INTRODUCTION
The mango tree is believed to have evolved in the subtropical north-east Indo-Burmese region, where it is found growing as a canopy-layer species in the rainforests. Mango has been cultivated for at least 4000 years in India, where it occupies a pre-eminent place amongst fruit crops and is acknowledged as the ‘King of the Fruits’ (Majumder and Sharma, Reference Majumder, Sharma, Bose and Mitra1990). The mango fruit can be eaten fresh, frozen, dehydrated, canned or made into jellies, jams, chutneys, pickles and juices. The leaves can be eaten as a vegetable, or used as stock fodder.
Mango trees were introduced into the humid tropics of the Malay Archipelago and South-east Asia 2500–2600 years ago, where the mango became naturalised. It was only in the 16th and 17th centuries that the mango reached Africa, and afterwards Brazil, with the aid of Portugese traders and travellers. The first introduction into the United States (Florida) was in 1861 (Mukherjee and Litz, Reference Mukherjee, Litz and Litz2009).
Some mango cultivars evolved in tropical areas and these differ from those that originated in the subtropics. The tropical cultivars, which came from the hot and humid regions of south-east Asia (5–6°N), produce seed with several genetically identical embryos (poly-embryonic Indo-Chinese group,). In contrast, those that evolved in the subtropical monsoonal regions of the Indian sub-continent (Assam/Burma border area, 24–26°N), with hot summers but cooler and dry winters, are mono-embryonic (Indian group) (Mukherjee and Litz, Reference Mukherjee, Litz and Litz2009; Schaffer et al., Reference Schaffer, Urban, Lu, Whiley and Litz2009; Whiley and Schaffer, Reference Whiley, Schaffer and Litz1997). Hybridisation occurs readily between cultivars from both groups. A considerable degree of genetic diversity is probably responsible for the adaptation of mango to a wide range of tropical and subtropical environments. The mango is now grown throughout the tropics (latitude range between 25°N and S) and subtropics (35°N and S), and as far north as latitude 35–37°N in southern Spain (Crane et al., Reference Crane, Bally, Masqueda-Vazquez, Tomer and Litz1997). It can be found at altitudes of up to 1400 m in the tropics. The optimum air temperature for mango growth is in the range of 24–27 °C. Mango trees have limited cold tolerance and are damaged when temperatures fall below 0 °C. The mono-embryonic cultivars tend to be better adapted to low temperatures than the poly-embryonic ones (Schaffer et al., Reference Schaffer, Whiley, Crane, Schaffer and Andersen1994). Although mango is considered to be drought-tolerant, and may survive for many months without rain or irrigation, water deficits during the reproductive cycle can adversely affect fruit retention and early fruit growth (Schaffer et al., Reference Schaffer, Urban, Lu, Whiley and Litz2009; Whiley and Schaffer, Reference Whiley, Schaffer and Litz1997).
India is by far the biggest producer of mango, with 2.31 million ha yielding (in 2010) about 15 million t of fruit annually. China is next with 470,000 ha producing 4.35 million t. These countries are followed by Thailand (310,000 ha; 2.55 million t), Pakistan (170,000 ha; 1.85 million t) and Mexico (170,000; 1.63 million t). The world totals are 4.95 million ha producing 37.1 million t (FAO, 2012). Mango is now traded internationally all the year round.
In this paper, the results of selected water management-related experiments are summarised, and an attempt is made to draw generic conclusions from an independent perspective. Starting with a description of the stages of development (including roots) of the mango trees in relation to water availability, this paper reviews plant water relations, water requirements, water productivity, and water management. A similar format has been used in previous published reviews in this series on other fruit crops, including banana (Carr, Reference Carr2009), coconut (Carr, Reference Carr2011) citrus (Carr, Reference Carr2012a) and pineapple (Carr, Reference Carr2012b). A book reviewing the water relations and irrigation of the major plantation crops has been published (Carr, Reference Carr2012c).
Majumder and Sharma (Reference Majumder, Sharma, Bose and Mitra1990) published a general review of mango, while Davenport (Reference Davenport2007, Reference Davenport and Litz2009) described in great detail its reproductive physiology. An overview paper by Léchaudel and Joas (Reference Léchaudel and Joas2007) on the influence of pre-harvest factors, including water availability, on post-harvest issues, including fruit quality, is of limited value in the context of this paper. The second edition of a book devoted to the mango (Litz, Reference Litz1997) has been recently published (Litz, Reference Litz2009).
CROP DEVELOPMENT
There are hundreds of mango cultivars in the world, indeed about 1000 cultivars are known to exist in India alone, nearly all of which are of the monoembryonic-type. Salient characteristics of the more important of these have been described by Menzel and Simpson (Reference Menzel, Simpson, Schaffer and Anderson1994) and Knight et al. (Reference Knight, Campbell, Maguire and Litz2009). Cultivars referred to in this paper include Carabao (originally from the Philippines), Chok Anan (Thailand), Haden (Florida), Irwin (Florida), Julie (West Indies), Keith (Florida), Kensington Pride (Australia), Nam Doc Mai (Thailand), Osteen (Florida), Sensation (Florida) and Tommy Atkins (Florida). Rootstocks can be used to limit excess vegetative growth and to impart dwarfing characteristics suitable for intensive, high-density production systems (Oosthuyse, Reference Oosthuyse2009; Reddy et al., Reference Reddy, Kurian, Ramachander, Singh and Kohli2003).
Vegetative growth
The mango is a large evergreen tree that can reach a height of 30–40 m and live for more than 100 years. The trees are either grown from seed, or by vegetative propagation with the scion grafted on to seedling rootstocks. In south-east Brazil, the recommended plant spacing, under rain-fed, dry conditions, is 10 × 10 m (100 trees ha−1), with field planting recommended at the start of the rains. By contrast, in the semi-arid north-east of the country, where the crop is irrigated, the density is increased to 250 trees ha−1 (8 × 5 m) (Pinto et al., Reference Pinto, Silva, Pinto and Johnston2007). Very high-density planting (e.g. 1600 trees ha−1) is now being encouraged, for example in India (Sharma, Reference Sharma2012).
A terminal meristem produces an indeterminate trunk bearing tiers of branches. Shoots grow in periodic flushes, lasting three to six weeks, during which time the apex produces 10–20 leaves (Davenport, Reference Davenport and Litz2009; Davenport and Núñez-Elisea, Reference Davenport, Núñez-Elisea and Litz1997). A period of ‘dormancy’ then follows. With mango, the time taken for leaves to become net exporters of carbon, rather than net importers, is relatively long (c. six weeks) compared with avocado (Schaffer et al., Reference Schaffer, Urban, Lu, Whiley and Litz2009; Whiley and Schaffer, Reference Whiley, Schaffer and Litz1997). Vegetative flushes occur one or more times a year on individual stems, the exact number depending upon the age of the tree, the cultivar and the growing conditions.
For example, shoot extension ceases at daily mean air temperatures less than c. 15 °C, while at 27.5 °C, the number of growth ‘flushes’ over a 20-week period can be between 2.3 (cv. Nam Dok Mai), 3.3 (cv. Carabao) and 4.7 (cv. Kensington Pride). The critical variable is the duration of the period between flushes, which in this example varied from 36 to 17 to 5 days respectively (Whiley, Reference Whiley1993).
Flowering
The mango tree produces 300–4000, small (5–10 mm diameter) pink flowers on, predominantly, many-branched, terminal panicles. The panicles are initiated in dormant apical buds on stems that have developed from lateral buds on shoots that flowered the year before. Both male and hermaphrodite (commonly known as ‘perfect’) flowers are found on a single inflorescence. Mango flowers begin to open early in the morning and anthesis has generally been completed by noon. Mango flowers are cross-pollinated, mainly by insects (Iyer and Degani, Reference Iyer, Degani and Litz1997). Under tropical conditions the period between floral initiation and anthesis can be as little as four weeks. The stimulus that induces flowering in mango trees has been the subject of much debate and study. There are two contrasting situations to consider: (1) the low-latitude tropics, and (2) the high-latitude tropics and subtropics, although the two situations obviously overlap, and altitude also needs to be taken into consideration.
Low latitude tropics: In these areas, the initiation of flower buds generally occurs after at least six to 12 weeks of water stress is ended by rain or irrigation. Water deficits prevent vegetative shoots from flushing. The longer this water stress period lasts, the more time there is available for a possible (unidentified) floral stimulus to accumulate (or for the quantity of an inhibitor to diminish). This initiation process has still not been demonstrated consistently. For example, in a glasshouse experiment in Florida (USA; 25°28′N 80°28′W), Núñez-Elisea and Davenport (Reference Núñez-Elisea and Davenport1994) were unable to demonstrate that flowering was stimulated by an extended period of water stress. This was thought to be due to the small size of containers (12 L), which dried out too quickly. Subsequently, Lu and Chacko (Reference Lu and Chacko2000) reported a similar experiment conducted in the open air in Darwin, Australia (12°25′S 130°52′E), but with larger containers (200 L). A controlled water deficit lasting five weeks promoted earlier and more intense flowering in both cultivars Kensington Pride (72% of the shoots flowered on water-stressed trees but only 13% on well-watered trees) and Irwin (67% and 4% respectively). The number of fruits (large and medium size) harvested from the water-stressed trees was also greater than those from the well-watered trees. The one exception was cultivar Nam Dok Mai, which appeared not to need an external stimulus, such as low temperature or water stress, to induce flowering (41% of the shoots flowered on well-watered trees).
However, research in Colombia suggests that, under tropical conditions, the primary factor controlling flower initiation in mango trees is the age of the last flush (Ramírez and Davenport, Reference Ramírez and Davenport2010). The older the flush, the greater the accumulation of a florigenic promoter, which is being synthesised continuously in mango leaves and translocated from the leaves to the buds through the phloem. Water stress, by delaying shoot development (cool conditions during winter in subtropical areas serve a similar purpose), extends the period over which the florigenic promoter is being formed until a critical concentration is reached that induces flower formation (see Ramírez et al., Reference Ramírez, Davenport and Fischer2010a, Reference Ramírez, Davenport, Fischer and Pinzónb and others for full discussion of this topic).
High-latitude tropics and subtropics: In these areas, flower buds are initiated during the cool winter months (night temperatures below 15 °C, day temperatures below 20 °C, for a minimum period of three weeks, cultivar-specific). Flowering then occurs in the early spring. Reporting a detailed, controlled-environment experiment in Florida, Núñez-Elisea and Davenport (Reference Núñez-Elisea and Davenport1995) showed that it was cool temperatures (around 15 °C) during bud dormancy rather than a short photoperiod (11 h) that caused floral induction (for cv. Tommy Atkins). Similarly, warm conditions (near 30 °C) rather than a long photoperiod (13 h) inhibited flowering, and the non-differentiated buds became vegetative. In a similar study in Queensland, Sukhvibul et al. (Reference Sukhvibul, Whiley, Smith, Hetherington and VIthanage1999) showed how the floral biology of all four cultivars studied (Kensington Pride, Irwin, Nam Dok Mai and Sensation) was affected, in different ways, when inflorescences developed under low temperatures (day/night temperatures at or below 20 °C/10 °C). The potential impact on yield was greatest with cv. Kensington Pride, a polyembryonic ecotype. As well as these external factors, phytohormones (auxins from leaves and cytokinins from roots) may also be involved in the initiation and induction of reproductive cycle in mango (Davenport, Reference Davenport2007, Reference Davenport and Litz2009; Davenport and Núñez-Elisea, Reference Davenport, Núñez-Elisea and Litz1997).
To confound the situation further, it is possible for both low temperatures and water stress to act together to induce flowering in mango trees. In a field experiment in a reasonably high-latitude tropical site in Queensland, Australia (19°S), Bally et al. (Reference Bally, Harris and Whiley2000) compared the responses of 20-year-old trees (cv. Kensington Pride), in terms of flowering and yield, to deficit irrigation against a well-watered control treatment. The two deficit treatments involved withholding irrigation for specific periods of time from the first vegetative flush following harvest until either (1) 90% of the buds were judged to be anatomically floral or (2) 70% of the inflorescences had emerged. In both cases where water was withheld, the number of terminals that flowered was increased (by an average of 20% over three years). In two out of the three years yields were also increased (by an average of 17%). However, since the minimum temperatures during the floral induction period were low enough to induce flowering (10–15 °C), the only valid conclusion is that water deficits had an additive effect on flowering that was initiated by low temperatures prior to the emergence of the inflorescences. It was not possible to explain any of the observed yield responses in terms of the measured parameters.
In Thailand (c. 13°N 100°E; alt. 5 m), Pongsomboon et al. (Reference Pongsomboon, Subhadrabandhu and Stephenson1997) monitored the changes in, and relationships between, a number of variables associated with flowering in four-year-old mango trees (cv. Nam Dok Mai) during the cool dry season. Although the changes were relatively small, there was a positive correlation (r = 0.78) between (pre-dawn) leaf xylem water potentialFootnote 1 (range −0.3 to −1.0 MPa) and the relative water content (range 98 to 87%). The proportion of terminal shoots that produced flowers increased (from about 40 to 90%) as the xylem water potential (r = 0.72) (and also the relative leaf water content, r = 0.65) declined. Similarly, there was a positive correlation between the total non-structural carbohydrate content of the terminal shoots and the flowering intensity. Although no causal relationship was established, this response was considered to be due to the combined effect of water stress together with low temperatures suppressing vegetative growth, resulting in carbohydrate accumulation. Flowering intensity was also associated with a decline in giberellic acid-type substances (GA3-) in the shoot tips.
Out-of-season flowering: Producers are interested in reliable out-of-season flowering in order to provide fruits for market at times of maximum value, especially in the tropics. In the northern hemisphere mango prices are highest in March and April and, as it takes four months from flowering to fruit maturity, flowering induction time needs to be shifted back to October (from February). Successful floral management in the tropics therefore means discouraging the initiation of new shoots, since they are likely to be vegetative, until the resting stems have matured enough to induce flowering shoots to develop (Davenport, Reference Davenport2007; Ramírez et al., Reference Ramírez, Davenport, Fischer and Pinzón2010b). As trees mature, this extended ‘rest’ period occurs naturally. At high altitudes in the tropics, cool temperatures provide an additional stimulus for flowering in stems of a given age.
According to Davenport (Reference Davenport2007), the first step in the initiation of flowering is to synchronise vegetative growth. This is usually achieved through tip-pruning. An adequate supply of water is essential at this time. With localised irrigation, there is still a risk of a second vegetative flush occurring when the rains start since some roots will have been in dry soil. Reducing nitrogen levels in the leaf discourages a second flush in the rainy season.
Fruiting
The mango fruit is a large fleshy drupe containing edible mesocarp. It is very variable in terms of size and shape, and the colour at maturity is cultivar-dependent. The fruit is rich in vitamins C and A. Many fruitlets form on each panicle, but more than 80% are shed (fruit drop) during the first four weeks after fruit set. Water stress should be kept to a minimum during the first four to six weeks after anthesis. This is when cell division is occurring and the cell walls are being synthesised (Schaffer et al., Reference Schaffer, Whiley, Crane, Schaffer and Andersen1994). Between 8 and 13% of the flowers set fruit but less than 1% of these fruits reach maturity (Davenport, Reference Davenport and Litz2009; Davenport and Núñez-Elisea, Reference Davenport, Núñez-Elisea and Litz1997). Some cultivars produce only one mature fruit on each panicle. Fruits take from three to four months to mature. By year 10, individual trees can produce 400–600 fruits annually and by year 40, 2500 fruits can be produced, depending on tree spacing (Menzel and Simpson, Reference Menzel, Simpson, Schaffer and Anderson1994). Mango is normally harvested green, and the fruit then ripens during the post-harvest period.
Roots
The primary purpose of a pioneering investigation in Pusa, New Delhi, India was to investigate the extent to which grass adversely affects the growth, including roots, of a range of fruit tree crops (Howard, Reference Howard1925). Root systems of trees established in the field during 1914 were exposed at intervals over a three-year period (1921–1923), and the extent and periodicity of root growth were recorded in great detail. The results were expressed in a series of line drawings. The monsoon lasted from mid-June to mid-October. The water table was at a depth of about 6 m for six months, but it rose rapidly after the rains began and came within ‘a few feet’ of the surface in August and September, before falling again after the monsoon ended. Flowering in mango trees occurred in February/early March, after which new vegetative shoots were produced. The mango fruit was ripe at the start of the rains.
To give examples of the detailed observational skills exhibited by Howard (Reference Howard1925), the following quotes about mango are taken from the text:
The large superficial roots give off smaller branches to the deep soil layers (followed in 1921 to 4.7 m depth) . . . the gradual downward movement of root activity after the rains has been observed on several occasions. On October 22, 1921, absorbing roots were not found below 1.12 m . . . the next year an exposure was made a month later . . . root activity had proceeded as far as 1.37 m from the surface, but below this point the roots were dormant. At the end of January, when the flower buds were beginning to swell, root activity had reached 2.36 m. By March 10, 1923, at the beginning of the hot season (during the flowering period), the lower roots were active down to 4.7 m.
It was also observed by Howard (Reference Howard1925) that
The root hairs of the mango were short, stiff and dark, reddish brown in colour, and did not readily decay; on June 9, 1922 new roots were abundant, the longest being 1 cm (six days after the rains began); August 14–22, 1921, many aerotropic active roots in the upper 0.30 m of soil, new roots growing horizontally at 0.51 m, going downwards at 0.66 m and 0.76 m; October 22–24, 1921, after the fall of the ground water, below 1.12 m and down to 3.96 m the root system was dormant; many active roots in upper 0.25 m; at 0.25 m many new roots growing towards the surface; January 27–February 2, 1923, flower buds swelling, exposure made to 2.8 m, new roots found at various depths down to 1.8 m, the root system was dormant below this depth.
In Florida (USA), Willis and Marler (Reference Willis and Marler1993) adopted a different approach. By tracing each root growing against the glass wall of an observation chamber, they recorded root growth of two cultivars (Keitt and Julie), both grafted on to Turpentine rootstock, over a period of 12 months. Roots grew fairly continuously, with only brief periods when there was little or no root extension. Both cultivars behaved in similar ways. By contrast, shoot growth was cyclic with distinct periods of shoot extension (during the year, cv. Keitt had four vegetative flushes and cv. Julie had five flushes), followed by periods of inactivity. There were no consistent relationships between root and shoot extension rates.
In an investigation of feeder root distribution in Bengaluru, India, Bojappa and Singh (Reference Bojappa and Singh1975) found that the greatest concentration of roots occurred within 0.60 m radius of the trunk and within 0.15 m of the soil surface in both young and mature mango trees. For young trees, 90% of the roots were within a radius of 1.8 m from the trunk, and for older trees within 3.6 m radius. There is little doubt that the roots of the mango tree can reach considerable depths. For example, Singh (Reference Singh, Alvim and Kozlowski1977) reported that roots of mango had been recorded at depths of 5.5 m and, in the case of a 60-year-old tree in Bihar (India), 4 m.
Summary: crop development
1. The mango is a large, long-lived, indeterminate evergreen tree that can reach a height of 30–40 m.
2. Cultivars that evolved in hot, humid conditions (Indo-Chinese group) differ from those that originated in subtropical regions (Indian group).
3. The mango is adapted to a wide range of tropical and subtropical environments.
4. The shoots grow in periodic flushes, lasting three to six weeks, during which 10–20 leaves are produced.
5. The base temperature for shoot growth is about 15 °C.
6. Flowers are formed on panicles that are initiated in dormant apical buds, which develop from lateral buds on shoots that have flowered the year before.
7. In the low-latitude tropics, flower buds are initiated after a period of water stress (six to 12 weeks duration) is ended by rain or irrigation (the age of the shoot also plays an important role).
8. In the high-latitude tropics and subtropics, flower buds are initiated during the cool winter months (night temperature below 15 °C and day temperature below 20 °C for a minimum of three weeks, cultivar-dependent).
9. Only about 10% of the flowers set fruit and then more than 90% of the fruitlets that form are shed during the following four weeks. After flowering, it takes the fruit three to four months to mature.
10. In contrast, warm conditions (30 °C) result in undifferentiated buds becoming vegetative.
11. Roots extend in depth to at least 5 m. Roots are particularly active in the top 0.25 m. There are no consistent relationships between shoot growth and root growth: roots grow more or less continuously.
PLANT WATER RELATIONS
In a review paper, Whiley (Reference Whiley1993) wrote the following:
Despite the importance of this fruit crop, there is little published data on the basic physiology of the (mango) tree in respect of gas exchange and water relations and their interactive response with the environment.
He then went on to describe the research that was underway at the time. Some progress has been made since then. This is now described under the following headings: stomata and gas exchange:
Stomata
According to Purseglove (Reference Purseglove1968), stomata are present on both leaf surfaces but with a greater number on the lower (abaxial) surface. By contrast, Wahdan et al. (Reference Wahdan, Abdelsalam, El-Nagar and Hussein2011), in a comparison of two new genotypes (both mono-embryonic) in Egypt, counted the stomata on the lower surface only (it is not stated whether they looked on the upper surface). For one cultivar, the average density was 384 stomata mm−2, and for the other 678 stomata mm−2. Similarly, Urban and Jannoyer (Reference Urban and Jannoyer2004), citing Ali et al. (Reference Ali, Koeda and Nii1999), reported densities of 700 stomata mm−2 on the lower surface of fully expanded mango leaves, but 1900 stomata mm−2 on partially expanded young leaves. There is clearly a range of values.
In Australia, Lu (Reference Lu and Malik2006) found that the stomata opened rapidly from about 0700 h with conductance reaching a maximum at about 0900 h. This was then followed by a steady but slow decline in stomatal opening until about 1800 h, after which the stomata closed rapidly.
In an unusual laboratory experiment on the island of La Réunion, Urban and Jannoyer (Reference Urban and Jannoyer2004) monitored the transpiration rate from excised mango leaves of three cultivars (Haden, Heidi and Lirfa) at three leaf development stages. A reduction of only 2% in the leaf water content resulted in complete stomatal closure. This was taken as an indication of the capacity of the mango to protect itself against excessive water loss.
In Northern Australia, Goodfellow et al. (Reference Goodfellow, Eamus and Duff1997) studied the impact of carbon dioxide enrichment of the air (700 μmol mol−1) on stomatal conductance and assimilation by mango saplings (cv. Kensington Pride) over a 28-month period. Reduced stomatal conductance in response to the elevated CO2 was attributed to a reduction in both stomatal density (by about 17%) and stomatal aperture. At both normal and enhanced CO2 levels, stomatal conductances declined curvilinearly with increasing leaf-to-air saturation deficits (range 1.5 to 5.0 kPa). In contrast, light-saturating assimilation declined linearly. Total plant biomass was substantially increased in the elevated CO2 treatment throughout the experiment.
Gas exchange
In a paper reporting the results of measurements made 20 years earlier, Lu et al. (Reference Lu, Chacko, Bithell, Schaper, Wiebel, Cole and Müller2012) compared the responses of five mango cultivars in the seasonally wet–dry tropics of northern Australia in terms of photosynthesis and stomatal conductance. The five cultivars belonged to the two distinct groups, poly-embryonic (cvs. Kensington Pride and Strawberry) and mono-embryonic (cvs. Haden, Irwin and Tommy Atkins). Measurements were made at two contrasting sites: one near Darwin (12°S 130′E; alt. 13 m) could be described as humid-hot, with a short dry season; and the other, which was located at Katherine (14°S 132′E; alt. 108 m), was more semi-arid with distinct wet and dry seasons. Maximum values occurred during the wet season, but it was during the dry season that the largest differences between the cultivars were observed. Net photosynthesis was then greater in the three mono-embryonic cultivars than in the two poly-embryonic cultivars. Both photosynthesis rates and stomatal conductances were negatively correlated (linear) with the saturation deficit of the air (range 1.5 to 4.0 kPa) with all five cultivars. The two poly-embryonic cultivars were particularly sensitive to dry air, especially cv. Kensington Pride (of Australian origin). A similar negative relationship between stomatal conductance and saturation deficit had previously been reported by Whiley and Schafer (Reference Whiley, Schaffer and Litz1997), but over a narrow range of saturation deficit (0.5 to 1.5 kPa).
The shapes of the diurnal curves (for conductance and photosynthesis) were similar in both wet and dry seasons but, for conductance, were at a lower level in the dry season, even when irrigated. In contrast, transpiration rates in the wet season increased during the morning, before peaking in mid-afternoon and then declining rapidly. In the dry season, photosynthesis rates, conductance and transpiration rates were less for cultivar Kensington Pride than for those recorded for other cultivars throughout the day. In the case of sap flow measurements (Granier's heat dissipation method), it was necessary to make an allowance for spatial variation in the sap flux density within the sap wood (Lu et al., Reference Lu, Chacko, Bithell, Schaper, Wiebel, Cole and Müller2012; see below).
The fruit setting and fruit development period was the time of maximum environmental stress, with gas exchange remaining low despite a wet (irrigated) soil. Net photosynthesis and stomatal conductance were both positively correlated on a diurnal and a seasonal basis, and during the wet and dry seasons. Because of excessive latex exudation, leaf water potential measurements (with a pressure chamber) were not considered to be a reliable indicator of tree water status (Lu, Reference Lu and Malik2006).
Urban et al. (Reference Urban, Jegouzo, Damour, Vandame and Francois2008) working in La Réunion (20°52′S 55°31′E), investigated why net photosynthesis rates of leaves of mango situated close to an inflorescence were lower than those of leaves on vegetative shoots. Measurements were made on recently matured leaves on vegetative terminals and on floral terminals of four-year-old trees growing in large lysimeters. These showed that net photosynthesis was lower on leaves close to a developing inflorescence as a result of reduced stomatal and mesophyll conductances. The photosynthetic capacity of the leaf was also reduced. The authors inferred that this reduction in the photosynthetic capacity, and also in the nitrogen content (per unit leaf area), was the result of sink limitation. This suggested that perhaps nitrogen was reallocated at the expense of the photosynthetic process. Parameters measured on leaves close to panicles bearing set fruits were intermediate in value to those on vegetative shoots and on leaves close to an inflorescence, suggesting that the changes in net photosynthesis associated with flowering are reversible.
Mango trees can maintain a high water status when under water stress through osmotic adjustment, which is attributed to the presence of latex (Schaffer et al., Reference Schaffer, Whiley, Crane, Schaffer and Andersen1994). Similarly, Whiley (Reference Whiley1993) concluded, after citing others, including Pongsomboon et al. (Reference Pongsomboon, Whiley, Stephenson and Subhadrabandhu1992), that the reason why mango is relatively drought-tolerant was because it maintained turgor in its leaves when subjected to a water deficit. Zero turgor occurred at a leaf water potential of −1.75 MPa, while permanent leaf damage only occurred when the relative water content declined to 77%, which is much higher than the values reported for other tree crops (e.g. macadamia).
Summary: plant/water relations
1. Stomata occur mainly on the lower (abaxial) surface of mature leaves at densities of 400–700 mm−2.
2. When well-watered, the stomata open rapidly in the morning. Maximum conductance in the low-latitude tropics is reached at about 0900 h, followed by a slow but steady decline until 1800 h when the stomata closed.
3. A reduction in the leaf water content of only 2% results in complete stomatal closure.
4. Raising the CO2 concentration of the ambient air (to 700 μmol mol−1) reduced the stomatal conductance (due to fewer, smaller stomata), but total biomass production was increased.
5. Rates of photosynthesis and stomatal conductance are correlated and both are negatively correlated with the saturation deficit of the air (range 0.5 to 4.0 kPa).
6. There is some evidence that cultivars differ in the sensitivity of the responses of stomata to dry air.
7. During the dry season, net photosynthesis rates by cultivars from the Indian group exceeded those by cultivars from the Indo-Chinese group.
8. Net photosynthesis rates of leaves close to an inflorescence are less than those of leaves on vegetative shoots.
CROP WATER REQUIREMENTS
Several different methods have been used to determine the water use of mango trees with mixed success. These include the sap flow, Bowen ratio, eddy correlation and soil water balance techniques. Unfortunately, in several cases, there is a lack of clarity in the way the results have been reported. Research on crop water requirements has been conducted in three countries: Australia, Brazil and South Africa.
Australia: Lu and Chacko (Reference Lu and Chacko1997) successfully evaluated the suitability of Granier's sap flow system for measuring transpiration by 10-year-old mango trees (cv. Kensington Pride) in the seasonally wet–dry tropics in northern Australia (12°25′S 130°52′E). During the dry season, water use averaged 100 kg tree−1 d−1 when irrigated, and 60 kg tree−1 d−1 without irrigation. During the rains the corresponding value was 126 kg tree−1 d−1. They compared these results with those obtained gravimetrically, and with the ‘cut tree’ method. The results from Granier's sap flow method were believed to be within 6% of the ‘true value’.
This evaluation found evidence of circumferential variation in sap flow rates (different readings between the east and west sides of the tree) and also radial variability. This was largely due to the following three characteristics of a mature mango tree:
• There is no visibly distinct heartwood (even when the tree is 20–30 years old).
• Patterns of sap flow may be influenced by orchard management practices such as grafting, pruning and localised irrigation.
• Training a mango tree to have a very short trunk means that there may be branch scars or branches close to where the sensors are sited.
Lu et al. (Reference Lu, Müller and Chacko2000) subsequently addressed these complications. They found that, under changing soil water conditions, correlations between different aspects (i.e. the radial position of the sensor probes on the trunk relative to the compass) and between the depths of insertion of the sensors were not constant. This meant that a large number of sensor probes were necessary to get a realistic estimate of total sap flow. However, over a period when soil water was freely available, the depth profiles remained relatively constant. As a result, a method for calculating total sap flow in a mango tree from sap flux density measurements made 0–20 mm below the cambium was developed and successfully evaluated.
Brazil: The mango is widely grown in Brazil, particularly in the semi-arid north-east region, where the mean annual rainfall is about 400 mm. Using the Bowen ratio-energy balance method, as well as the water balance approach, Silva et al. (Reference Silva, Azevedo and Silva2007) monitored evapotranspiration from a mango orchard over two successive seasons, June to November 1998 and 1999, in this region at Petrolina (09°09′S 40°22′W; alt. 366 m). The trees were spaced 8 × 5 m (250 trees ha−1). They were 5.2 m tall with a leaf area index of (very large) 13–15. Irrigation (drip) was applied daily to keep the soil profile close to field capacity. The total rainfall over each dry season was only about 50 mm, while 950 and 1145 mm of irrigation water were applied during 1998 and 1999 respectively. The proportion of the net radiation dissipated as latent heat was greater at times of low evaporative demand than when evaporation rates were high, exceeding 70% in both years. Over the season, actual evapotranspiration rates (ET) averaged 4.5 ± 0.4 mm d−1 in 1998 and 4.3 ± 0.6 mm d−1 in 1999. The corresponding reference crop evapotranspiration values (ETo, Penman–Monteith) were 5.3 ± 1.03 mm d−1 and 4.9 ± 1.01 mm d−1 respectively. Assuming that, for a well-watered crop, ET was equal to potential evapotranspiration (ETc), the values of the crop coefficient (Kc) were 0.85 in 1998 and 0.88 in 1999. The peak ET rates in each year were 5.2 and 5.5 mm d−1 respectively.
Azevedo et al. (Reference Azevedo, Silva and Silva2003) had previously published a very similar account of the same experiment, but with more details of the methodologies used four years earlier, and only reporting the results for 1999. One surprising outcome was the very close agreement in the estimates of ET between the two methods used (Bowen ratio and water balance). For example, in 1999, the cumulative totals over the period of measurement were 552 mm (Bowen ratio) and 555 mm (water balance), both with a mean water use of 4.1 mm d−1 (Azevedo et al., Reference Azevedo, Silva and Silva2003). Considering the differences in the two methodologies this agreement had to be fortuitous resulting, perhaps, from a cancellation of errors. There was further confusion in that the cumulative ET totals reported in the subsequent paper (Silva et al., Reference Silva, Azevedo and Silva2007) were (presumably) the averages for both methods (it was not made clear), namely 676 mm in 1998 (4.6 mm d−1) and 719 mm in 1999 (4.8 mm d−1). These figures are different from the ones reported by Azevedo et al. (Reference Azevedo, Silva and Silva2003) and summarised above. No clear explanation was offered for these discrepancies. Perhaps, the most that can be taken from these two papers is that ETc from mature mango trees in this region of Brazil, between flowering and fruit maturation, are between 4 and 5 mm d−1. Subsequently, Teixeira and Bastiaanssen (Reference Teixeira and Bastiaanssen2012) evaluated several methods for determining and interpreting field measurements of energy fluxes over a micro-sprinkler-irrigated mango tree orchard, including the eddy correlation and Bowen ratio techniques. Depending on the method used, growing-season ET totals (or are they annual totals? It's not made clear) varied between 965 and 1552 mm (2003/2004), and between 1127 and 1440 mm (2004/2005).
Teixeira et al. (Reference Teixeira, Bastiaanssen, Moura, Soares, Ahmad and Bos2008) researched this topic further by using the eddy covariance technique to measure ET in a mango orchard in the semi-arid region of the Sao Francisco River basin in north-east Brazil (9°22′S 40°34′W). Measurements were made from 2003 to 2005; the cultivar was Tommy Atkins, 12-year-old in 2003, the trees were spaced 10 × 10 m; the tree height was 5.5 m; the leaf area index was 5.6; the soil was sandy (red-yellow Latossoil); the water table was at a depth of 2.5 m; the effective root zone was about 1.2 m deep; and the orchard was irrigated with micro-sprinklers. The authors plotted the outputs from the eddy covariance method (turbulent energy flux = H + λE) against the energy balance (available energy = Rn – G). The slope of the line gave the energy balance ratio (0.88). The latent heat flux (λE) was always greater than the sensible heat flux (H) during daylight hours, which in turn exceeded the soil heat flux (G). The net radiation term (Rn) is the amount of energy available at the crop surface (incoming solar radiation less reflected short wave radiation and less re-emitted long wave radiation) that can be used to heat the air (H), evaporate water (λE) or heat the soil (G). A very small proportion of solar radiation is utilised in photosynthesis.
The aim of these field measurements was to quantify how much of the net radiation contributed to the evaporation process. In this experiment, 89% of Rn was used to evaporate water (transpiration and evaporation) in the first year and 80% was used in the second year. When converted to the equivalent depths of water, these represented annual ET totals of 1492 mm in 2003/2004 and 1346 mm in 2004/2005, a combined average daily ET rate of 3.7 mm d−1, with peaks of 6.3 mm d−1 and 5.1 m d−1 in the two years, values close to those cited above. Minimum ET values were 0.6 mm d−1. When averaged over 20-day periods, the crop coefficient, Kc = ETc/ETo, where ETo is the reference crop evapotranspiration (Penman–Monteith equation; weather data obtained from an automatic weather station), varied between 0.65 and 1.05. The high values occurred during periods when the soil surface was frequently wetted by rain or irrigation (micro-sprinklers were used to irrigate the trees, not drip). When evapotranspiration was partitioned between transpiration and evaporation, the mean values of each in year 1 were 3.06 mm d−1 and 0.75 mm d−1, and in year 2 the mean values were 2.79 mm d−1 and 0.85 mm d−1 respectively.
Using λE (evaporation) flux profile relationships, Teixeira et al. (Reference Teixeira, Bastiaanssen, Moura, Soares, Ahmad and Bos2008) calculated the seasonal changes in the aerodynamic (ra) and crop canopy (rc) resistances. Relatively high rc values were associated with dry air conditions, while high ra values occurred during the rains when rc was low. The 24-hour annual mean rc and ra values were 135 s m−1 and c. 37 s m−1 respectively. The orchard could be described as aerodynamically ‘rough’.
Silva et al. (Reference Silva, Campos and Azevedo2009) reported the results of a deficit irrigation experiment in northeastern Brazil (Petrolina) that was designed to identify the irrigation regime that gave the highest water productivity. The soil was sandy (90% sand), classified as a red-yellow Latosol, with an available water content of only about 8%. The water table was 4–6 m below the surface. The 12-year-old trees (cv. Tommy Atkins) were spaced 10 × 5 m (200 trees ha−1), and irrigated by sprinklers, with one sprinkler per tree. There were four levels of water application: 0.70, 0.80, 0.90 and 1.00 times ETo, the reference crop evapotranspiration (Penman–Monteith). The experiment lasted for two years, 2005 and 2006. Actual water use (ET) was estimated using the soil water balance approach. This included estimates of drainage from and/or capillary rise into the root zone (based on a profile of tensiometer readings from 0.2 to 1.2 m depth). The average total irrigation amounts applied in a season were between 365 and 550 mm. In addition, there were 154 mm of rain.
Cumulative evapotranspiration totals (ET) averaged over both seasons (from flowering to fruit maturity) were between 370 mm (0.70 × ETo) and 480 mm (1.00 × ETo, the control treatment). Daily ETc rates in the well-watered control treatment were between 3.6 and 5.6 mm d−1. Yields of fresh fruit were similar ranging between 28.0 t ha−1 (the control) and 31 t ha−1 (0.90 × ETo). The authors plotted a not-very-convincing quadratic curve through the four data points. Water productivities based on irrigation water applied were between 5.1 kg fruit m−3 (control) and 8.0 kg m−3 (0.70 × ETo). The corresponding values for ET were 5.8 and 7.9 kg m−3. These values are considered again below in the context of other work on water productivity.
South Africa: Over a six-year period, Mostert and Hoffman (Reference Mostert and Hoffman1997) monitored the water use of 12-year-old (initially) mango trees (cv. Fascell; tree density = 210 ha−1) in the Eastern Lowveld in South Africa (25°33′S 30°58′E; alt. 1000 m). Tensiometers were installed at depths of 300, 600 and 900 mm. The amount of water needed to bring the soil profile (to a depth of 900 mm) back to field capacity when the average readings at all three depths had reached either −30 kPa (or −60 kPa) was monitored with flow meters. It was assumed that only 70% of the orchard area was watered. Similarly, it was also assumed that 70% of the rainfall was effective. The total annual potential water use (ETc) averaged over the six years for the frequently irrigated treatment was 1200 mm (range 1050 to 1390 mm). These figures include 0.70 × the annual rainfall, which averaged 494 mm (range 293 to 639 mm). It is not clear whether these were net or gross figures. The ETc totals needed to be adjusted in order to give values representative of the total ground area (i.e. divided by 0.7). Unfortunately, this was all rather poorly explained in the text. Peak rates of water use reached about 4.4 mm d−1 in October/November, falling to about 2.1 mm d−1 in June (these are assumed to be net values; the equivalent gross values would be 6.3 and 3.0 mm d−1 respectively).
Yields of fruit were recorded in this experiment, but with large coefficients of variability (average 24%). The yield differences were only significant in two years, when the frequently irrigated treatment out-yielded the rain-fed treatment by 51% (158 kg tree−1 cf. 104 kg tree−1). Similarly, in one year only, the two treatments that were not irrigated in the winter months (May to August, the time of flower bud development) but were afterwards irrigated frequently out-yielded the control rain-only treatments. They also out-yielded (marginally) those that were not stressed in the winter months. Over the six years, the average yield from trees that were stressed in the winter months was 30.5 t ha−1 compared with 27.8 t ha−1 from those trees that were watered at that time. This yield advantage in favour of a water deficit treatment was thought to be the result of a vigorous flush of flowers following the relief of water stress. Caution is recommended when interrogating the data reported in this paper (Mostert and Hoffman, Reference Mostert and Hoffman1997). After making several assumptions, the water productivity for the incremental irrigation application was estimated to be only about 1.0 kg m−3.
Summary: crop water requirements
1. A range of techniques has been used to measure/estimate water use by mango trees, with some success: unfortunately the results of this research have not always been well reported.
2. After allowing for complications associated with the properties of the trunk, the sap flow method is believed to be capable of monitoring transpiration by a mango tree.
3. There was surprisingly good agreement between the seasonal ET totals derived from the Bowen ratio method and the soil water balance method.
4. The best estimates of water use by mango trees in tropical humid areas suggest mean seasonal ETc rates of 4–5 mm d−1 with peak rates of 5–6 mm d−1.
5. The value of Kc varies between 0.65 and 1.05, depending on the frequency and extent of wetting (linked to the method of irrigation) of the soil surface, and the tree density.
WATER PRODUCTIVITY
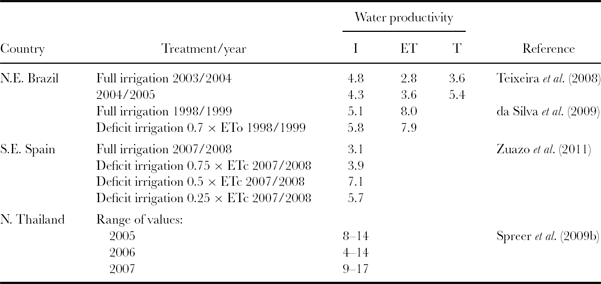
This section considers the evidence from field experiments of the yield response to irrigation water by mango. This is quantified in terms of the mass of fresh fruit (kg) for each unit of water applied (m3). It can also be expressed on a unit of evapotranspiration and/or transpiration basis. Local customs also have their own units for measuring the same thing. For example, in Australia, water productivity, as it is also known, is recorded commercially as the weight (t) of packed class 1 fruit per ha per ML (Bithell, Reference Bithell2012). Water productivity is not an easy parameter to measure, especially with a long-term tree crop prone to biennial bearing. Research on this topic has been undertaken in Brazil, Thailand and Spain.
Brazil: The recommended fertilizer levels (N:P:K) in Brazil for the mango crop vary according to the expected productivity (from <10 to >50 t fruit ha−1), the nutrient content of the leaf, the element itself, and whether or not the trees are irrigated (Pinto et al., Reference Pinto, Silva, Pinto and Johnston2007). The timing and proportions of the total annual application also vary with whether or not the crop is irrigated. Maximum recommended nutrient inputs for a rain-fed crop are 50 kg N ha−1, 34 kg P ha−1 and 66 kg K ha−1. For an irrigated crop these figures are increased to 120 kg N ha−1, 64 kg P ha−1 and 208 kg K ha−1. ‘Fertigation’ is encouraged with drip or micro-sprinklers. In Brazil, yields of up to 40 t ha−1 are possible with irrigation, but average yields under rain-fed conditions are in the range 8–12 t ha−1.
The recorded yields of fresh fruit from the commercial mango orchard in north-east Brazil, where Teixeira et al. (Reference Teixeira, Bastiaanssen, Moura, Soares, Ahmad and Bos2008) measured actual evapotranspiration and its components (summarised above), were 41.5 t ha−1 in 2003/2004, and 48.4 t ha−1 in 2004/2005. The corresponding values for water productivity for each of the two years were 4.8 and 4.3 kg m−3, when based on the volume of irrigation water applied; 2.8 and 3.6 kg m−3, when based on evapotranspiration; and 3.6 and 5.4 kg m−3, when based on transpiration respectively.
Thailand: The most important mango producing country in south-east Asia is Thailand. Apart from being a common house garden tree, mango is produced on medium to large plantations all over the country. The majority of fruit is grown for local consumption. One cultivar is particularly popular (Chok Anan). It is unusual in that in addition to the main harvest in May there are also two additional out-of-season harvests in June and August as a result of off-season flowering. This has certain advantages to the farmer but it does lead to biennial bearing. Most of the on-season fruit development occurs in the dry season when farmers need to apply supplementary irrigation to ensure high yields of good quality, but there is competition for water from other users (Spreer et al., Reference Spreer, Ongprasert, Hegele, Wünsche and Müller2009b).
This therefore was the context in which Spreer et al. (Reference Spreer, Nagle, Neidhart, Carle, Ongprasert and Müller2007, Reference Spreer, Ongprasert, Hegele, Wünsche and Müller2009b) compared the responses of mango (cv. Chok Anan grafted on to Talap Nak rootstock) to four irrigation treatments: well-irrigated (100% ETc), rain-fed only (no irrigation), regulated deficit irrigation (50% replacement of ETc) sustained over the dry season (when rainfall ranged from 70 to 333 mm) and partial root zone drying (50% replacement of ETc, water applied to alternate sides of the tree at two-week intervals). The experiment was located in an orchard near Chiang Mai (18.53°N 100.03°E; alt. 350 m). The trees were 10 years old at the beginning of the experiment. The soil was classified as a Regosol, characterized by a high stone content and a low water holding capacity. The experiment continued for four years (2004–2007). Unusually, the authors used 0.75 and 0.90 as the levels of confidence needed to determine statistically significant differences in fruit yields as well as 0.95 (the usual minimum level). The yield response curves (production functions) were also misleading. Quadratic curves were plotted for each of the four years with only four data points. These relationships were then used to identify a (false) optimum water input (irrigation plus rain). From a visual assessment, it would have been equally justified to draw a two-stage linear response curve, or even a single straight line.
Over the four years (which included two ‘on’ or good-yielding years and two ‘off’ years) between 38 and 75% of the trees were alternate bearing, the average annual yields were similar for all three irrigated treatments, at about 81 kg tree−1. The rain-fed trees averaged 19% less at 66 kg tree−1 (the tree density was not specified). As a result, the water productivity was considerably higher in the two deficit treatments than it was for the well-watered trees. In a separate paper, Spreer et al. (Reference Spreer, Muller, Hegele and Ongprasert2009a) reported the results for the first two years, 2004 and 2005, of what appears to be the same experiment. It is not easy to reconcile the results as presented in the three papers by Spreer et al.
Spain: The provinces of Granada and Malaga in south-east Spain represent the northern limit of the commercial production of mango. This is a subtropical, Mediterranean climate characterised by dry, hot summers and wet autumns and winters. The average annual rainfall is about 450 mm. Zuazo et al. (Reference Zuazo, Pleguezuelo and Tarifa2011) reported the results of a field irrigation experiment conducted near Granada (36°48′N 3°38′W; alt. 195 m) over three seasons (2006–2008). With the aim of identifying the most productive irrigation schedule, the treatments were based on four different levels of replacement of water lost by ETc. These were 100% ETc (the control); 75% ETc; 50% ETc and 25% ETc, where ETc is the evapotranspiration from a well-watered mango crop. When less than the maximum amount of water is applied, this is known as ‘sustained deficit irrigation’. Unfortunately, there was not a rainfall-only (unirrigated) control treatment. ETc was calculated from the Penman–Monteith estimate of ETo, with Kc derived from drainage lysimeter data. These were adjusted for tree size (viz. Kc = 0.51 at flowering, 0.72 at fruit set and 0.60 during fruit expansion) (ETc = Kc × ETo). The 12-year-old trees (their age at the start) were grown on bench terraces (cultivar Osteen; density 600 trees ha−1), the soil texture was 68% sand, 24% silt and 8% clay.
The average depths of water applied with drip irrigation to each treatment over the three seasons were 474 mm (control), 342 mm, 258 mm and 168 mm respectively. The frequency of irrigation was not reported. The corresponding fruit yields were 24.1, 22.5, 30.7 and 16.0 kg tree−1. Only yields from the lowest yielding treatment (25% ETc) were significantly different (p ≤ 0.05) from the other three. This may have been a chance result. In the same order, water productivities averaged 3.1, 3.9, 7.1 and 5.7 kg m−3 (overall mean 5.0 kg m−3) with the 50% ETc treatment apparently more than twice as productive as the well-irrigated 100% ETc treatment. Using a derived binomial function (y = −3.42x2 + 32.34x – 47.13; R2 = 0.85, n = 12, where y is the yield of fresh fruit (kg tree−1) and x is the volume of irrigation water applied (m3 tree−1)), the authors identified the optimum seasonal water application (for fresh fruit yield) as being between 4.5 and 5.5 m3 tree−1 (270–330 mm). Yields were positively correlated with the number of fruits per tree.
Summary: water productivity
1. Experiments intended to quantify the yield response to water by mango trees have produced results of limited value due in part to poor design and/or because they were badly reported.
2. The long-term nature of the crop and the tendency for biennial bearing are added complications.
3. The range of tree densities (from 100 to >3000 trees ha−1) now used commercially adds another dimension of complexity to the challenge of determining water productivity.
4. The range of water productivities reported for fully irrigated crops extended from 3 to 5 kg (fresh fruit) m−3 (irrigation). The overall mean value was 4.3 kg m−3.
5. For deficit irrigated crops the spread was from 3 to 6 kg m−3. The overall mean was slightly higher than it was for well-watered crops (5.6 kg m−3) (excluding the results from Thailand; Table 1).
6. This difference in water productivity provides (very) limited evidence that deficit irrigation of mango may be worthwhile.
7. Only one experiment allowed yield responses to evapotranspiration and to transpiration to be determined.
Table 1. Water productivity (kg m−3) for mango based on irrigation water applied (I), evapotranspiration (ET) and transpiration (T). Please see text for further details of each experiment.

WATER MANAGEMENT
A number of different topics are included under this heading. These include irrigation methods, irrigation scheduling, salinity and intensification. Only research that is specific to mango is described here.
Irrigation methods
Any method of irrigation can be adapted for use in a mango orchard, but the tree density will strongly influence the final choice. In a modern orchard, drip and micro-sprinkers are likely to be the preferred options. In the deficit irrigation experiment reported above, Spreer et al. (Reference Spreer, Ongprasert, Hegele, Wünsche and Müller2009b) divided some of the plots into two halves, one of which was irrigated with drippers and the other half with micro-sprinklers. Yields of fruit and water productivities resulting from the two irrigation methods were similar. Lu (Reference Lu and Malik2006) reported that drip irrigation was not commonly used in mango orchards, farmers in Australia preferring under-tree micro-sprinklers.
Irrigation scheduling
In order to induce flowering, irrigation is usually withheld from mango trees from the end of the wet season in the low-altitude tropics (April in the southern hemisphere) until flowering. Irrigation then recommences when 75% of the canopy is in flower (Lu et al., Reference Lu, Müller and Chacko2000).
In Australia, Lu (Reference Lu and Malik2006) compared three irrigation scheduling methods in a field trial with mango trees:
(1) A control treatment, which was irrigated according to local authority recommendations.
(2) Irrigation determined by measurements made with a micro-dendrometer, which monitors changes in twig diameter.
(3) Soil water monitoring (with a capacitance probe). Actual water use was recorded by recording xylem sap flow.
A ‘shrinkage index’ based on the micro-dendrometer readings was found to be an excellent indicator of the onset of water stress. This was because a much higher proportion of the water applied to a tree was actually transpired (as opposed to evaporated) when the micro-dendrometer was acting as the indicator (shrinkage index = 77%) of when to irrigate. This compared with the values of 31% and 38% for the other two scheduling methods respectively. Monitoring sap flow was found to be a less sensitive indicator of when to irrigate (Lu, Reference Lu and Malik2006).
Lu (Reference Lu and Malik2006) recognised that both dendrometer method and sap flow measurements were far from being practical for growers to use for scheduling irrigation. Instead, a wetting front detector, developed in Australia and known as ‘FullStop’, was recommended to farmers (CSIRO, 2007).
Salinity
In a review of the sensitivity of crops to salinity, Ayers and Westcot (Reference Ayers and Westcot1985), using the best available information, classified mango, on a four-grade scale,Footnote 2 as being ‘sensitive’ to salinity. Based on the results of a four-year field experiment (1996–1999) in a mature mango orchard (12-year-old) in south-east Spain, this classification was later challenged by Zuazo et al. (Reference Zuazo, Raya and Ruiz2004). Their results suggested that mango was more tolerant of salinity than the analysis suggested by Ayers and Westcot (Reference Ayers and Westcot1985).
In this part of Spain, which includes the coastal provinces of Malaga and Granada, seawater intrudes into the ground water, especially in dry years. When this saline water is used for irrigation visible (and serious) damage occurs to the mango trees, the chloride ion being particularly harmful. In the experiment (Zuazo et al., Reference Zuazo, Raya and Ruiz2004) mango tree performance (cv. Osteen) was evaluated for two rootstocks (Gomera-1 and Gomera-3). The yield responses to four levels of water salinity (from 1.02 to 2.50 dS m−1) were compared, and the results were presented in the form of a salt-tolerance model as used by Mass and Hoffman (Reference Mass and Hoffman1977). This includes a threshold electrical conductivity value at which yield loss begins, followed by a linear regression the slope of which is a measure of the rate of yield decline as salt levels increase. Although there were small differences between the two rootstocks (Gomera-1 was slightly more tolerant than Gomera-3), one salt-tolerance model fitted both sets of data. For the first two years, the threshold electrical conductivity of the saturated soil extract (ECe) was 0.88 dS m−1, but this increased to 1.81 dS m−1 in years 3 and 4 of the experiment. The corresponding slopes were 17.1% yield loss, for each unit increase in ECe (r2 = 0.66, N = 48) and 12.5% (r2 = 0.76, N = 48). By extrapolation of the straight line, zero yields were predicted at ECe values of 6.75 and 9.78 dS m−1 respectively. With a leaching fraction of about 0.20, the corresponding values for the electrical conductivity of the irrigation water were 3.7 and 4.1 dS m−1. Based on the Mass and Hoffman (Reference Mass and Hoffman1977) model, these parameters are characteristic of a crop that is at the interface between being classified as moderately sensitive or moderately tolerant to salinity (Zuazo et al., Reference Zuazo, Raya and Ruiz2004).
Intensification
As like many tree crops, intensification is now the name of the game in the case of mango. Pioneering work in South Africa has highlighted some of the benefits that can result from ultra-high-density planting (Oosthuyse, Reference Oosthuyse2009). This means planting trees in hedgerows at a spacing of 3 m (between rows) × 2 m (or even 1 m) between trees within a row. This corresponds to planting densities of 1666 trees ha−1 or 3330 trees ha−1. This is very different from the traditional 10 × 10 m spacing (100 trees ha−1) or even 10 × 5 m (200 trees ha−1). In the ultra-high densities, it is important to restrict the size of the canopy, and to control canopy shape, by selective branch removal, and to keep the height of the trees below 2 m to facilitate easy manual harvesting of the fruit (and spraying). Other advantages of high-density planting include a reduction in the time taken from planting for the trees to reach the optimum canopy cover (therefore fewer weeds), and to come into full production (Oosthuyse, Reference Oosthuyse2009). The system lends itself to drip irrigation and fertigation. This system of production is now being promoted in India (see the video: Sharma, Reference Sharma2012), where the emphasis is on the production of uniform high-quality fruit for export. The trees are mulched with coconut coir waste or with black plastic for weed control and water conservation.
Summary: water management
1. In the tropics, after the induction of flowering following a period of water stress, irrigation recommences when 75% of the canopy is in flower.
2. Micro-sprinklers and drip irrigation are probably the two most effective ways of irrigating mango.
3. A micro-dendrometer (which measures the diameter of a twig) has been successfully used to monitor the onset of water stress in mango, but is not suitable for scheduling irrigation commercially.
4. Mango is moderately sensitive/moderately tolerant of salinity. Rootstocks may differ in their sensitivity to salinity.
5. Intensification of mango production will increase the need for irrigation and affect the way the crop is managed.
CONCLUSIONS
Less than 25 years ago, Rao and Chacko (Reference Rao and Chacko1989) wrote the following in a summary paper at a symposium on mango organised by the International Society for Horticultural Science (ISHS): ‘Studies on water relations in mango trees (are) a topic totally neglected so far and (their) effect(s) on various aspects of growth and development of the trees need attention’. The question that naturally follows is ‘how much progress has been made since then?’
Some progress has been made in our understanding of the flowering process, specifically the role of water in the initiation of flowering of mango in the tropics, although the mechanisms responsible have yet to be fully understood. Similarly, some progress has been made in our understanding of the processes of gas exchange, and the sensitivity of stomata (conductance and photosynthesis) to the dryness of the air. The sap flow method has proved to be a useful way of measuring transpiration of a mango tree, and attempts have been made to monitor water use in the orchard using a range of techniques. Unfortunately, these experiments (and others on the water relations of mango) have not always been well reported. Some progress has therefore been made, but it is probably not something that the commercial grower would recognize as being helpful in the short/medium term. The big change is the intensification of production, specifically the increases in tree density. This will impact on the water relations and irrigation requirements of mango, and should be the focus of future research on this topic.