-lactams and quiescin sulfhydryl oxidase 1 (QSOX1), a highly conserved disulphide bond generating enzyme that is overexpressed in diverse tumour types by reacting with cysteine residues and forming a selenium-sulphide (Se-S) bond or covalently modifying cysteine residues (Ref. Reference Lieberman66), including Cys-111 (Refs Reference Capper67, Reference Chantadul68), Cys-221 (Refs Reference Chen69, Reference Chiou70), and Cys-165 and Cys-237 (Ref. Reference Hanavan71). Joice et al. have reported that ebselen can modify Cys-327 in Trypanosoma brucei hexokinase via thiol oxidation (Ref. Reference Joice72). Leroux et al. also have demonstrated that ebselen can mediate by disrupting Insulin-Degrading Enzyme (IDE) dimerisation, and reduce the HD exchange of Cys-(181–198) (Ref. Reference Leroux73). The most striking finding is that ebselen exhibits promising antiviral activity against COVID-19: it inhibits the enzymatic activity of Mpro through covalent bonding to the catalytic cysteine (Refs Reference Jin74, Reference Sies and Parnham75, Reference Menéndez76). Mpro is a key enzyme of COVID-19, that plays a pivotal role in mediating viral replication and transcription, making it an attractive target (Refs Reference Anand77, Reference Yang78). Besides, the papain-like protease (PLpro) from the human coronavirus is a protease that plays a critical role in virus replication, Tomczak et al. have reported that ebselen and its analogues are able to modify Cys-111 of PLpro in the active site (Ref. Reference Weglarz-Tomczak79). In summary, ebselen reacts with the thiol group in cysteine and the reactivity between ebselen and cysteine makes it an effective modulator of this essential amino acid.
Targeting GSH
GSH is a tripeptide that contains a thiol group and is the most abundant low molecular-weight thiol compound in mammalian cells. It is essential as an antioxidant and in maintaining homeostasis (Refs Reference Kovacs-Nolan80, Reference Xiao81). Due to its important role in the cell, many studies have indicated that GSH can be a therapeutic target (Refs Reference Laird29, Reference Thangamani48). When ebselen is present, it can react with the SH group on GSH and affect GSH levels (Ref. Reference Ren, Zou and Holmgren82) (Fig. 2). As a target of ebselen, GSH plays different roles in various diseases. The regulation of GSH by ebselen in different diseases is bidirectional. The main effect of ebselen on GSH is to regulate its levels to maintain micro-environmental homeostasis. For example, ebselen increases the steady-state level of GSH against (arsenite)-induced nephrotoxicity in female rats (Ref. Reference Al-Brakati83). Ebselen protects against manganese toxicity to male reproductive organs and male fertility was proven to be achieved by increasing the GSH activity (Ref. Reference Mohammed, Ebraheim and Metwally84). The myocardial GSH level is preserved by oral ebselen in an ischaemia-reperfusion model (Ref. Reference Baljinnyam85). Ebselen can also directly remove intracellular GSH by binding to its SH group, leading to apoptosis (Refs Reference Yang, Shen and Ong4, Reference Shi33). Ebselen and silver ions react with the SH group of GSH in gram-negative bacteria and rapidly deplete GSH thiols (Ref. Reference Zou47). GSH itself is a redox buffer but also supplies electrons to glutathione reductases and other proteins. When oxidised, GSSG can be reduced by glutaredoxin, which utilises NADPH as its ultimate electron source. Thus, ebselen can regulate the amount of GSH in the cell and influence a broad range of biological processes.

Fig. 2. Mechanisms of ebselen action on TrxR and GSH.
Targeting other thiol proteins
Ebselen is an effective inhibitor of TrxR in both tumour cells and bacteria. TrxR is now being studied clinically as a molecular marker for some tumours. Ebselen blocks electron transport in tumour cell TrxR (Ref. Reference Meinerz86). Ebselen inhibits TrxR activity by binding the cysteine residue at the active site (Ref. Reference Yu87). Bacteria are classified as gram-positive or gram-negative bacteria; the former lacks GSH and glutaredoxin, while the Trx system is essential for bacterial DNA synthesis. The bacterial Trx system is particularly sensitive to ebselen (Ref. Reference Lu88). Escherichia coli TrxR, similar to other low molecular weight bacterial TrxRs, possesses only the FAD and NADPH binding domains and lack the interface domains found in mammalian TrxRs. Mammalian TrxRs are larger and contain an N-terminal active site motif, CVNVGC, and C-terminal amino-acid extension with a selenocysteine (U) in a GCUC active site motif. For mammalian TrxR, ebselen reacts with the selenocysteine in the C-terminal GCUG- active motif. And the adjacent cysteine can resolve the diselenide bonds formed between ebselen and Sec, indicating that mammalian TrxR cannot be inhibited by ebselen easily (Ref. Reference Ren, Zou and Holmgren82). Ebselen is a competitive inhibitor of E. coli TrxR with a K i of 0.52 ± 0.13 μM, reacting with the active site dithiol (Ref. Reference Sandalova89). In vitro, ebselen was found to inhibit E. coli TrxR through forming a difficult-to-reduce selenosulphide bond (Se-S). And ebselen blocked the electron flow from TrxR to Trx, then to its substrates, such as RNR and Msr (Ref. Reference Lu88). Therefore, ebselen is a highly effective antibiotic that can act on the Trx system and against different kinds of bacteria (Refs Reference Thangamani, Younis and Seleem44, Reference Thangamani, Younis and Seleem45). A recent study sheds light on the antioxidant mechanism of ebselen, revealing that form ebselen selenol and diselenide by reacting with Trx and TrxR rather than with GSH (Ref. Reference Zhao and Holmgren90). Multiple studies suggest that ebselen inhibits TrxR in bacteria, such as Staphylococcus aureus (Ref. Reference Dong91), Aspergillus fumigatus (Ref. Reference Marshall16), Bacillus anthracis (Ref. Reference Gustafsson19) and Deinococcus radiodurans (Ref. Reference Maqbool92). Additionally, ebselen is more effective in combination with other compounds. Silver can act synergistically with ebselen against multidrug-resistant gram-negative bacteria by directly reacting with the SH group in TrxR (Ref. Reference Zou47). Ebselen also can cooperate with PX-12, targeting microbial Trx system (Ref. Reference May18). These results demonstrate that ebselen targets the Trx system, exhibiting unprecedented antimicrobial activity, and provide a proof of concept of ebselen's efficacy against a variety of bacterial infections.
Involvement In biological processes
Ebselen is a polytropic molecule that interacts with many thiol-containing targets inside the cell, directly or indirectly regulating the expression of proteins or the transcription of genes. Due to its interaction with a broad range of biomolecules, ebselen is likely to influence several pathophysiologic processes.
Anti-inflammatory
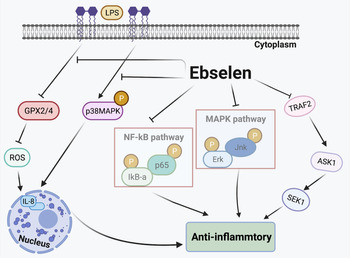
Inflammation Is caused by acute trauma and the invasion of the host by different pathogens (Ref. Reference Kumar93). The classic inflammation-related pathways are the nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, which have recently been shown to also regulate different aspects of innate or adaptive immune responses (Refs Reference Sun94, Reference Huang95). In vivo and in vitro studies have demonstrated that ebselen exhibits anti-inflammatory effects by regulating the expression of inflammatory cytokines. It has been suggested that selenium modulates inflammation by suppressing the NF-κB and MAPK signalling pathways in RAW264.7 macrophages. Ebselen also downregulates Erk, Jnk and p38 phosphorylation levels through MAPK signalling pathways in Staphylococcus aureus-induced mastitis (Ref. Reference Bi21). In endothelial cells, results suggest that ebselen affects the TRAF2-ASK1-SEK1 signalling pathway to reduce inflammation especially in atherosclerosis (Ref. Reference Yoshizumi42). In human GC cell lines, ebselen has been shown to decrease the generation of IL-8 by inhibiting p38 MAPK phosphorylation. Helicobacter pylori lipopolysaccharide suppresses (GPx2/4) the activation of ROS, which also mediates IL-8 expression, and ebselen reverses these effects (Ref. Reference Xu20). By regulating gene and protein expression, and modulating the responses of pro- and anti-inflammatory cytokines, the combination of ebselen and γ radiation is able to decrease inflammation by suppressing TNF-α, IL-2, interferon (INF-γ), TGF-β expression levels and significantly increase IL-10 levels in breast cancer cells (Ref. Reference Thabet and Moustafa27). In animal models, ebselen treatment significantly reduces the levels of Toll-like receptor 4 (TLR4) and p-p38 MAPK, highlighting the role of the TLR4-p38 MAPK signalling pathway in anti-inflammatory processes in traumatic brain injury (Ref. Reference Wei30). Ebselen is also reported to inhibit the expression of TNF-α and attenuate bronchiolar inflammation in a sephadex-induced lung inflammation rat model (Ref. Reference Belvisi96). Taken together, the results of these studies suggest that ebselen exerts anti-inflammatory effects on various stimuli and targets in different biological pathways (Fig. 3).

Fig. 3. Anti-inflammatory effects of ebselen. Selenium inhibits the MAPK and NF-κB signalling pathways. Ebselen suppresses the TRAF2-ASK1-SEK1 signalling pathway, inhibiting inflammation. Helicobacter pylori lipopolysaccharide (LPS) suppresses glutathione peroxidase (GPX2/4) activation of reactive oxygen species (ROS), which also mediates IL-8 expression; ebselen reverses these effects. TRAF2, TNF receptor-associated factor 2; ASK1, apoptosis signal-regulated kinase 1; SEK1, phosphorylation of stress-activated protein kinase ERK kinase 1; p38 MAPK, p38 mitogen-activated protein kinase; LPS, lipopolysaccharide; GPX, glutathione peroxidase; IL-8, interleukin 8.
Apoptosis
Apoptosis is the spontaneous and orderly death of cells that is controlled by genes and plays an important role in maintaining normal homeostasis and the pathophysiological processes of various diseases. Ebselen is confirmed to prevent apoptosis caused by exposure to harmful environments or injuries. Ebselen attenuates oxidative stress-induced neuronal cell apoptosis by inhibiting the JNK and activator protein-1 (AP-1) signalling pathways in PC12 cells (Ref. Reference Yoshizumi24). In these cells, apoptosis is also induced by NO, and ebselen can stimulate the activation of p44/42 MAPK, which may antagonise the activity of p38 MAPK and JNK, leading to a mitochondrial permeability transition and release of cytochrome C (Ref. Reference Sarker97). Similarly, in H2O2-induced endothelial cell apoptosis, ebselen decreases the expression of p38 MAP kinase, caspase-3 activation and cytochrome C release leading to reduced apoptosis (Ref. Reference Ali98). Through attenuating ischaemia-reperfusion injury and pancreatic β-cell apoptosis, ebselen inhibits the expression of IL-1β and TNF-α (Ref. Reference Park31). Mitochondria are also intracellular apoptosis targets of ebselen (Ref. Reference Azad and Tomar99). Ebselen can downregulate the expression of cytochrome C to rescue mitochondrial apoptosis in a liver mitochondria damage model induced by Fe2+/citrate (Ref. Reference Boireau28). Ebselen can also increase the Bcl-2:Bax ratio, inhibit the release of cytochrome C and Smac, and increase the activation of caspase-3, suppressing the mitochondrial apoptosis pathway after acute spinal cord injury (Ref. Reference Jia100). These studies all show ebselen's effects on apoptotic pathways under different experimental conditions (Fig. 4).

Fig. 4. The effects of ebselen on apoptosis. Ebselen actives p44/42MAPK and Bcl-2 to inhibit cell apoptosis, possibly by antagonizing the ASK1-p38MAPK-p53-MPT-CytC-caspase3 and ASK1-JNK-c-Jnk-MPT-CytC-Caspase3 signalling pathways. Ebselen increases the Bcl-2:Bax ratio, releasing cytochrome C, Smac and activating caspase3 to inhibit mitochondrial apoptosis. p44/42MAPK, p44/42 mitogen-activated protein kinase; MPT, mitochondrial permeability transition; CytC, cytochrome C; JNK: c-Jun N-terminal kinase; AP-1: activator protein-1; IL-1β: interleukin 1β.
Oxidative stress
Oxidative stress is an imbalance between ROS and their elimination. It is triggered by excessive production of highly reactive molecules (ROS and reactive nitrogen species) when the body is exposed to various harmful stimuli (Ref. Reference Leuti, Maccarrone and Chiurchiù101). Ebselen is a potent antioxidant and is very effective in maintaining redox balance in the body. CXCR4 has been shown to activate Akt through ROS-dependent pathways and CD40 activates JNK, p38 and Akt via signalling pathways that are largely ROS-dependent. But ebselen can deplete intracellular ROS, reducing oxidative stress and modulating B cell activation (Ref. Reference Lee, Westendorf and Gold102), and attenuates oxidative stress by inhibiting the JNK and AP-1 pathways (Ref. Reference Yan103). Cisplatin-induced ROS generation is reduced by ebselen through the Nrf2-ARE pathway in auditory cells (Ref. Reference Kim25). Ebselen protects NIH/3T3 cells against oxidative stress by regulating the expression of Bcl-2 and p53 (Ref. Reference Tak and Park104). These results demonstrated that ebselen regulates oxidative stress through various signalling pathways and is a promising antioxidant (Fig. 5).

Fig. 5. The effects of ebselen on oxidative stress. CXCR4 actives Akt through ROS-dependent pathways, CD40 activates JNK, p38, and Akt via signalling pathways that are largely ROS-dependent, and ebselen inhibits the ROS in these signalling pathways to suppress oxidative stress. Ebselen inhibits the c-JNK-AP-1 signalling pathways and p53 protein to attenuate oxidative stress. Ebselen activates the Nrf2-ARE signalling pathway and upregulates Bcl-2 protein expression, countering oxidative stress. CXCR4, chemokine receptor 4; Nrf2, nuclear factor erythroid 2-related factor 2; ARE, antioxidant response element.
Cell differentiation
Cell differentiation refers to the process whereby cells from the same source gradually produce cell groups with different morphologies and functions. The essence of cell differentiation is the selective expression of a subset of the genes present in the genome in time and space. Some studies have shown that ebselen can regulate the differentiation of various cell types. Stimulation with ebselen may activate the intracellular tyrosine kinase-MEK1/2-ERK1/2 signalling pathway in PC12 cells and lead to neuronal differentiation (Ref. Reference Nishina105). Ebselen also promotes osteogenic differentiation, in part by modulating the PI3K-Akt signalling pathway (Ref. Reference Li26). Treatment with ebselen in the early stages of osteoclast differentiation negatively controls the formation and survival of osteoclasts by targeting the Akt/NF-κB pathway and preventing trabecular bone matrix degradation as well as osteoclast formation in bone tissues (Ref. Reference Baek106). As an inhibitor, ebselen suppresses TGF-β induced α-SMA mRNA and protein expression involved in myofibroblast differentiation in nasal polyps (Ref. Reference Park32). In short, ebselen influences cell differentiation by regulating the expression of various proteins (Fig. 6).

Fig. 6. The effects of ebselen on cell differentiation. Ebselen stimulates the intracellular tyrosine kinase MEK1/2-ERK1/2 and PI3 K-Akt signalling pathways to induce cell differentiation. Ebselen inhibits the Akt-NF-κB and TGF-β-α-SMA signalling pathways against cell differentiation. MEK1/2, MAPK kinase; ERK, extracellular-signal-regulated kinase; PI3K, phosphatidylinositol 3-kinase; Akt, AKR thymoma protein kinase; TGF-β, transforming growth factor β; α-SMA, smooth-muscle α actin.
Immune regulation
In immune regulation, ebselen acts as either a cytokine inducer or immune stimulant (Refs Reference Steinbrenner, Speckmann and Klotz107, Reference Allingstrup and Afshari108). Under certain conditions, ebselen may have anti-immunosenescent potential in clonal T cells in vitro and ex vivo in polyclonal culture models (Ref. Reference Marthandan109). There are several reports that suggest that many inflammatory factors are activated and released when cells are treated with ebselen, including IL-2, IL-8, and IL-10 (Refs Reference Xu20, Reference Thabet and Moustafa27, Reference Wei30). Additionally, ebselen mediates the expression of intercellular adhesion molecule-1, TNF-α, INF-γ and TGF-β, which affect the adhesion and migration of immune cells and promote their anti-inflammatory properties (Refs Reference Thabet and Moustafa27, Reference Vera43). All of these studies demonstrate that ebselen affects immune regulation and suggest it has anti-inflammatory activity.
Involvement in disease
Due to excessive production of free radicals leading to oxidative stress, the biological processes result in age-related diseases such as AD, Parkinson disease (PD) and others. As an antioxidant, ebselen supports the free radical scavenging potential of the cell and has important pharmacological effects. Ebselen is also reported to be promising as an anti-microbial compound, and affects immune regulation, detoxification and oncotherapy.
Neurodegenerative disease
Neurodegenerative disease is caused by the loss of neurons or their myelin sheaths, such as in AD, PD, ALS, ischaemic stroke, etc. As one of the most common chronic neurodegenerative diseases, AD is the single biggest cause of dementia (Ref. Reference Lane, Hardy and Schott110). Recent studies indicate that ebselen may be an excellent candidate AD treatment (Ref. Reference Luo111). Ebselen can work effectively against oxidative stress through different actions such as increasing the activity of GPx and SOD, as well as reducing the levels of β-amyloid in an AD model (Refs Reference Martini52, Reference Klann53, Reference Xie112). The neuroprotective effect of ebselen is also related to iron-induced τ phosphorylation that is reduced by inhibiting DMT1 (Ref. Reference Xie113). PD is the second most common neurodegenerative disorder after AD, affecting millions of people worldwide (Ref. Reference Zesiewicz114). Recent findings revealed that one common cause of PD is mutations in CHCHD2 and LRRK2, and that ebselen may be a useful neuroprotective compound for carriers of CHCHD2 (Ref. Reference Tio115) and LRRK2 mutations (Ref. Reference Angeles116). Ebselen also has a protective effect in ALS, as the compound can react with SOD1 mutant variants, including those with unaltered enzymatic activity, that are known to cause ALS (Refs Reference Capper67, Reference Oh-Hashi and Hirata117).
Ischaemic stroke is the most common type of stroke, in which blood vessels in the neck or brain are blocked (Ref. Reference Randolph118). Ebselen has shown some clinical efficacy in ischaemic stroke by blocking the production of superoxide radicals and inhibiting inducible NO synthase (Ref. Reference Martínez-Vila119). In vivo experiments suggest that ebselen alleviates stroke through its antioxidant activity (Ref. Reference Parnham and Sies120). Most published results indicate that ebselen works in the treatment of neurodegenerative diseases by reducing neuronal damage (Refs Reference Yamagata121, Reference Imai122, Reference Lapchak and Zivin123, Reference Koizumi124, Reference Seo125) and decreasing the activity of both malonaldehyde and NO (Ref. Reference Sui55). Collectively, these results suggest that the neuroprotective activity of ebselen is unprecedented.
Microbial infection
The inhibition of several microorganisms by ebselen shows its strong anti-microbial activity. For instance, ebselen inhibits biofilm formation and disrupts mature biofilms of vancomycin-resistant enterococci (Ref. Reference AbdelKhalek126). Ebselen inhibits Mycobacterium tuberculosis antigen 85 by covalently binding to a cysteine residue in the active site (Refs Reference Favrot127, Reference Favrot, Lajiness and Ronning128). In recent years, our laboratory has been committed to studies of ebselen's action against Gram-positive and Gram-negative bacteria, and we have been looking for compounds that can act synergistically with ebselen. We have found that ebselen is an effective topical antibacterial agent in an animal model of multidrug-resistance (MDR) S. aureus LT-1 skin infection (Ref. Reference Dong91). A combination of silver ions and ebselen might be developed for novel treatment against MDR Gram-negative bacterial infections (Ref. Reference Zou47). These treatments have been shown to work against MDR Acinetobacter baumannii urinary tract infections (Ref. Reference Dong129) and in an MDR uropathogenic E. coli (UPEC) BC1-induced mouse cystitis model (Ref. Reference Wang57). All the above-mentioned results are recent findings from our laboratory and we are still investigating the specific mechanisms of ebselen's anti-bacterial activity. Other studies have shown that ebselen does not affect mitochondrial function and that it inhibits translation, including the synthesis of toxic proteins (Ref. Reference Thangamani, Younis and Seleem45), in methicillin-resistant S. aureus (Ref. Reference Thangamani, Younis and Seleem44). Additionally, the inhibitory effects of ebselen on T. brucei hexokinase 1 (Ref. Reference Joice72) and P. falciparum growth (Ref. Reference Harris130) further support the notion that ebselen is effective against microorganisms. Together, these studies reveal that ebselen is a promising agent for use against pathogenic bacteria and that it can be used to prevent the occurrence of infectious diseases.
Anti-tumour activity
A tumour is produced by the local proliferation of tissue cells in the body under the action of various tumorigenic factors. Many studies indicate that ebselen acts as an anti-tumour agent, targeting various tumours. The combination of ebselen and allopurinol was chemoprotective and increased the anti-tumour activity of cisplatin in rat breast and ovarian cancer models (Ref. Reference Lynch36). Ebselen and γ radiation induce apoptosis and inhibit cancer progression in breast cancer cells (Ref. Reference Thabet and Moustafa27). Ebselen also activates the apoptosis pathway and induces cell death in AR42J tumour cells (Ref. Reference Santofimia-Castaño131). Ebselen inhibits pancreatic and renal cancer cell lines tumour growth in vitro and their invasion of matrigel (Ref. Reference Hanavan71). Additionally, ebselen ameliorates ovarian damage by reducing oxidative stress caused by cisplatin (Ref. Reference Soyman37). The administration of ebselen can induce apoptosis in multiple myeloma (MM) cell lines by enhancing the production of endogenous ROS (Ref. Reference Zhang132). Ebselen inhibits the activity of human methionine aminopeptidase 2 in solid tumour cancers (Ref. Reference Węglarz-Tomczak, Giurg and Mucha133). Ebselen was shown to block ADAM9 (a disintegrin and metalloprotease, pro-cancer protein) through the inhibition of ROS production in human prostate cancer cells (Ref. Reference Sung134) and suppresses cancer biomarker α-methylacyl coenzyme A racemase levels (Ref. Reference Wilson135). The dose-dependent cell death of multiple tumour cell lines after treatment with ebselen results from its inhibition of histone deacetylases (Ref. Reference Wang136). Thus, the anti-tumour activity of ebselen is outstanding.
Toxicity reaction
Recent studies suggest that ebselen can reduce the toxicity of some compounds or metals. For example, ebselen is highly effective in reducing the toxicity of the metals cadmium, manganese, iron and mercury (Refs Reference Ardais, Santos and Nogueira137, Reference Avila138, Reference Oliveira139, Reference Davis and Bartfay140). Ebselen can also reverse cisplatin-induced nephrotoxicity by rescuing GSH depletion in the kidney in a rat model (Ref. Reference Husain141). The level of GSH has been shown to rise after ebselen treatment in acute spinal cord injury (Ref. Reference Jia100). Detoxification of 3-nitropropionic acid-induced toxicity by ebselen in rats has been reported (Ref. Reference Wilhelm, Jesse and Luchese142). Ebselen decreased the toxicity of mechlorethamine in A-431 cells by inhibiting apoptosis (Ref. Reference Lulla143). Hyperphosphorylation of cytoskeletal proteins induced by the neurotoxic agent diphenyl ditelluride in the cerebral cortex of young rats is prevented by ebselen treatment (Ref. Reference Moretto144). In sum, ebselen is a powerful detoxifying compound.
Future directions and conclusions
With the worldwide outbreak of COVID-19, there is an urgent need to find a drug to fight the virus. A recent study reported that ebselen was the strongest inhibitor of viral Mpro activity among the compounds tested (Ref. Reference Jin74). Ebselen has now entered clinical trials in COVID-19 patients; with proven efficacy, it could save drug developers much time in dealing with this sudden public health issue. Sometimes developing new targets for old compounds can be a good research direction.
Ebselen can affect a variety of biological processes, and has been shown to be effective against numerous pathological conditions in clinical trials, animal models and in vitro experiments. Some studies have reported that combining ebselen with other existing compounds, such as silver nanoparticles or curcumin, may lead to enhanced medicinal properties (Refs Reference Dong91, Reference Chen145). Thus, the exploration of ebselen combinations in disease treatment is promising.
Although ebselen is recognised as a compound with low toxicity in vivo, at high doses, it can cause toxic effects, which vary a lot according to the species, exposure time and route of administration. In acute exposure, the toxicity of ebselen varies depending on the route of administration, age and species in rodents (Ref. Reference Meotti146). In vivo chronic toxicity data with ebselen show that chronic subcutaneous administration of the compound (10 mg/kg, for 21 days) to suckling rats culminated with lipid peroxidation and non-protein thiol depletion in the liver (Ref. Reference Farina147). To date, the toxicological effects of acute and chronic exposures to ebselen in mammalian models have not been widely reported in the literature, and the specific mechanism remains to be fully elucidated.
Recently, there have been numerous reports of ebselen derivatives, such as the organoselenium compound (N-allyl-1,2-benzisoselenazol-3(2H)-one (N-allyl-BS)) and novel selenocyanates, having anti-tumour activity (Refs Reference Nie148, Reference Kaczor-Keller149). Seeking novel ebselen derivatives for the treatment of disease and to determine the molecular mechanism of their action is a promising line of research. These new ebselen derivatives or compounds acting synergistically with ebselen may open new alternatives for the treatment of different diseases. Further studies of ebselen will continue to provide new ideas for disease prevention and treatment, with good prospects for broad application.
Acknowledgements
We are grateful for the support of the National Natural Science Foundation of China (81903105, 32170191), Yichang Medical Treatment and Public Health Foundation (A19-301-50), and the Health Commission of Hubei Province Foundation (WJ2019H528).
Conflict of interest
None.