INTRODUCTION
Animal manure is a key input in agriculture, particularly under the constraints of organic certification. Besides its direct effect on crop production as a nutrient source, manure also provides indirect benefits by enhancing the soil organic matter (SOM) pool and thus improving key soil properties, such as soil structure and, water and nutrient retention (Rasool et al., Reference Rasool, Kukal and Hira2008). Manure decomposition in the soil is mainly accomplished by soil microorganisms and is strongly determined by the composition of organic matter, the soil microbial community and environmental factors, such as temperature and moisture (Knacker et al., Reference Knacker, Förster, Römbke and Frampton2003; Wichern et al., Reference Wichern, Müller, Joergensen and Buerkert2004). The composition of animal manure is influenced by fodder composition and quality (Powell et al., Reference Powell, Wattiaux, Broderick, Moreira and Casler2006), which comprises not only nutrient contents and forms, but also secondary plant metabolites such as tannins (Mafongoya et al., Reference Mafongoya, Barak and Reed2000). Tannins belong to the chemical group of polyphenols and are present in many browse species around the world. In desert regions, tannin-containing browse species, such as Acacia gerrardii Benth. and Dodonaea viscosa Jacq., were found to be important feed sources in traditional animal husbandry (Schlecht et al., Reference Schlecht, Dickhoefer, Predotova and Buerkert2011). Although tannins are known to reduce feed digestibility and cause toxicities, at lower dosages tannins may have beneficial effects on animal health, feed utilisation and productivity (Waghorn, Reference Waghorn2008). In addition, the reduced feed digestibility and the shift of N excretion from urine to faeces by protein complexation and protection during digestion, lead to higher N concentrations and more stable N forms in faeces (Al-Kindi, Reference Al-Kindi2015; Makkar, Reference Makkar2003; Powell et al., Reference Powell, Fernández-Rivera and Hös1994).
In intensive irrigated agriculture of semi-arid and arid regions such as in Oman mineralization is particularly fast and nutrient losses via leaching and gaseous emissions can be severe (Siegfried et al., Reference Siegfried, Dietz, Schlecht and Buerkert2011). Under these conditions, the input of more stable N forms can be expected to release N slowly, thus benefitting crop production in the long term (Makkar, Reference Makkar2003). In addition, the protection of organic substances by tannins can contribute to improving soil properties, such as cation exchange capacity, soil structure and moisture retention (Mafongoya et al., Reference Mafongoya, Giller and Palm1998) and reducing nutrient losses via gaseous emission and leaching (Zingore et al., Reference Zingore, Mafongoya, Nyamugafata and Giller2003). On the other hand, slow N release or N immobilization may trigger N deficiencies in crops. Therefore the effects of the inclusion of tannin-containing feedstuff or feed additives in animal diets on the quality of animal manure need to be addressed. In an overview of leaf and litter characteristics and decomposition patterns of multipurpose agroforestry trees given by Mafongoya and Hove (Reference Mafongoya, Hove, Nair, Jose and Gordon2008), higher polyphenol contents and protein-binding capacities are often correlated with a reduced decomposition rate and N immobilization. While this has been shown for tannins from leaf litter and prunings directly applied to soils (Powell et al., Reference Powell, Fernández-Rivera and Hös1994), their effect on soil processes after passing the digestive system of animals has rarely been examined.
This study aimed to investigate the effect of tannins from a Quebracho extract (Schinopsis balansae Engl.), as a well-characterized tannin source with consistent quality, either fed to goats (QTf) or added to manure in a water suspension creating a QT-coat (QTc), on the turnover of goat manure under irrigated sub-tropical conditions using the litterbag method.
MATERIALS AND METHODS
Site description
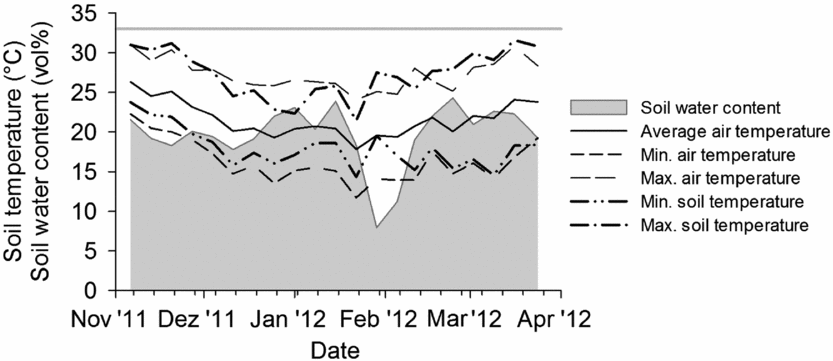
A litterbag experiment was conducted within a two-year field experiment on a private farm near Sohar (24°22′ N, 56°34′ E) in the Al-Batinah Plain of the Sultanate of Oman. Litterbags were installed in the experimental plots during two periods, from November 2011 to January 2012 under sweet corn (Zea mays L., var. Honey Jean No. 2) cultivation and from February 2012 to April 2012 under radish (Raphanus sativus L., var. Early 40 days) cultivation. Daily average air temperature during both periods was about 22 °C (Figure 1). The sandy soil of the flood-irrigated field was classified as a hyperthermic Typic Torrifluvent (US Taxonomy; Al-Farsi, Reference Al-Farsi2001) with pH 8.7 (H2O 1:2.5), a soil organic carbon (SOC) content of 8 mg g−1, total N of 0.4 mg g−1 and a C/N ratio of 19 (Ingold et al., Reference Ingold, Dietz, Sradnick, Joergensen, Schlecht and Buerkert2015a). Following plant demand the soil was irrigated every three to nine days during sweet corn cultivation and two to five days during radish cultivation, resulting in average soil moisture contents of 20% to 22% at 6-cm soil depth (ThetaProbe ML2x, Delta-T Devices, Cambridge, UK). Mirroring air temperatures, during the first period initial soil temperature at 6-cm depth ranged from 14 °C to 24 °C in the morning (at 6 a.m.) and from 21 to 31 °C at noon, while during the second period these ranges were 17 to 18 °C and 28 to 32 °C (Figure 1).
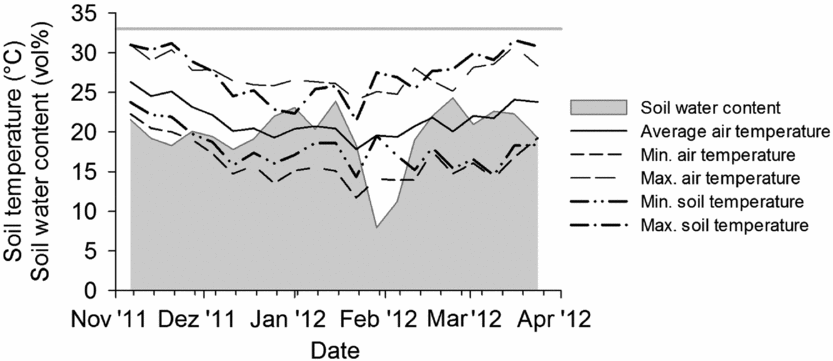
Figure 1. Weekly volumetric water content, air temperature and soil temperature (6 a.m. and 12 p.m. representing the coolest and hottest daytime during the two cropping periods) at the experimental site near Sohar, Sultanate of Oman. The grey line at 33% soil water content indicates the water holding capacity of the soil.
Manure treatments and experimental setup
The Quebracho tannin extract (QT; Schinopsis balansae, Silva Team S.p.a., San Michele, Italy) used in this study was either fed to 12 goats (QTf) or added to manure in a water suspension creating a coating (QTc) to compare its direct or indirect effects on manure mineralization. A non-amended goat manure collected from 36 goats served as control (Co). Manure was collected from male Jabal Akhdar goats (average body weight of 23 kg) kept in individual crates and fed a basal diet of 50% Rhodes grass hay (Chloris gayana Kunth), 47% maize and 3% soya (Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b). One group of goats received additionally 3.6% QT extract (QTf), the maximum that goats took up voluntarily. After an adaptation period, manure was collected on a daily basis and air-dried for five days until constant weight.
The water soluble QT extract had a total phenol concentration of 75%, of which 95% were classified as total tannins (mainly condensed tannins), and pHH2O 4.7. Quebracho tannin extract contained 53% C, 0.1% N, 0.03% P, 0.1% K and 2.4% Na. As the analysis of tannin in manure is difficult, the tannin content in manure was estimated by dividing the daily QT tannin intake by the daily faecal output, assuming that the amendments were not digested nor absorbed by the goats (Terrill et al., Reference Terrill, Waghorn, Woolley, Mc Nabb and Barry1994; Makkar, Reference Makkar2003). For QTc, a water suspension of QT was added to dry pure goat manure in an equivalent amount as estimated for QTf (11.4% QT extract). The solution was mixed well until it was completely attached to manure droppings, creating a QT coat, and air-dried again.
Litterbags containing the three described manure types were installed in the second year of a two-year completely randomized field experiment with four replications. The plots were set up as basins of 2.5 × 6.5 m with separate access for flood irrigation. Sweet corn and radish were cultivated consecutively. Applied manure was roto-tilled into the top 12 cm of the soil one day before sowing of sweet corn and radish or with rakes after emergence of plants (25 and 45 days after sowing of sweet corn). Application rates of manure were adjusted to 200 kg ha−1 of N for sweet corn and 135 kg ha−1 for radish with alternating application rates for the other nutrients depending on nutrient concentrations (Supplementary Table S1 available online at https://doi.org/10.1017/S0014479717000291). The total ash-free DM (OM) input was 7.9 Mg ha−1 for Co, 8.4 Mg ha−1 for QTf and 8.8 Mg ha−1 for QTc during sweet corn cultivation and 5.1 Mg ha−1 for Co, 5.5 Mg ha−1 for QTf and 5.7 Mg ha−1 for QTc during radish cultivation. Litterbags of 1-mm mesh and 15 × 20 cm size were installed horizontally in 10-cm depth within the corresponding manured plots two days after sowing of sweet corn and radish. To account for spatial variability, plots were split into two halves and one litterbag per sampling date was placed in each half. Distances between litterbags and plant rows were 10–20 cm. During sweet corn cultivation, 12 litterbags containing 27–30 g manure DM per litterbag (equivalent to 200 kg ha−1 N) were installed in each plot and recovered after 2, 4, 6, 8, 12 and 20 weeks (S2, S4, S6, S8, S12 and S20; n = 144). During radish cultivation six litterbags containing 18–20 g manure DM per litterbag (equivalent to 135 kg ha−1 N), were installed in each plot followed by a recovery after 2, 4, and 6 weeks (R2, R4, and R6; n = 72).
Litterbag sampling and analysis
At each sampling date, two litterbags per plot were picked randomly from each split area and extracted from the soil. Samples were cleared of bigger soils particles, roots and termites, and placed in dark paper bags before air drying to constant weight. As mineral soils contain only a small portion of OM, ash content is often set equivalent to soil contamination accepting a slight underestimation (Potthoff and Loftfield, Reference Potthoff and Loftfield1998). Results are therefore based on ash-free dry matter, representing the OM. In addition, the nutrient content of the samples was corrected for contamination from soil by subtracting the product of ash content and the nutrient concentration of adherent soil. The relative C and nutrient releases were calculated relating their contents in the litterbag samples to the initial C and nutrient contents of manure. For the small amounts of remaining root parts and dead termites in the samples, no such correction could be made which may have led to slight overestimations of remaining OM and nutrients. As all treatments were affected by roots and termites, a comparison between the treatments seems to be admissible. The presence of the subterranean termite Microcerotermes diversus Silvestri and/or its foraging signs were noticed. To estimate the impact of termite activity on OM disappearance, samples showing signs of the termite activity were statistically compared with samples without any termite activity, regardless of the treatment.
Dried manure samples were weighed, pooled per plot and sampling date, ground, ashed and extracted with 32% HCl for the determination of P, K and Na. Phosphorus was measured colourimetrically (Hitachi U-2000 spectrophotometer, Hitachi Ltd., Tokyo, Japan), and K and Na by flame photometry (Instrument Laboratory 543, Instrumentation Laboratory, Bedford, MA, USA). Total C and N concentrations were determined by means of a CN analyser (VarioMax® CHN, Elementar Analysensysteme GmbH, Hanau, Germany). The data of the remaining OM was fitted to a negative exponential model, widely used for decomposition studies (Olson, Reference Olson1963):
where OMt represents the remaining OM at the time t in days and k is the decomposition rate constant. This model is based on the assumption that all fractions of the OM are decomposed at the same rate, irrespective of their specific decomposability. In comparison to this model, an asymptotic model was used. This model is based on the assumption that the OM is made up of a decomposable compartment (A), which degrades at a constant rate k’ and a recalcitrant compartment (100 – A) that is usually used to describe long-term decay processes (Kurz-Besson et al., Reference Kurz-Besson, Coûteaux, Thiéry, Berg and Remacle2005)
Statistics
Results are presented as arithmetic means with their respective standard deviations or standard errors. The analysed parameters (remaining OM, C and nutrient concentrations, their relative and absolute release as well as the C/N ratio) were statistically analysed with ANOVA and repeated measures ANOVA followed by Tukey HSD test at p < 0.05. Prior to statistical analysis, data were screened for outliers and winsorised with the next located value. The data were analysed for homogeneity of variances and normal distribution of residuals. The Games–Howell test was used when homogeneity of variances was violated. Where normal distribution of residuals was not given, the non-parametric Kruskal–Wallis test was employed for single sampling dates. When the assumption of sphericity tested with Mauchly's test was significantly violated, the Greenhouse–Geisser correction was applied. For all statistical analysis, the software package SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) was used.
RESULTS
Remaining organic matter
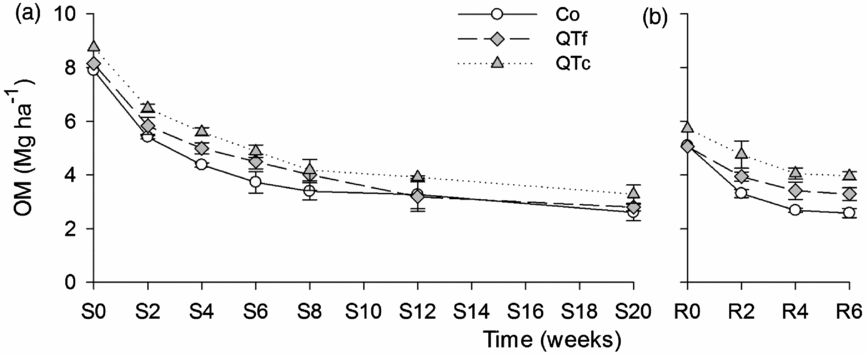
Across all treatments, the OM disappearance was highest during the first two weeks after litterbag installation, with about 30% of OM removed from litterbags during both sweet corn and radish cultivation (Figures 2a and b). After six weeks, up to 54% of the OM had disappeared under both crops and up to 64% after 12 weeks under sweet corn. The repeated measure ANOVA revealed significant time and treatment effects on the remaining OM under sweet corn [F(1.9, 17.4) = 92.09, p <0.001 and F(2, 9) = 124.38, p <0.001, respectively], and under radish [F(2, 18) = 27.2, p <0.001 and F(2, 9) = 128.59, p <0.001, respectively]. Even though the assumption of the normal distribution of residuals was violated on sampling dates S2, S4, R2 and R4, additionally conducted non-parametric Kruskal–Wallis tests revealed significant treatment effects [H(2) = 8.0–9.3, p <0.05]. The remaining OM under sweet corn for QTc was 20–26% higher than for the other two treatments (p <0.001), whereas under radish QTf had 14% (p <0.01) and QTc 51% (p <0.001) more remaining OM compared with Co.

Figure 2. Remaining OM in litter bags (Mg DM ha−1) under the cultivation of sweet corn (a) and radish (b) at the experimental site near Sohar, Sultanate of Oman, sampled in 2- to 8-week intervals (n = 4). Symbols represent means, whiskers represent ± one standard error.
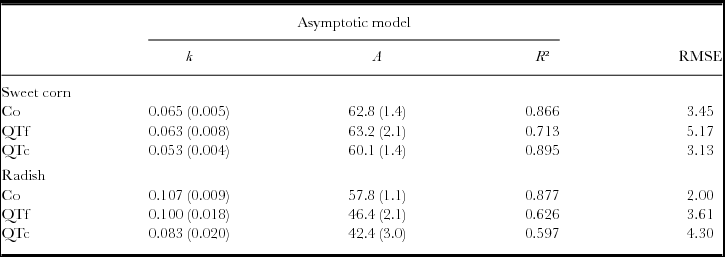
The negative exponential model did not give a good fit to the remaining OM. In contrast, the asymptotic model resulted in R² values between 0.7 and 0.9, and 0.6 and 0.9 for the remaining OM data, collected during sweet corn cultivation and radish cultivation, respectively (Table 1). Under sweet corn, the decomposable compartments A and the decay constants k of Co and QTf were similar, whereas QTc had a 18% smaller decay constant than Co with similar A. Under radish, A was reduced in QTf and QTc by 27% and 14%, respectively, compared with the control, whereas the decay constant was only reduced by 22% for QTc (Table 1).
Table 1. Parameters of the asymptotic model fits on remaining OM during 12 weeks of sweet corn cultivation (n = 25) and 6 weeks of radish cultivation (n = 13) in a litterbag experiment on an experimental field near Sohar, Sultanate of Oman.
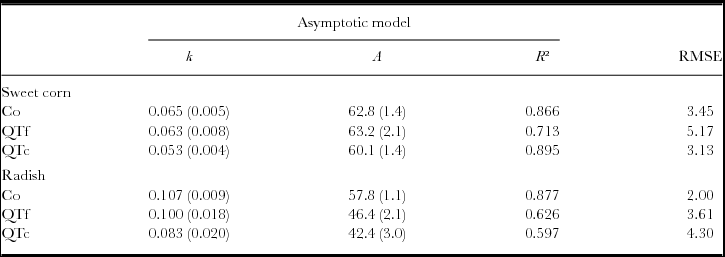
Numbers in brackets represent standard errors, the decay constant k is expressed in days−1, A represents the decomposable compartment and RMSE refers to the Root-Mean-Square Error.
Carbon and nutrient concentrations
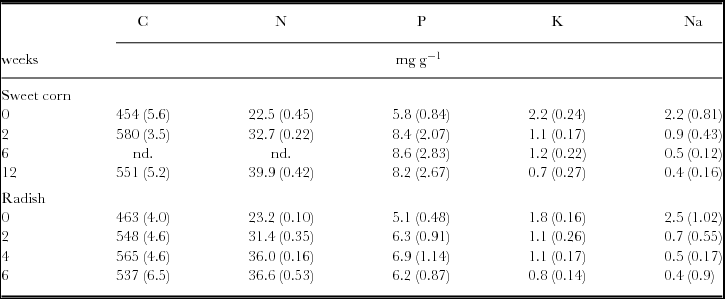
To show the general trend for C and nutrient concentrations during the course of the experiment, results were averaged across all treatments for each sampling date under sweet corn and radish (Table 2). Carbon and nutrient concentrations significantly changed during the course of the experiment (p-values ranging from 0.001 to 0.03), with two exceptions. While C concentrations changed significantly during sweet corn cultivation (p <0.05), no significant time effect was observed (p >0.4) under radish. On the contrary, P was unchanged under sweet corn (p >0.4), whereas it significantly increased (p <0.01) under radish. While C and P concentrations increased within the first two to four weeks by 22–28% and 35–48%, respectively, and declined slightly thereafter, N concentration continuously increased (up to 58–77%) until the end of the sampling period (Table 2). K and Na concentrations strongly dropped within the first two weeks (by 40–70%) and remained relatively constant or declined slightly until the end of each sampling period.
Table 2. Carbon and nutrient concentrations of litterbag samples (n = 20) during sweet corn (12 weeks) and radish cultivation (6 weeks) averaged across all treatments at the experimental field near Sohar, Sultanate of Oman.

Nd: Not determined. Standard deviation is given in brackets.
Carbon and nutrient release
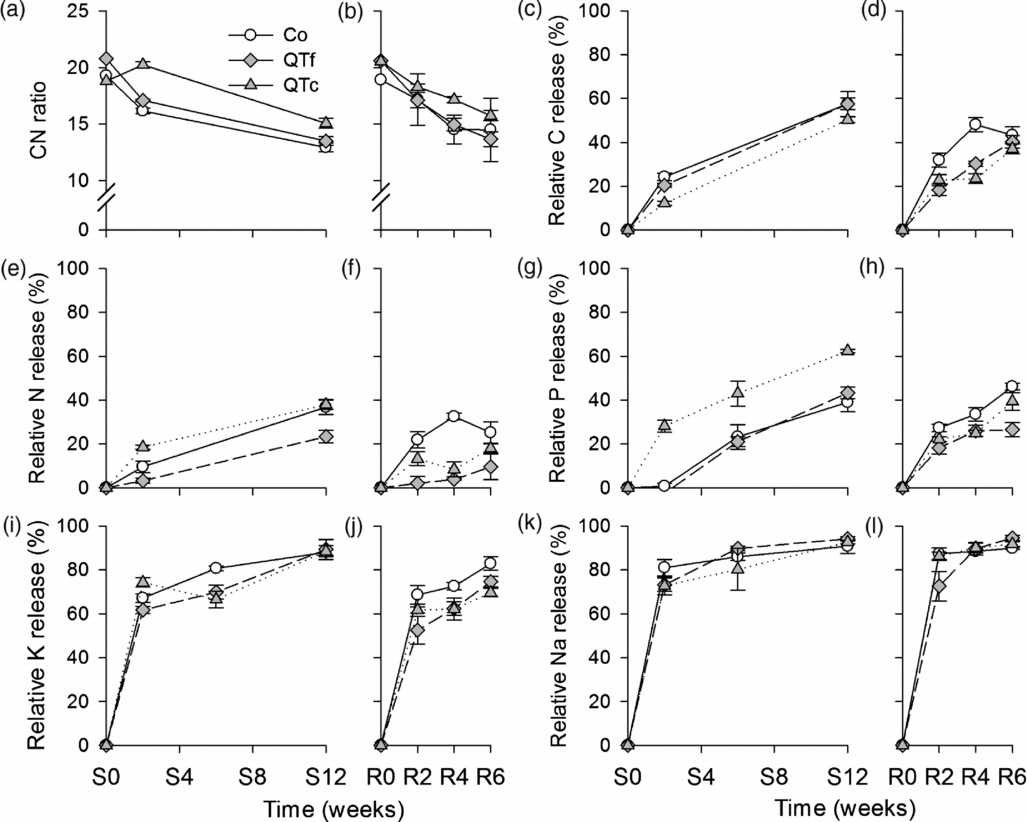
The C/N ratio of goat manure was significantly affected by sampling time [F(1, 9) = 733, p <0.001], treatment [F(2, 9) = 266, p <0.001], and their interaction [F(2, 9) = 16.4, p <0.01]. Under both crops, C/N ratios of control manure declined by around 32%, whereas QTf manure showed a reduced C/N ratio of 34% and QTc of 22% (Figures 3a and b). The C/N ratio was significantly lower in Co compared with QTc (p <0.001) under sweet corn and radish, whereas Co and QTf were only significantly different under sweet corn.
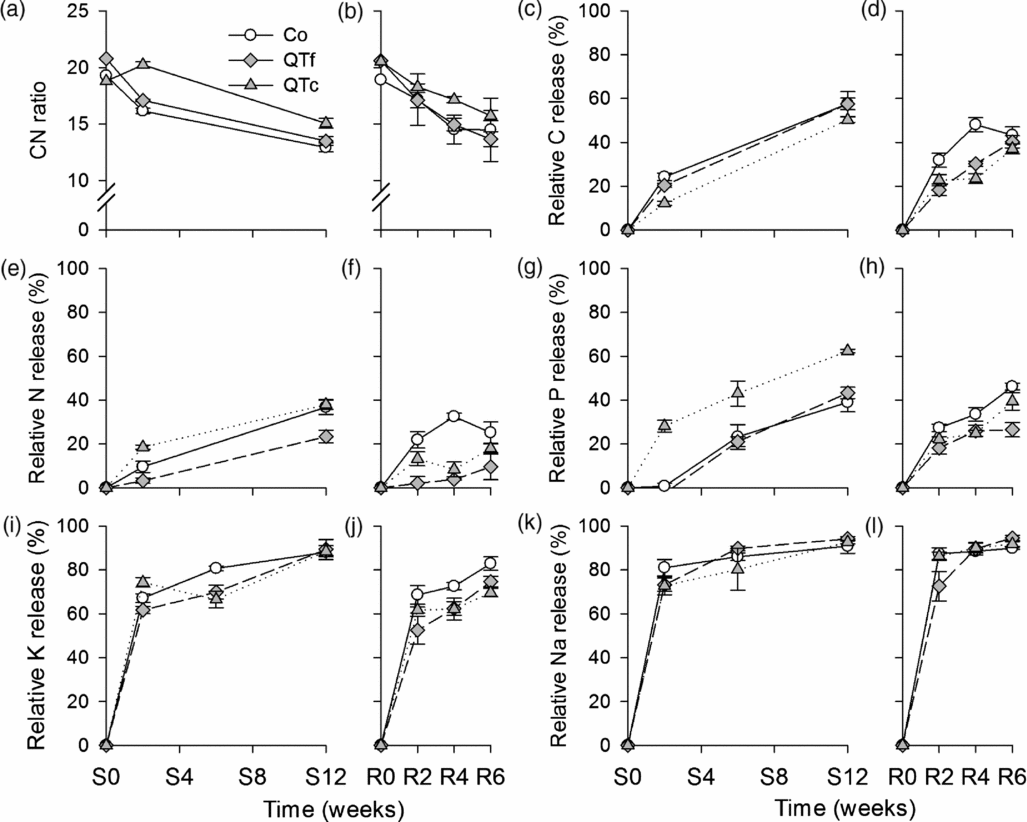
Figure 3. C/N ratios (a, b) and relative C (c, d), N, (e, f), P (g, h), K (i, j) and Na (k, l) release from manure (n = 4) during sweet corn (a, c, e, g, i, k) and radish (b, d, f, h, j, l) cultivation at the experimental field near Sohar, Sultanate of Oman. Symbols represent means, whiskers represent ± one standard error.
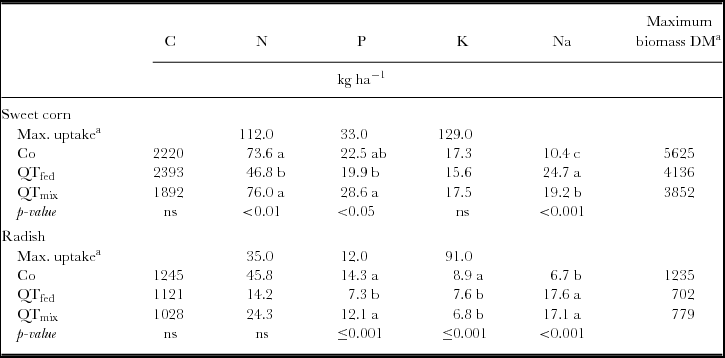
The repeated measures ANOVA revealed significant effects of time and treatment on the relative C and nutrient release from litterbags during both cultivation periods (Figures 3c–l, Table 2; Table S2). Additionally, the absolute C and nutrient release were significantly affected by QT treatments (Table 3). Within the first four weeks, the relative C loss was on average by 16–50% lower from QT manure than from control manure, whereas at the end of the experimental periods, the difference to control was only 0–14%. In contrast, the absolute C release was not significantly altered (Table 3). The relative N release was significantly affected by treatments under sweet corn and radish, respectively (Figures 3e and f). The absolute N release was only significant under sweet corn, with F(2, 9) = 8, p <0.01 (Table 3). Relative and absolute N releases were significantly lower for QTf by 37–67% and 36%, respectively, under sweet corn and by 62–90% under radish as compared with Co. Coating QT increased N release under sweet corn (p <0.05), while under radish no effect was observed (Table 3). Relative and absolute P releases were significantly affected under sweet corn and under radish, respectively (Figure 3g; Table 3). Similar to N, P release was higher (p <0.05) in QTc compared with Co and QTf under sweet corn, whereas P release was slightly reduced (15–26%) compared with the control (p <0.05) under radish. In the initial two weeks after installation, P release in QTc was 40-times higher than in Co (Figure 3g). K release was only affected during radish cultivation, whereas no significant treatment effects were observed under sweet corn (Table 3; Figures 3i and j). Both QT treatments reduced relative K release under radish by 10–16% (p <0.01) and absolute K release by 15–24% (p <0.05). The higher Na application rates in the QT treatments resulted in up to 2.6-fold higher Na release under sweet corn and under radish compared with the control (Table 3), while the relative Na release was not significantly affected by the treatments (Figures 3k and l).
Table 3. Means of total C and nutrient release from goat manure in kg ha−1 until crop harvest in a litterbag experiment (n = 4), maximal biomass and nutrient uptake of crops in the experimental field near Sohar (Sultanate of Oman)Footnote a, and results from statistical analysis (ANOVA followed by Tuckey HSD test at p < 0.05).
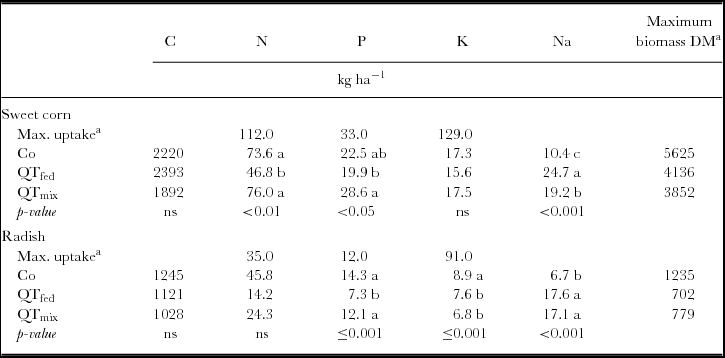
ns = not significant. Values followed by different letters are significantly different at p < 0.05.
a Results published in Ingold et al. (Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b).
DISCUSSION
Disappearance of organic matter
Quebracho tannin extract altered the turnover of goat manure under both sweet corn and radish in diverse ways. On the irrigated sandy soil, the observed OM disappearance was 60–65% of the applied OM which is in the range of results (25 to 95%) reported for sheep, goat and cattle manure after 10–12 weeks of litterbag installation in Niger and Burkina Faso (Esse et al., Reference Esse, Buerkert, Hiernaux and Assa2001; Ouédraogo et al., Reference Ouédraogo, Mando and Brussaard2004). The turnover of OM in litterbags highly depends on manure quality, position of litterbags (surface or in the soil) and accessibility of litterbags to meso- and macrofauna (Esse et al., Reference Esse, Buerkert, Hiernaux and Assa2001; Knacker et al., Reference Knacker, Förster, Römbke and Frampton2003; Ouédraogo et al., Reference Ouédraogo, Mando and Brussaard2004). Herein, goat manure with comparatively high N and P contents, and medium C/N ratio of 19–21 was used (Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b). Placed at 10-cm depth in an irrigated field, litterbags were located in a permanent moist environment (>45% of the water-holding capacity) with daily soil temperature varying between 14 and 32 °C and providing an ideal environment for the decomposer community. The fast turnover of OM under the hot and moist conditions led to a quick decline of the easily decomposable fraction in all treatments, approaching the asymptotic value of the more stable or recalcitrant fraction within 4 to 8 weeks.
The QT extract either directly applied (QTc) or defecated by goats (QTf) increased the remaining OM of manure in soils by 27% to 52% and 5% to 14%, respectively (Figures 2a and b). The stronger effect of QTc indicates different mechanisms of inhibition caused by application methods. Haettenschwiler and Vitousek (Reference Haettenschwiler and Vitousek2000) pointed out two basic mechanisms by which polyphenols, and thus tannins, may interact with OM decomposition: (i) effects on the activity of soil organisms by enzymatic inhibition and toxicity (Triebwasser et al., Reference Triebwasser, Tharayil, Preston and Gerard2012); and (ii) physico-chemical effects on OM and nutrient pools, protecting organic molecules from degradation by complexation (Mutabaruka et al., Reference Mutabaruka, Hairiah and Cadisch2007). In QTc under both crops, the calculated decomposable fraction (A) and the decay constant (k) of the asymptotic model were lower when compared with the control (Table 2). The smaller A may indicate a protection of organic matter from degradation by the formation of tannin-protein complexes (Mafongoya et al., Reference Mafongoya, Giller and Palm1998; Powell et al., Reference Powell, Fernández-Rivera and Hös1994). This agrees with the reduced net C and N mineralization of QT complexes with bovine serum albumin (BSA), indicating a reduced accessibility of complexed C by microorganisms while un-complexed BSA or QT was metabolized as C sources (Mutabaruka et al., Reference Mutabaruka, Hairiah and Cadisch2007). The lower k might be explained by the direct inhibition of the microbial activity, reducing the decomposition of the total OM. Condensed tannins, the major tannin type in QT extract, were found to reduce activity of several enzymes involved in C and N mineralization in soils (Triebwasser et al., Reference Triebwasser, Tharayil, Preston and Gerard2012). However, there are differences in the susceptibility of microorganisms to tannins (Mutabaruka et al., Reference Mutabaruka, Hairiah and Cadisch2007). Interestingly, the remaining OM from QTf manure was only higher than control during radish cultivation, being explained by a lower A (Table 2). Unlike QTc, with its soluble and reactive tannins on the manure surface, ingested QT did not alter k, but seemed to protect organic matter by complexation and changes in manure composition (Al-Kindi, Reference Al-Kindi2015; Makkar, Reference Makkar2003; Powell et al., Reference Powell, Fernández-Rivera and Hös1994). However, the differences in OM decomposition from QTf and control were relatively small, as noticed in C, N, acid detergent fibre (ADF) contents and C/N ratio from goats fed with 2% to 4% QT extract (Al-Kindi, Reference Al-Kindi2015; Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b). The results indicated that OM decomposition of QT-amended manure is inhibited by direct effects on microbial activity rather than by physico-chemical stabilization of the substrate. The presence of termites in the experimental field, which can play a crucial role in OM decomposition particularly in semi-arid and arid regions (Esse et al., Reference Esse, Buerkert, Hiernaux and Assa2001; Ouédraogo et al., Reference Ouédraogo, Mando and Brussaard2004), reduced the remaining OM contents in litterbags by only 0–6% and was thus considered negligible.
Carbon and nutrient release during sweet corn and radish cultivation
After 6–12 weeks, the relative N and K releases from buried goat manure were with about 10–35% and 80%, respectively, in a similar range as the release from surface applied sheep and goat manure in Niger in absence of termites (Esse et al., Reference Esse, Buerkert, Hiernaux and Assa2001). The relative P release from buried goat manure after 6–12 weeks was higher (25–60%) than what was found in surface applied sheep and goat manure (Esse et al., Reference Esse, Buerkert, Hiernaux and Assa2001).
In soil incubation experiments, N mineralization was found to be reduced by polyphenols in many sub-tropical and tropical prunings and leaves, and negatively correlated with the polyphenol/N ratio and the lignin+polyphenol/N ratio (Mafongoya et al., Reference Mafongoya, Giller and Palm1998). The general inhibition of OM decomposition and N mineralization, in addition to increases in cation exchange capacity found after tannin application to soil (Halvorson et al., Reference Halvorson, Gonzalez and Hagerman2013) indicate that QT in goat's diets or directly applied to manure reduces C and nutrient releases from goat manure. However, in fertilization trials with manure from sheep fed polyphenol-containing fodder sources, such as Acacia trachycarpa E.Pritz, Combretum glutinosum Perr. ex DC. and Guiera senegalensis J.F.Gmel. N uptake by millet (Pennisetum glaucum (L.) R.Br.) was not affected by polyphenol content of fodder sources (Powell et al., Reference Powell, Ikpe and Somda1999) as was the case for Brachiaria grass when Vigna unguiculata Walp., Calliandra calothyrsus Meisn. and Flemingia macrophylla (Willd.) Merr. were used in sheeps’ diet (Tiemann et al., Reference Tiemann, Hincapie, Frossard, Kreuzer and Hess2009). In contrast, N release from QTf manure was reduced by 36–62% during both sweet corn and radish cultivation and was accompanied by yield reductions of 26% to 43% (Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b), while C and other nutrient releases were less affected. By binding of tannins to N-containing fodder residues in goats’ digestive system (Makkar, Reference Makkar2003), N compounds are protected from microbial degradation (Waghorn, Reference Waghorn2008) and may thereafter be protected also in the soil (Mutabaruka et al., Reference Mutabaruka, Hairiah and Cadisch2007; Powell et al., Reference Powell, Fernández-Rivera and Hös1994). While the effect of tannin feeding on C and N contents of excreted faeces was diverse (Al-Kindi, Reference Al-Kindi2015; Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b; Powell et al., Reference Powell, Wattiaux, Broderick, Moreira and Casler2006), tannins in ruminant diets lead to more recalcitrant C and N forms in manure (Al-Kindi, Reference Al-Kindi2015; Powell et al., Reference Powell, Wattiaux, Broderick, Moreira and Casler2006). This could explain the slow N release from QTf under both crops.
While the effects of QT amendment on remaining OM were relatively similar under sweet corn and radish, C and nutrient release patterns differed considerably (Figure 3). The inhibitory activity of condensed tannins on enzymes involved in C and N mineralization was found to depend on the cropping history of the soil (Triebwasser et al., Reference Triebwasser, Tharayil, Preston and Gerard2012). This was explained by an adaptation of soil microbial community towards enzyme isoforms less susceptible to naturally occurring tannins. Herein, varying C and nutrient release during sweet corn and radish cultivation may result from a different susceptibility of predominating soil microbial community to tannins (Mutabaruka et al., Reference Mutabaruka, Hairiah and Cadisch2007; Scalbert, Reference Scalbert1991). It is known that different decomposer communities affect mineralization processes under Poaceae and Brassicaceae (Appuhn and Joergensen, Reference Appuhn and Joergensen2006), with differing climatic conditions such as daily temperature fluctuations and wet/dry cycles affecting microbial community structure and/or its activity (Knacker et al., Reference Knacker, Förster, Römbke and Frampton2003). The different mineralization patterns and effects of tannins indicate an effect of the cultivation period. While tannins can inhibit or favour the development and activity of bacteria and fungi (Mutabaruka et al., Reference Mutabaruka, Hairiah and Cadisch2007; Scalbert, 1991), the QT used seems to favour fungal and inhibit bacterial development in the digestive tract as well as in the soil (Al-Kindi, Reference Al-Kindi2015; Mutabaruka et al., Reference Mutabaruka, Hairiah and Cadisch2007; Sradnick et al., Reference Sradnick, Ingold, Marold, Murugan, Buerkert and Joergensen2014).
Relative N and P releases were increased by QTc under sweet corn, whereas both tannin treatments significantly reduced its release under radish as compared with the control (Figures 3e–h). Kwabiah et al. (Reference Kwabiah, Palm, Stoskopf and Voroney2003) did not find significant effects of polyphenols on the microbial biomass, P uptake and plant P availability in a laboratory soil incubation experiment. Similarly, leaves and manure derived from goats fed Acacia nilotica and Acacia karro did not affect P mineralization in an incubation experiment (Mafongoya et al., Reference Mafongoya, Barak and Reed2000). The reduction of K release may indicate an increase in cation exchange capacity by tannins, which was observed by Halvorson et al. (Reference Halvorson, Gonzalez and Hagerman2013). While under radish, no significant differences between QTc and QTf were found, there were differences between these two treatments under sweet corn (Figures 3i and j). The data indicate complex interactions with the cultivated crop or environmental factors, which merit further research. In general, QT-containing goats’ diets may be a promising way not only to reduce N losses within the animal system (via urine), but also to stabilize N in manure and reduce N losses via leaching and volatilization in intensive agricultural systems.
Fertilizer value of QT-amended manure
Under the given conditions in Oman, the mineralization of manure was expected to be very high, so that most of the applied nutrients would be available for crops within the cropping period. However, this was not true. N, P and K uptake of well-fertilized sweet corn on the same experimental field accounted to 112, 33 and 129 kg ha−1, respectively (Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b), while N, P and K release from the control treatment provided only 66%, 68% and 13% of this demand (Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b). For the amended treatments, the ratio between crop demand and release of N, P and K was 42–68%, 60–87% and 12–14%, respectively. The discrepancy between N and P demand and release was likely the cause for yield reduction (−24%) in QTf, whereas QTc exhibited higher N and P release compared with control and caused yield reduction of 32% (Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b). It is likely that N and P released from QTc was not completely available for plants and may have been relocated by leaching. However, Zingore et al. (Reference Zingore, Mafongoya, Nyamugafata and Giller2003) showed that N leaching from polyphenol-rich prunings with a high protein-binding capacity was lower and maize yields higher than for prunings low in polyphenols and protein-binding capacity. In contrast, the nutrient supply from control manure seemed to be sufficient for radish, as yields were twice as high as on mineral fertilized plots (Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b). Regardless of the QT application method, tannins reduced sweet corn and radish yields likely reflecting N deficiency, which was indicated by reduced chlorophyll SPAD values measured during the cropping period (Ingold et al., Reference Ingold, Al-Kindi, Jordan, Dietz, Schlecht and Buerkert2015b). However, contributions of other factors such as a direct inhibition of germination and root development by tannins cannot be ruled out.
CONCLUSIONS
Fast organic matter decomposition in irrigated production systems in northern Oman may lead to major losses of SOM and nutrients. In contrast to our expectations, only 60% of organic material in goat manure was easily decomposable and it approached an asymptotic value after 4–8 weeks. The addition of QT to goat manure via feed additives or coated to the manure's surface increased remaining organic matter after application to the soil. It partly reduced relative C and nutrient release by the end of the cropping period. There are indications that during the cultivation of sweet corn and radish, different groups of soil microbes and soil fauna dominated the mineralization processes. The observed contrasting effects for the nutrient release during sweet corn and radish cultivation may result from the different effect of QT on specific groups of soil organisms. It is likely that there were interactions of the tannin effect with the cultivated crop, the environmental conditions and the QT application method. Feeding QT reduced N release under both crops, allowing a long-term N accumulation in soil. However, the stabilization of manure by QT may also lead to nutrient immobilization, causing deficiencies in crops that should be further studied.
Acknowledgements
We thank Royal Gardens and Farms, Sultanate of Oman, for its support and the German Research Foundation (DFG) for funding this research within the Research Training Group 1397 ‘Regulation of Soil Organic Matter and Nutrient Turnover in Organic Agriculture’ at Universität Kassel, Germany.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S0014479717000291