INTRODUCTION
The oil palm (Elaeis guineensis) has been taken from its natural forest habitat in West Africa to become a large-scale commercial tree crop now centred in southeast Asia. This is an example of a transfer process that is continuing to this day as the crop moves away from the humid tropics into drier regions, where water stress becomes an important yield limiting factor (Gerritsma and Wessel, Reference Gerritsma and Wessel1997). The main economic product is palm oil obtained from the mesocarp in the fruit. Palm kernel oil, which has a different fatty acid composition, is produced in smaller quantities. Kernel cake, produced after the kernel oil has been extracted, is used in animal feeds. Oil palm is the highest yielding oil crop (t ha−1) in the world (Corley and Tinker, Reference Corley and Tinker2003).
In contrast to coconut, oil palm production is dominated by the large-scale plantation sector, with an estimated 80% of oil production coming from estates. When grown as a smallholder crop, for example in Africa, oil palm contributes much more than oil to the communities living in the areas where it is cultivated. For, example, the sap can provide raw material for sugar and alcoholic beverages. The palms are also a source of building materials, and some of the tissues are important sources of fibre.
A great deal of research has been reported in the last 30–40 years on the water relations of oil palm, and more recently their irrigation needs. This review attempts to synthesize this research from an independent perspective, and to do this in practically useful ways. It follows the format used in previous reviews in this series, notably on coffee (Carr, Reference Carr2001), banana (Carr, Reference Carr2009), tea (Carr, Reference Carr2010a; Reference Carr2010b), coconut (Carr, Reference Carr2011) and sugar cane (Carr and Knox, Reference Carr and Knox2011). First, as an introduction, the origin and centres of production of oil palm are summarized. This is followed in turn by descriptions of crop development processes and detailed reviews of plant water relations, crop water-use and water productivity. Various aspects of this topic have previously been reviewed (e.g. by Corley, Reference Corley1996 (irrigation); Lim et al., Reference Lim, Goh, Kee and Henson2008 (climate); Henson, Reference Henson and DaMatta2009a (ecophysiology)), and in an excellent book by Corley and Tinker (Reference Corley and Tinker2003, all aspects). Emphasis here is placed on those publications describing research that has international relevance.
CENTRES OF PRODUCTION
The centre of origin of the oil palm is the tropical rainforest of West Africa where its natural habitat is believed to be in swamps (although it may not withstand permanent flooding) and along river banks. These areas are too wet for many of the dicotyledonous trees of the rainforest, and hence there is less competition. Because of its slow rate of growth in height, oil palm is unable to compete effectively with rainforest trees, and it does not grow well in the deep shade of the under-storey vegetation (Corley, Reference Corley, Corley, Hardon and Wood1976a, citing Zeven, Reference Zeven1967).
The oil palm has long been important to village economies throughout its area of distribution in the forests of West Africa, where semi-wild palm groves became established around homesteads (Nouy et al., Reference Nouy, Baudouin, Dejégul and Omoré1999). The export of palm oil and kernels from Africa grew rapidly in the late 19th century, whilst the first large-scale plantations in Malaysia and Indonesia were established in the early years of the 20th century. These were followed in the 1920s by plantations in the Congo and later in other parts of West Africa (Corley, Reference Corley, Corley, Hardon and Wood1976a). It is now grown between latitudes 19°N (Dominican Republic) and 15°S (Brazil). In 2009, the two largest palm oil producers by far were Indonesia (85 million t fresh fruit bunches from 5 million ha) and Malaysia (83 million t from 3.9 million ha). This represents a substantial proportion of the estimated world total area of 14.7 million ha. In terms of fruit production, these two countries produce 81% of the world total, with fruit bunch yields averaging 17–21 t ha−1 (containing 3.4–4.2 t ha−1 of mesocarp oil) (FAOSTAT, 2010).
The international importance of oil palm as a source of vegetable oil is a relatively recent phenomenon, mainly the result of Malaysian enterprise and initiative over the last 30–40 years, not only in establishing the crop but also through progressive research support to the developing industry by both the state and, particularly, the private sector.
Climate
The climatic conditions prevailing in the highest yielding regions (Indonesia and Malaysia) can be summarized as follows (Hartley, Reference Hartley1988; Lim et al., Reference Lim, Goh, Kee and Henson2008):
• Annual rainfall of at least 2000 mm spread evenly during the year;
• Mean maximum and minimum air temperatures of 29–33 °C and 22–24 °C respectively;
• Relative humidity >85% (equivalent to saturation deficits <0.6 kPa);
• Bright sunshine averaging 5 h d−1 throughout the year, rising to 7 h d−1 in some months (or solar radiation of 16–17 MJ m−2 d−1).
Palms are also grown successfully in less favourable conditions than these, including those found in Thailand, and parts of West Africa and South America, where there are regular dry seasons. The books by Hartley (Reference Hartley1988) and Corley and Tinker (Reference Corley and Tinker2003) include tables summarizing temperature, sunshine and rainfall data for some of the main oil palm growing centres, together with typical yields.
CROP DEVELOPMENT
The oil palm has a single stem with a single apical vegetative growing point (meristem). It can eventually grow to a height of 30 m or more, although old palms become difficult to harvest and are usually replaced in commercial plantations once they start to exceed 12 m. In a mature palm, the meristem produces a new leaf primordium about every two weeks. This leaf then takes about two years to develop and for the leaflets to unfold in the centre of the palm crown. In each leaf axil there is an inflorescence primordium. These develop into separate male and female inflorescences. Following pollination, the female inflorescence develops into a fruit bunch from which mesocarp and kernel oil can be extracted (Corley and Gray, Reference Corley, Gray, Corley, Hardon and Wood1976a).
Canopy
A mature oil palm (> eight years) can produce 20–25 leaves (fronds) each year (more when younger). These may live for two years after emergence, remaining photosynthetically active for at least 21 months (Corley, Reference Corley1985). The crown consists of 40–50 open fronds, each up to 5 m in length, with another 50–60 in various stages of development. During the dry season, leaflet opening is delayed and the rate of spear leaf extension is reduced (see e.g. Henson Reference Henson1991b; Henson et al., Reference Henson, Noor, Harun, Yahya and Mustakim2005). Although new leaves continue to be initiated and to elongate slowly, they accumulate unopened as ‘spear’ leaves. When the rains start, there is a flush of leaf opening followed by a return to the normal pattern (Corley and Gray, Reference Corley, Gray, Corley, Hardon and Wood1976a). As the fronds age, they collapse downwards and are then usually removed. Drought does not have much effect on leaf area (and light interception) of mature palms, but may slow development in young palms (Corley and Tinker, Reference Corley and Tinker2003). According to Corley (Reference Corley, Corley, Hardon and Wood1976b), the optimum leaf area index for yield varies with location from, for example, about four in west Cameroon to 6–7.5 in Malaysia, the precise figure depending on the leaf pruning regime (removal of unproductive leaves) and planting density.
Flowering
The early development of the inflorescence, when it is completely enclosed by leaves, takes on average 2½–3 years. Shortly before anthesis the inflorescence emerges from the leaf axil (Corley and Gray, Reference Corley, Gray, Corley, Hardon and Wood1976a). Pollination is mainly by insects, in particular by a weevil (Elaeidobius kamerunicus) that was introduced into southeast Asia from Africa in 1981. Previously in southeast Asia hand pollination was practiced.
Fruiting
The fruit (botanically a drupe) consists of an orange-coloured mesocarp which contains ‘palm oil’, a hard lignified shell (endocarp) and a white kernel containing ‘kernel oil’. There are three fruit forms, dura, pisifera and tenera. The dura, originally introduced into southeast Asia from Africa, has a thick endocarp with correspondingly less oil-bearing mesocarp tissue. By contrast, the pisifera has little or no endocarp and is commonly female sterile. The discovery of single gene inheritance of shell thickness allowed duras (female) to be successfully crossed with pisferas (male) to produce high oil yielding teneras. Today, all commercially produced seed and all estate-scale plantings are Dura × Pisifera crosses (Hardon, Reference Hardon, Smart and Simmonds1995; Corley and Tinker, Reference Corley and Tinker2003). The individual fruits are tightly packed in large ovoid bunches.
Yield
The first fruits normally ripen during the third year after planting. Yields per palm rise for the next few years and then slowly decline as interplant competition occurs. The two principal components of yield are bunch number and bunch weight; bunch weight increases with plant age, whilst bunch number declines. Bunch number per palm depends on the leaf production rate (each leaf subtends one inflorescence), the sex ratio (the ratio of female inflorescences to the total, which is reduced by drought), the abortion rate (also associated with dry conditions) and the bunch failure rate (the sudden failure of bunches to develop 2–4 months after anthesis). Each of these components is determined sequentially from about 25 months before harvest (sex differentiation) to 1–3 months before (bunch failure). The components of bunch weight are probably determined in the following sequence: spikelet number (16–18 months before harvest), flowers per spikelet (12–15 months before), inflorescence abortion (10–11 months before), fruit set (at anthesis, 5–6 months before) and mean fruit weight (2–5 months before). Young palms develop fruit faster than older ones (Turner, Reference Turner, Earp and Newall1977). The yield of oil depends on the oil weight to bunch weight ratio (Corley and Gray, Reference Corley, Gray, Corley, Hardon and Wood1976b; Corley and Tinker, Reference Corley and Tinker2003), which normally ranges between 19% and 26%, depending on genotype and harvesting standards. In addition, palm kernels constitute about 5% of bunch weight and contain about 50% oil (2.5% of bunch weight).
The effect of water stress on fruit yield varies depending on its timing and severity relative to each of these development stages, a process further complicated by the fact that the potential for fruiting is continuous. Water stress also influences the distribution of yield during the year. This too is of commercial importance and is largely governed by bunch number. In countries like Malaysia, with less marked seasonal changes, the yields are relatively uniform, with only up to 12–15% of the annual crop being harvested in the peak months. By contrast, in regions where there is a marked dry season, like Benin, this figure can increase to 35–40% with as little as 1% of the crop being harvested in the dry months. This has cost implications in terms of the harvesting and processing facilities needed to cope with the yield peaks (Corley and Tinker, Reference Corley and Tinker2003; Nouy et al., Reference Nouy, Baudouin, Dejégul and Omoré1999). In a recent paper, Legros et al. (Reference Legros, Mialet-Serra, Caliman, Siregar, Clement-Vidal and Dingkuhn2009a) suggested that daylength (photoperiod) may play a role in controlling seasonal peaks in flowering, even in regions near the equator. They based this suggestion on observations made at two sites, one very close to the equator 0°55′N (Sumatra), where water is not a limiting factor, and the other located at 3°12′S (South Kalimantan), where droughts occur. The photoperiod-sensitive phase was estimated to occur nine months before bunch maturity (or a function of this time interval), while the sensitive phase for drought was estimated to be 29 months before bunch maturity. It needs to be demonstrated that the palm is sensitive to differences in daylength of as little as one minute.
Roots
The depth and distribution of roots affect the amount and availability of water in the soil. Oil palm has an adventitious root system categorised by Tinker (Reference Tinker, Corley, Hardon and Wood1976) as follows: primary roots (6–8 mm in diameter) that develop from the base of the trunk (or bole) and either spread horizontally or descend into the soil; secondary roots (2–4 mm) that develop on the primary roots; tertiary roots (1.7–1.2 mm) arising on the secondaries; and unlignified quaternaries (0.1–0.3 mm) on the tertiaries. By making a number of assumptions, Tinker (Reference Tinker, Corley, Hardon and Wood1976) estimated the total effective nutrient absorbing root length (taken to be restricted to the tertiary and quaternary roots) in the top few centimetres of soil to be about 60 km palm−1. This analysis ignored roots deeper in the profile, which are important for water uptake in dry weather. Roots have been traced to depths of 5 m where there were no physical restrictions or high water tables. The greatest mass of fine roots is generally found in the surface layers, down to 0.60 m (variable). Root distribution is influenced by irrigation. For example, in the Côte d'Ivoire, irrigation virtually doubled the density of fine roots, below 0.20 m down to at least 1.0 m, the limit of measurement, compared with the unirrigated control (Prioux et al., Reference Prioux, Jaquemard, Franqueville and Caliman1992).
Also in the Côte d'Ivoire, Dufrêne et al. (Reference Dufrêne, Dubos, Rey, Quencez and Sauugier1992) traced primary, secondary, tertiary and quaternary roots of 14-year-old palms, growing in a deep ferralitic sandy soil, to depths of 4.8 m. The fine roots (<1 mm diameter) were mainly found in the top layers (49% within 0.40 m of the surface). The total root biomass (dry) equated to 30 t ha−1.
Subsequently, in a remarkably detailed study, also in the Côte d'Ivoire, Jourdan and Rey (Reference Jourdan and Rey1997a) identified eight morphologically different types of roots on mature oil palms growing in what is commonly known there as a ‘tertiary sand’. The relative positions and growth characteristics of all these roots were described. The horizontal (plagiotropic) primary roots grew at an average rate of 30 mm d−1, reaching a maximum length of 25 m, whilst the primary vertical (orthotropic) roots, growing at a similar rate, reached depths of at least 6 m (the limit of observation). At the same site, Rey et al. (Reference Rey, Quencez, Dufrêne and Dubos1998) traced roots (secondary, tertiary and quaternary), gradually declining in numbers, to depths of at least 4 m, although water extraction still occurred at depths below 5.0 m. Primary (horizontal?) roots were found in the surface horizons to depths of 0.60 m, 50% being in the top 0.20 m.
Based in part on these field observations and measurements, Jourdan and Rey (Reference Jourdan and Rey1997b) developed a 3-D model of the complete root system of oil palm with which they were able to (1) simulate the spatial distribution of roots under plantation conditions, (2) estimate root biomass and (3) locate and quantify root-absorbent surfaces. The results, some of which were presented in elegant illustrations, were compared with actual root distribution data (to depths of 1.0 m). Examples of the outputs from the model included: (1) root competition (horizontal primary roots) between neighbouring plants (triangular 9 m spacing) occurred in the topsoil as early as five years after planting; (2) total root biomass was estimated to be 3 t ha−1, after four years, and, after 16 years, 55 t ha−1, (this is an exceptionally high figure) at a planting density of 143 palms ha−1; and (3) quaternary and tertiary roots provided 84% of the total absorbent root surface (total = 1480 m2 ha−1).
In Papua New Guinea, Nelson et al. (Reference Nelson, Banabas, Scotter and Webb2006) used soil water depletion as a measure of root activity beneath palms on two soil types. Water uptake was greatest under the weeded zone close to the stem. Water was extracted to a depth of 1.6 m, the limit of measurement. The total dry mass of roots within this depth equated to 20 t ha−1. In the weeded zone, 75% of the total root mass occurred in the top 0.39 m. This, and other similar observations, probably explain why oil palm is often described (mistakenly?) as shallow rooted, although, as noted above, roots can reach depths of 5–6 m.
Summary: crop development
1. The oil palm has a single stem with one growing point which, in ‘mature’ palms, initiates one leaf primordium every two weeks. It takes about two years for a leaf to emerge and fully expand.
2. During dry weather, leaflet opening is delayed and ‘spears’ accumulate in the crown. When the rains start, there is a flush of leaf opening. The rate of leaf initiation is thought to be relatively unaffected by drought.
3. The optimum leaf area index for yield varies with location (range 4–6.5).
4. There is one inflorescence in each leaf axil. The flowers are borne on separate male and female inflorescences. Sex differentiation occurs about 25 months before harvest.
5. The proportion of female inflorescences (the sex ratio) is an important determinant of yield and is sensitive to water stress. Maleness is favoured by stress conditions.
6. Abortion of inflorescences is associated with dry conditions.
7. Fruiting is a continuous process; the timing of water stress relative to stages of development is important for predicting its impact on yield. Daylength, as well as rainfall, may play a role in determining seasonality of yield.
8. The oil palm has an adventitious root system.
9. Plagiotropic roots can spread laterally >25 m from the trunk. Orthotropic roots can extend to depths of at least 6 m. The greatest concentration of roots is in the 0–0.6 m layer.
10. Primary roots can grow at rates of about 30 mm d−1.
PLANT WATER RELATIONS
Gas exchange
In order to understand better how the oil palm responds to water stress, factors influencing stomatal conductance and rates of photosynthesis have been studied using a range of techniques. Stomatal conductance, in particular, has been found to be a sensitive indicator of plant water status.
The juvenile palm has stomata on both leaf surfaces but, as the plant develops, there is a progressive loss of adaxial stomata on the later-formed leaves, and an increase in abaxial stomata number and size. For an older palm (>2 years after planting), stomatal densities are in the range 130–200 mm−2 (lower, or abaxial, surface only; mean 175 mm−2) depending in part on the position of the leaflet within the frond (Henson, Reference Henson1991a).
Factors influencing the degree of stomatal opening in oil palm were first studied in Benin by Wormer and Ochs (Reference Wormer and Ochs1959). In a series of measurements using the infiltration technique (which gives a measure of the relative stomatal opening), summarized by Ochs and Daniel (Reference Ochs, Daniel, Corley, Hardon and Wood1976), they found that:
• Changes in stomatal opening as the soil dried were related to changes in transpiration;
• The critical soil water deficit at which the stomata first began to close varied with soil type;
• The rate of unfolding of new leaves declined two to three months after the stomata first began to close (at midday);
• Relative stomatal opening could be used to quantify the number of ‘dry days’ (see below);
• Relative stomatal opening could be used to schedule when to irrigate (see below).
Some of the related research in the Côte d'Ivoire, undertaken by IRHO/CIRAD, was summarized by Caliman (Reference Caliman1992), citing Dufrêne (Reference Dufrêne1989). In particular, the relationship between photosynthesis rates and stomatal conductance and the close match between stomatal opening (infiltration score) and the relative depletion of available soil water were described.
The infiltration technique was also used by Rees (Reference Rees1961) to monitor diurnal and seasonal changes in stomatal opening in oil palm in Nigeria. In the wet season, the stomata opened early in the morning and remained wide open throughout the day before closing in the early evening. In the dry season, partial closure occurred during the middle of the day. A detailed analysis showed that stomata began to close rapidly when the air temperature (as measured in the shade of the leaf canopy) exceeded about 32 °C, with almost complete closure occurring at 35–36 °C. Closure was also observed during periods of very low humidity, when it was cooler. This was associated with the Harmattan (dessicating, dust-laden winds from the Sahara desert). Subsequently, Corley (Reference Corley1973), using a diffusion porometer, observed a similar pattern of midday stomatal closure in drought-affected palms in Malaysia.
Later, a combination of field studies (in Colombia) and controlled environment measurements (in the UK) by Smith (Reference Smith1989) showed how stomatal conductance (measured with an infra-red gas analyser) declined when the saturation deficit of the ambient air exceeded 1.7 to 2.0 kPa, even when the soil was well-watered. These observations were, however, unable to explain the diurnal changes in stomatal opening observed in irrigated palms in the field. By contrast to the results reported above, in the irrigated palms partial closure during the morning was interrupted by partial re-opening in mid-morning followed by partial closure again in mid-afternoon. In the unirrigated treatment, stomatal conductance reached a minimum at midday before increasing in the late afternoon. It is not clear whether the atmospheric conditions were similar for irrigated and droughted plants when the comparisons were made.
In Malaysia, Henson and Chang (Reference Henson and Chang1990) and Henson (Reference Henson1991a) found that stomatal conductance (measured with a diffusion porometer or by infra-red gas analysis) rose to a peak in mid-morning and then progressively declined to low values in the afternoon. The same diurnal pattern, obtained under clear sky conditions, was followed regardless of the age of the palm, although actual conductances increased with age. Photosynthesis rates followed a similar trend.
Similarly, Henson (Reference Henson1991b) found that on clear days stomatal conductance in young palms declined progressively from early morning to 13:00 hours (the limit of reported measurements) under both wet and particularly under dry soil conditions. In contrast to previous measurements, there was no mid-morning peak. The decline was linearly related to increases in the saturation deficit of the air (range 0–2.5 kPa). After allowing for the effect of the saturation deficit at the time of measurement, there was a linear decline in conductance as the potential soil water deficit increased (up to 150 mm) during the dry season. Photosynthesis rates also declined in a similar way. Previously, Henson and Chang (Reference Henson and Chang1990) had observed a recovery in conductance following a mid-day minimum, on a day when humidity increased (i.e. saturation deficit declined) in mid-afternoon, thus replicating the mid-day partial closure observed by Rees (Reference Rees1961), Corley (Reference Corley1973) and Smith (Reference Smith1989).
Care has to be taken when making direct comparisons of stomatal behaviour when different measurement techniques are used. For example, the relationship between infiltration score and conductance (depending on the porometry method used) is complex and inconsistent, as Burgess (Reference Burgess1992) demonstrated with the tea crop.
However, Dufrêne and Saugier (Reference Dufrêne and Saugier1993), during detailed measurements in the Côte d'Ivoire, confirmed the sensitivity of stomatal conductance to changes in the saturation deficit of the air. They found an exponential decline over a range of 1.0–4.5 kPa, with a corresponding, but linear, reduction in transpiration rate. By contrast, photosynthesis of individual light-saturated leaflets did not decline until the saturation deficit exceeded 1.8 kPa. This decrease in transpiration with no corresponding reduction in net assimilation implies an increase in water-use efficiency at moderate saturation deficits (<1.8 kPa), a climatic condition commonly observed in the riparian forests of West Africa where the oil palm originated. Subsequently, Henson (Reference Henson and Basiron1995) extended this observation on leaflets to the whole canopy. Canopy photosynthesis was halved when the saturation deficit of the air increased from 0.8 to 2.0 kPa.
In Sumatra (Indonesia), Lamade and Setiyo (Reference Lamade and Setiyo1996) compared photosynthetic rates and stomatal conductances of three contrasting clones (MK04, MK10 and MK22). All three were sensitive to the saturation deficit of the air, with net photosynthesis declining linearly, and stomatal conductance exponentially, over the range 1–5 kPa. Clone MK22 was the most sensitive to the saturation deficit and also to high temperatures. For all three clones, maximum rates of both photosynthesis and stomatal conductance occurred at 33 °C. Although fully exposed leaves can reach temperatures 10 °C above ambient (Hong and Corley, Reference Hong and Corley1976), the importance of this in relation to whole canopy photosynthesis is not known. However, as Corley and Tinker (Reference Corley and Tinker2003) stated: ‘the inhibitory effects of temperatures between 33 °C and 40 °C on photosynthesis may be largely due to saturation deficit induced stomatal closure’. There is evidence that short-term imbalances in source (assimilation rates restricted by drought) to sink (demands of the developing fruit) relations can be compensated for by mobilization of starch and glucose reserves in the stem (Legros et al., Reference Legros, Mialet-Serra, Clement-Vidal, Caliman, Siregar, Fabre and Dingkuhn2009b).
There is some evidence obtained from plants grown in containers that abscisic acid, generated in roots growing in a drying soil, may play a role in mediating stomatal responses (and photosynthesis) to water stress (Henson et al., Reference Henson, Jamil and Dolmat1992).
Drought impact assessment
Finding ways of detecting and quantifying the level of water stress in oil palms early in the extended fruit development process could assist in yield forecasting and irrigation decision making. Various approaches have been proposed.
Although oil palm exhibits some definite symptoms of water stress during the dry season, there is no visible wilting because of the nature of the leaves (fibrous, thick hypodermis and well-developed cuticle) (Rees, Reference Rees1961). An index for determining the effects of extreme drought on oil palm was developed by Maillard et al. (Reference Maillard, Daniel and Ochs1974) based on a weighted assessment of the visible effects of drought on the foliage of individual palms within a population (N). Three stages of water stress were identified (S1–S3), with a fourth comprising any palms that had died (D). In order of severity these stages were used to label palms in which:
• Five or six spear leaves had accumulated in the centre of the crown – S1;
• Four to six green leaves had collapsed or broken, accompanied by drying of fruit bunches – S2;
• All the leaves at the base of the crown had dried, and foliage in the centre of the crown had collapsed – S3.
From this labelling procedure, a Stress Index was calculated in the following way:
In this way, it was possible to compare numerically seasons, sites and cultivars. It is not known how widely this index has been used, or how it relates to productivity. Stages S2 and S3 are rarely seen outside the driest parts of West Africa.
Although leaf water status is used for other tree crops (e.g. cocoa and coconut) as a measure of water stress, there are few published values for oil palm. In Costa Rica, Villalobos et al. (Reference Villalobos, Umaňa and Chinchilla1992) recorded leaf water potentials with a pressure chamber in adult (17-year-old) irrigated palms as low as −1.7 MPa. Surprisingly, the corresponding values for unirrigated palms were higher (−1.0 MPa) due to stomatal closure. This stomatal control of leaf water status was considered to be the reason why oil palm can survive long dry periods. By contrast, young palms (10 months after field planting) were unable to maintain a high leaf water potential under severe drought conditions (midday values were down to −1.95 MPa). Subsequently, Kallarackal et al. (Reference Kallarackal, Jeyakumar and George2004) monitored the diurnal changes in leaf water potential at three sites in India, also using a pressure chamber. Under well-watered conditions, values declined from −0.1 MPa early in the morning to minima of −1.4 to −1.5 MPa by the middle of the day, before recovering in the late afternoon. Similarly, Henson and Chang (Reference Henson and Chang1990) in Malaysia reported values typically ranging between −0.4 MPa early in the morning to −1.4 MPa at midday. The seasonal changes in stomatal conductance due to drought were much greater than the corresponding changes in leaf water potential (only 0.3 MPa). A larger range of leaf water potentials is obtained when container-grown plants are subjected to water stress (Henson et al., Reference Henson, Jamil and Dolmat1992).
Other methods of assessing crop water status have been tried. For example, Henson (Reference Henson1991c) demonstrated the potential value of measuring the leaf:air temperature difference for detecting and partly quantifying crop water stress, provided that there was a well-watered crop nearby to act as a control. Henson (Reference Henson1998), following Dufrêne (Reference Dufrêne1989), also found that sap flux probes provided a sensitive means of assessing relative, but not absolute, transpiration rates in relation to soil water availability and potential evapotranspiration.
With the objective of identifying a suitable assessment method, Henson et al. (Reference Henson, Noor, Harun, Yahya and Mustakim2005) listed a selection of possibilities and evaluated some of them over one dry season in Malaysia. In terms of time scale (minutes to years) and sensitivity of measurement (high to low), the selection included: leaf or canopy temperature, leaf or canopy gas exchange, leaf water potential, sap flux, evapotranspiration, soil water depletion, spear leaf extension, spear leaf accumulation, frond production rate, inflorescence abortion, reduced sex ratio, bunch number reduction, reduced fruit yield and death of the palm. Based on the sensitivity of the response, ease of detection and simplicity of measurement, spear leaf extension rate (relative rate over a 24 h period, simple but with accessibility issues) and the canopy–air temperature difference (ΔT, which requires instrumentation, but which can be automated) were selected as being the best practical options. The next stage is to relate yield to these measurements, which is not an easy task.
Summary: plant water relations
1. Stomata occur on the lower surface of leaves of mature palms at densities of 130–200 mm−2.
2. Relative stomatal opening is a good indicator of soil water availability, provided allowance is made for levels of solar radiation and saturation deficit.
3. Stomata begin to close rapidly if the air temperature exceeds 32–33 °C and/or when the saturation deficit of the air exceeds a critical value (there is uncertainty about the exact value, which ranges from zero to 1.7 kPa).
4. In a wet soil, stomata open early in the morning and remain fully open throughout the day before closing in the evening OR partially close mid-morning, then reopen, then partially close mid-afternoon, OR open in the morning and then progressively close during the day.
5. In a drying soil, stomata partially close during the middle of the day OR open in the morning and then progressively close during the day.
6. Conductance declines linearly (or exponentially) with increases in the saturation deficit above the critical value.
7. Some of the differences in stomatal responses reported are probably in part linked to the measurement technique used.
8. Rates of photosynthesis decline in concert with declines in stomatal conductance once a critical value (ca. 1.8 kPa) has been exceeded.
9. There is some (limited) evidence that maximum instantaneous water use efficiency occurs at saturation deficits of <1.8 kPa.
10. In a wet soil, the leaf water potential of field-grown palms can fall to −1.5 MPa in the middle of the day. Differences in leaf water potential between watered and droughted palms are often small in comparison with differences in conductance or photosynthesis.
11. Leaf to air temperature differences and spear leaf extension rates have been identified as practical options for rapidly assessing crop water status in the field.
CROP WATER USE
In this section, attempts to measure the actual water use of palms in the field are reviewed, together with estimates of the value of the crop coefficient (Kc) that relates actual water use (ETc, transpiration plus evaporation from the soil surface) to potential reference crop evapotranspiration (ETo). Problems with interpretation arise due in part to the different terminology and abbreviations, which are not always well defined, used by researchers. Similarly, the ways of calculating and defining evaporation are not always clearly specified (for example, which version of the Penman equation is used). These may be some of the reasons why Corley (Reference Corley1996) could not identify a consensus view on the value of the crop coefficient. A further complication is that a proportion of rainfall is intercepted by the foliage and evaporated directly from the crop surface (measured as the equivalent of 1 mm d−1 by Nelson et al., Reference Nelson, Banabas, Scotter and Webb2006), thereby substituting for transpiration. For mature oil palms, this can represent up to 22% of potential evaporation (Henson and Chang, Reference Henson, Chang, Basion, Jalani and Chan2000, citing others), whilst 15% of the rainfall volume can appear as stem flow (Nelson et al., Reference Nelson, Banabas, Scotter and Webb2006). For comparison, measurements in the Côte d'Ivoire on mature palms, made over 2½ years, suggested a much smaller value for stem flow, namely 4% of incident rainfall, with throughfall at 82%, and, by difference, intercepted rainfall at 14% (Dufrêne et al., Reference Dufrêne, Dubos, Rey, Quencez and Sauugier1992). In Malaysia, Squire (Reference Squire1984) found that intercepted rainfall represented 11% of the total. Clearly there is a range of values for interception and stem flow depending on the size of the palm, and the size and intensity of the rainfall event.
Radersma and Ridder (Reference Radersma, Ridder and de1996) allowed for canopy interception of rainfall when computing the water-use of oil palms in La Mé, Côte d'Ivoire (ca. 5°20′N 4°02′W; alt. 35 m). The ET estimates were derived from the Penman-Monteith equation (Allen et al., Reference Allen, Pereira, Raes and Smith1998), using published values for the key parameters (including crop, aerodynamic and surface resistances) controlling transpiration and evaporation from crop and soil surfaces. With an annual rainfall of 1500 mm, of which 13% was intercepted by the palms, daily transpiration rates (T) were estimated to be between 3.3 and 6.5 mm d−1 during the rains, depending on net radiation levels and the saturation deficit of the air, and from 1.3 to 2.5 mm d−1 during the dry season. The corresponding seasonal and annual ET totals were 623 mm (wet season), 395 mm (dry season) and 1118 mm (total).
Previously, at the same site, Dufrêne et al. (Reference Dufrêne, Dubos, Rey, Quencez and Sauugier1992) had also used the Penman-Monteith equation to assess the transpiration rate (T) of mature palms. When soil water was freely available, the ratio (Kc) between actual evapotranspiration (ETc, derived from a water balance analysis) and potential evaporation (ETo = 3.2 mm d−1, Penman-van Bavel) averaged 0.81 but, during the dry season (ETo = 3.5 mm d−1) this declined to 0.56. The corresponding T/ETo ratios were about 0.70 and 0.35 respectively. ETc first fell below ETo when 40% of the available water in the top 0.80 m of soil had been depleted (equivalent to a soil water deficit of ca. 30 mm), with the ET/ETo ratio then falling, on a daily basis, to 0.10–0.20. The maximum depth of water extraction was about 5 m. This decline in ET/ETo was matched by corresponding reductions in the stomatal conductance (measured with a diffusion porometer) from about 6 to 1.5 mm s−1.
In Kedah, Malaysia, Henson et al. (Reference Henson, Noor, Harun, Yahya and Mustakim2005) monitored the water use of young (4–5 years) palms over one dry season (2002/03) with a Delta-T Profile moisture probe. The ratio of actual ET (estimated using the water balance method) to potential evapotranspiration rates (ETo, Penman) declined from about 1.0 to 0.1 as the dry season progressed before returning to 1.0 following the start of the rains. ETo varied between 2.7 and 4.5 mm d−1. The crop coefficient fell below unity when about 15% (c. 15–20 mm) of the available water in the top metre of soil had been depleted. On the deep sandy clay loam soil, water was extracted from depths below 0.60 m. In a follow-up study, Henson and Harun (Reference Henson and Harun2007) monitored gas exchange and water use at the same site during a later dry season (2005/06), which was interrupted by appreciable rain (up to 40 mm) at approximately monthly intervals. The impact of the dry conditions on gas exchange and growth was mitigated by the rain, with actual evapotranspiration rates for different drought periods varying between 3.9 and 2.7 mm d−1, and the corresponding ET/ETo ratios between 0.85 and 0.50.
At the same site, Henson and Harun (Reference Henson and Harun2005) had earlier used a micrometeorological approach (the eddy correlation or covariance method) to monitor fluxes of carbon dioxide and water vapour above oil palms (60% ground cover). As might be expected, canopy conductance, photosynthesis and evapotranspiration were all lower during the dry season than during the rains. Thus, for example, evapotranspiration rates averaged 1.3 mm d−1 (0.3ETo, Penman) compared with 3.3–3.6 mm d−1 (0.8–0.9ETo) in the rains. On a daily basis, the carbon dioxide flux peaked early in the morning and then generally declined, quite sharply during dry periods. This response was again associated with changes in the saturation deficit of the air, with negative correlations (linear) being found between carbon dioxide flux and saturation deficit over the approximate range 1 to 4 kPa. Using a similar approach, Henson (Reference Henson1999) had previously distinguished evapotranspiration and carbon dioxide flux from the soil and ground flora (below canopy) from that originating from the oil palm canopy. Over a ten day period in December when the soil was wet, below-canopy evapotranspiration averaged 0.47 mm d−1 and above-canopy ETc 3.84 mm d−1, with an average ratio of 0.13:0.87. For comparison, the ETo over the same period averaged 4.10 mm d−1.
A similar approach was used by Kallarackal et al. (Reference Kallarackal, Jeyakumar and George2004) to assess the water use of irrigated oil palms (4–5 years old) in three relatively dry areas of India. The microclimate above the crop was monitored and the data used to calculate potential evapotranpiration (ETo, Penman, as modified by Van Bavel), whilst the Penman-Monteith equation was used to estimate actual transpiration (T). Photosynthesis and stomatal conductance were measured with an infra-red gas analysis system. Diurnal changes in stomatal conductance followed a pattern similar to that described above, with the stomata opening in the early morning but then progressively closing from about 08:00 hours onwards. This closure was again associated (negative exponential) with increases in the saturation deficit of the air over the range <1 to 4 kPa. Transpiration rates varied between 2.0 and 5.5 mm d−1 (equivalent to 140–385 l palm−1 d−1), with lower values being obtained in the dry season (associated with dry air) despite irrigation. The corresponding actual transpiration (T) to potential evapotranspiration (ETo) ratio varied between 0.70 and 0.90.
A large scale catchment water balance approach was used by Yusop et al. (Reference Yusop, Chong, Garusu and Ayob2008) to estimate ET for oil palm in Johor, Malaysia. Rainfall and run-off were monitored over eight months in three catchments, each of which was planted with oil palms of different ages – two, five and nine years old, together with cover crops. Although there were inconsistencies between catchments in the estimates of ET, when averaged across catchments and expressed on an annual basis, estimated ET was a realistic 1200–1300 mm. Earlier, a similar catchment scale study was undertaken in Pahang, Malaysia. In a comparison of a natural forested catchment with an adjacent one converted to oil palm, the annual ET (1525 mm) for 7–9-year-old palms represented 71% of the annual rainfall (2150 mm), equivalent to 4.2 mm d−1 (Henson, Reference Henson, Singh, Lim and Chan2009b, citing others).
On a completely different scale, Foong (Reference Foong1993) used a drainage lysimeter (8.8 m diameter, 1.5 m deep) containing one irrigated palm to measure evapotranspiration rates over a 15-year period (1976–1990) in Peninsular Malaysia. On an annual basis, ETc during the first seven years averaged 4.5–5.0 mm d−1, and for later years, 5.0–5.5 mm d−1. During the monsoon season, monthly mean values fell to 3.0–3.5 mm d−1 and reached 6.5–7.5 mm d−1 in the dry season. The corresponding values for ETo or Kc were not reported.
In the Côte d'Ivoire, 15-year-old palms extracted water from depths below 5 m during the dry season when actual ET rates averaged 2.5 mm d−1 (range 4.6–0.6 mm d−1) (Rey et al., Reference Rey, Quencez, Dufrêne and Dubos1998). The total depth of available water in the 5-m deep profile was estimated to be only 250 mm. Based on stomatal conductance measurements (diffusion porometer), about 70% (175 mm) of this was considered to be easily available.
For ease of reference, the crop water use data are summarized in Table 1.
Table 1. Oil palm crop water use summary table. Further details are in the text.
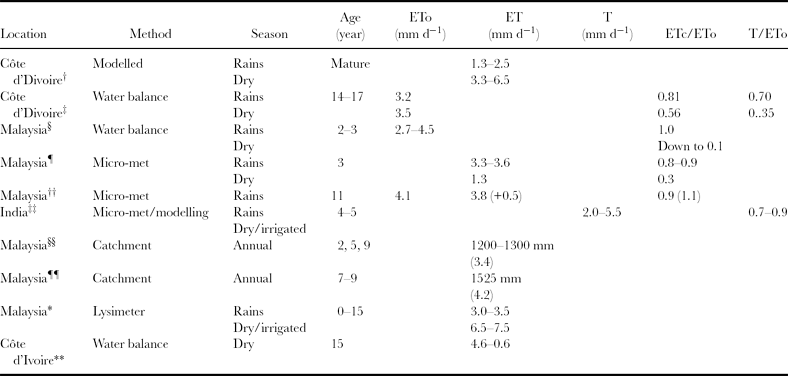
†Radersma and Ridder (Reference Radersma, Ridder and de1996); ‡Dufrêne et al. (Reference Dufrêne, Dubos, Rey, Quencez and Sauugier1992); §Henson et al. (Reference Henson, Noor, Harun, Yahya and Mustakim2005); ¶Henson and Harun (Reference Henson and Harun2005); ††Henson (Reference Henson1999); ‡‡Kallarackal et al. (Reference Kallarackal, Jeyakumar and George2004); §§Yusop et al. (Reference Yusop, Chong, Garusu and Ayob2008); ¶¶Henson, Reference Henson, Singh, Lim and Chan2009b; *Foong (Reference Foong1993); **Rey et al. (Reference Rey, Quencez, Dufrêne and Dubos1998).
Summary: crop water use
1. Evapotranspiration rates averaged 4.1 mm d−1 (range 3.5–5.5 mm d−1) during the rains (equivalent to an annual total of 1500 mm) and 1.9 mm d−1 (range 0.6–2.9 mm d−1) in the dry season at various locations.
2. Under well-watered conditions for mature palms, the crop coefficient (Kc) averaged 0.9 (range 0.8–1.0). During the dry season, Kc values for young palms can fall to 0.1 (range 0.1–0.7).
3. A significant proportion of evapotranspiration (13%) can come from below the palm canopy (soil and ground flora).
4. Water can be extracted from depths >5 m in the dry season.
5. Both single leaf and canopy level measurements confirmed that stomatal conductance, photosynthesis and transpiration are all reduced when the air is dry (saturation deficits from <1.0 to 4.0 kPa), even if the soil is wet.
6. The limiting soil water deficits above which transpiration is reduced, on sandy clay loam/sandy soils, are in the range of 15–30 mm.
WATER PRODUCTIVITY
Yield forecasting
As Corley and Tinker (Reference Corley and Tinker2003) made clear in their review of the role of water in the productivity of oil palm, there is no simple relationship between rainfall totals and yield. The commonly accepted way of comparing sites and seasons is to use the concept of a soil water deficit, a measure of the relative dryness of the soil. The critical value of the deficit above which yield is lost varies with the soil type and the depth and density of rooting, and also with the stage of growth. Citing the work of others, including a review by Turner (Reference Turner, Earp and Newall1977), Corley and Tinker (Reference Corley and Tinker2003) suggest that there is broadly a 10% reduction in fresh fruit bunch yield for each 100 mm increase in the soil water deficit. The responses may differ between locations depending on whether dry seasons occur annually (e.g. West Africa), or at irregular intervals (e.g. Indonesia). As fruiting is continuous, there are always delayed effects of drought on yield. The yield loss for an individual harvest depends on when it occurs in relation to the stage of inflorescence development, the most critical stages being floral initiation, sex differentiation and the abortion-sensitive period (Turner, Reference Turner, Earp and Newall1977). Using data from Indonesia and West Africa, Caliman and Southworth (Reference Caliman, Southworth and Jatmika1998) developed statistical relationships between water deficits at certain growth stages and subsequent yields with the intention of being able to forecast the effects of drought on monthly yields. This followed a similar approach to yield forecasting, also based on water deficits, developed by Dufour et al. (Reference Dufour, Frêre, Caliman and Horns1988) in West Africa. How transferable these outputs are is not known.
For north Kedah, Malaysia (6.27°N, 100.29°E), a region with a distinct and sometimes prolonged dry season, Henson et al. (Reference Henson, Yahya, Noor, Harun and Mohammed2007) used a mechanistic computer model (OPRODSIM) to simulate, with mixed success, the growth and fruit yield of oil palm for the first six years after planting and provide predictions for the next four years. The model provided a useful first approximation of the effects of climate on dry matter production, yield and water use in a seasonally dry environment. It was also used to predict the likely yield increases from irrigation, which averaged c. 20–25 kg fresh fruit bunch ha−1 mm−1 when 200 mm or more (effective) irrigation was applied during the dry season (Henson, personal communication). The same model was also used to compare different approaches to scheduling irrigation, namely only applying varying proportions of the amount of water needed to return the soil profile to field capacity, or applying the full amount at different threshold soil water deficits (Henson, Reference Henson2006). The effect on yield of these two approaches was similar. The yield response per unit of irrigation was greater when irrigation was only applied during the dry season rather than over the whole year. To quote the author: ‘the model results do not seem too improbable’.
Yield responses to irrigation
Corley (Reference Corley1996) reviewed the results of 13 oil palm irrigation experiments conducted in several countries since 1967, four of which are described below as examples. Although the quality of the results reported is very variable, and generally of limited generic value, making direct comparisons difficult, Corley attempted to develop relationships between fruit yield responses to irrigation (YR, t ha−1) and soil water deficit (WD, mm, as calculated using the IRHO methodFootnote 1) in the unirrigated control treatments. Assuming that a realistic target yield of fresh fruit with irrigation was 30 t ha−1, the following equation was proposed as a basis for planning:
In an early oil palm irrigation experiment at Grand Darwin in the Côte d'Ivoire, two irrigation treatments were compared with an unirrigated control over the two-year period (1966/67 and 1967/68). Applying 150 mm each month (total 1130 mm) increased annual bunch yields from 10.5 t ha−1 to 23.5 t ha−1, whilst a similar yield increase was realized (but with much less water, total 650 mm) by scheduling irrigation using the degree of stomatal opening (infiltration score maintained >10) to decide when to irrigate. Based on these data, Ochs and Daniel (Reference Ochs, Daniel, Corley, Hardon and Wood1976) concluded that a yield response of 25–30 kg bunches ha−1 mm−1 was possible in areas where the potential soil water deficit was 200–600 mm (IRHO method). The yield benefit from irrigation was largely the result of an increase in leaf production, fewer abortions and an improved sex ratio, all leading to increased bunch number.
In Benin, Taffin and Daniel (Reference Taffin and Daniel1976) reported the preliminary results of an evaluation of drip irrigation of oil palm. Although no direct comparisons were possible, drip irrigation providing virtually a full alleviation of the soil water deficit was judged to be an effective way of applying water to palms (despite problems with clogging of the emitters). Apart from days when temperature exceeded 33 °C and the air was dry, the stomata were fully open during the dry season, and good yields were obtained (30 t ha−1 fresh fruit in the third year of treatment). When irrigation was delayed, the stomata took several weeks to respond to water application and then failed to reopen fully.
Following this experience, a 900 ha estate was established in a marginal area (for oil palm) of Benin (potential soil water deficit = 800 mm, IRHO method) (Chaillard et al., Reference Chaillard, Daniel, Houeto and Ochs1983). The first 400 ha section was irrigated in 1977, and the remainder in 1979. Water was applied directly to the sandy clay soil through a low level pipe network into rills (small trenches) at least 6 m in length alongside each palm. The scheme was designed to apply 5 mm d−1 (later restricted to 2.5 mm d−1 because of water shortages). Maintenance of the pipelines and rills was a constant problem. Because of the long time delay between sex-differentiation of the flowers within the inflorescences and harvesting the ripe fruit, it is only possible to begin to judge the effectiveness of irrigation from about 28 months after it begins, providing it is sustained over the whole time period. The target fresh fruit yields, believed to be achievable, were only 18 t ha−1. The economics of the project were not considered in their paper.
In the Côte d'Ivoire, Prioux et al. (Reference Prioux, Jaquemard, Franqueville and Caliman1992) summarized the results of several trials conducted over a 12-year period on a commercial estate. On average, irrigation (with two micro-sprinklers per tree) increased fresh fruit bunch yields from about 18 to 22 t ha−1, and mesocarp oil from 3.99 to 4.84 t ha−1. Irrigation from planting onwards also advanced the first harvest by about one year, and evened out crop distribution.
Following a five-year trial (1974–79) in Central Johore, Malaysia, Corley and Hong (Reference Corley, Hong, Pushparajah and Chew1982) concluded that irrigation was unlikely to be economic in such areas where dry seasons only occur infrequently. A small increase in oil yield (average over three years = 0.52 t ha−1 y−1; or +8%) as a result of an increase in bunch number, which was attributed to a lower abortion rate, and in the mesocarp oil to bunch ratio, was not enough to justify the costs of irrigation.
In southern Thailand, where there is a three to four month long annual dry season, four different methods of irrigation (furrow, sprinklers, microsprinklers and drip) were compared in a commercial context (Palat et al., Reference Palat, Smith, Corley and Pushparajah2000; Reference Palat, Chayawat, Clendon and Corley2009). Of these, drip was considered to be the ‘best’ from a practical perspective, i.e. in terms of operating cost and ease of management, although fresh fruit yields were similar for each method. With drip irrigation, different application rates were compared, from 150 to 450 l palm−1 d−1 at two levels of fertilizer. Irrigation was applied daily during the dry season whenever the soil water deficit exceeded 30 mm. Fruit yield increases of up to 10 t ha−1 (from 18 to 28 t ha−1) were obtained, the highest yield coming from the treatment receiving 450 l palm−1 d−1 (equivalent to 6.4 mm d−1) at the high fertilizer level (twice the commercial rate). Over the ten-year period (1995–2005) the cumulative soil water deficit during the dry season averaged 235 mm (based on pan evaporation, 4.2 mm d−1, less rainfall). There was a linear relation between yield and daily application rates. Unfortunately, the total quantities of water applied were not reported. The yield response (following an increase in bunch number) in any one year was statistically related to the soil water deficit in the first quarter of that year (when abortion might have occurred), and to the deficit two years earlier (the time of sex differentiation).
When installing the drip irrigation, adverse effects on palms were observed when the tubing was buried within one metre of the trunk (causing damage to roots) but not when the distance was increased to 2 m. Emitters were spaced at one metre intervals along the pipe, with the expectation that pipes between alternate rows of palms would be sufficient. The economics of irrigation were sensitive to the price of palm oil.
Irrigation in such areas has other advantages, for example, allowing young palms to come into production early. Thus, irrigated palms in progeny trials in Thailand (Rao et al., Reference Rao, Palat, Chayawat and Corley2009) started yielding fruit six months earlier than unirrigated palms (within two years of planting compared with 30 months). Production of fruit in the first harvest year, recorded from 30 months after planting, averaged 18 t ha−1. increasing to 32 t ha−1 for the years four to seven, with the best progeny yielding over 40 t ha−1 in some years. For comparison unirrigated palms averaged 22 t ha−1 over the same four- to seven-year period.
Excess water
In some poorly drained locations, such as coastal areas and valley bottoms, there may be excess water associated with a high water table. Little is known about its impact on the productivity of oil palm, although prolonged flooding is known to reduce stomatal conductance and gas exchange processes (photosynthesis and transpiration) and to kill young palms (Lamade et al., Reference Lamade, Setiyo, Purba, Jatmika, Bangun, Asmono, Sutarta, Pamin, Guritno, Prawirosukarto, Wakyono, Herawan, Hutomo, Darmosarkoro, Adiwiganda and Poeloengan1998). The optimum depth of a water table for different soils has yet to be defined, as has the maximum allowable duration of exposure to flooding without yield loss (Henson et al., Reference Henson, Harun and Chang2008).
Drought mitigation
For reasons unknown (but presumably due to better root growth), removal of young inflorescences appeared to increase the drought resistance of young oil palms in Benin (Ochs and Daniel, Reference Ochs, Daniel, Corley, Hardon and Wood1976, citing Bénard and Daniel, Reference Bénard and Daniel1971). Disbudding increased vegetative growth including roots and, in the first 24 months of harvesting, disbudded palms produced as much fruit as the controls yielded in 42 months. The duration of ‘physiological drought’, defined as when the relative stomatal opening was below 5 (recorded using the infiltration technique at midday on a scale from 0, closed, to 12, wide open) was reduced from 50 to 30 days. Keeping the soil surface weed-free also reduced the number of ‘dry days’ experienced. Planting Stylosanthes as ground cover resulted in 10–15% more ‘dry days’ during the dry season than using Brachiaria. At the end of five years, cumulative fruit yield from trees growing on bare soil was double that from trees in soil planted with a legume cover crop.
Based on experience in the Côte d'Ivoire (Caliman, Reference Caliman1992) and Benin (Nouy et al., Reference Nouy, Baudouin, Dejégul and Omoré1999), the following drought mitigation measures have been identified:
• Selecting a good site with deep water-retentive soils.
• On sloping land, planting on the contour together with adopting appropriate soil and water conservation measures (e.g. bunds, terraces) can lead to improved growth in young palms.
• Where the soil is compacted subsoiling can improve root growth and distribution, both vertically and laterally, and water availability, and fruit yield increases averaging 8% have been obtained.
• Leaving strips of bare soil along the planted row, killing off the cover crop between the rows at the start of the dry season, and leaving small patches to regrow when it rains. Pueraria phaseoloides regrows quickly, while Calopogonium mucunoides is drought resistant and develops well under the shade of oil palms. Yield benefits of >30% have been achieved from bare soil treatments.
• Mulching with organic materials (including empty fruit bunches at 30 t ha−1) reduces evaporation from the soil surface (Lim et al., Reference Lim, Goh, Kee and Henson2008, citing others). However, as 30 t ha−1 is about five times the annual production of empty bunches, this cannot be widely applied.
• In very dry locations (e.g. Benin) reducing the plant density to 100 palms ha−1 can reduce damage by drought.
• Removing some or all the inflorescences on young oil palms stimulates vegetative growth, particularly root development, and may improve drought tolerance (Bénard and Daniel, Reference Bénard and Daniel1971).
Summary: water productivity
1. Because of the long period between the initiation of the inflorescence and fruit maturity, the full response to irrigation will only be seen in the third year.
2. The allowable soil water deficits at different crop development stages are not known.
3. There are no absolute figures quantifying the yield responses to irrigation but they are in the region of 20–25 kg fresh fruit ha−1 mm−1, equivalent to a yield loss of about 10% for every 100 mm increase in the potential soil water deficit.
4. The main effect of irrigation is to increase the number of bunches by increasing the sex ratio and reducing abortion losses.
5. Irrigation can advance the time when fruiting begins in young palms by at least six months and increase yields in the early years (e.g. years 4 to 7) after planting.
6. Irrigation offers an opportunity to improve crop distribution during the year (still to be confirmed).
7. Drip irrigation and micro-sprinklers are considered to be suitable methods for irrigating oil palm.
8. Recommended drought mitigation measures include: maintaining bare soil along the rows, mulching, reducing plant populations, and removing young inflorescences.
CONCLUSIONS
The oil palm originated in West Africa where there are annual dry seasons of variable duration. The principal centres of production are now Indonesia and Malaysia, where dry seasons are less regular and intense, although the crop is moving into drier regions of Malaysia and north into Thailand and elsewhere in the world. Much of the research reported on the physiology and water relations of oil palm has been done in Malaysia and previously in Francophone West Africa. Central to understanding how water stress impacts on yield is a detailed knowledge of the development stages of the inflorescence, which are spread over a long time. While these have been well defined, their timing can be variable. Quantifying the relation between water availability at each stage and yield has yet to be achieved. There is convincing evidence at both the single leaf and canopy levels that dry air reduces stomatal conductance, even when the soil is wet, with similar reductions in the rates of photosynthesis and transpiration. Less is known about the actual water use of oil palm at the field level, and the minimum amount of water needed to obtain good yields.
With respect to irrigation, the following view expressed by Henson in (Reference Henson2006) still applies:
‘Despite many trials, what constitutes an optimum or adequate water supply to maintain yield is still poorly defined. The issue has been complicated and deductions hindered by the variability of conditions under which the trials have been conducted, by poor experimental design and inadequate controls, by a lack of comprehensive monitoring of soil and atmospheric conditions, and by differences in methodology relating to definitions of soil water status.’
All irrigation experiments are notoriously difficult to perform, including interpretation of the data in ways that are of practical value beyond the place and time that they were undertaken. For a tree crop like oil palm, it is particularly difficult, and compromise is always necessary between what is desirable and what is practical. Nevertheless, there is scope to develop functional relations between yield and crop water-use to aid rational irrigation planning and water management in the dry areas where oil palm is now being increasingly grown.
Acknowledgements
the support provided by Dr Hereward Corley and Dr Ian Henson during the preparation of this paper, and the contribution of two referees, are gratefully acknowledged.



