INTRODUCTION
As consumers seek healthy and more diverse food products, the demand for tropical fruit has increased significantly during the last 15 years with an estimated value of production at $18 billion in 2009 (Food and Agriculture Organization of the United Nations, 2011). Mamey sapote (Pouteria sapota) is native to Mexico and central American countries as far south as northern Nicaragua (Balerdi and Shaw, Reference Balerdi, Shaw, Shaw, Chan and Nagy1998; Mossler and Crane, Reference Mossler and Crane2009). It is also cultivated in the Caribbean, Florida and other tropical and subtropical regions of the world (Tellez et al., Reference Téllez, Saucedo, Arévalo and Valle2009). The tree thrives from sea level to 900 m in elevation and under an annual rainfall of 2000 mm. It adapts to a wide range of soil types including sandy or heavy soils; however, it does not withstand dry periods or waterlogged soils (Goenaga and Jenkins, Reference Goenaga and Jenkins2012). Depending on the cultivar, fruit shape varies from round to elliptical; it has a leathery brown skin and contains one to three large seeds (Goenaga and Jenkins, Reference Goenaga and Jenkins2012). Fruit pulp is sweet, soft and orange or deep red in color when ripe, and it is consumed fresh or processed to prepare ice cream or milkshakes. The fruit is high in vitamins A and E, minerals and carotenoid content (U.S. Department of Agriculture, 2011).
As with many other tropical fruit crops, there is a scarcity of information on the best management practices and optimum growing conditions for mamey sapote trees. For example, little is known about the adaptability of mamey sapote to high acidic soils. The most productive soils of the world are already under cultivation, and those available for agricultural expansion, particularly in the tropics, are often strongly acidic, possessing toxic levels of soil aluminum (Al) (Kamprath, Reference Kamprath and Adams1984; Samac and Tesfaye, Reference Samac and Tesfaye2003). The mechanism by which soil acidity reduces the yield of many crops has been studied extensively (Horst et al., Reference Horst, Wang and Eticha2010; Kochian et al., Reference Kochian, Pence, Letham, Pineros, Magalhaes, Hoekenga and Garvin2002). A high concentration of Al restricts root growth and hence exploitation of soil/subsoil by roots for moisture and nutrients. Soil Al concentrations as high as 15 cmol kg−1 can be found in tropical acid soils; in the tropical Americas, about 50% of the soils with potential for agricultural use have been diagnosed with Al toxicity problems (Committee on Sustainable Agriculture and the Environment in the Humid Tropics et al., 1993; Hoekenga et al., Reference Hoekenga, Maron, Piñeros, Cancado, Shaff, Kobayaski, Ryan, Dong, Delhaize, Sasaki, Matsumoto, Yamamoto, Koyama and Kochian2006; Zheng, Reference Zheng2010). We are not aware of published studies conducted to screen mamey sapote germplasm for acid soil tolerance under field conditions. The objective of this investigation was to determine the critical soil Al concentrations that affect growth of mamey sapote germplasm on acid soils under field conditions and to identify potential sources of tolerance to this stress.
MATERIALS AND METHODS
Field experiments were established on 10 June 2010 (year 1) and 12 July 2011 (year 2) at the Corozal Research Station of the University of Puerto Rico. The study was conducted on a deep, well-drained Ultisol (Aquic Tropudult) in seven 3.65 × 3.65 m blocks arranged in a randomized complete block design. Blocks differed in soil acidity due to differential applications of calcitic limestone many years prior to the experiment to achieve levels of soil Al. Soil from each block was sampled before planting by taking 15 borings at a depth of 0–20 cm from each plot. The samples were air-dried and passed through a 20-mesh screen. Soil pH in water and 0.01 M calcium chloride (CaCl2) (soil: water ratio = 1:2) was measured with a glass electrode. Potassium chloride(KCl) extractable Al was determined using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES), and exchangeable cations, extracted with neutral 1 M ammonium acetate (NH4OAc), was also determined similarly. Percentage Al saturation of the soil was calculated on the assumption that exchangeable calcium (Ca) + magnesium (Mg) + potassium (K) + Al + hydrogen (H) was the effective cation exchange capacity of the soil (Kamprath, Reference Kamprath and Adams1984).
All blocks were sown with open-pollinated seeds from mamey sapote seedlings, i.e., ‘Copan’, ‘Magaña’, ‘Pantin’ and ‘Tazumal’. Literature on the exact origin of these cultivars is non-existent, however, the original source of these materials are presumed to be from Mexico, Guatemala and Belize and selections made later in Florida by scientists at the Tropical Research Education Center of the University of Florida and by nursery owners in Florida (Balerdi and Shaw, Reference Balerdi, Shaw, Shaw, Chan and Nagy1998). To our knowledge, these cultivars have never been field-tested under a wide range of soil Al concentrations, which in this study ranged from 3.5 to 14.02 cmol kg−1. The pHH2O and pHCaCl2 in these plots ranged from 4.11 to 4.46 and 3.44 to 3.89, respectively. Soil Ca ranged from 151 to 595 mg kg−1 and soil Mg from 46 to 80 mg kg−1. Seed of each cultivar was planted in each block at a depth of 5 cm in a 3 m row. Upon germination, rows were thinned to 10 plants row−1. Rows were 91 cm apart with plants being 30.5 cm apart within the row.
Plants in each row were side-dressed with a commercial mixture of nitrogen (N)–phosphorus (P)–K–Mg (10–2.2–12.5–1.8%, respectively) applied at a rate of 670 kg ha−1 two weeks after planting. Sources of these nutrients were ammonium sulfate, phosphoric acid, potassium oxide and magnesium oxide, respectively.
Trees were harvested for biomass accumulation about 13 months after field planting during the weeks of 24 June 2011 in year 1 and 22 August 2012 in year 2. At each harvest, stem diameter was measured with a digital caliper at 25 cm from the soil. Soil was then loosened with a garden fork, and eight plants from each cultivar in each row were pulled from the soil, washed and separated into leaves, stem and roots. Plant parts from each variety were dried at 70 °C to constant weight for dry matter determination. The dry samples were ground to pass a 1.0-mesh screen using a Wiley mill and analysed for N, P, K, Ca, Mg, Al and manganese (Mn). For this purpose, tissue samples were incinerated in crucibles at 500 °C for 4 h, and allowed to cool overnight. The incinerated samples were digested with 20 mL 33% HCl until 10 mL of solution remained in the crucible. After digestion was completed, each sample was filtered through Whatman No. 541 filter paper into a 100 mL volumetric flask. The solution was used for nutrient determination (Perkin-Elmer, 1994; USEPA, 2014) using a PE 7300DV inductively coupled plasma-optical emission spectrometer (Perkin-Elmer, Shelton, CT, USA). Total nitrogen was determined by a modification of the micro-Kjeldahl method (Foss, 2002). For this purpose, 0.2 g of tissue was weighed and transferred to a Kjeldahl tube. The following compounds were added to each tube: Hengar granules for smooth boiling; one catalyzing tablet (1.5g K2SO4 + 0.15g CuSO4); 5mL concentrated H2SO4 and 3 mL 30% H2O2. Samples were digested in a digestion block for 2 h at 380 °C.
Analyses of variance and regression analyses were done using the GLM procedure of the SAS program package (version 9.4, SAS Institute, Inc., Cary, NC, USA). Only coefficients at p ≤ 0.05 were retained in the models.
RESULTS AND DISCUSSION
Differences among soil Al treatments were highly significant (p ≤ 0.01) for total, leaf, stem and root dry weight at the end of the experimental period (analysis of variance not shown). Cultivars and the cultivar × year interaction were not significant. Therefore, results were averaged over cultivar and years.
Increasing soil Al concentration from about 3.5 cmol kg−1 to 7.8 cmol kg−1 resulted in an increase in total dry weight by more than 25 % (Figure 1a). Trees attained maximum total by weight at a soil Al concentration of 7.8 cmol kg−1. Soil Al concentrations higher than 7.8 cmol kg−1 resulted in a significant reduction of dry weight in all plant parts (Figure 1b–d). At this Al concentration, leaf, stem and root dry weight accounted for 37 %, 34 % and 29 %, respectively, of the total dry weight. At the highest soil Al concentration (14.0 cmol kg−1), leaf, stem and root dry weights were reduced by 30 %, 42 % and 35 %, respectively, when compared to maximum dry weights attained at a soil Al concentration of 7.8 cmol kg−1. However, as a percentage of total dry weight at this soil Al concentration (14.0 cmol kg−1), leaf, stem and root dry weight allocations were 40 %, 31 % and 29 %, respectively, of the total dry weight, which are not too different from allocations at a soil Al concentrations of 7.8 cmol kg−1. This response contrasts that found in longan (Dimocarpus longan) and rambutan (Nephelium lappaceum), in which high soil Al significantly reduced the dry weight of all plant parts but root dry weight was less affected than that of other plant organs (Goenaga, Reference Goenaga2011; Reference Goenaga2013).

Figure 1. Dry weight of plant organs of mamey sapote as influenced by soil aluminum. Each point is the mean value of two replications and eight trees per replication.
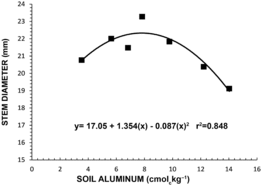
As with dry matter accumulation, stem diameter significantly increased until soil Al concentration reached 7.8 cmol kg−1 and then declined (Figure 2). The increase in plant dry weight and stem diameter with increasing levels of soil Al up to 7.8 cmol kg−1 (Figures 1 and 2) indicates that mamey sapote has some tolerance to high soil Al. In a similar study with longan, all varieties tested were found to be very susceptible to soil Al concentrations higher than 5.1 cmol kg−1 (Goenaga, Reference Goenaga2013). Studies conducted with corn, wheat, soybean, sweet potato and Brachiaria grown in acid soils showed that growth of plants under 60 % Al saturation was reduced to half when compared to plants grown in limed soils (Kamparath, Reference Kamprath and Adams1984). In this experiment, plants reached maximum dry matter production and stem diameter at Al concentration of 7.8 cmol kg−1, which corresponded to 56 % Al saturation (Figure 3) and provided additional evidence that mamey sapote has some tolerance to high soil Al. Accordingly, Goenaga and Jenkins (Reference Goenaga and Jenkins2012) evaluated six mamey sapote cultivars in an acid Ultisol (pH 4.75) at Corozal and an Oxisol (pH 6.62) at Isabela, Puerto Rico. Average yield at Corozal was significantly higher (+31 %) at Corozal than at Isabela, demonstrating adaptability of mamey sapote to acidic soils.

Figure 2. Stem diameter of mamey sapote as influenced by soil aluminum. Each point is the mean value of two replications and eight trees per replication.

Figure 3. Relationship between soil aluminum and aluminum saturation in an Ultisol in Corozal, Puerto Rico.
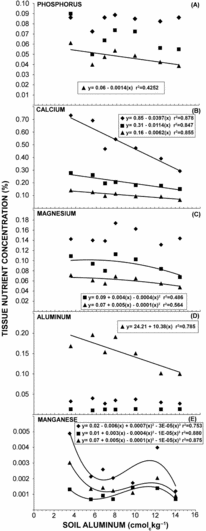
Increments in soil Al significantly increased the concentration of leaf N and K but had no significant effect on the concentration of leaf P, Mg, Fe, Zn and Al (data not shown). Although increases in soil Al content did not significantly affect the concentration of P in leaf and stem tissues, it significantly reduced the concentration of root P (Figure 4a). Increments in soil Al also resulted in a significant reduction in the concentration of leaf, stem and root Ca and in stem and root Mg (Figure 4b,c). It is noteworthy that in the present study increments in soil Al content did not significantly affect the Al concentration in leaf and stem tissues but it significantly declined in root tissue (Figure 4d). These results are in contrast to those found by others (Goenaga and Smith, Reference Goenaga and Smith2002; Goenaga, Reference Goenaga2013; Perez and Goenaga, Reference Pérez and Goenaga2015), in which the concentration of leaf Al increased significantly with increases in soil Al and suggests an Al-exclusion mechanism rendering Al tolerance to mamey sapote trees. Plants have evolved two major mechanisms for Al tolerance: (i) internal tolerance and (ii) Al exclusion from the root apex (Eckhard et al., Reference Eckhard, Horst, Neumann and Marschner2012; Kochian et al., Reference Kochian, Pineros and Hoekenga2005; Langer et al., Reference Langer, Cea, Curaqueo and Borie2009). The fact that the Al concentration in leaf and stem tissues was not significantly affected with increments in soil Al and that there was a significant decline in root Al concentration suggest that mamey sapote may exclude Al from roots. Various investigators have suggested the role of organic acid anion exudation from the root apex as a mechanism for Al exclusion (Hoekenga et al., Reference Hoekenga, Maron, Piñeros, Cancado, Shaff, Kobayaski, Ryan, Dong, Delhaize, Sasaki, Matsumoto, Yamamoto, Koyama and Kochian2006; Kochian et al., Reference Kochian, Pence, Letham, Pineros, Magalhaes, Hoekenga and Garvin2002; Magalhaes et al., Reference Magalhaes, Liu, Gimaraes, Lana, Alves, Wang, Schaffert, Hoekenga, Piñeros, Shaff, Klein, Carneiro, Coelho, Trick and Kochian2007). Mn toxicity symptoms are also often observed in plants growing in acidic soils. It is noteworthy that the concentration of Mn in leaf, stem and root tissues decreased with increases in soil Al up to about 7.8 cmol kg−1 (Figure 4e) suggesting also organic acid exudation to exclude Mn. Rosas et al. (Reference Rosas, Rengel and Mora2007) found that roots of white clover exuded organic acids when plants were grown at high Mn concentration. These results showed the formation of organic acid-Mn complexes in response to increases in Mn concentration in the nutrient solution but the authors could not prove conclusively whether exudation of organic acids is a plant response for overcoming Mn toxicity as it is for Al toxicity.

Figure 4. Leaf (♦), stem (■) and root (▲) nutrient concentration of mamey sapote as influenced by soil aluminum. Absence of curve fitting denotes lack of a significant response. Each point is the mean value of 2 replications and eight trees per replication.
CONCLUSIONS
The results of this study demonstrate that dry matter production and stem diameter in young trees of mamey sapote were not significantly affected when grown at soil Al concentrations ranging from 3.5 to as high as 7.8 cmol kg−1. The concentration of leaf and stem Al was not significantly affected but the Al concentration in roots declined sharply with increases in soil Al, suggesting the activation of an Al-exclusion mechanism.
Disclaimer
Mention of trade names or commercial products in the paper is solely for the purpose of providing specific information and does not imply recommendation or endorsement of the U.S. Department of Agriculture.
Acknowledgements
The author thanks Jose Luis Rodriguez, Angel Marrero and Delvis Perez for assistance in data collection, tissue processing and chemical analyses of samples.