INTRODUCTION
In tea trade, African black teas are classified as plain to medium flavoury. Such teas are valued for taste and colour characteristics; factors attributed to the non-volatile components of black tea. The black tea theaflavins contribute to the astringency (briskness) and brightness while thearubigins contribute to the colour and thickness (mouth-feel) of plain black tea (Biswas et al., Reference Biswas, Biswas and Sarkar1971; Reference Biswas, Biswas and Sarkar1973). The theaflavins and thearubigins are products of polyphenols oxidation during black tea processing (Davis et al., Reference Davis, Lewis, Cai, Powell, Davis, Wilkins, Pudney and Clifford1997; Jhoo, Reference Jhoo, Shahidi and Ho2007). The polyphenols, (flavanols, flavonol glycosides, polyphenolic acids and depsides) make up to between 30% and 40% of the dry weight in tea shoots (Harbowy and Balentine, Reference Harbowy and Balentine1997). Successful relationships have been demonstrated between total theaflavins levels of Central and Southern African plain black teas and sensory evaluations or prices (Ellis and Cloughley, Reference Ellis and Cloughley1981; Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973; Wright et al., Reference Wright, Mphangwe, Nyirenda and Apostolides2002). Consequently, total theaflavins levels were suggested as objective quality parameter for plain black teas (Ellis and Cloughley, Reference Ellis and Cloughley1981). Such relationships were positive but insignificant for Kenya (Owuor et al., Reference Owuor, Reeves and Wanyoko1986) and Sri Lanka (Roberts and Fernando, Reference Roberts and Fernando1981). When the contributions of the individual theaflavins were normalised to account for their differences in astringencies, the normalised factor, theaflavin digallate equivalent, showed good relationship with sensory evaluation for both Kenyan and Central/Southern African black teas (Owuor et al., Reference Owuor, Obanda, Nyirenda, Mphangwe, Wright and Z.2006). This confirmed that theaflavins are indeed black tea quality parameter. Although high polyphenols levels in green tea leaf were assumed to lead to high black tea levels of theaflavins and thearubigins (Erturk et al., Reference Erturk, Ercisli, Sengul, Eser, Haznedar and Turan2010; Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973; Yao et al., Reference Yao, Caffin, D’Arcy, Jiang, Shi, Singanusong and Xu2005), the composition of the polyphenols has been shown to be more critical to black tea quality than total polyphenols per se. The flavan-3-ols levels and composition successfully predicted black tea quality (Owuor and Obanda, Reference Owuor and Obanda2007; Wright et al., Reference Wright, Mphangwe, Nyirenda and Apostolides2000).
The flavan-3-ols, comprising of (+)-catechin (C), epicatechin (EC), epicatechin gallate (ECG), gallic acid (GA), epigallocatechin (EGC) and epigallocatechin gallate (EGCG) dominate green leaf polyphenols (Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973). The simple catechins (dihydroxyflavan-3-ols) undergo oxidative dimerization with the gallocatechins (trihydroxyflavan-3-ols) to produce theaflavins (Table 1). In Central and Southern African black teas, high levels of EC and EGC (Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973) and EC and ECG levels (Wright et al., Reference Wright, Mphangwe, Nyirenda and Apostolides2000) were associated with high quality black teas. But in Kenya, high EGCG and low EC levels were indicators of high black tea quality potential of clonal tea bushes (Owuor and Obanda, Reference Owuor and Obanda1997; Reference Owuor and Obanda2007; Owuor et al., Reference Owuor, Obanda, Nyirenda, Mphangwe, Wright and Z.2006). The green leaf EGCG levels correlated significantly with black tea total theaflavin, liquor brightness and sensory evaluation, while EC correlated positively with thearubigins and negatively with theaflavin digallate equivalent and sensory evaluation (Owuor and Obanda, Reference Owuor and Obanda2007). The sum of gallated flavan-3-ols (flavan-3-gallates), trihydroxyflavan-3-ols (gallocatechins) and ratios of trihydroxyflavan-3-ols:dihydroxyflavan-3-ols in green tea leaf predicted plain black tea quality potential (Owuor and Obanda, Reference Owuor and Obanda2007). Indeed, flavan-3-ols composition was a better quality indicator than total polyphenols or total flavan-3-ols (Owuor and Obanda, Reference Owuor and Obanda1997; Reference Owuor and Obanda2007). These results demonstrated the importance of the flavan-3-ols composition and levels as indicators of black tea quality potential of green tea leaf. Large clonal differences were observed in the distribution of the flavan-3-ols in green leaf and individual theaflavins in Kenyan (Owuor et al., Reference Owuor, Obanda, Nyirenda, Mphangwe, Wright and Z.2006) and Southern and Central African plain black teas (Wright et al., Reference Wright, Mphangwe, Nyirenda and Apostolides2002). Earlier, it was suggested that sources of plain teas could be predicted from the distribution pattern of the individual theaflavins (McDowell et al., Reference McDowell, Feakes and Gay1991). The composition and/or levels of the flavan-3-ols from which the individual theaflavins arise (Table 1) could therefore be dependent on location of production. Results from Kenya showed that the composition of the individual theaflavins were dependent on cultivars (Owuor and Obanda, Reference Owuor and Obanda1997). For cultivars grown in one location, the flavan-3-ol composition pattern was clonal specific at a set plucking standard (Magoma et al., Reference Magoma, Wachira, Obanda, Imbuga and Agong’2000) suggesting their composition could be genetically controlled. The pattern further remained constant although the levels varied in the same cultivars subjected to uniform agronomic inputs in different locations (Cherotich et al., Reference Cherotich, Kamunya, Alakonya, Msomba, Uwimana, Wanyoko and Owuor2013; Kwach et al., Reference Kwach, Owuor, Kamau, Wanyoko and Kamunya2013). However, it was not known if varying agronomic inputs on same cultivar could change the levels and composition of the flavan-3-ols.
Table 1. Formation of theaflavins from flavan-3-ols.

Nitrogenous fertilizer application is the most costly agronomic input in tea production, after harvesting (Bonheure and Willson, Reference Bonheure, Willson, Willson and Clifford1992). The expense is justified as nitrogen application increases yields (Msomba et al., Reference Msomba, Kamau, Uwimana, Muhoza and Owuor2014) though high rates reduces black tea quality (Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973; Owuor et al., Reference Owuor, Othieno, Horita, Tsushida and Murai1987b) even in the same cultivar under same management practices at various locations (Owuor et al., Reference Owuor, Kamau, Kamunya, Msomba, Jondiko and Uwimana2013). But it is not known if the variations were due to changes in polyphenols levels or composition of the flavan-3-ols in green tea leaf. Caffeine is an important black tea quality parameter (Ashihihara and Crozier, Reference Ashihihara and Crozier2001; Spiller, Reference Spiller1998). At a single site, caffeine levels increased with nitrogenous fertilizer (Owuor et al., Reference Owuor, Othieno, Horita, Tsushida and Murai1987b), varied with year of prune (Owuor and Lang’at, Reference Owuor and Lang’at1988), season (Yao et al., Reference Yao, Caffin, D’Arcy, Jiang, Shi, Singanusong and Xu2005) and location of production (Akhlas et al., Reference Akhlas, Ahmad, Siyar and Khanum2003). However, it is not known if the extent in caffeine increase with nitrogenous fertilizer rates varies with location of production. The objective of this research was to determine whether the composition of flavan-3-ols varies with location, and whether there is an interaction between nitrogen fertilizer and location on levels of caffeine and polyphenols in the Eastern Africa tea growing regions.
MATERIALS AND METHODS
Experimental set up
Clone TRFK 6/8 is the most widely grown tea variety in Eastern Africa, constituting 80% of Rwanda tea, 60% of Kenya clonal tea and 35–40% of Tanzania tea (Kwach et al., Reference Kwach, Kamau, Msomba, Muhoza and Owuor2014; Msomba et al., Reference Msomba, Kamau, Uwimana, Muhoza and Owuor2014). A fertilizer trial on clone TRFK 6/8 was set in seven tea estates in the tea growing locations of Eastern Africa in 2008 (Table 2). At each site, nitrogenous fertilizer rates (0, 75, 150, 225 and 300 kg N ha−1 year−1) as NPKS 25:5:5:5 trial was laid out in a Randomized Complete Block Design replicated 3 times (Kwach et al., Reference Kwach, Kamau, Msomba, Muhoza and Owuor2014; Msomba et al., Reference Msomba, Kamau, Uwimana, Muhoza and Owuor2014). Each plot comprised 30 tea plants of clone TRFK 6/8. The plants at each site were pruned between April and May 2008 before the first fertilizer treatment applications so that all the plants were in same pruning cycle. The first fertilizer treatments were applied in September/October 2008, depending on the onset of rain at the individual site. Subsequently, fertilizer was applied annually as single dose in September. The plots were plucked on a 14 days harvesting intervals.
Table 2. The study sites coordinates and altitude in metres above mean sea level (m amsl).

Leaf sampling, extraction and HPLC analysis of flavan-3-ols
In May 2012, leaf (100 g) samples were collected by random hand plucking of two leaves and a bud for the determination of caffeine and polyphenols contents. The leaf was steamed for 1 min, and then dried in an oven at 80 °C to a constant weight. The dried leaf was cooled and crushed to a powder (Owuor and Obanda, Reference Owuor and Obanda2007). About 125 mg of the powder from each sample was extracted in 25 mL acetonitrile water (1:1 v/v) mixture at room temperature for 30 min with constant shaking. Total polyphenols was determined as outlined in the International Organization for Standardization method (ISO-14502-1, 2005). Flavan-3-ols and caffeine levels were determined using a solvent gradient Shimadzu HPLC with UV detector as described in the International Organization for Standardization (ISO-14502-2, 2005) method. The statistical analyses were done using MSTAC as a split plot design, with locations as main treatment split for rates of nitrogen.
RESULTS
There was good baseline HPLC chromatogram resolution for caffeine and the individual flavan-3-ols (Figure 1). Caffeine levels varied significantly (p ≤ 0.05) with locations (Table 3), with Rwanda sites recording the highest levels, while sites in Tanzania had the lowest levels. At all locations, caffeine increased significantly (p ≤ 0.05) with rise in nitrogen rates. There were no significant interactions effects between location of production and nitrogen rates on caffeine levels in tea.

Figure 1. HPLC profile of green tea leaf caffeine and favan-3-ols at 278 nm.
Table 3. Variations in caffeine levels (mg g−1 DM) in 2 + a bud of clone TRFK 6/8 with location of production and nitrogen fertilizer rates.

Significant (p ≤ 0.05) changes in total polyphenols (Table 4), simple catechins (dihydroxyflavan-3-ols) (C, EC, and ECG (epicatechin gallate) (Table 5), GA and EGC (Table 6), trihydroxy:dihydroxyflavan-3-ols ratio, gallated:non gallated flavan-3-ols (flavan-3-gallates: flavan-3-ols) ratio (Table 7), total catechins, total flavan-3-ol gallic acid esters and simple (non-ester) flavan-3-ols (Table 8) in green leaf of clone TRFK 6/8 were recorded due to location of production and nitrogen fertilizer rates. The Kenya sites recorded higher (p ≤ 0.05) values of total polyphenols, EGCG, total gallocatechins, gallated:non gallated catechins, total catechins and total gallated catechins than in Tanzania and Rwanda sites which were close.
Table 4. Variations in total polyphenols levels with location of production and nitrogenous fertilizers rates (% gallic acid equivalent (GAE)).

Table 5. Variations in clone TRFK 6/8 dihydroxyflavan-3-ols levels (mg g−1 DM) with location of production and rates of nitrogenous fertilizer.
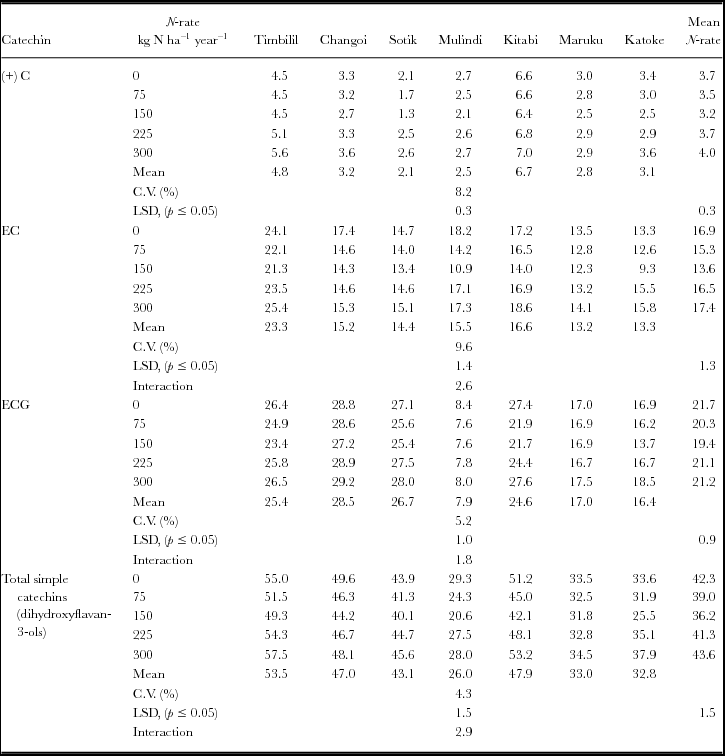
Table 6. Variations in trihydroxyflavan-3-ols levels (mg g−1) DM with location of production and nitrogen rates.
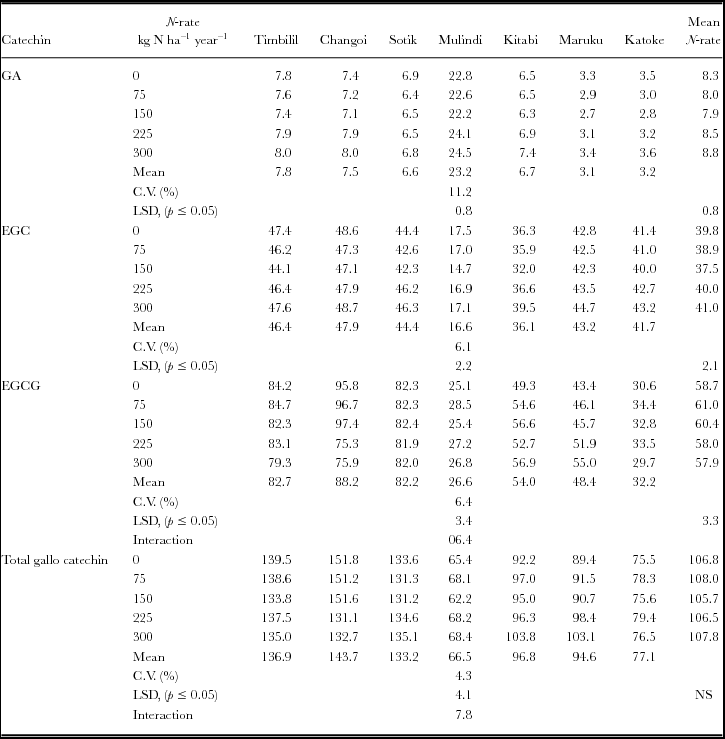
Table 7. Variations in flavan-3-ols ratios with location of production and N rates.

Table 8. Variations in sum of flavan-3-ols (mg g−1 DM) with location of production and nitrogen rates.

Generally, the total polyphenol (Table 4), dihydroxyflavan-3-ols (Table 5), trihydroxyflavan-3-ols (Table 6), and total flavan-3-ols and flavan-3-gallate (Table 8) levels decreased with rates of nitrogen up to 150 kg nitrogen ha−1 year−1. Above this fertilizer rate, there were increases in these parameters with nitrogenous fertilizer rates. For total polyphenol, dihydroxyflavan-3-ols and trihydroxyflvan-3-ols, this pattern was clearer in Timbilil, Mulindi, Kitabi and Katoke. But in Changoi, there was decline in levels of total polyphenols up to beyond 225 kg nitrogen ha−1 year−1, while in Maruku the levels increased with increase in rates of nitrogen fertilizer. The gallated:non gallated flavan-3-ols and trihydroxy/dihydroxy falvan-3-ol ratios (Table 7) however, showed an inverse response curve. There were positive quadratic responses peaking up at about 150 kg nitrogen ha−1 year−1 and thereafter declining. In Maruku, there were linear increases in the two ratios with rise in nitrogen fertilizer rates. EGCG, however, showed different patterns (Table 6). In Kenya, the levels decreased with increase of nitrogen rates while in Rwanda and Tanzania sites the patterns were not clear. Although all parameters changed significantly (p ≤ 0.05), due to nitrogenous fertiliser rates, the changes in total trihydroxyflavan-3-ols (Table 6) were not significant. The extent of the variations in these parameters due to nitrogenous fertilizer rates changed with location of production, causing significant (p ≤ 0.05) interactions effects between nitrogen rates and location of production.
DISCUSSION
Caffeine in tea is a stimulant (Bokuchava and Skobeleva, Reference Bokuchava and Skobeleva1969) and plays an important role in cream formation in black tea (Roberts, Reference Roberts and Geissman1962). The levels of green leaf caffeine at different locations were in similar range to those in clones grown in different locations in Kenya (Kwach et al., Reference Kwach, Owuor, Kamau, Wanyoko and Kamunya2013) and Northeast India black teas (Dev Choudhury et al., Reference Dev Choudhury, Rahman and Barbora1991). The variation in black tea quality as measured by caffeine is therefore partly dependent on location of production in Eastern Africa.
Similar increases in caffeine levels with increase in nitrogenous fertilizer rates had been recorded in black teas at single locations (Owuor et al., Reference Owuor, Othieno, Horita, Tsushida and Murai1987b) and at different agro ecological zones in Pakistan (Akhlas et al., Reference Akhlas, Ahmad, Siyar and Khanum2003). Thus, irrespective of region of production, increase in nitrogen fertilizer rates enhances caffeine levels in tea leaves. Cultural practices such as year of prune (Owuor and Lang’at, Reference Owuor and Lang’at1988), plucking standards (Owuor et al., Reference Owuor, Obanda, Othieno, Horita, Tsushida and Murai1987a), plucking intervals (Owuor and Odhiambo, Reference Owuor and Odhiambo1994) and season of production (Yao et al., Reference Yao, Caffin, D’Arcy, Jiang, Shi, Singanusong and Xu2005) previously influenced caffeine levels in tea produced at single sites. Agronomic inputs are therefore critical in the determination of levels of caffeine in tea. The lack of significant interactions effects between nitrogen fertilizer rates and locations of production demonstrated that the patterns of changes in caffeine levels due to nitrogen fertilizer rates were not influenced by environmental factors at location of production, or nitrogen fertilization.
The levels of all polyphenols were within the limits reported in clonal tea grown in Kenya (Cherotich et al., Reference Cherotich, Kamunya, Alakonya, Msomba, Uwimana, Wanyoko and Owuor2013; Kwach et al., Reference Kwach, Owuor, Kamau, Wanyoko and Kamunya2013). The green tea leaf total polyphenols (Table 4) and flavan-3-ols levels (Tables 5, 6 and 8) varied (p ≤ 0.05) due to location of production and nitrogenous fertilizer rates. These variations may in part explain the previously observed black tea quality changes with location of production (Owuor et al., Reference Owuor, Kamau and Jondiko2010a) and nitrogen fertilizer rates in Kenya (Owuor et al., Reference Owuor, Wachira and Ng’etich2010b; Reference Owuor, Kamau, Kamunya, Msomba, Jondiko and Uwimana2013) and Pakistan (Akhlas et al., Reference Akhlas, Ahmad, Siyar and Khanum2003). The quality variations were therefore in part arising from the composition of the precursor compounds as demonstrated herein. These variations (p ≤ 0.05) in total polyphenols and flavan-3-ols with location of production show that the potential of clone TRFK 6/8 to make black tea of high quality varies with locations even when agronomic inputs are identical. Black tea quality variations with location of production had been attributed to several factors including soil types, soil fertility (Bonheure and Willson, Reference Bonheure, Willson, Willson and Clifford1992), temperatures (Tanton, Reference Tanton1982), rainfall and rainfall distribution (Othieno et al., Reference Othieno, Stephens and Carr1992) and altitudes (Mahanta et al., Reference Mahanta, Baruah, Owuor and Murai1988). These factors could be partly responsible for the variations in the polyphenols observed in this study.
The levels and composition of the dihydroxy- and trihydroxyflavan-3-ols control the composition of the individual theaflavins (Owuor and Obanda, Reference Owuor and Obanda2007; Owuor et al., Reference Owuor, Obanda, Nyirenda, Mphangwe, Wright and Z.2006). The relative astringencies of the four predominant theaflavins in black tea i.e. theaflavin digallate, theaflavin-3-gallate, theaflavin-3′-gallate and theaflavin, are 6.4:2.2:2.2:1, respectively (Sanderson et al., Reference Sanderson, Ranadive, Eisenberg, Farrel, Simon, Manley and Coggon1976). High ECG and EGCG content in fresh leaf lead to the formation of high amounts of gallated theaflavins levels in black tea (Madanhire, Reference Madanhire1995), a parameter associated with higher black tea quality (Owuor and Obanda, Reference Owuor and Obanda1997; Reference Owuor and Obanda2007). The total theaflavins per se may not be critical to plain black tea quality estimation as the ratios and sum of the individual theaflavins (Owuor et al., Reference Owuor, Obanda, Nyirenda, Mphangwe, Wright and Z.2006; Wright et al., Reference Wright, Mphangwe, Nyirenda and Apostolides2002). Total trihydroxyflavan-3-ols and EGCG levels in green tea leaf were indicators of Kenyan black tea quality (Owuor and Obanda, Reference Owuor and Obanda2007; Owuor et al., Reference Owuor, Obanda, Nyirenda, Mphangwe, Wright and Z.2006) while total theaflavins in black tea and EGC in green leaf were Central Africa black tea quality indicators (Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973; Wright et al., Reference Wright, Mphangwe, Nyirenda and Apostolides2000). Tea leaf combining high flvan-3-ol levels with high trihydroxy:dihydroxy flavan-3-ols ratio have potential to produce high quality black tea quality through formation of high amounts of theaflavins (Owuor and Obanda, Reference Owuor and Obanda2007; Owuor et al., Reference Owuor, Obanda, Nyirenda, Mphangwe, Wright and Z.2006). Since trihydoxyflavan-3-ols have lower redox potentials than dihydroxyflavan-3-ols (Bajaj et al., Reference Bajaj, Anan, Tsushida and Ikegaya1987), low levels of trihydroxyflavan-3-ols may limit formation of theaflavins.
It had been anticipated that the ratio gallated:non gallated flaval-3-ols would be constant as formation of the flavan-3-ols were claimed to be genetically controlled (Magoma et al., Reference Magoma, Wachira, Obanda, Imbuga and Agong’2000). However, the recent literature (Cherotich et al., Reference Cherotich, Kamunya, Alakonya, Msomba, Uwimana, Wanyoko and Owuor2013; Kwach et al., Reference Kwach, Owuor, Kamau, Wanyoko and Kamunya2013) and these results demonstrate that the flavan-3-ols levels vary widely with location of production in a single cultivar. Environmental conditions of production is therefore a major factor controlling flavanols formations and hence the quality of black tea. The variations in the polyphenols, especially flavan-3-ols levels and flvan-3-ols ratios with location of production observed show that even with a single cultivar, it may not be possible to produce same quality black tea in different locations, further supporting previous observations and conclusion on clonal black teas (Owuor et al., Reference Owuor, Obanda, Nyirenda and Mandala2008; Reference Owuor, Kamau and Jondiko2009; Reference Owuor, Kamau and Jondiko2010a; Reference Owuor, Wachira and Ng’etich2010b; Wright et al., Reference Wright, Mphangwe, Nyirenda and Apostolides2000).
Variations in black tea quality due to nitrogen fertilizer rates at single sites have been widely documented (Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973; Owuor et al., Reference Owuor, Kamau, Kamunya, Msomba, Jondiko and Uwimana2013; Venkatesan and Ganapathy, Reference Venkatesan and Ganapathy2004; Venkatesan et al., Reference Venkatesan, Murugesan, Ganapathy and Verma2004). Similar variations were observed in the polyphenols (Tables 4–8) at different locations. Increasing nitrogen fertilizer rates reduced black tea quality both at single locations (Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973; Owuor et al., Reference Owuor, Othieno, Horita, Tsushida and Murai1987b) and in various locations (Akhlas et al., Reference Akhlas, Ahmad, Siyar and Khanum2003; Owuor et al., Reference Owuor, Kamau and Jondiko2010a; Reference Owuor, Kamau, Kamunya, Msomba, Jondiko and Uwimana2013). Total theaflavins and thearubigins levels declined with increase in nitrogen fertilizer rates at a single site in clone TRFK 6/8 (Owuor and Odhiambo, Reference Owuor and Odhiambo1994) and in clone BBK 35 at different locations (Owuor et al., Reference Owuor, Kamau, Kamunya, Msomba, Jondiko and Uwimana2013) in Kenya. In clone AHP S15/10 in Kenya, such decline was only up to 159 kg nitrogen ha−1 year−1 but thereafter increased with rise in the fertilizer rates (Owuor et al., Reference Owuor, Othieno, Horita, Tsushida and Murai1987b). Similar decline in flavan-3-ols (Tables 4–6, 8) with increasing nitrogenous fertilizer rates up to 150 kg nitrogen ha−1 year−1 were observed. Although these results contradict observations from India where theaflavins, thearubigins (Venkatesan and Ganapathy, Reference Venkatesan and Ganapathy2004), total polyphenols and flavan-3-ols (Venkatesan et al., Reference Venkatesan, Murugesan, Ganapathy and Verma2004) increased with rise in nitrogen fertilizer rates, the results demonstrate that pattern of change in flavan-3-ols production with rates of nitrogen direct the formation of theaflavins and thearubigins.
Application of above 180 nitrogen ha−1 year−1 reduced dihydroxyflavan-3-ols in Pakistan (Akhlas et al., Reference Akhlas, Ahmad, Siyar and Khanum2003) and (–)-EGC and (–)-EGCG levels in Malawi (Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973). Results presented herein contradict these observations as levels of EGC and other flavan-3-ols declined up to 150 kg N ha−1 year−1, except EGCG, that increased linearly with increasing rates of nitrogen. Indeed total flavan-3-ols increased at higher rates of nitrogen fertiliser, while total flavan-3-ol gallates declined. The contradicting results may be attributed to differences in growing conditions and/or cultivars used in the studies. However, the pattern of the variations in the trihydroxy:dihydroxyflavan-3-ols and gallated:non gallated flavan-3-ols ratios due to nitrogenous fertilizer rate (Table 7) explain the earlier reports (Hilton et al., Reference Hilton, Palmer-Jones and Ellis1973; Owuor et al., Reference Owuor, Othieno, Horita, Tsushida and Murai1987b; Venkatesan and Ganapathy, Reference Venkatesan and Ganapathy2004) in which high rates of nitrogenous fertilizer reduced black tea quality. The reduction in plain black tea quality at higher nitrogen rates may be as a result of depression of gallated flavan-3-ols and enhanced levels of non gallated flavan-3-ols that would lead to lower levels of theaflavin digallate equivalent, a reliable black tea quality parameter (Owuor and Obanda, Reference Owuor and Obanda1997; Reference Owuor and Obanda2007; Owuor et al., Reference Owuor, Obanda, Nyirenda, Mphangwe, Wright and Z.2006).
The extents of variations in the polyphenols due to nitrogen fertilizer rates changed with locations causing significant (p ≤ 0.05) interactions effects. Thus, even in the same clone, nitrogenous fertilizer rate that ensures optimum black tea quality may be region specific within the tea growing regions of Eastern Africa. In a recent study (Msomba et al., Reference Msomba, Kamau, Uwimana, Muhoza and Owuor2014) optimal nitrogen fertilizer rate for realization of optimal yield varied with location of production. The optimum nitrogen fertiliser rate for production of high quality black tea and yields may therefore be region specific. The response in polyphenols production in Maruku Estate did not follow the same pattern as responses at the other sites. The differences were noted even between Maruku and Katoke that were within 30 km away from each other. Further investigations are necessary to understand the causes of this change in pattern of response.
In conclusion, caffeine and flavan-3-ols levels changed significantly (p ≤ 0.05) with location of production and nitrogen fertiliser rates. The extent of the responses to nitrogen rates varied with locations. It is recommended that region specific agronomic inputs, especially nitrogen rates need to be developed to ensure production of tea leaves with optimal green leaf precursor levels that culminate to production of high black tea quality.
Acknowledgements
We thank the Inter University Council for Eastern Africa (VicRes) and the National Council for Science and Technology, Kenya for financial support. The management of tea estates in which the trials were conducted are gratefully acknowledged.












