INTRODUCTION
Poor seedling establishment is a major constraint to production and productivity in direct seeded rice production systems (Harris et al., Reference Harris, Pathan, Gothkar, Joshi, Chivasa and Nyamudeza2001). Poor seed quality is a major cause of poor seedling establishment, seedling abnormalities or even failure of emergence (Groot et al., Reference Groot, Surki, de Vos and Kodde2012). Then, farmers can ensure sufficient production by planting high-quality seed or sowing seed of poor quality at high rates to compensate for seed deaths and seedling abnormality. However, since sowing poor quality seed at increasing rates has cost implications for rice production and weed control, the use of high-quality seed remains a more viable option. The absence of a quality rice seed production and distribution system in Ghana has led to the use of farmer-saved seeds from previous harvests as the main seed source among farmers. However, such seed is stored in uncontrolled storage environments in which they are exposed to air humidity ranging between 29 and 95% (Bam et al., Reference Bam, Craufurd, Dorward, Asiedu, Kumaga and Ofori2007). Under such fluctuating environmental conditions, rice seeds deteriorate at a faster rate than those stored in controlled environments, for example, at −20 °C and 2 to 4 °C (Hay et al., Reference Hay, de Guzman, Ellis, Makahiya, Borromeo and Hamilton2013).
Seed storage and potential longevities are affected by amongst other things, seed production environment, genotype and crop maturity (Rao and Jackson, Reference Rao and Jackson1996). Prevailing weather conditions during maturity determine the extent of field weathering and, hence, the quality of harvested seed (Nagata et al., Reference Nagata, Sasaki and Ohdaira2013; TeKrony et al., Reference TeKrony, Egli and Phillips1979) and its subsequent storage potential. Rainy or humid and warm weather conditions have been suggested to favour field weathering and physiological deterioration of seeds (TeKrony et al., Reference TeKrony, Egli and Phillips1979), which is the case for seed production in tropical rice producing countries including Ghana. This is largely a consequence of crack formation during the early ripening stage in response to high temperatures (Nagata et al., Reference Nagata, Sasaki and Ohdaira2013). Varietal differences in endospermic cracking propensity exist (Nagata et al., Reference Nagata, Sasaki and Ohdaira2013) and can benefit hybridization programs if cracking is successfully reduced in high-yielding cultivars. Also, additional cracks may develop due to alternating drying and wetting cycles of paddy in the field (Kunze, Reference Kunze2008), which may reduce vigour and viability of seeds.
A common post-harvest practice among rice farmers in Ghana is to leave (cure) harvested rice in the field for a number of weeks (Bam et al., Reference Bam, Craufurd, Dorward, Asiedu, Kumaga and Ofori2007), up to 5 weeks, before threshing (Nyaaba, Reference Nyaaba2015), mainly due to competition with harvesting of other crops for labour. As commented previously, exposure to diurnal changes in air temperature and relative humidity (RH) and the wet and dry cycles typical of a humid tropical environment during field curing of harvested panicles could influence seed quality and storage longevity by promoting fissure and crack development. Furthermore, this is likely to be accompanied by seed microflora activity, particularly, fungi since fungal sporulation and grain mold severity in many crop species are greatly influenced by seed water activity and storage temperature (Mousa et al., Reference Mousa, Ghazali, Jinap, Ghazali and Radu2011; Topani et al., Reference Topani, Rachana, Navi, Thakur, Bandyopadhyay, Varanavasiappan and Seetharama2007). Mycotoxigenic fungal invasion and subsequent contamination of rice with mycotoxins begins in the field and is exacerbated by poor post-harvest practices (Mousa et al., Reference Mousa, Ghazali, Jinap, Ghazali and Radu2011).
The seed moisture content (MC) of newly harvested grain can compromise the allowable storage time of seeds with dryer seeds keeping longer. Farmers generally achieve this reduction in MC via field dry down. During this field drying, commonly termed ‘natural curing,’ biochemical processes continue within a seed until the simple sugars are converted into starch and other components such as oil and protein. Water is released during some of these processes and farmers prefer this natural water loss to high-heat drying, which compromises seed quality. Though moisture release from curing is not reversible, increases of seed MC can occur with increases of air humidity. For these reasons, we have termed as ‘field curing’ the farmers practice of maintaining harvested rice panicles in the field for weeks before threshing, where they are exposed to drying-wetting cycles.
Delayed field curing is an unavoidable practice in the developing countries due to lack of mechanization. At the time of this study, there were no published reports on how controlling seed MC more precisely in the field (e.g., by reducing water uptake) can influence seed longevity and subsequent survival. Furthermore, the exact mechanism(s) via which seed vigour and viability are lost during delayed field curing (Nyaaba, Reference Nyaaba2015) are unclear. Given that delayed field curing is likely to persist in many developing countries, it would be prudent to identify strategies that can alleviate the potential negative effects of this practice on seed vigour and viability.
Given the above, the present study tests the following hypotheses: (1) the seeds exposed to wetting and drying (wet cured seeds) cycles during field curing develop more fissures and cracks than those that are field cured within a dry environment (dry cured seeds); (2) wet cured seeds exhibit higher levels of physiological deterioration of both embryo and endosperm prior to, and during storage than dry cured seeds; (3) wet cured seeds exhibit higher levels of microbial infection than dry cured seeds; (4) species differ in terms of their susceptibility to curing induced cracking, embryo and endosperm physiological deterioration and microbial infection. The study was conducted on three contrasting rice species Oryza sativa L., Oryza glaberrima Steud and an O. sativa × O. glaberrima interspecific hybrid, in a humid tropical environment in Ghana. Comparisons of seed moisture relations, endosperm integrity, microflora activity, germinability and vigour are made within and across species, between wet and dry cured seeds.
MATERIALS AND METHODS
Plant material
Three upland rice species, namely O. sativa L. subsps. japonica, O. glaberrima Steud. and an interspecific hybrid between O. glaberrima Steud. and O. sativa L. were planted at the CSIR-Crops Research Institute, Kumasi, Ghana (06°43.018 ′N, 01°989 ′W) on 8 September 2014, and harvested at maturity on 3 February 2015.
Field curing
Harvested panicles of each species were heaped in the field in six approximately equal amounts and a heap of each species was immediately threshed, while the remaining five were left in the open field to cure (referred to as ‘wet cured,’ henceforth). The hand threshed, winnowed and cleaned seed of each species were placed in sealed plastic containers and immediately transported to the laboratory for further drying; these seeds served as the controls. Also, panicles of each species were placed in transparent plastic containers (50 cm long, 33 cm high and 32 cm wide) with lids in place and incubated in the field. Holes of approximately 2 × 2 cm2 were cut into all four sides of the bowls to facilitate gaseous exchange with the atmosphere. The rainproof, yet ventilated containers prevented the seeds from being exposed to the wetting (i.e., rain or dew) and drying cycles typical of tropical environments; this treatment is referred to as ‘dry cured,’ henceforth. The containers were fixed to a wooden pole at about 1.3 m above ground to safeguard them and their contents from tropical windstorms. Thereafter, panicles of the three species were taken from both wet and dry cured environments at weekly intervals (for 5 weeks), hand threshed, air dried to about 12% MC (fresh mass basis [fmb]) and stored hermetically at 4 °C.
Measurement of environmental parameters
Air temperature and RH were logged hourly for 5 weeks using Tiny tag data loggers (Gemini Data Loggers, UK) placed within each container. For comparative purposes, data on air temperature and RH (from 3 February 2015 to 10 March 2015) was obtained for the wet cured environment from the CSIR-Crops Research Institute's weather station (Davis instruments, France) located c. 200 m from the study site. To minimize the confounding effects of increased temperatures within the containers used for dry curing, these were fixed in the field prior to harvesting, and temperature and RH monitored within them for a month using Tiny tag data loggers. These data were compared to data from the weather station at weekly intervals for 4 weeks. After each measurement interval, additional holes were inserted into the containers until the temperature (t = 0.77, d.f. = 135; p = 0.442); and RH (t = 1.63, d.f. = 230; p = 0.105) were statistically comparable between the wet and dry cured environments.
Seed moisture content (MC) and water activity
Seed samples from wet (n = 648) and dry (n = 648) cured environments were taken at 08h00 and 15h00 daily during the 5-week curing period for MC determination. Seed MC was first estimated using a grain moisture tester (Kett Electric Laboratory, Tokyo, Japan): MC was determined on seed samples with MC < 17% on a three 5 g milled samples instead of two 5 g samples at 130 °C for 2h but for seed samples with MC ≥ 17%, the two-stage drying method was used (International Seed Testing Association, 2005).
Sub-samples of seeds taken for MC determinations (n = 1296) were also used for water activity measurements. Water activity was determined at 20 °C on triplicates of milled seed samples using the AquaLab 3TE (Decagon Devices Inc., Pullman, Washington, USA) after seeds were incubated in sealed containers and left overnight in the laboratory to equilibrate.
Assessment of damage to endosperm
At harvest, a batch of panicles of each of the three species were hand threshed and hand de-hulled, non-fissured seeds (of each species) were placed in the wet (n = 2500/seeds/species) and dry (n = 2500 seeds/species/bowl) cured environments. Dry curing was achieved by placing seeds within ventilated rainproof bowls as described earlier. The wet cured seeds were placed in muslin bags and tied onto 2 m high poles in the field to avoid rodent attack. Four replicates of 20 seeds of each species were randomly drawn daily at 08h00 from the wet and dry cured environments to determine the number of cracked seeds using a Grainscope TX-200 (Kett Electric Laboratory, Tokyo, Japan).
Seed microflora studies
Seed microflora were characterized in vitro on seed samples taken weekly from wet and dry cured environments using the blotter method (International Seed Testing Association, 2001). Two hundred seeds (8 replicates of 25 seeds) per species, per environment, per week were randomly selected and surface sterilized with 1% NaOCl for 3–5 min, washed three times with distilled water, and placed on wet blotters within plates (n = 25 seeds per plate) and incubated for 7 days at 28 ± 2 °C under a 12 h near ultraviolet light and 12 h darkness cycle (Marthur and Kongsdal, Reference Marthur and Kongsdal2003). Habit characteristics of fungal colonies found on the infected seeds were examined under the stereoscopic microscope (Leica MS 5). A compound microscope (Leica DMLS) was then used to identify fungal species based on morphological characteristics (Marthur and Kongsdal, Reference Marthur and Kongsdal2003).
Seed germination and vigour
Thirty-six seed lots of the three species (18 each from wet and dry environments) were sampled for MC after field curing for 5 weeks and the remaining seed were stored at 4 °C (for close to 21 days) until they were withdrawn from storage and held in sealed bags overnight at 25 °C to equilibrate before germination assessments. Four replicates of 50 seeds of each wet cured seed sample and 25 seeds (because of limited quantity of seeds from the dry cured environment) of each dry cured seed sample for each sampling time were tested for germination between moist rolled anchor seed germination paper towels measuring 10 in. × 15 in. (Anchor paper Co., USA) at alternating temperatures of 34/11 °C (16 h day/8 h night) (Ellis et al., Reference Ellis, Hong and Roberts1983) for 14 days. The first germination counts were made on day 7 and all hard, non-germinated seeds were de-hulled to remove any effect of seed coat dormancy. Final germination counts were made on day 14 of germination. The criterion for germination was normal seedling development (International Seed Testing Association, 2005).
Seed vigour was based on radicle length and seedling dry weight. Seedlings were selected for radicle length and dry weight measurements using randomized allocation and selection of numbers. The lengths of five randomly selected seedlings per replicate, per treatment (n = 20) were measured 7 days after initiation (DAI). Seedling dry mass for each replicate of a treatment was measured on 10 randomly selected seedlings at 14 DAI. Seedlings were dried at 80 °C for 72 h before being weighed to four decimals.
Data analyses
For both wet and dry cured environments, weather data collected at 08h00 and 15h00 from the weather station and Tiny tag data loggers for the wet and dry cured environments were used. Relationships between air temperature and RH were compared within and between the wet and dry cured environments using Simple Linear Regression with Groups (Genstat Release 17, VSI International, UK). The relationship between seed MC and water activity of the three species was also compared between the wet and dry cured environments. Differences in the relationships between seed MC and water activity in the wet and dry cured environments were determined using a comparison of regressions. Cubic models were fitted to the data to quantify the relationships. For each curing environment, the data for MC and water activity for the three species were pooled for the analysis.
The number of seeds infected with fungi was compared within each isolated fungal species between the curing environments using a chi-square test of independence (with data for the various sampling times being pooled). Where the number of isolates was less than 5, the CHIPERMTEST procedure was followed to calculate the chi-square value and probability. Data on total number of seeds infected per species was also compared between curing environments using chi-square test of independence (Genstat Release 17, VSI International, UK). Germination and seed vigour (radicle length and seedling dry weight) data were compared within and across species, wet cured and dry cured, and within sampling times using analysis of variance (ANOVA; GLM Multivariate) (IBM SPSS version 24). Germination data were normalized using arcsine transformation, while seedling length and dry weight data were normalized using the common logarithm (log10) before analysis. The Bonferroni post-hoc test was used to separate means at the 5% level of significance. Standard error (SE) was calculated for all means shown.
RESULTS
Field curing environments
The mean air temperature for the wet cured environment was 28.8 ± 0.6 °C and 31.1 ± 1.1 °C for the dry cured environment. The mean air RH was 71.8 ± 7.5% and 61.5 ± 6.9% for the wet and dry cured environments, respectively. Air temperature and RH were significantly and positively related in both environments (Figure 1). Comparison of regressions also showed significant (r 2 = 0.75; p < 0.001) differences between the wet and dry cured environments.
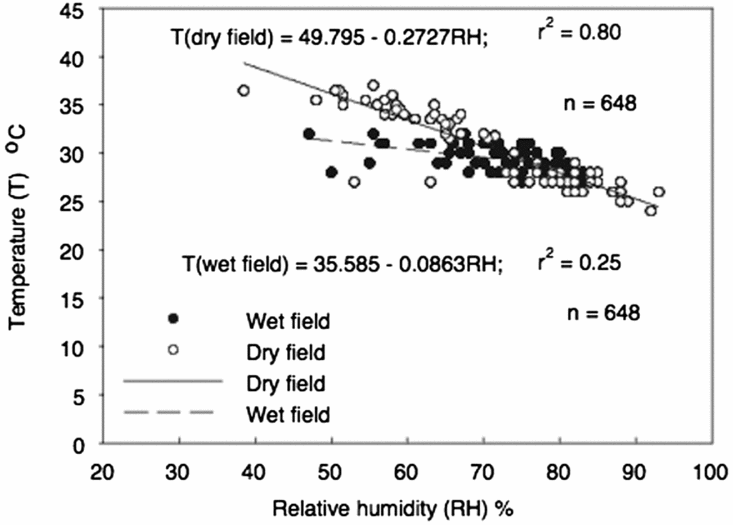
Figure 1. Relationships between air temperature and relative humidity of three rice species (O. sativa, O. glaberrima and O. sativa × O. glaberrima) dry cured (n = 648) (open symbols) and wet cured (n = 648) (filled symbols) over 5 weeks. Comparison of regressions showed significant (r 2 = 0.75 ± 0.146; p < 0.001, n = 1296) differences between wet and dry field cured environments.
Water activity and seed moisture content
Seed water activity at 08h00 and 15h00 was similar between curing environments for all species (Figure 2A and B). A similar trend was observed for seed MC in both environments (Figure 2C and D). Water activity in dry cured seeds was significantly (p < 0.001) lower than in wet cured seeds (Figure 2A and B). The mean water activity ranged from 0.55 ± 0.15 in the dry cured to 0.67 ± 0.23 in the wet cured seeds, repectively. The mean MC was 11.32 ± 1.98% and 14.23 ± 5.50% for dry and wet cured environments, respectively. The data also showed that the wet cured seeds were subjected to a more pronounced daily drying (day) and wetting (night) cycles over the 5 weeks (Figure 2).
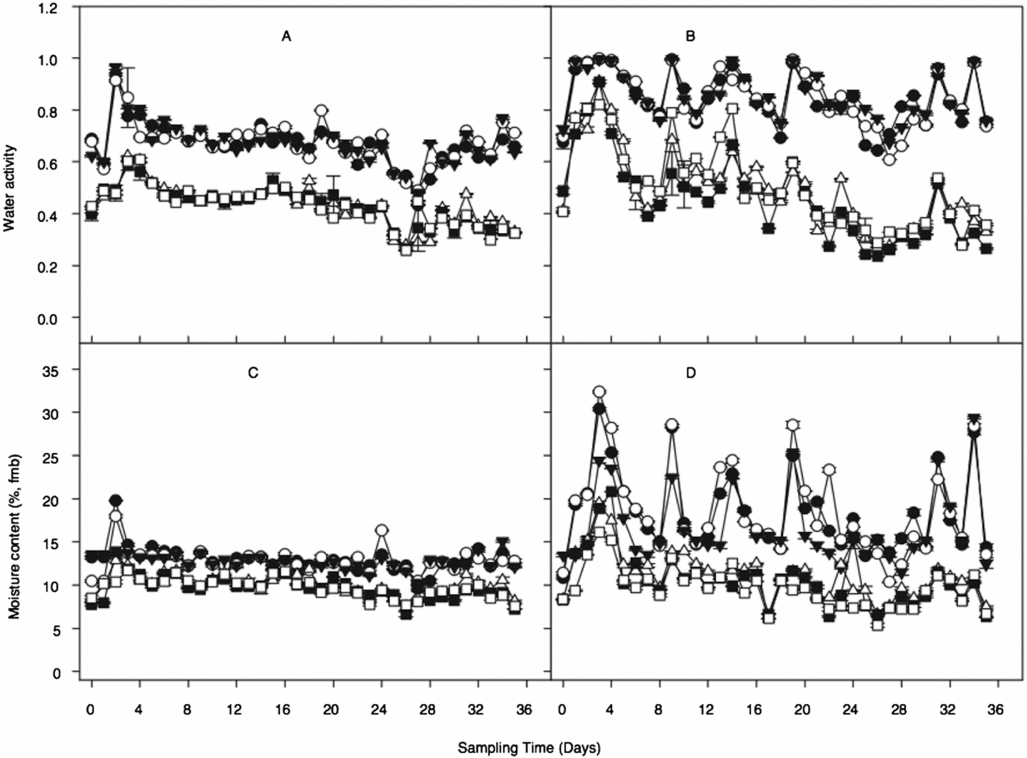
Figure 2. Diurnal changes in water activity and seed moisture content of O. sativa (open circles at 08h00; filled squares at 15h00), O. glaberrima filled inverted triangles at 08h00; open squares at 15h00 and O. sativa × O. glaberrima (filled circles at 08h00; open inverted triangles at 15h00) seeds dry and wet cured for 5 weeks. A: Water activity of seeds sampled from dry cured environment, n = 648; B: Water activity of seeds sampled from wet cured environment, n = 648; C: Moisture content of seeds sampled from dry cured environment, n = 648; D: Moisture content of seeds sampled from wet cured environment, n = 648. Values represent mean of three replicates ± SD.
Relationships between seed moisture content and water activity
The relation between seed MC and water activity of the three species was sigmoidal and described by a cubic model in both wet and dry cured field environments (Figure 3; Table 1). The sorption characteristics in the wet and dry cured environments could not show the inflection point (water activity values between 0 and 0.20) but showed linear (water activity values between 0.20 and 0.85) and reflection points (water activity > 0.85) similar to an isotherm. The reflection point was more pronounced in the wet than dry cured environment. Comparison of the relationships between seed MC and water activity in the dry and the wet field cured environments showed significant (r 2 = 0.82; p < 0.001) differences between environments. To test if there were differences in the relationships between seed MC and water activity between the dry and wet cured environments at water activity below 0.85, water activity values 0.85 and above were removed from data sets for both dry and wet cured seeds before analysis. Comparison of relationships between seed MC and water activity showed significant (r 2 = 0.77; p < 0.001, n = 1124) differences between both environments. Within both environments, differences among species in their sorption characteristics were not significant (p > 0.05).

Figure 3. Relationships between seed moisture content and water activity of seed samples of O. sativa, O. glaberrima and O. sativa × O. glaberrima taken from dry cured (A) (n = 648) and wet cured (B) (n = 648) at 08h00 and 15h00. The relationship is described by cubic model in the dry (A) and wet (B) field cured environments. Comparison of regressions showed significant (r 2 = 0.822 ± 0.185; p < 0.001, n = 1296) differences in the sorption characteristics of the three species among the dry and wet field cured environments.
Table 1. The relationship is described by a cubic model (y = a + bx + cx 2 + dx 3) for wet and dry field cured environments: y = moisture content (%, fmb) and x is water activity of seed.

Harvested panicles of three rice species were field cured in wet and dry field environments for 5 weeks. Samples of seed were taken at 08h00 and 15h00 each day in sealed bottles. Cubic models were fitted to the data from both curing environments. Values represent mean ± SE. Comparison of regressions showed significant differences in the relationship between dry and wet field cured environments (r 2 = 0.822 ± 0.185; p < 0.001, n = 1296).
Endosperm crack frequency
The effect of field curing on the percentage seed exhibiting endosperm cracking (labelled ‘% cracking’ henceforth) in the three rice species in wet and dry cured environments is shown in Figure 4. In all the three species percentage cracking increased with curing time, irrespective of the environment. Percentage cracking in the dry cured environment was initially below the wet cured environment in all species but seeds in both curing environments eventually reached 100% (Figure 4). At harvest, crack frequency was initially low (4.0 ± 0.7%) for all the three species, however, after just 3 days of curing in the wet environment this reached 40.4 ± 4.5% in all the three species and by 2 weeks of curing percentage cracking reached 100% in all the three species. Within the wet cured environment, cracking percentage reached 100% in 9 days in O. sativa and 10 days in O. sativa × O. glaberrima and O. glaberrima. However, within the dry cured environment, percentage of cracks reached 100% in 11 days in O. sativa, 12 days in O. sativa × O. glaberrima and 14 days in O. glaberrima (Figure 4).

Figure 4. Time course of crack development in harvested seed samples of O. sativa (A), O. sativa × O. glaberrima (B) and O. glaberrima (C) dry cured (open symbols) and wet cured (filled symbols). Values represent mean of four replicates ± SD.
Seed microflora
A number of fungal species were isolated from wet (22) and dry (23) cured environments (Supplementary Material Table S1). The frequency of occurrence of some of the fungal species such as Aspergillus niger, Curvularia pallenses, Curvularia oryzae, Fusarium equisetti, F. oxysporium, F. solani, Nigrospora oryzae, Penicillium sp and Myrothecium sp was, however, low and thus all subsequent analyses of the occurrence of fungal species was limited to 13 species.
Two categories of fungi were isolated from the samples taken from wet and dry cured environments: saprophytic and potential pathogenic colonizers. The saprophytic colonizers were Rhizopus sp, Botrytis sp, Phoma sp and Trichoderma sp, while the remaining isolates were potential pathogenic colonizers (Table S1). The most frequently occurring fungal species across the three species were Rhizopus sp, Aspergillus flavus, Bipolaris oryzae, Curvularia lunata, Fusarium pallidoroseum, Fusarium monilliforme, Melanospora sp, Phoma sp, Sclerotium rolfsii and Verticillium sp. Except for Botrytis sp, F. pallidoroseum, Melanospora sp, S. rolfsii and Verticillium sp, which preferentially colonized the wet cured seeds, the remaining species preferentially colonized the dry cured seeds of all the three species (Table S1). Botrytis sp and S. rolfsii were confined to wet cured samples. Except for the 4th week of sampling when Verticillium sp was isolated from dry cured O. sativa seeds, these species were not isolated in dry cured O. glaberrima and O. sativa × O. glaberrima seeds. Across the three species, A. flavus, B. oryzae, C. lunata, Fusarium molliniforme and Phoma sp were associated with dry cured seeds. The number of seeds in which Alternaria sp, S. rolfsii, Trichoderma sp and Verticillium sp occurred in the wet and dry cured samples were low, and generally associated with wet cured seeds and where they occurred in the dry cured environment, they were only isolated from 4 and 5 week cured seeds. Except for O. glaberrima, where fungal infection was comparable between curing environments, a significantly higher number of seeds infected with fungi occurred in dry cured O. sativa (p = 0.016) and O. sativa × O. glaberrima seeds (p < 0.001; Table 2). Across species, significantly (p < 0.001) higher fungal infection was associated with the dry cured environment (Table 2).
Table 2. Total number of seed samples from three rice species infected with fungal species isolated in wet and dry cured environments during various sampling times (weeks).
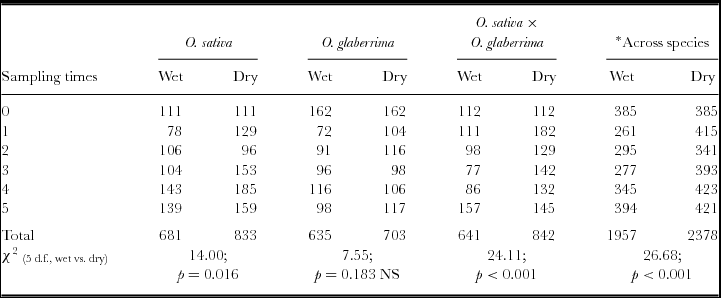
O. sativa, O. glaberrima and O. sativa × O. glaberrima were assessed from the day of harvest (week 0) and thereafter weekly for 5 weeks from wet and dry cure panicles for the occurrence of seed mycoflora. Total number of seeds from which fungal species were isolated from each rice species were pooled for wet and dry curing environments. Identical number of seeds of O. glaberrima from wet and dry cured environments were infected with fungi. Significantly higher number of seeds of O. sativa (p = 0.016) and O. sativa × O. glaberrima (p < 0.001) from dry cured environment were infected with fungi.
*Total number of seeds from which fungal species were isolated from the three rice species in wet and dry cured environments was pooled for each sampling time.
NS = Not significant.
Seed germination and vigour
Differences in seed quality measured as normal seedling production (root and shoot production) differed significantly between species (p < 0.001), sampling time (p < 0.001) and curing environments (p = 0.023) (Table S2). O. glaberrima and O. sativa generally exhibited greater seed germinability than O. sativa × O. glaberrima, being higher in the dry than wet cured environment. Compared with the control, seed germinability started declining from week 2. By week 5, percentage germination had declined significantly from 91% in the control to 76% (Table 3). Seed germinability was significantly influenced by species × sampling time and species × curing environment interactions. There were, however, no significant interactions among species × sampling time × curing environment (Table S2).
Table 3. Main effects of species, environment and sampling time (weeks) on seed germinability (%) and vigour (seedling root length (cm) and dry weight (mg)) of three rice species during delayed field curing in wet and dry cured environments.
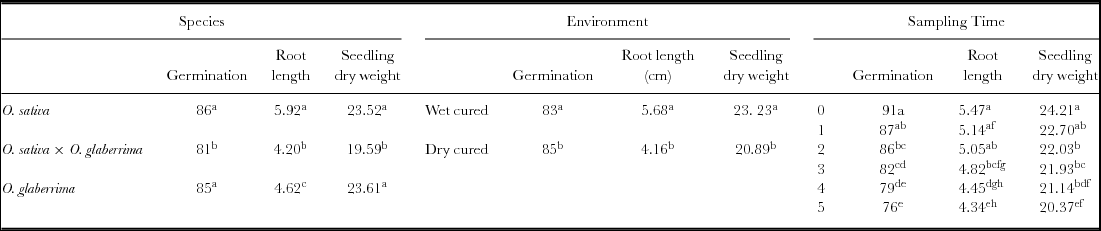
Different lower case letters in a column show significant differences in germination, root length and seedling dry weight across species, curing environment and sampling time.
Seedling vigour was evaluated in terms of radicle length and seedling dry weight. Significant differences in root length (p < 0.0001) and dry weight (p < 0.0001) was observed between species, sampling times and field cured environments (Table S2). Within the species, O. sativa produced the longest roots followed by O. glaberrima and then O. sativa × O. glaberrima. Dry cured seeds produced seedlings with shorter roots and lower dry weight compared with wet cured ones. Compared with the control, root length began declining in cured seeds from week 3 onwards. In contrast, seedling dry weight declined relative to the control from week 2 onwards (Table 3). There were significant species × sampling time (p = 0.027) and sampling time × curing environment interactions. No significant species × curing environment interactions was observed for root length and seedling dry weight. There was also no significant interaction between species × sampling time × curing environment (Table S2).
DISCUSSION
Though delayed field curing of rice has been, and is very likely to continue to be, an unavoidable practice in many developing countries (e.g., Ghana), no published reports on how changes in seed MC during curing influences seed vigour and viability are available at the time of this study. Herein, we showed how field curing seeds in a dry environment could potentially improve subsequent seed viability by improving MC management. Furthermore, the results show that the benefits of dry field curing may be largely based on the minimization of structural damage to the endosperm, which is promoted during wet field curing.
Much work has explored the theory of hygroscopic behaviour of seeds under controlled environments (Hay and Timple, Reference Hay and Timple2016; Hogan and Karon, Reference Hogan and Karon1955), but fewer report the effect in the field, particularly, from a large sample as in our study. However, in the field environment in which seeds are most often cured in tropical rice growing regions air temperature and RH do not remain constant. The present study evaluated the sorption behaviour of rice using climatic data during field curing in the wet and dry environments for 5 weeks. The relationship between MC and water activity for the three rice species was similar and sigmoidal in the wet and dry cured environments (Figure 3). However, the reflection point or multi-molecular (water-to-water molecules) water binding sites (Vertucci and Leopold, Reference Vertucci and Leopold1987) dominated the sigmoid curve more in the wet cured than the dry cured environment. The difference in the shape of the relationships between the wet and dry cured environments might be due to inter-environment differences in temperature and hydration levels. When compared with dry cured environment, lower temperatures in the wet environment could be due to evaporation from soil and plant tissues. In contrast, heat produced from respiratory activities of seed and fungi within the dry cured environment could have led to higher temperatures. Differences in hydration levels suggested hydration induced structural changes in cellular components due to fewer number of multilayer of water molecules (water-to-water molecules) or tighter molecular packing of polymer chains resulting from the higher temperatures in the dry cured environment (Vertucci and Leopold, Reference Vertucci and Leopold1987). The dominance of multi-molecular water binding sites in the wet cured environment would have implications for seed storage as high-water activity could result in deteriorative processes leading to vigour and germination decline. The similarity of the sorption characteristics for the species in the wet and dry curing environments (Figure 3) suggested that these species had similar chemical composition (Vertucci and Leopold, Reference Vertucci and Leopold1987).
At harvest, 4% of the seeds exhibited endospermic cracks in all the three species (Figure 4), which probably occurred during grain filling or maturation. Alternate drying and wetting cycles during field curing, especially during unfavourable climatic conditions, resulted in the development of additional cracks (Kunze, Reference Kunze2008). Higher percentage cracks observed in the wet cured seeds might be as a result of higher magnitudes of diurnal moisture change resulting from moisture desorption and re-adsorption and large moisture differentials between the internal and surface of seeds in the wet cured environment. Such moisture differentials can induce tensile stresses at the centre of the grain as accumulated moisture induces seed surface layer expansion (Kunze and Choudhury, Reference Kunze and Choudhury1972). In contrast, within the dry cured environment (where diurnal changes in seed MC remained almost constant) (Figure 2C), lower rate of moisture adsorption presumably allowed for moisture to diffuse deeper and at a much slower rate into the grain thereby reducing the intensity of tensile stresses at the centre of the grain (Kunze, Reference Kunze2008). This difference may also have accounted for the displacement of the time course for cracking in the dry cured below the wet cured environment until they all reached 100% (Figure 4).
B. oryzae and C. lunata are two seed-borne fungi that were most frequently isolated across the three species at harvest (Table S1) and suggested that infection occurred during crop growth or after maturation, before harvest. These two fungal pathogens were also significantly more predominant in the dry cured than wet cured seeds (Table S1). The total number of seeds infected with specific fungal species in the wet cured environment was lower than the dry cured environment (Table 2) probably due to the release of anti-fungal proteins and hydrolytic enzymes, which accumulate in dead seed coats or pericarps, upon hydration of the wet cured seeds (Raviv et al., Reference Raviv, Aghajanyan, Granot, Maover, Frenkel, Gutterman and Grafi2017). The water activity of wet cured seeds was also more frequently above 0.85 (Figure 3B), and this may have facilitated higher activity of such anti-fungal enzymes, if present. Rossi et al. (Reference Rossi, Scandolara and Battilani2009) also suggested that reduction in number of seeds infected with fungi in environments subjected to precipitation might be due to washing off of conidia by rain or heavy dew or by dispersal of conidia by wind. They showed that sampling stalk residues of maize from the field after rainfall decreased the number of fungal spores in field exposed stalk residues compared with maize stalks kept in a dry condition. In contrast, wide temperature range within the dry cured environment (Figure 1) probably presented a more conducive environment for fungal sporulation, and hence higher fungal infection from seeds cured in this environment.
Soil-borne fungal species, e.g., Botrytis sp, Alternaria sp, S. rolfsii, Trichoderma sp and Verticillium sp (Bonner et al., Reference Bonner, Vozzo, Elam and Land1994; Notteghem et al., Reference Notteghem, Roux-Cuvelier, André, Roumen and Chataigner1997), were mostly isolated from wet cured seed samples (Table S1). The absence of these fungal species in the control seed samples at harvest, their complete absence or occurrence in weeks four and five samples only in the dry cured environment, and occurrence mainly in wet cured seeds (Table S1) demonstrated the impact of prolonged field curing on the invasion of soil-borne fungal species. Pyricularia oryzae was not isolated in this study, despite its reputation as a seed-borne pathogen of rice, and this is consistent with the findings of Fisher and Petrini (Reference Fisher and Petrini1992), who did not also isolate P. oryzae from rice seeds. The work of Ou (Reference Ou1985) suggests that this may be because the fungus requires rice plant extracts in culture media to grow.
Germination, seedling root length and dry weight all declined with delayed curing (Table 3). This was to be expected based on the increased endosperm cracking, and fungal infection with delayed curing. Fungal activity has a deleterious effect on seed germination and vigour (Nghiep and Gaur, Reference Nghiep and Gaur2004). Unfavourable climatic conditions such as high temperature, RH and rainfall during post-harvest field curing impacted negatively on the physiological quality of seeds (TeKrony et al., Reference TeKrony, Egli and Phillips1979). The effect of deteriorated or poor quality seed is manifested in vigour loss, slower, uneven rate of germination, reduced seedling vigour and seedling abnormalities (Groot et al., Reference Groot, Surki, de Vos and Kodde2012).
Seed germination was higher in the dry cured compared with the wet cured environment (Table 3). In contrast, seedling root length and dry weight were lower in the dry cured compared with the wet cured environment (Table 3). Germination count was made 7 DAI of germination, whereas measurement of seedling root and dry weight determination were made 14 DAI. By 14 DAI, there were mycelia growth mostly observed on seedlings from the dry cured environment and may explain the observed trend. Despite lower MC and water activity in the dry cured environment, high temperature (Figure 1) was probably supportive of fungal sporulation and mycotoxin contamination of seeds and this may explain why higher numbers of seeds were infected with fungi in this environment. Decline in seed germinability, and seedling vigour, fungal sporulation and grain colonization have been reported as a consequence of increasing temperature and RH levels (Topani et al., Reference Topani, Rachana, Navi, Thakur, Bandyopadhyay, Varanavasiappan and Seetharama2007). The presence of some seed-borne pathogens in the germinating seeds resulted in seedling decay thereby affecting the seedling survival. B. oryzae, Alternaria padwickii and C. lunata were isolated from rice seedlings with decayed roots and shoots, while B. oryzae, C. curvularia and Aspergillus spp were also isolated from seedlings with short roots in previous studies (Nghiep and Gaur, Reference Nghiep and Gaur2004). In our study, B. oryzae, C. lunata and Aspergillus sp preferentially colonized dry cured seeds and might have also been responsible for the observed decline in seedling weight and root length observed in seedlings arising from dry cured seeds (Tables S1 and 3).
In this study, prolonged field curing compromised the integrity of the endosperm by promoting multiple cracks in the endosperm. The dry cured environment reduced seed MC and the amount of active water in seeds, and delayed cracking, implying that it has greater potential to minimize seed deterioration during curing and subsequent storage than wet curing. Curing environment did not influence the number of seeds with fungal infection in O. glaberrima but dry curing was associated with higher fungal infection in O. sativa and O. sativa × O. glaberrima. Fungal infection of seeds in the dry cured environment was higher compared with wet cured seeds. Prolonged field curing also increased the vulnerability of wet cured seeds to soil-borne fungal infection. Species, curing environment and delayed field curing significantly influenced seed germination and seedling vigour. Germination and seedling vigour declines with prolonged field curing have implications not only for seed quality, but also subsequent seedling establishment and grain yield. These findings suggest that dry curing could be an option for improving farmers’ current post-harvest practices due to its positive effect on the management of MC, seed germinability and its ability to delay endosperm cracking; however, research on how fungal infection could be curbed under such conditions is needed.
Acknowledgements
The authors are grateful to Kofi Lelabi Kota, Samuel Reyes Tandoh, Daniel Gamenya and Isaac Tawiah, research technicians at CSIR-Crops Research Institute (CRI), Kumasi, Ghana, for assisting in the field and laboratory work in Ghana, and Miss Nombali Gumede, Philani Ndlovu and Prince Sandile Khuzwayo formerly research assistants at the Plant Germplasm Conservation Research, School of Life Sciences, University of KwaZulu-Natal (UKZN), South Africa for assisting in the laboratory work at UKZN. The authors acknowledge the financial support through the National Research Foundation (NRF), South Africa and CSIR-Crops Research Institute, Ghana to RKB.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S001447971800008X