INTRODUCTION
Aluminium (Al) toxicity is one of the greatest limitations to plant productivity in acidic soils in the tropical and subtropical areas of the world (Kochian et al., Reference Kochian, Hoekenga and Piñeros2004). In these soils, with pH values below 5.5, toxic forms of Al, particularly Al3+, become soluble and are absorbed by plants (Kochian et al., Reference Kochian, Hoekenga and Piñeros2004), resulting in growth reductions, poor plant development and low plant productivity (Chen et al., Reference Chen, Qi, Jiang and Yang2010; Kochian et al., Reference Kochian, Hoekenga and Piñeros2004; Silva et al., Reference Silva, Pinto-Carnide, Martins-Lopes, Matos, Guedes-Pinto and Santos2010). The primary symptom of Al toxicity is a rapid inhibition of root growth, resulting in a limited water and mineral nutrient uptake. Most of the absorbed Al remains in the roots (Kochian et al., Reference Kochian, Hoekenga and Piñeros2004), but a small proportion can be translocated to the leaves.
Some of the effects of Al on the photosynthetic process are apparently initiated as a consequence of its toxic effects, which are primarily manifested at the root level. Several studies show that Al interferes with the absorption and/or transport of essential mineral nutrients to the leaves (Giannakoula et al., Reference Giannakoula, Moustakas, Mylona, Papadakis and Upsanis2008), resulting in low rates of net CO2 assimilation (A) and reduced biomass accumulation (Jiang et al., Reference Jiang, Chen, Zheng, Han, Tang and Smith2008). In any case, the mechanisms by which Al may affect the photosynthetic apparatus remain unclear. In fact, impairments to A, which can occur through stomatal and non-stomatal factors (Jiang et al., Reference Jiang, Chen, Zheng, Han, Tang and Smith2008; Peixoto et al., Reference Peixoto, DaMatta and Cambraia2002), may vary both inter- and intra-specifically; additionally, these impairments also depend on such factors as the age of the plant, Al concentration and exposure time to Al. In Citrus grandis and Thinopyrum bessarabicum, for example, Al treatment provoked stomatal closure and the inhibition of electron transport rates (ETRs; Jiang et al., Reference Jiang, Chen, Zheng, Han, Tang and Smith2008; Moustakas et al., Reference Moustakas, Eleftheriou and Ouzounidou1997), suggesting both stomatal and photochemical limitations to A. In Citrus reshni, Al led to decreases in A, but, intriguingly, Al increased or did not affect the activity of the enzymes of the Calvin cycle (Chen et al., Reference Chen, Qi, Smith and Liu2005). In this case, it is likely that the main toxic effects of Al were manifested in the chloroplast ultrastructure (Moustakas et al., Reference Moustakas, Eleftheriou and Ouzounidou1997) rather than in the CO2-fixation enzymes per se, which ultimately could result in depressed ETRs. Decreases in the ETRs were also observed in isolated chloroplasts from Al-treated corn hybrids (Mihailovic et al., Reference Mihailovic, Drazic and Vucinic2008). The ETR was depressed in Citrus grandis exposed to varying Al concentrations, with no effect on the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco; Jiang et al., Reference Jiang, Chen, Zheng, Han, Tang and Smith2008), findings that are in contrast to the results obtained with rye in which the Rubisco activity was impaired due to Al application (Silva et al., Reference Silva, Pinto, Dias, Correia, Moutinho-Pereira, Pinto-Carnide and Santos2012).
Rice (Oryza sativa) has been reported to be the most Al-tolerant cereal crop under field conditions and is capable of withstanding significantly higher concentrations of Al than other major cereals (Foy, Reference Foy1988). Although the genetic variability of some rice cultivars in terms of Al toxicity has been documented (Mendonça et al., Reference Mendonça, Cambraia, Oliva and Oliveira2005), the mechanisms for the high Al resistance of rice are not well understood (Famoso et al., Reference Famoso, Clark, Shaff, Craft, McCouch and Kochian2010; Ryan and Delhaize, Reference Ryan and Delhaize2010). Thus, there is an urgent need to increase our understanding of the mechanisms that govern rice tolerance to Al stress. The objective of this work was, therefore, to evaluate the effects of Al on the photosynthetic apparatus in two rice cultivars with contrasting tolerances to Al.
MATERIAL AND METHODS
Plant material and growth conditions
Two rice cultivars (Oryza sativa L.), Fernandes (CNA-1158) and Maravilha (CNA-6843-1), considered tolerant and sensitive to Al, respectively, were used in this study. Seeds, obtained from the Brazilian Center for Rice and Bean Research (EMBRAPA, Goiânia, GO, Brazil), were selected for size uniformity and form and were treated with concentrated sulphuric acid for 1 min. After being washed in running water, the seeds were surface sterilized with 2% (v/v) sodium hypochlorite for 15 min and washed again in running, deionized water. The seeds were germinated in rolls of neutral paper dipped in Clark's nutrient solution (Clark, Reference Clark1975), pH 4.0, at one-third of the original ionic strength under continuous aeration (Mendonça et al., Reference Mendonça, Cambraia, Oliva and Oliveira2005). After nine days, the seedlings were selected and transplanted into polyethylene pots containing 1.8 L of Clark's nutrient solution (pH 4.0) and then exposed to 0 and 1.0 mM Al, applied as anhydrous AlCl3. The nutrient solutions were maintained under continuous aeration, and the pH was adjusted daily to 4.0 using NaOH 0.1 N or HCl 0.1 N. The experiment was performed in a temperature-controlled growth chamber (25 ± 3 °C), under a photosynthetic photon flux of 230 μmol m−2 s−1 and a 16 h photoperiod. After applying the Al treatments for 10 days, the photosynthetic parameters were measured in the early morning (see below), and the plants were harvested and washed thoroughly in deionized water for biomass determination and further analyses.
Al content
The dry, powdered plant material was mineralized in a nitric-perchloric mixture (3:1, v/v). The Al content was determined using a modified aluminon method (Wang and Wood, Reference Wang and Wood1973).
Biomass
The plant tissues were oven-dried at 70 °C for 72 h, and the dry weights of the leaves and roots were determined.
Leaf gas exchange and chlorophyll a fluorescence parameters
The net carbon assimilation rate (A), stomatal conductance to water vapour (g s), transpiration rate (E) and internal CO2 concentration (C i) were always measured on the second fully developed attached leaves in the morning (8:30–9:30 h) using a portable, open-system infrared gas analyser (Portable Photosynthesis System LI-6400, LI-COR Inc., Lincoln, NE, USA) equipped with a blue/red light source (LI-6400–02B). The measurements were made under artificial irradiance of 800 μmol photons m−2 s−1 at the leaf level and 400 μL CO2 L−1 of air. All measurements were performed at 25 °C, and the vapour pressure deficit was maintained at approximately 1.0 kPa, while the amount of blue light was set to 10% of total irradiance to optimize the stomatal aperture.
The kinetics of the chlorophyll (Chl) a fluorescence induction were measured using a 5.5-mm fibre optic probe interfaced with a portable pulse amplitude modulation fluorometer (MINI-PAM, Heinz WALZ, Effeltrich, Germany), in parallel to the gas exchange measurements described above (using the same leaf). The probe, conducting saturating pulses, measuring and actinic light (see below), was held 1.2 cm from the surface of leaf blades at a 60° angle using a standard leaf clip. Following a dark adaptation for 30 min using leaf clips, the leaf tissue was illuminated with a weak modulated measuring beam (0.03 μmol m−2 s−1) to obtain the initial fluorescence (F 0). A saturating white light pulse of 6000 μmol m−2 s−1 was applied for 0.8 s to ensure the maximum fluorescence emission (F m) from which the variable-to-maximum fluorescence ratio F v/F m = [(F m–F 0)/F m)] was calculated. This ratio has been used as a measure of the potential photochemical efficiency of photosystem II (ΦPSII). The leaf tissue was exposed to actinic photon irradiance (800 μmol m−2 s−1) for 30 s to obtain the steady-state fluorescence yield (F s). Subsequently, a saturating white light pulse (3000 μmol m−2 s−1; 0.8 s) was applied to achieve the light-adapted maximum fluorescence (F m′). The light-adapted initial fluorescence (F 0′) was estimated according to Oxborough and Baker (Reference Oxborough and Baker1997). Using these parameters, the photochemical (q P) and non-photochemical quenching (NPQ) coefficients, the quantum yield of PSII electron transport (ΦPSII) and the apparent ETR were calculated, as described elsewhere (Cruz et al., Reference Cruz, Mosquim, Pelacani, Araújo and DaMatta2003).
Chloroplast pigments
The chlorophylls (a and b) and carotenoids were extracted by grinding the leaves in aqueous acetone 80% (v/v), and the absorbances of the extracts were spectrophotometrically measured at wavelengths of 470.0, 646.8 and 663.2 nm, according to Lichtenthaler (Reference Lichtenthaler1987).
Statistical analysis
The treatments were arranged in randomized blocks following a 2 × 2 factorial scheme (two cultivars and two Al levels), with three plants in individual pots per treatment combination serving as conditional replicates. The data were subjected to an analysis of variance, and the means were compared using the F test at a 5% probability.
RESULTS
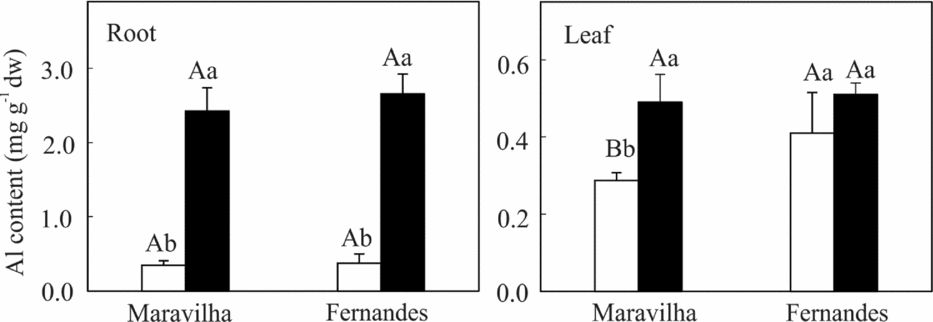
Compared with the control individuals, the Al-treated plants (both the Al-sensitive cv. Maravilha and the Al-tolerant cv. Fernandes) displayed dramatic increases (approximately 700%) in the Al concentration in their root tissues. In contrast, the Al concentration in the leaves increased to a lesser extent (approximately 70%) only in Maravilha (Figure 1). Notably, the Al concentrations in both the roots and leaves were essentially similar between the cultivars.

Figure 1. Aluminium content in the roots and leaves of two rice cultivars exposed to 0 (□) and 1 mM (■) aluminium for 10 days. The means followed by the same capital letter between the cultivars and the same small letter between the Al levels within each cultivar do not differ significantly (p ≥ 0.05, F test). The bars represent the mean ± SD of triplicates.
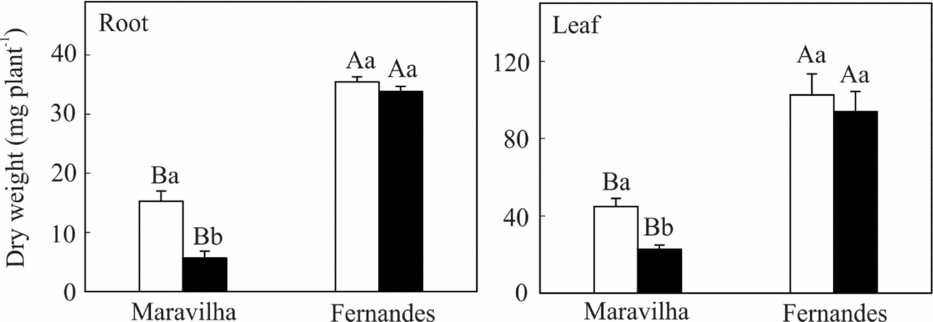
The Al treatment resulted in significant decreases in the biomasses of the roots (62%) and leaves (50%) for Maravilha, whereas no noticeable effect of Al on the biomass was found for Fernandes (Figure 2). Irrespective of the Al treatment, Fernandes accumulated biomass in both roots and leaves to a greater extent than did Maravilha.

Figure 2. Root and leaf dry weights of two rice cultivars exposed to 0 (□) and 1 mM (■) aluminium for 10 days. The means followed by the same capital letter between the cultivars and the same small letter between the Al levels within each cultivar do not differ significantly (p ≥ 0.05, F test). The bars represent the mean ± SD of triplicates.
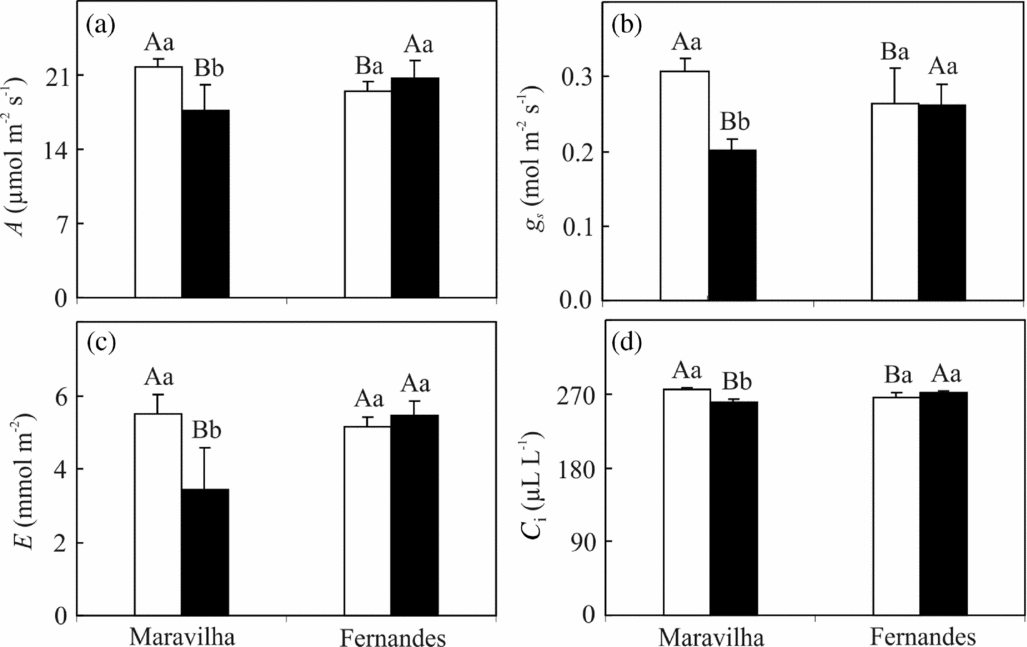
Under the control conditions, Maravilha showed slightly higher (though significant) values of the net carbon assimilation rate (A), stomatal conductance (g s) and internal CO2 concentration (C i) compared to Fernandes, whereas the transpiration rate (E) did not differ between the cultivars (Figure 3). In response to Al treatment, these leaf gas-exchange parameters were all depressed in Maravilha yet remained unchanged in Fernandes. It should be noted that, in the former cultivar, the value of g s decreased proportionally more than A; therefore, the observed decreases in C i suggest stomatal limitations to photosynthesis.

Figure 3. Net carbon assimilation rate, A (a), stomatal conductance, gs (b), transpiration rate, E (c) and internal CO2 concentration, Ci (d) in two rice cultivars exposed to 0 (□) and 1 mM (■) aluminium for 10 days. The means followed by the same capital letter between the cultivars and the same small letter between the Al levels within each cultivar do not differ significantly (p ≥ 0.05, F test). The bars represent the mean ± SD of triplicates.
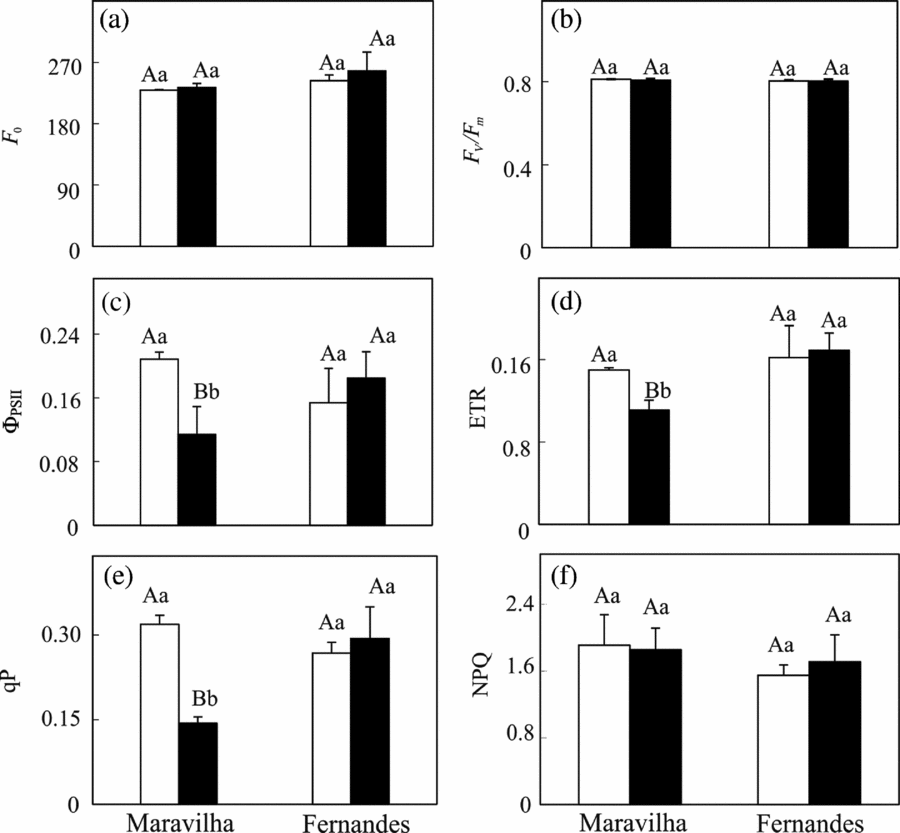
Both the initial fluorescence (F 0) and potential photochemical efficiency of PSII (F v/F m) were unresponsive to the Al treatment, independently of the cultivar (Figures 4a and b). In Maravilha, the addition of Al provoked significant decreases in the photochemical quenching coefficient (q P), quantum yield of PSII electron transport (ΦPSII) and apparent ETR, in parallel to an unchanging NPQ coefficient (). These parameters all remained at the control levels in Fernandes (Figures 4c–f).

Figure 4. Minimum chlorophyll a fluorescence (F 0) (a), variable-to-maximum chlorophyll fluorescence ratio (F v/F m) (b), effective quantum yield of PSII (ΦPSII) (c), electron transport rate (ETR) (d), photochemical quenching coefficient (q p) (e) and non-photochemical quenching coefficient (NPQ) in two rice cultivars exposed to 0 (□) and 1 mM (■) aluminium for 10 days. The means followed by the same capital letter between the cultivars and the same small letter between the Al levels within each cultivar do not differ significantly (p ≥ 0.05, F test). The bars represent the mean ± SD of triplicates.
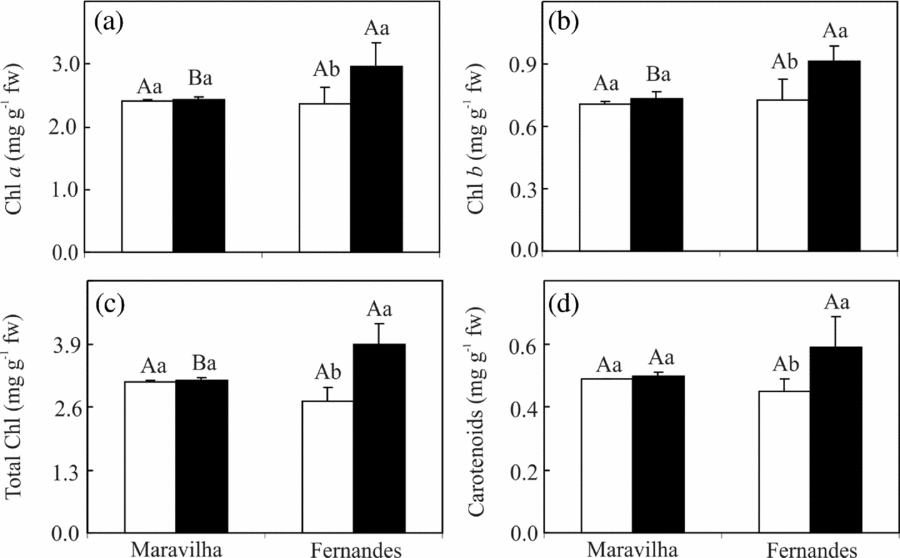
The Al treatment did not affect the concentrations of the photosynthetic pigments in Maravilha; in contrast, it increased the concentrations of total Chl and total carotenoids, coupled with an unaltered Chl a/b ratio, were noted in Al-treated Fernandes compared with its control (Figure 5).

Figure 5. Total chlorophylls, Chl (a + b), Chl a/b ratio (a–c) and carotenoid concentration (d) in two rice cultivars exposed to 0 (□) and 1 mM (■) aluminium for 10 days. The means followed by the same capital letter between the cultivars and the same small letter between the Al levels within each cultivar do not differ significantly (p ≥ 0.05, F test). The bars represent the mean ± SD of triplicates.
DISCUSSION
The inhibition of root growth is one of the earliest and key toxic effects of Al on plants, and, therefore, most research has focused on the toxicity of Al in root tissues (Chen et al., Reference Chen, Qi, Smith and Liu2005). Although some recent efforts have been undertaken to improve our understanding on the effects of Al on the leaves, significant uncertainties remain whether Al may directly or indirectly affect the photosynthetic apparatus. Here, we show evidence that Al may constrain the photosynthetic performance, and thus biomass accumulation, through indirect factors, as the toxic effects of Al were manifested only in the Al-sensitive cultivar compared to its tolerant counterpart, despite the quite similar contents of Al in the cultivars. Therefore, others Al-tolerance mechanisms, rather than Al-exclusion mechanisms, should have played increased roles in explaining the differential genotypic abilities to cope with Al stress in this study (Ryan and Delhaize, Reference Ryan and Delhaize2010). It should be emphasized that we had already demonstrated that the cultivar Fernandes not only produces more biomass than Maravilha but it also exhibits a higher tolerance to Al under varying Al levels and exposure times (Justino et al., Reference Justino, Cambraia, Oliva and Oliveira2006; Mendonça et al., Reference Mendonça, Cambraia, Oliva and Oliveira2005).
However, the differences in A do not fully explain the genotypic differences in biomass because A decreased to a lesser extent than biomass in Al-treated Maravilha. Possibly, this cultivar possesses a lower inherent ability to redirect biomass to construct a more robust leaf area, as evidenced by its lower overall biomass accumulation, despite the larger A relative to Fernandes, as demonstrated under the control conditions.
Although the mesophyll resistance to CO2 flux into the chloroplasts or the biochemical limitations to CO2 fixation cannot be ruled out as factors in this study, we demonstrated that the stomatal factors played a key role in limiting A in the Al-sensitive cultivar, particularly because g s decreased to a greater extent than A in parallel to the decreases in C i. Aluminium-induced decreases in g s, through an as-yet unresolved mechanism (Chen et al., Reference Chen, Qi, Jiang and Yang2010), have been reported for other plant species (Akaya and Takenaka, Reference Akaya and Takenaka2001; Peixoto et al., Reference Peixoto, DaMatta and Cambraia2002). One possible mechanism concerns the reduced root water uptake that would trigger stomatal closure, as suggested for rice (Mendonça et al., Reference Mendonça, Cambraia, Oliveira and Oliva2003), even though this effect has been considered unimportant in Quercus glauca (Akaya and Takenaka, Reference Akaya and Takenaka2001). Recent information has noted that an Al-activated malate transporter in guard cells may be involved in stomatal closure (Meyer et al., Reference Meyer, Mumm, Impes, Endler, Weder, Al-Rasheid, Geiger, Marten, Martinoia and Hedrich2010), suggesting a direct effect of Al on stomatal movements. In any case, the unresponsiveness of g s to Al in the Fernandes plants may largely explain the maintenance of its gas exchange rates and biomass at the control values.
In addition to the stomatal limitations to A, photochemical constraints also possibly limited the actual A in the Al-treated Maravilha plants, particularly because ΦPSII and, consequently, the ETR decreased to greater extents than A. Considering that carbon fixation, the usual main sink for the absorbed light in chloroplasts, was depressed in Maravilha under Al stress, adjustments in the capture, use and dissipation of light are required to provide photoprotection to the photosynthetic apparatus. Because the Chl pools (largely associated with light capture) were unaltered in response to Al in Maravilha, the decreases in A should lead to a surplus excitation energy, that could potentially lead to photoinhibition given the limited ability of Maravilha to safely dissipated such excess as heat, as evidenced by its unchanged NPQ values in response to Al stress (Krause and Weis, Reference Krause and Weis1991). In addition, the portion of oxidized Q A (analysed as q p) decreased remarkably in Al-treated Maravilha, thus representing a fraction of PSII centres prone to suffer photoinhibitory damage (Lima et al., Reference Lima, DaMatta, Pinheiro, Tótola and Loureiro2002). Irrespective of these facts, the F v/F m ratio was maintained at high values (~0.80), coupled with an unchanging F 0 and, therefore, we argue against the possibility of occurrence of photoinhibitory damages under the present experimental conditions. The unresponsiveness of both F v/F m and F 0 to the Al stress contrasts with the results reported for Thinopyrum bessarabicum (a wild relative of wheat; Moustakas et al., Reference Moustakas, Eleftheriou and Ouzounidou1997) and sorghum (Peixoto et al., Reference Peixoto, DaMatta and Cambraia2002) plants (that were grown under light intensities similar to those of this current study) in which these parameters decreased in response to Al. In any case, it must be emphasized that the rice plants were grown under relatively low photon irradiances; had they been grown under field conditions where irradiances can reach values higher than 2000 μmol photons m−2 s−1, photoinhibitory damages caused by Al stress should be expected.
In contrast to several plant species (e.g., sorghum (Peixoto et al., Reference Peixoto, DaMatta and Cambraia2002), soybean (Milivojevic and Stojanovic, Reference Milivojevic and Stojanovic2003) and corn (Mihailovic et al., Reference Mihailovic, Drazic and Vucinic2008)) in which a decrease in the concentrations of chloroplastidic pigments has been noted due to Al stress, we found unaltered (Maravilha) or enhanced (Fernandes) pigment concentrations. The reported decreases in pigment pools have often been associated with the impacts of Al on the uptake and/or transport of several essential mineral nutrients required for chloroplastidic pigment biosynthesis (Giannakoula et al., Reference Giannakoula, Moustakas, Mylona, Papadakis and Upsanis2008). Nonetheless, in Fernandes leaves, Justino et al. (Reference Justino, Cambraia, Oliva and Oliveira2006) have previously demonstrated an increase in the nitrogen content in response to Al, which could circumstantially explain the increased Chl concentrations, considering that Chl biosynthesis is highly responsive to the nitrogen content in the leaves (Mihailovic et al., Reference Mihailovic, Drazic and Vucinic2008). Furthermore, studying the effects of Al on the same rice cultivars used in this study, Mendonça et al. (Reference Mendonça, Cambraia, Oliveira and Oliva2003) found an improved macronutrient (Ca, Mg, P and K) use efficiency in Fernandes compared to Maravilha, lending additional support to explain the increases in Chl in Fernandes.
CONCLUSION
In summary, our data indicate that Al affects both the growth of the roots and also the growth of the leaves. In the Al-sensitive Maravilha, Al induced stomatal, and most likely photochemical, constraints on photosynthesis, with no apparent signs of photoinhibition. In contrast, no alterations in biomass accumulation and photosynthetic performance due to the Al supplementation were evident in the Al-tolerant Fernandes, despite the similar Al levels of the cultivars. These results highlight a higher intrinsic ability to cope with Al stress in Fernandes.
Acknowledgements
We gratefully acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) for financial support and fellowships.