INTRODUCTION
Mulberry (Morus spp.) is a potential tree crop for agroforestry yielding fodder, fruit, fuel, wood and medicine. It has been exploited commercially for the silk industry to rear silkworms (Bombyx mori). The crop has a transcontinental distribution and is cultivated in more than 50 countries both in temperate and tropical climates (Biasiolo et al., Reference Biasiolo, Da Canal and Tornadore2004). In India, the total area under mulberry cultivation is around 171 952 ha (Lakshmanan, Reference Lakshmanan2007). Leaf area, the major economic unit of mulberry crop is a direct component of yield (Susheelamma and Dandin, Reference Susheelamma and Dandin2006). Hence, the target of growers is production of more mulberry foliage throughout the year with succulent leaves of high nutritive value.
Mulberry growth and productivity are remarkably influenced by climatic variations. Water availability is one of the most important determinants of leaf yield in mulberry (Chaitanya et al., Reference Chaitanya, Masilamani and Reddy2002). High yielding mulberry cultivars have a tremendous water demand due to faster growth rate, large cumulative leaf area and canopy size; hence water deprivation can arrest the growth and leaf yield performance of elite mulberry genotypes as a consequence of severely down-regulated photosynthesis and carbon assimilation. Only a few reports provide preliminary information on photosynthetic characteristics in mulberry during water stress (Chaitanya et al., Reference Chaitanya, Jutur, Sundar and Reddy2003; Ramanjulu et al., Reference Ramanjulu, Sreenivasalu, Giridara and Sudhakar1998; Thimmanaik et al., Reference Thimmanaik, Giridara, Jyothshna and Suryanarayan2002). Moreover, data on the physiological growth responses and leaf yield contributing traits of mulberry under moisture stress conditions are limited.
The ability to maintain key physiological processes, such as photosynthesis during water stress, is indicative of sustainable productivity under water shortage. However, attempts to measure the degree of tolerance with a single parameter have limited scope because of the multiplicity of factors and their interactive contribution to drought tolerance under field conditions. To understand various factors controlling and determining the growth and leaf yield of mulberry under water stress, it is imperative to identify certain morpho-physiological traits which are directly associated with drought stress tolerance. Further, it is necessary to evaluate various leaf yield components to understand their contribution to optimizing leaf yield production under drought. Mulberry has great genetic diversity, including extensive variation for stress tolerant traits. However, efficient and dependable screening tools are required to evaluate and screen the existing gene pool to identify drought-tolerant mulberry genotypes through non-destructive assessment of the plants during their growing phase. A physiological approach can complement such screening tests for testing large number of plant genotypes and discriminate reliably among tolerant or sensitive strains (Sayar et al., Reference Sayar, Khemira, Kameli and Mosbahi2008). In principle, physiological measurements are all sensitive to water stress and offer practical means for the early detection of drought stress in higher plants. Hence, keeping the sensitivity and reliability of these physiological stress indicators in mind, we framed three major objectives for our present investigation: i) to evaluate the applicability of the leaf gas exchange characteristics to determine drought stress responses in mulberry and distinguish drought tolerant strain(s) from a diverse group of mulberry genotypes; ii) to investigate and compare the photochemical performance of photosystem II (PSII) in mulberry genotypes under drought stress conditions; iii) to link different growth and leaf yield characteristics with the overall physiological performance of mulberry genotypes under low water regimes.
MATERIALS AND METHODS
Plant materials
The experimental material consisted of 15 mulberry genotypes from varied agro-climatic conditions selected on the basis of putative differences in leaf yield under drought conditions (Table 1). Among the tested genotypes, S-13 was selected as a drought tolerant check as it was reported to perform well under water stress conditions (Chaitanya et al., Reference Chaitanya, Jutur, Sundar and Reddy2003; Kotresha et al., Reference Kotresha, Prabhakar, Srinivas and Vidyasagar2007; Ramanjulu and Sudhakar, Reference Ramanjulu and Sudhakar1997; Sushelamma et al., Reference Susheelamma, Jolly, Giridhar and Sengupta1990; Thimmanaik et al., Reference Thimmanaik, Giridara, Jyothshna and Suryanarayan2002). Cuttings of the tested genotypes were obtained from different sericultural research units of the country and propagated in nursery for one year, prior to the initiation of experiments.
Table 1. Mulberry genotypes (Morus spp) used in the study with their place of origin and climatic conditions.

†CSR & TI- Central Sericultural Research and Training Institute.
Experiment no. 1: Field assays
The study was conducted in the experimental fields of the University of Hyderabad, India (17.3°10´N to 78°23´E; altitude 542.6 m asl). Fifteen mulberry genotypes were evaluated under two irrigation regimes: well-watered (control) and water-limited (drought) during February to July (which constituted two dry summer seasons of the study zone) for two consecutive years (2007 and 2008). Rainfall during the experimental period was sporadic and considered negligible; the mean air temperature recorded during daytime was 32.7 °C; mean monthly photosynthetically active radiation (PAR), measured at peak photosynthetic time (09:00–11.00 hours) ranged from 1230 to 2500 μmol m−2 s−1 (Figure 1A, B). The soil of the experimental site was sandy loam with pH of 7.5. The experimental design was a randomized block with split-plot arrangement of treatments (Chaturvedi and Sarkar, Reference Chaturvedi and Sarkar2000) including four replications in a net plot size of 14.4 m × 14.4 m (12.6 m rows, 0.9 m apart). Healthy rooted saplings were removed from the nursery and transplanted in the experimental field using a pit system of plantation, as recommended for rainfed mulberry gardens (Dandin et al., Reference Dandin, Jayaswal, Giridhar, Dandin, Jayaswal and Giridhar2003). After six months of establishment, the plants were uniformly pruned at a basal height of 30 cm. After 10 days, a dose of mixed fertilizer was applied at the rate of 50:50:50 kg NPK ha−1 with subsequent irrigation. Once complete leaf sprouting was achieved in all the plants, the treatments for water stress were imposed. The control plot was irrigated twice per week (20 to 24 irrigations in each growing season depending upon the edaphic and climatic conditions), whereas the stressed plot was irrigated once a fortnight in a growing season. As genotypic variation in growth rates, leaf area, canopy size, stomatal conductance and transpirational water loss were apparent in the tested genotypes, in order to prevent local differences in the degree of water availability, the duration of irrigation was increased in the control plot during the hottest months (April–June) to maintain soil moisture content within a range of 70–80%. Such treatments compensated for higher evapotranspirational moisture loss and maintained enough soil moisture in the control plot for normal growth of mulberry genotypes. To verify the degree of drought stress, soil moisture content at 30 cm and 45 cm depth of the control and stressed plots were determined periodically by taking wet weight and oven-dried (105 °C for 2 days) weight of soil according to Ritchie et al. (Reference Ritchie, Nguyen and Holaday1990) (Figure 1C).

Figure 1. Monthly averages of (A) rainfall, air temperature, relative humidity; (B) photosynthetically active radiation (PAR); (C) soil moisture contents (expressed as percentage of oven-dried weight) recorded at two depths (30 cm and 45 cm) at the study site during the experimental period (February to July) for 2007 and 2008. Values are mean ± s.d.
The recommended package of practices were followed uniformly throughout the growing seasons for both the treatments, which included the application of 20 t farmyard manure (FYM) ha−1yr−1 (within a fortnight after first pruning) and 280:120:120 kg NPK ha−1yr−1 (within a month after each pruning) in equal splits. The plantations were maintained as bushes by pruning after every experimental harvest at a basal height of 30 cm. All other agronomic practices were also kept normal and uniform for both treatments. Measurements were made and data were collected for two years from four growing seasons: season I (February 2007–April 2007), season II (May 2007–July 2007), season III (February 2008–April 2008) and season IV (May 2008–July 2008), covering four experimental harvests.
Field assays: Measurements of leaf gas exchange traits and leaf water status
The rate of leaf gas exchange was measured using a portable infrared CO2/H2O gas analyser (IRGA) (LCpro-32070, ADC Bioscientific Ltd. U.K.) equipped with a broad leaf chamber. The air humidity in the leaf chamber was about 40%, with CO2 concentration of 360–370 μmol mol−1, air temperature of 26–28°C and flow rate of 500 μmol s−1. The gas analyser was used to measure instantaneous net photosynthetic rates (Pn), stomatal conductance of CO2 (gs) and transpiration rates (E), periodically during each growing season between 10:00 and 11:00 hours on clear sunny days under natural PAR. Each measurement was made when Pn was stabilized; this process typically took 1–2 min.
To calculate instantaneous water use efficiency (WUEi = Pn/E), we considered the values for the days on which vapour pressure deficit (VPD) conditions were same or quite uniform. All photosynthetic measurements were performed in situ on young, well-expanded and light-exposed leaves, randomly chosen from the upper half of the plant canopy of uniform plants in each replicate.
Leaf water status of the field grown genotypes (taking fully expanded leaves of the third or fourth positions from the apex of top branches and sampled between 10:00 and 11:00 hours) was determined by measuring the relative water content (RWC). The RWC was calculated by the formula: RWC(%) = [(fw − dw)/(sw − dw)] × 100 where, fw is fresh weight, sw is the mass after rehydration obtained by storing leaf samples for 24 h in distilled water and dw is oven-dried weight (105 °C) of leaves (Castillo, Reference Castillo1996).
Field assays: Measurements of leaf yield and determination of tolerance index
At the end of each growing season, the plants were harvested to obtain yield measurements. Leaves of each plant were harvested separately and the weight was recorded as fw of leaves in grams. For determining tolerance index (TI), the mean leaf yield (LY) derived from the average of four experimental harvests was considered. Tolerance index was calculated according to the formula: TI = LY(D)/LY(C) where, LY(D) and LY(C) are the leaf yields under drought (D) and control (C) conditions, respectively (Szira et al., Reference Szira, Bálint, Börner and Galiba2008).
Experiment no. 2: Glasshouse assays
Two drought-tolerant and two drought-susceptible genotypes were selected for glasshouse experiments for further studies. Three-month-old healthy potted (pot size 35l) saplings of the four mulberry genotypes were selected in a completely randomized block design (CRBD) with four replications. The plants were randomly submitted to two watering regimes: in stressed plants water stress was imposed progressively by gradual decrease in pot water holding capacity (PC) (from 100% PC to 25% PC), whereas the control plants were maintained at 100% PC (Said and Hugh, Reference Said and Hugh2005). The water regimes maintained in the glasshouse experiments were similar to those experienced in the field trials. The study was undertaken from 20 August to 30 October 2008 (71 days). Photosynthetic photon flux density (PPFD) measured inside the glasshouse (09:00–11.00 hours) ranged from 900 to 1200 μmol m−2 s−1, air temperature ranged from 22 ± 1 °C (early morning) to 34 ± 4 °C (early afternoon) and relative humidity from 36 ± 5% to 48 ± 2%. All the sampling and measurements were made using fully expanded leaves of the third or fourth positions from the apex of top branches. Samplings were conducted periodically at an interval of 15–18 days and the results reported were the mean of all periodic data.
Glasshouse assays: Measurements of leaf water status, leaf gas exchange and chlorophyll a fluorescence
Leaf water status was determined by calculating the RWC. In addition to preliminary photosynthetic gas exchange functions, i.e. Pn, gs, E and WUEi, leaf temperature (TL) of plants was measured by an integral leaf thermistor probe (ADC, M.PLC-011, LICOR) attached to the leaf chamber. The internal CO2 concentration (Ci) and internal to ambient CO2 ratio (Ci/Ca) ratio were determined using IRGA. While measuring leaf gas exchange, the cuvette conditions were maintained at 40% air humidity, 360 μmol mol−1 CO2 concentration and flow rate of 500 μmol s−1. Each leaf was incubated for 1–2 min inside the microclimate of the leaf chamber until Pn was stabilized and thereafter data were recorded. All the measurements for leaf gas exchange parameters were conducted between 10:00 and 11:00 hours under natural PPFD inside the glasshouse. Chlorophyll a fluorescence of the non-detached leaves (carried out in the same leaves used for gas exchange records) was measured at predawn (05:00–05:30 hours) using a portable plant efficiency analyser-fluorometer (Handy PEA-2126, Hansatech Instruments, Kings Lynn, UK). Minimal (Fo) and maximum (Fm) fluorescence yield were measured in the leaves, dark-adapted for 30 min. Fm was estimated by illuminating the dark-adapted leaves with PPFD pulse of 3000 μmol m−2 s−1 for 1 s with the help of light-emitting diodes (650 nm). Variable fluorescence (Fv) was calculated as the difference between Fm and Fo values (Fv = Fm − Fo). Maximum quantum yield of PSII in the dark-adapted leaves was estimated by the ratio Fv/Fm = (Fm − Fo)/Fm according to Genty et al. (Reference Genty, Briantais and Baker1989). Measurements were made in 10 replicates. A single leaf per plant constituted each replicate.
Glasshouse assays: Growth and leaf yield measurements
Periodically, some of the potted mulberry plants were completely harvested (both shoots and roots) while the remaining plants were harvested at the end of the experiment (75th day) to obtain periodic as well as final growth and yield records. The height of all primary shoots of each plant was measured to obtain total shoot length. Relative plant height growth rate (RHGR) was calculated based on periodic data according to Hunt (Reference Hunt1982). Leaves and stems of each plant were harvested separately and the weight recorded as fw in grams. Leaf and stem weight were added to get above ground biomass. Leaf area (cm2) was recorded using a LICOR leaf area meter (LI 1600, LICOR Biosciences, USA). Root weight was recorded as fw of roots in grams. Root volume (cm3) was measured following the water displacement method (Burdett, Reference Burdett1979). Leaf mass ratio (LMR), stem mass ratio (SMR), root mass ratio (RMR) and root:shoot ratio were calculated using the leaf, stem and root weights in the final harvest. The periodically as well as finally harvested plant tissues were oven-dried at 70 °C for 72 h to determine the dry matter of leaves, stems and roots, and based on these data, the growth parameters of crop growth rate (CGR) net assimilation rate (NAR) and biomass duration (BMD) were calculated (Nagakura et al., Reference Nagakura, Shigenaga, Akama and Takahashi2004).
Data analysis
Results were represented as mean ± s.d. The significance of the differences between mean values of control and water-stressed plants was determined using the t-test and analysis of variance (ANOVA). Data analyses were performed using the statistical package SigmaPlot 11.0.
RESULTS
Field assays: Leaf gas exchange and leaf RWC
Significant genotypic variation was recorded for Pn among the mulberry genotypes in both water regimes (Figure 2A). In control conditions, genotype PNG exhibited highest Pn (15.08 μmol m−2 s−1) followed by AR-12 (13.2 μmol m−2 s−1), V-1 (12.58 μmol m−2 s−1) and Bogurai (12.52 μmol m−2 s−1). Pn was minimum for M Local (9.03 μmol m−2 s−1) and S-1 (7.98 μmol m−2 s−1). Water stress apparently reduced Pn in all mulberry genotypes, and a large reduction in Pn was observed in genotypes DD (73.6%), Bogurai (77.1%) and PNG (80.1%). However, higher Pn under water deficit conditions was recorded in genotypes V-1 (7.9 μmol m−2 s−1), S-13 (7.17 μmol m−2 s−1) and S-1(6.32 μmol m−2 s−1). The gs ranged from 0.57 to 0.27 mol m−2 s−1 and from 0.19 to 0.012 mol m−2 s−1 in control and stress treatments, respectively (Figure 2B). In well-watered conditions, S1635 exhibited the maximum gs (0.57 mol m−2 s−1) followed by KS-2 and V-1 (0.46 mol m−2 s−1), whereas gs was lower in Jhoropakari (0.29 mol m−2 s−1) and S-1 (0.27 mol m−2 s−1). However, under water stress, genotype V-1 (0.19 mol m−2 s−1), S-13 (0.16 mol m−2 s−1) and S-1 (0.12 mol m−2 s−1) exhibited maximum gs. Drought caused drastic reduction in E of all genotypes (Figure 2C) with an average decline of 70%. Genotypes V-1 (1.65 mmol m−2 s−1), S-36 (1.58 mmol m−2 s−1), S-1(1.57 mmol m−2 s−1) and S-13 (1.48 mmol m−2 s−1) maintained relatively higher rates for E under low water regimes. Relative to unstressed plants, drought led to an increase in WUEi in most of the mulberry genotypes, except PNG and Bogurai (Figure 2D). PNG had highest WUEi (4.3 μmol CO2 mol−1 H2O) under well-watered conditions, but during water stress its WUEi decreased substantially (1.87 μmol CO2 mol−1 H2O) compared to the control. In Bogurai there was a 13.4% reduction in WUEi under water stress. Genotypes V-1, S-13 and S-1 performed best in terms of WUEi under drought regimes and the maximum percentage increase in WUEi was recorded in S-1 (61.2%) followed by S-13 (57.9%).

Figure 2. (A) Net photosynthetic rate (Pn); (B) stomatal conductance of CO2 (gs); (C) transpiration rate (E); (D) instantaneous water use efficiency (WUEi); (E) leaf yield (LY)/plant; (F) tolerance index (based on leaf yield) of 15 mulberry genotypes grown under two water regimes (control and drought) during February to July for 2007 and 2008. Data represented are the average over four growing seasons. Values are mean ± s.d.
The RWC of the drought-stressed mulberry genotypes ranged from 72.1% to 66.6% and it was higher in V-1 followed by TR-10, S-1 and S-1635. Lower values of RWC were observed in DD and PNG (Table 2).
Table 2. Effect of drought stress on relative water content (RWC) in the three-month old leaves of mulberry genotypes grown under field conditions. Data represented are the average over four growing seasons.

Values are mean ± s.d. Effects of drought were tested by t-test (p < 0.001).
Field assays: Leaf yield performance
The mulberry genotypes exhibited wide genetic variance in leaf yield production (Figure 2E). Under well-watered conditions, the highest leaf yield plant−1 was recorded in V-1 (1190 g plant−1) followed by MR-2 (1138 g plant−1) and S-36 (1024g plant−1). Genotypes Bogurai (452 g plant−1), K-2 (558 g plant−1) and TR-10 (580 plant−1) were found to be low leaf yielders amongst tested mulberry genotypes under high water availability. A marked decline in leaf yield was recorded in all the genotypes under water-stressed treatment. However, the maximum leaf yield plant−1 was recorded in V-1 (450 g plant−1) followed by S-13 (375 g plant−1) under drought, whereas the minimum leaf yield was obtained in DD (164 g plant−1) and PNG (150g plant−1) followed by Bogurai (112 g plant−1).
The TI calculated on leaf yield plant−1 basis, revealed conspicuous genotypic variation among the tested mulberry accessions in respect of drought tolerance (Figure 2F). The TI ranged from 0.51 to 0.16. Highest TI value was obtained with S-13 (0.51) followed by V-1 (0.4) and S-1635 (0.34), whereas the value was much lower for the genotypes S-36 (0.16), MR-2 (0.16), DD (0.18) and Bogurai (0.2).
Glasshouse assays: Leaf water status, leaf gas exchange and chlorophyll a fluorescence
Under water stress RWC was reduced in all mulberry genotypes compared to control (Table 3). However, V-1 presented the highest RWC of 71.2%, whereas the decrease in RWC was stronger in Bogurai and dropped to 66% during periods of water deprivation. Water stress caused a similar trend of inhibition in the functional gas exchange characteristics of four mulberry genotypes evaluated under glasshouse conditions. Suppression of Pn, gs and E was more pronounced in stressed leaves of drought-susceptible (DD and Bogurai) than drought-tolerant (V-1 and S-13) genotypes. Genotypes V-1 and S-13 exhibited less inhibition in Pn, gs and E and maintained better gas exchange functions during drought periods (Figure 3A, B, C). V-1 and S-13 exhibited higher WUEi in both treatments (Figure 3D). Compared to well-watered counterparts, a reduction in Ci/Ca ratio was observed in the stressed leaves of all four mulberry genotypes (Figure 3E). The reduction in Ci/Ca was more conspicuous in DD (21%), whereas the ratio decreased only 12.3% and 13% in V-1 and S-13, respectively. The TL under control conditions ranged from 31.4 °C to 35.9 °C. Among the four mulberry genotypes, V-1 had minimal TL under well-irrigated conditions. Water stress resulted in an increase in TL for all the genotypes (Figure 3F). The highest TL of stressed mulberry leaves was observed in Bogurai (37.7 °C) followed by DD (37 °C), whilst V-1 again maintained the lowest value for TL (~32.6 °C) throughout the periods of water deprivation.
Table 3. Estimated leaf relative water contents (RWC %) in four mulberry genotypes grown under glasshouse conditions under two water regimes (control and drought).
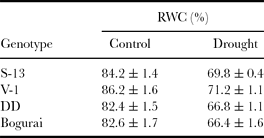
Values are mean ± s.d. Effects of drought were tested by t-test (p < 0.001).

Figure 3. (A) Net photosynthetic rate (Pn); (B) stomatal conductance of CO2 (gs); (C) transpiration rate (E); (D) instantaneous water use efficiency (WUEi); (E) internal CO2/ambient CO2 ratio (Ci/Ca); (F) leaf temperature (TL); (G) minimal fluorescence (Fo); (H) maximal fluorescence (Fm); (I) maximal quantum yield of PSII (Fv/Fm) of four mulberry genotypes grown under two water regimes (control and drought) in glasshouse conditions. Values are mean ± s.d.
Chlorophyll a fluorescence parameters were significantly affected by drought stress. In all the mulberry genotypes studied, drought stress induced an increase in Fo, which was significantly more in DD and Bogurai than in V-1 and S-13 (Figure 3G). Unlike Fo, a reduction in Fm was encountered in the water-stressed genotypes compared to the well-watered counterparts (Figure 3H). Reduction in Fm was more conspicuous in Bogurai (16%), followed by DD (9.7%), whereas a reduction of 2% in Fm was recorded in the stressed leaves of S-13. In V-1, a marginal increase in Fm (0.8%) was observed in water-stressed conditions, compared to control. The Fv/Fm ratio in the four mulberry genotypes remained above 0.8 in control treatment throughout the experiment. The ratio was also practically not altered in the drought-tolerant genotype V-1 (0.812) and S-13 (0.809) under water stress. However, significant drawdown in Fv/Fm was recorded in the susceptible genotypes, particularly in Bogurai (0.653) followed by DD (0.726) (Figure 3I).
Glasshouse assays: Growth and leaf yield characteristics
The RHGR was analysed for the four mulberry genotypes according to the increment over periodic observations under both the watering regimes. Drought stress significantly slowed down plant height growth rate in all the mulberry genotypes compared to control. Genotype V-1 and S-13, however, had greater height growth rate than the drought-sensitive genotypes DD and Bogurai (Figure 4A). Drought stress also led to decrease in total biomass accumulation in both the tolerant and susceptible genotypes. In V-1 and S-13, there was no significant alterations in biomass allocation in shoots since LMR and SMR were unaffected by drought. By contrast, the group of drought-susceptible genotypes allocated more biomass to stems and significantly less to leaves, as deduced from the decrease in LMR (~38.5%) at the expense of an increase in SMR (~28.4%) (Figure B, C). No significant variation was encountered in RMR within the genotypes; however, within the treatments there was significant variation. Higher RMR was evident in all the mulberry genotypes under low moisture regimes (Figure 4D). Water deprivation significantly increased the root:shoot ratio in the tolerant as well as in susceptible mulberry genotypes (Figure 4E). Crop growth rate ranged from 1.5 to 2.07 g m−2 day−1 and from 0.33 to 0.61 g m−2 day−1 under control and drought treatments, respectively (Figure 4F). Under well-watered conditions, V-1 had higher CGR (2.07 g m−2 day−1) while in Bogurai the rate was minimal (1.5 g m−2 day−1). Deprivation in soil moisture led to a severe reduction in CGR in all the mulberry genotypes. Under well-watered conditions, the genotypes did not differ much in respect to NAR; however, drought stress led to a significant drawdown in NAR in all groups (Figure 4G). V-1 exhibited relatively higher values for NAR (0.64 g m−2 day−1) followed by S-13 (0.6 g m−2 day−1) under low-water regimes, whereas in Bogurai the NAR was lowest (0.2 g m−2 day−1). Regardless of the watering regimes, V-1 maintained relatively higher values for BMD, whilst drought caused dramatic reduction of BMD in all the mulberry genotypes with an average decline of 73.1% (Figure 4H).

Figure 4. (A) Mean relative plant height growth rate (RHGR); (B) leaf mass ratio (LMR); (C) stem mass ratio (SMR); (D) root mass ratio (RMR); (E) root:shoot ratio; (F) crop growth rate (CGR) (G) net assimilation rate (NAR); (H) biomass duration (BMD) of four mulberry genotypes grown under two water regimes (control and drought) in glasshouse conditions. Values are mean ± s.d.
The four mulberry genotypes also varied significantly in respect to root characteristics (Table 4). Enhancement in root fw was observed in the tolerant genotypes (~15.5%) under water stress treatments. Vertical proliferation of roots was retarded in all the mulberry genotypes under low water regimes, which was reflected in reduction of root length in the mulberry genotypes of both groups. However, the drought-tolerant group maintained larger root length irrespective of the watering regimes. V-1 had the largest root length of 104.1 cm and 88.9 cm in control and water stress treatments, respectively. A significant increase in root volume was also recorded in V-1 (39%) and in S-13 (49.6%) compared to control; however, the root volume was reduced to 22.2% and 48.3% in DD and Bogurai, respectively, in response to water stress.
Table 4. Morphology and leaf yield characteristics of four mulberry genotypes grown under glasshouse conditions under two water regimes (control and drought).

Effects of drought were tested by t-test. *p< 0.05, **p< 0.01, ***p < 0.001, n.s., not significant.
The mulberry genotypes differed significantly in shoot morphology and leaf yield characteristics regardless of watering regimes (Table 4). Under well-watered conditions, V-1 and DD had greater plant height of 97.7 cm and 85.2 cm, respectively. The total shoot length was also higher in V-1, whereas in the remaining three genotypes no significant variation was encountered in total shoot length under well-watered conditions. V-1 and DD also performed well in leaf yield and total leaf area under control conditions. Maximum leaf weight plant−1 was recorded in V-1 (154.5 g), whereas Bogurai had lowest leaf weight of 122 g plant−1 under high water availability. Genotypes V-1 and S-13 had larger accumulation of above ground biomass while DD and Bogurai had the minimum values for the same. However, in contrast to well-watered conditions, drought stress led to an apparent decrease in all leaf yield components, particularly in number of leaves, leaf weight, leaf area and above ground biomass of four mulberry genotypes, although to different extents. Bogurai and DD exhibited minimum leaf weight plant−1 with a concomitant sharp drawdown in cumulative leaf area. However, V-1 and S-13 maintained relatively higher leaf weight, leaf area and above ground biomass during water stress.
DISCUSSION
The present comprehensive study elucidates wide genetic variation for drought tolerance among different mulberry genotypes. Based on growth and leaf yield productivity in the four growing seasons under drought conditions, two mulberry genotypes (V-1 and S-1) were considered to be drought tolerant apart from the drought-tolerant control S-13. Leaf yield for V-1 and S-1 differed significantly under well-watered and drought stress conditions; however, these genotypes were able to maintain relatively higher and stable leaf yield throughout the growing seasons under drought conditions compared to other genotypes. The most important and immediate response of drought stress in mulberry was the reduction in leaf surface size that co-limited leaf area as well as leaf mass and ultimately led to low leaf yield. Limitation in leaf yield due to drought stress cannot be assigned to a single physiological process; rather it can be explained as a consequence of reduced Pn with a concomitant down-regulation in gs (Dias et al., Reference Dias, Araujo, Moraes, Barros and DaMatta2007). As a consequence of low soil moisture availability, a significant decline in the rates of photosynthesis (Pn) and stomatal conductance to CO2 (gs) were observed in all the studied mulberry genotypes. The carbon acquisition ability of the genotypes was hampered by the greater magnitude of reduction in gs compared to Pn under drought. Such high stomatal sensitivity can reduce the leaf gas exchange capacity by narrowing stomatal pore or by stomatal closure and in this process, Pn is also dramatically down-regulated (Buckley et al., Reference Buckley, Mott and Farquhar2003; Medrano et al., Reference Medrano, Escalona, Bota, Gulías and Flexas2002).
Our data highlights stomatal inhibition as the major cause of photosynthetic down-regulation in mulberry leaves under water stress. Although Pn and E had similar responses under drought, the relative decrease of E was greater, resulting in a significant increase of WUEi in most of the mulberry genotypes. However, in drought-tolerant genotypes, Pn was found to be higher and less sensitive to E compared to the sensitive genotypes under soil water deficit; hence, the WUEi was consequently much improved in the tolerant genotypes. Plants preferring ‘drought avoidance’ minimize water loss by substantial reduction in gas exchange parameters, mainly controlling gs. Such a strategy undoubtedly leads to reduced photosynthesis, which ultimately results in slow growth rate and poor leaf yield. However, a drought-tolerant genotype can maintain better physiological functions like photosynthesis and stomatal conductance of CO2 despite large water deficit (Tardieu, Reference Tardieu2005). The strategies noticed in V-1 and S-13 can be termed as ‘drought tolerance’ rather than ‘drought avoidance’ as they faced less inhibition in Pn and maintained higher leaf gas exchange rates under severe water restriction compared to other genotypes. Down-regulation in the gas exchange physiology exhibited by the drought-sensitive genotypes significantly limited photosynthetic carbon assimilation affecting quantitative traits and resulted in low leaf yield productivity under drought.
In the second phase of our study, we narrowed down to selected drought-tolerant and drought-susceptible mulberry genotypes and continued to investigate the evidence for yield losses in the susceptible compared to tolerant genotypes. Gas exchange measurements demonstrated that the susceptible genotypes with significantly reduced Pn and gs also exhibited significantly reduced Ci/Ca values under drought stress, indicating strong stomatal inhibition with negative reflexes on the photosynthetic CO2 uptake. There are uncertainties in calculating Ci in water-stressed leaves such as mulberry due to stomatal patchiness (Gomes et al., Reference Gomes, Oliva, Mielke, Almeida, Leite and Aquino2007; Nikolopoulos et al., Reference Nikolopoulos, Liakopoulos, Drossopoulos and Karabourniotis2002). As we did not measure patchy stomatal conductance (Mott and Buckley, Reference Mott and Buckley2000; Terashima, 1998), we cannot be sure that our Ci values were not subjected to the patchiness problems. However, it should be noted that: i) patchiness should not be an important problem under progressively drought exposed conditions and ii) since gs values measured during the water-stressed periods were always higher than 0.03 mol m−2 s−1, below which the patchiness phenomenon can be important (Flexas et al., Reference Flexas, Bota, Escalona, Sampol and Medrano2002; Grassi and Magnani, Reference Grassi and Magnani2005). Elevated TL (>35 °C), as recorded in control and stressed leaves of drought-susceptible mulberry genotypes grown under water deficit might adversely affect mesophyll conductance of CO2 and cause damage to the photosynthetic machinery (Bernacchi et al., Reference Bernacchi, Portis, Nakano, von Caemmerer and Long2002; Salvucci and Crafts-Brander, Reference Salvucci and Crafts-Brandner2004). Because of impaired leaf cooling by low E, TL of stressed leaves in sensitive genotypes showed significantly high values. Interestingly, the drought-tolerant genotypes were able to maintain low TL and therefore, heat-induced damage to the photosynthetic apparatus was presumably less critical. The overall photosynthetic gas exchange studies (including field assays and glasshouse experiments) highlight the reliability of such non-destructive gas exchange techniques for screening mulberry genotypes for drought tolerance. Among the various leaf gas exchange parameters measured in this study, rate of photosynthesis, stomatal conductance of CO2 and leaf temperature seems to be useful selection criteria for screening drought tolerant strain(s) from a diverse group of mulberry genotypes.
Measurements on chlorophyll a fluorescence were widely monitored to evaluate the direct effects of drought stress on PSII photochemistry. Light-harvesting complexes reduced their efficiency due to drought-induced damages to the antennae, as evidenced by an increase in Fo in stressed leaves of mulberry genotypes. In the stressed leaves of drought-tolerant mulberry genotypes, this increase was less and was balanced by the maintenance of Fm, which contributed to keeping a similar Fv/Fm. A decrease in Fm, as observed in the sensitive mulberry plants, may be related to the decrease in the activity of the water-splitting enzyme complex and perhaps a concomitant cyclic electron transport with or around PSII (Zlatev and Yordanov, Reference Zlatev and Yordanov2004). The significant increase of Fo and decrease of Fm under drought stress concurrently led to a strong decrease of Fv/Fm values in the sensitive mulberry genotypes suggesting a chronic photoinhibition due to photo-inactivation of PSII centres, possibly attributable to D1 protein damage (Ohnishi et al., Reference Ohnishi, Allakhverdiev, Takahashi, Higashi, Watanabe, Nishiyama and Murata2005). The ability to maintain high Fv/Fm under moisture stress thus indicates that the drought-tolerant genotypes had stabilized PSII photochemistry and better carbon assimilation capacity when compared to the sensitives. The relatively faster method of measuring photosynthetic activity via chlorophyll a fluorescence techniques seems to be promising for monitoring photosynthetic events and rapid screening of large quantities of mulberry germplasm for drought tolerance.
In the present study, drought stress decreased the root length in drought-susceptible mulberry genotypes. However, in the drought-tolerant genotypes, root length was less affected and a significant increase in root volume with simultaneous increment in root biomass was recorded compared to the susceptible genotypes. Such phenomena indicate that low soil water availability altered root growth pattern. Instead of vertical growth, horizontal proliferation of roots occurred to exploit the existing soil water in and around the rhizosphere. Better rooting vigour, as observed in the drought-tolerant genotypes, would have facilitated higher hydraulic conductance in those genotypes, which was reflected by their ability to maintain a higher RWC of their leaf tissues. It is often reported that under limited water supply the natural coherence among the growth characteristics is altered and the plants show a shift as well as selectivity in biomass allocation to specific parts (Dias et al., Reference Dias, Araujo, Moraes, Barros and DaMatta2007). In our investigation, the biomass allocation to leaves (LMR) under water deficit was severely affected in the drought-susceptible genotypes, which can be explained as an adaptive response in order to avoid excess irradiance and to prevent transpirational loss by dramatic reduction in leaf size and number. However, such adaptive phenotypic plasticity causing diminution of foliage cannot be linked to enhanced performance under drought as leaf yield is economically important in mulberry. In contrast to the susceptible genotypes, the LMR was maintained in the tolerant genotypes, which would contribute to higher leaf yield under low water regime. Allocation to roots remained unaffected, moreover, RMR and root:shoot ratios were improved in both tolerant and susceptible mulberry genotypes thus reflecting a common trend of higher biomass allocation to roots under water deficit conditions. A major loss in NAR, as observed in drought-sensitive mulberry genotypes under water-limited conditions might be ascribed to strong reduction in Pn, whilst superior photosynthetic functioning and better root characteristics of drought-tolerant genotypes would enable them to attain relatively higher NAR as well as CGR and BMD under water stress regimes compared to the susceptible genotypes (Dias et al., Reference Dias, Araujo, Moraes, Barros and DaMatta2007; Machado et al., Reference Machado, Bynum, Archer, Lascano, Wilson, Bordovsky, Segarra, Bronson, Nesmith and Xu2002).
CONCLUSIONS
The results reported here indicate substantial genotypic variation in morphophysiological and leaf yield traits among the tested mulberry genotypes. The reduced leaf yield under water limitation as observed for those genotypes which otherwise yielded highly when well-irrigated (in the experiments reported here) suggest that maintaining better yields in dry-land conditions may be possible through breeding strategies, specifically focused on minimizing the gap between potential yield and actual yield. The ‘drought tolerant’ characteristics as observed in the tested genotypes, such as higher rates of photosynthesis and stomatal conductance of CO2, low leaf temperature, less photoinhibition, stabilized photochemistry, higher biomass allocation to leaves and better root growth characteristics are likely to be beneficial in such breeding programme. In drought-tolerant genotypes, the relative variation in some of these characteristics tend to be consistent between well-watered and water-limited conditions, indicating low genotype × environment interaction, which could be exploited in breeding programmes for improving drought tolerance in mulberry.
Acknowledgements
We acknowledge Department of Science and Technology (DST), Government of India, New Delhi for financial assistance (Grant SR/SO/PS-27/05). We are grateful to Central Sericultural Germplasm Resources Centre (Hosur, India) and Regional Sericultural Research Stations (Anantapur and Salem, India) for providing the mulberry germplasm.










