TRP Channels make Brains
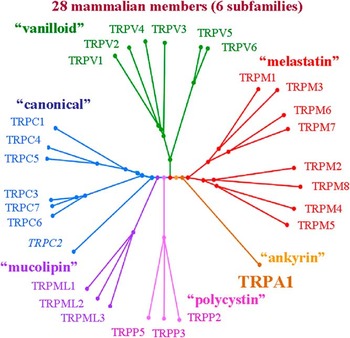
The transient receptor potential (TRP) multigene superfamily encodes integral membrane proteins that function as ion channels. Members of this family are conserved in yeast, invertebrates and vertebrates. All members TRP channels are subdivided into seven subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), TRPA (ankyrin) and TRPN (NOMPC-like), which is only found in invertebrates. Of the six mammalian subfamilies, 28 members are known, with only 27 in humans (TRPC2 is a pseudogene) (see Figure 1).

Figure 1 Phylogenetic tree of the mammalian TRP channel superfamily. TRPC (‘canonical’), TRPM (‘melastatin’), TRPV (‘vanilloid’), TRPA (‘ankyrin’), TRPP (‘polycystin’) and TRPML (‘mucolipin’) are the only identified subfamilies in mammals.
We have witnessed that almost all 28 members of mammalian TRP channel are involved in establishing the five classical senses described in De Anima (book II, 350 B.C.) by Aristotle, which allow humans to perceive the outside world: sight (visus), hearing (auditus), smell (olfactus), taste (gustus) and touch (contactus). And also for balance (or equilibrioreception), which is now also considered as a sixth exteroceptive sense, and the interoceptive senses, which provide information from within the body (e.g. proprioception informs the brain about the relative position of muscles and joints), TRP channels play an essential role.Reference Damann, Voets and Nilius1
Now, we recognize that TRP channels play a much wider role than once proposed. They are involved in many homeostatic functions and, importantly, play an essential role in our brain much beyond their function as cell sensors.Reference Nilius, Owsianik, Voets and Peters2
The Classic Canonical TRPCs
TRP channels play an important role in making connections in the brain, for example they play an essential role in growth cone guidance in the developing brain. In connection with Homer proteins, post-synaptic density proteins with known functions in receptor trafficking, calcium homeostasis and as key mediators of synaptic plasticity, they are also known to function in axon guidance. For this function they co-operate with the Ca2+ permeable channel TRPC1, form a component of the calcium signalling repertoire within motile growth cones, regulating guidance-cue-induced calcium release and maintaining basal cytosolic calcium.Reference Gasperini, Choi-Lundberg, Thompson, Mitchell and Foa3 In the striatum, the signalling compound, the Brain Derived Neurotrophic Factor, BDNF, is the important regulator of striatal neuron survival, differentiation, and plasticity. BDNF delivery to the striatum is implicated in Huntington's disease. The relative contributions of multipartite intracellular signalling pathways to the short-term induction of striatal gene expression by BDNF is connected to TRPC channels.Reference Gokce, Runne, Kuhn and Luthi-Carter4 TRPCs are highly expressed in the cerebellum. In rats, in the first three weeks, TRPC3 expression is increased, and TRPC4 and TRPC6 expression is decreased. TRPC3 is mainly expressed in Purkinje cells, TRPC4 is restricted to granular cells, and TRPC6 to Purkinje cell bodies. Obviously, TRPC cell-specific expression is required for a proper cerebellar development.Reference Huang, Young and Glitsch5 TRPCs are also essential in the entorhinal cortex. Persistent neuronal activity lasting seconds to minutes has been proposed to allow for the transient storage of memory traces in entorhinal cortex and plays a major role in working memory. This activity is coupled to the function of TRPC4 and TRPC5.Reference Zhang, Reboreda, Alonso, Barker and Seguela6 The role of TRPC in growth cone path finding has been described by several groups (for a review see Refs Reference Greka, Navarro, Oancea, Duggan and Clapham7–Reference Bezzerides, Ramsey, Kotecha, Greka and Clapham9). It is also know that bone morphogenic proteins (BMPs) are involved in axon pathfinding due to activation of the calcineurin phosphatase Slingshot (SSH). Both enzymes regulate actin dynamics by modulating the actin-depolymerizing factor (ADF)/cofilin-mediated actin dynamics. This interaction, seen in the attraction to repulsion switching, requires the expression of TRPC1, which mediated Ca2+ signals through SSH activation and growth cone repulsion.Reference Wen10–Reference Li, Hutchins and Kalil12 Another TRPC channel, TRPC3, plays an important role in the hippocampus: BDNF, the potent modulator of hippocampal neuronal structure and function, is released via theta burst stimulation of mossy fibres and elicits cationic current and intracellular Ca2+ elevations in CA3 pyramidal neurons via TRPC3 and lends further credence to the relevance of BDNF signalling for synaptic function in the hippocampus.Reference Li, Calfa, Inoue, Amaral and Pozzo-Miller13 The same channel, TRPC3, is important for the regulation of motor control. TRPC3 is needed for mGluR-dependent synaptic signalling in mouse cerebellar Purkinje cells, where it is most abundantly expressed. Mutant mice lacking TRPC3 exhibit an impaired walking behaviour.Reference Hartmann14 The TRPC channel, TRPC6, is localized to excitatory synapses and promotes their synapse formation. Over-expression of TRPC6 increases the number of spines in hippocampal neurons, which is enhanced in spine formation, a better spatial learning and memory, unravelling a previously unknown role of TRPC6 in synaptic and behavioural plasticity.Reference Zhou15 In addition, TRPC6 channel activity may play a yet unknown role for exploration behaviour.Reference Beis, Schwarting and Dietrich16
The ‘Spicy’, ‘Hot’ Vanilloid TRPVs
TRPV1, the best-studied TRP channels, which is critically involved in nociception via sensory C and Aδ fibres, is classically activated by the ‘hot’ and pungent capsaicin and heat. This channel plays a surprisingly very important ‘non-sensory role’ our brain. In the striatum, TRPV1 modulates GABA transmission, an inhibitory pathway, via endocannabinoids (eCBs) and offers alternative therapeutic routes in disorders of striatal neurotransmission.Reference Musella17, Reference Peng18 TRPV1 is also involved in growth cone guidance. It regulates growth cone morphology and growth cone movement. Activation of TRPV1 results in growth cone retraction and formation of varicosities along the neuritis.Reference Goswami, Schmidt and Hucho19 In the striatum, TRPV1 regulates the release of the excitatory messenger glutamate. It enhances the frequency of glutamate-mediated spontaneous (sEPSCs) and miniature excitatory postsynaptic currents (mEPSCs). TRPV1-mediated regulation of excitatory transmission in the striatum might be important for the final output to other basal ganglia structures (Parkinson).Reference Musella20 In the hippocampus, TRPV1 influences via eCBs various forms of synaptic plasticity at excitatory and inhibitory synapses.Reference Edwards21 The influence on synaptic plasticity is important in the Nucleus Accumbens (NAc) which plays a key role in goal-directed behaviours and reward-dependent learning. TRPV1 channels trigger long-term depression, an inhibitory form of synaptic plasticity, and modulate synaptic strength via endocannabinoids in a cell-type specific fashion via activation of distinct pre- and postsynaptic targets.Reference Grueter, Brasnjo and Malenka22, Reference Chávez, Chiu and Castillo23 Another vanilloid TRP channel, TRPV2, is also involved in growth cone guidance probably via sensing of membrane stretch during development.Reference Shibasaki, Murayama, Ono, Ishizaki and Tominaga24
The Long, ‘Strange’ Melastatin TRPMs
The TRPM subfamily has eight members. TRPM2 is a Ca2++-permeable channel, activated by reactive oxygen/nitrogen species (ROS/RNS) and ADP-ribose (ADPR) and is linked to cell death. Expression of TRPM2 is shown in hippocampal pyramidal neurons where it plays a role regulator of voltage-dependent Ca2+ channels and the NMDA receptors via a rise in the intracellular Ca2+ concentration, [Ca2+]i and its depolarizing effect.Reference Olah25 TRPM2 in Substantia Nigra neurons has a relation to the development of Parkinson's disease, probably via coupling to mitochondrial ROS production.Reference Freestone26 Another TRPM channel, TRPM3, is known for its activation by Pregnenolone Sulfate (PS), which acts as an excitatory neuromodulator and has a variety of neuropharmacological actions, such as memory enhancement and convulsant effects. PS increases glutamatergic spontaneous excitatory postsynaptic currents (sEPSCs) and affect the pathophysiological functions mediated by the hippocampus.Reference Lee27 TRPM3 also affects glutamate release in the brain. In developing cerebellar Purkinje cells (PCs) and developing hippocampal pyramidal neurons, the release of PS contributes to glutamatergic synapse stabilization and acts as important player in the neonatal cerebellar cortex, an effect that may play a role in the refinement of these synapses.Reference Zamudio-Bulcock and Valenzuela28 TRPM4, a Ca2+ activated but Ca2+ impermeable cation channels plays a role as a neuronal pacemaker. It generates neural-motor rhythms that often depend on specialized cellular or synaptic properties, such as pacemaker neurons or alternating phases of synaptic inhibition.Reference Rubin, Hayes, Mendenhall and Del Negro29
The Ugly TRPs: A Role in Diseases of the Brain
TRP Channels Play a Role in Neuronal Ca2+ Homeostasis
TRP channels have now been recognized as important players in neurodegenerative diseases and during brain ischemia. In neurons, excessive Ca2+ entry occurs via over-activation of glutamate receptors or of a range of channels and transporters (TRPM2, TRPM7, the Na+/ Ca2+ exchanger NCX, voltage-activated Ca2+ channels, and connexin/pannexin hemichannels). Both, TRPM2 and TRPM7 play a potentially toxic role via an increase in [Ca2+]i which in turn triggers a range of downstream neurotoxic cascades, including the uncoupling mitochondrial electron transfer from ATP synthesis, and the activation and overstimulation of enzymes such as calpains and other proteases, protein kinases, nitric oxide synthase (NOS), calcineurin and endonucleases. Blocking of these channels might become an important target for a useful therapeutic strategy for treating ischemic and excitotoxic disorders.Reference Szydlowska and Tymianski30 Alterations in Ca2+ homeostasis have been suggested in the onset/progression of neurological diseases, such as Parkinson's, Alzheimer's, bipolar disorder, and Huntington's. On the other hand, TRPC channels form Ca2+ entry pathways, which are essential in maintaining cytosolic, ER, and mitochondrial Ca2+ levels. Silencing of TRPC1 and TRPC3 has been shown to inhibit neuronal proliferation. Loss of TRPC1 is implicated in neurodegeneration. Thus, dysfunction or loss of function of TRPC channels negatively influences normal physiological processes and play also a role in neurodegeneration due to a defective Ca2+ signalling rather than Ca2+ overload.Reference Selvaraj, Sun and Singh31 However, a massive overactivation of TRPC1 can induce hippocampal glutamate-induced cell death. TRPC1 may represent a promising target for pharmacological interventions to prevent or reduce glutamate-induced neuronal damage.Reference Narayanan, Irmady, Subramaniam, Unsicker and von Bohlen Und Halbach32 Neurotrophins play a significant role in the brain as survival factors, modulators of synaptic transmission and plasticity and they participate in associative learning and memory. As mentioned, BDNF is important for hippocampal synaptic plasticity and neuronal Ca2+ homeostasis via TRPC3. This channel mediates changes in neuronal structure including dendritic spine density. Downregulation of TRPC3 prevents an increase in spine density caused by BDNF and is required for spine formation. Dysfunction in the BDNF-TRPC3 interaction may contribute a decrease in spine density that is similar to patterns seen in mental retardation, in particular Rett Syndrome.Reference Amaral, Chapleau and Pozzo-Miller33 As mentioned already, TRPC3 defects impair motocontrol. TRPC3 were identified as postsynaptic cation channels essential for metabotropic glutamate receptor1-dependent synaptic transmission in cerebellar Purkinje neurons. Motor coordination defects have been described in TRPC3 knockout mice.Reference Hartmann14 TRPCs play a role in hereditary ataxias, a complex group of neurological disorders characterized by the degeneration of the cerebellum. The dominant mouse model of cerebellar ataxia, moonwalker (Mwk), is caused by a gain-of-function mutation (T635A) in TRPC3. TRPC3 is highly expressed in Purkinje cells during the phase of dendritogenesis. Not unexpectedly (see above), growth and differentiation of Purkinje cell dendritic arbors are dramatically impaired in Mwk mice. TRPC3 therefore plays a crucial role in dendritic development and survival of Purkinje cells mediating cerebellar ataxia. The pathogenesis likely includes altered phosphorylation of TRPC3.Reference Becker34 Considering the similar effects in the trpc3-deficient mouse, obviously opposing aspects of TRPC3 channel activation can lead to the similar phenotype.Reference Trebak35 Spinocerebellar ataxia type 14 (SCA14), an autosomal dominant neurodegenerative disease, is caused by mutations in a protein kinase PKC. This mutant enzyme fails to phosphorylate TRPC channels, which results in a sustained Ca2+ entry and Purkinje cells degeneration in SCA14.Reference Adachi36 TRPC3 is also involved in the development of Parkinson's disease. Parkinsonian movement disorders are often associated with abnormalities in the Substatia nigra (SNr) GABA neuron firing, which is coupled to TRPC3 channels activity, which plays an important role in ensuring the appropriate firing intensity and pattern in SNr GABA projection neurons, which are crucial to movement control.Reference Zhou, Matta and Zhou37
TRP Channels and Epilepsy
Muscarinic stimulation generates prolonged depolarizations called plateau potentials (PP) in hippocampal pyramidal neurons, which are candidates for triggering of the ictal phase of seizures. The underlying mechanism seems to be a gain-of-function of TRPC5 channels due to an increased trafficking to the plasma membrane. Again, this completely novel mechanism, as for so many TRP channels, provides a new understanding of the pathology of epilepsy and defines a new pharmacological target.Reference Tai, Himes and MacVicar38 In addition, the vanilloid TRP channel, TRPV1, seems to be critically involved in the pathogenesis of epilepsy. TRPV1 is now identified as an antiepileptogenic target. Nerve growth factor (NGF) upregulates TRPV1 expression and triggers epileptogenesis. In addition, the endocannabinoid anandamide (AEA) activates TRPV1 endogenously and AEA levels are increased in epilepsy. Activation of TRPV1 also triggers apoptotic neuronal death in chronic epilepsy.Reference Fu, Xie and Zuo39 In general, TRPV1 activation selectively modifies synapses onto interneurons and may affect long-term changes in physiological and pathological circuit behaviour during learning and epileptic activity.Reference Gibson, Edwards, Page, Van Hook and Kauer40, Reference Alter and Gereau41
TRPM7 is also involved in the pathogenesis of epilepsy. Lowering extracellular Ca2+ and Mg2+ activates TRPM7, which results in a the paradoxical Ca2+ influx associated with epilepsy.Reference Chen42
Also, a polycystin channel, TRPP2 – better know as PKD2 (mutations lead to polycystic kidney disease) – is involved the pathogenesis of the Joubert syndrome, a rare genetic disorder that affects the cerebellum and includes ataxia, seizures and mental retardation.Reference Cevik43
TRPs, Neurodegenerative Diseases, Defects in Neurodevelopment and Mental Retardation
Because TRP channels are involved in brain development, it is not surprising that more and more evidence is being collected regarding a decisive role of these channels in neurodevelopmental disorders.
The best studied neurodegenerative disease is caused by mutations in the intracellular TRP channel TRPML1 (mucolipidin 1), which causes Mucolipidosis type IV (MLIV). This disease is characterized by severe CNS: developmental, degenerative disorder, motor impairment, mental retardation, optic nerve dystrophy, cornea opaqueness, corpus callosum degeneration, cerebellum degeneration.Reference Bach44, Reference Bach, Zeevi, Frumkin and Kogot-Levin45 TRPML1 fails to function correctly in lysosomal storage diseases (LSDs), which are caused by the inability of cells to process the material captured during endocytosis. While they are essentially diseases of cellular ‘indigestion’, LSDs affect large numbers of cellular activities.Reference Kiselyov, Yamaguchi, Lyons and Muallem46 The ‘biogenesis’ model for MLIV pathogenesis suggests that TRP-ML1 modulates postendocytic delivery to lysosomes by regulating interactions between late endosomes and lysosomes. The effects of TRP-ML1 loss on hydrolytic activity have a cumulative effect on lysosome function, resulting in a lag between TRP-ML1 loss and full manifestation of MLIV.Reference Miedel47 Disruption of the TRPML1 channel also causes a defective autophagy, which resulted in oxidative stress and impaired synaptic transmission.Reference Venkatachalam48, Reference Venugopal49 TRPML1 has also been described as an iron channel in LEL, which may contribute to the haematological and degenerative symptoms of Mucolipidosis type IV patients.Reference Dong50
Two other TRP channels, TRPM2 and TRPM7, are causative, involved in the pathogenesis of the Guamanian amyotrophic lateral sclerosis (ALS-G) and Parkinsonism dementia (PD-G, or Parkinsonism dementia complex, PDC), related neurodegenerative disorders that are found at a relatively high incidence on the Pacific Islands Guam and Rota.Reference Plato51 These disease result from a complex interaction between genetic predisposition and environmental factors. Two candidate environmental triggers have been identified: (a) altered mineral content of the soil and drinking water and (b) a neurotoxin derived from the cycad plant, a traditional food source in Guam. Channel mutations lead to a significant loss of nigral dopaminergic neurons due to a reduced Mg2+ influx in the neuronal cells.Reference Plato52 A TRPM7 missense mutation in the C-terminus increases the inhibition of TRPM7 by Mg2+ and worsens the Mg2+ homeostasis in a Mg2+ deficient environment. This defects leads to a reduced intracellular Mg2+ concentration described in both ALS-G and PD-G (PDC) patients.Reference Hermosura53 This discovery is especially important because both TRPM7 and TRPM2 have been also implicated in neuronal cell death pathways. TRPM2 senses oxidative stress and TRPM7 is required for cell viability.Reference McNulty and Fonfria54 TRPM7 and TRPM2 have been put forward as potential factors in neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, strokes and diseases associated with oxidant-mediated neuronal damage.Reference McNulty and Fonfria54–Reference Li, Westwick, Cox and Poll56 Mutations in TRPM2 have been discovered that result in rapidly inactivated channels that are unable to maintain sustained Mg2+ influx.Reference Hermosura57, Reference Hermosura and Garruto58 Via other pathomechanisms, TRPM7 is involved in the Familial Alzheimer's Disease (FAD) which is linked to aberrant PI(4,5)P2 metabolism which in turn causes a dysregulation of this channel. The PI(4,5)P2 turnover is affected by the presenilin mutants and the cellular PI(4,5)P2 content correlates with the contents of the 42-residue amyloid β-peptide (A β42). Thus, FAD mutant presenilin generates toxic A β42 levels and causes TRPM7 dysfunction.Reference Landman59 TRPM1, the founding member of the TRPM family and until now mainly know as a tumour suppressor and a retinal channel for light perception, could be linked to a complex neurodevelopmental disorder characterized by severe visual impairment, intellectual disability, and refractory epilepsy. This disease is caused by a micro-deletion in chromosome 15q13.3 carrying the trpm1 gene.Reference Lepichon, Bittel, Graf and Yu60
TRPs, Schizophrenia and Bipolar Disorders
Several TRP channels have been suggested as players in the pathogenesis of schizophrenia. TRPV1, in cooperation with the endocannabinoid system, influences GABAergic and glutamatergic synapses and play a modulatory function on dopamine transmission. Through these mechanisms TRPV1 and endocannabinoids have an important influence on various neurobiological processes (e.g., control of movement, motivation/reward) and, particularly, on different pathologies affecting these processes such as basal ganglia disorders, schizophrenia, and drug addiction.Reference Fernandez-Ruiz, Hernandez and Ramos61
Interestingly, some natural compounds that have been used in traditional medicine as anti-depressants have TRP channels as targets. One of these compounds is Incensole acetate, which is released by the burning of resin from the Boswellia plant and has been used for religious and cultural ceremonies for millennia. It activates TRPV3, which is expressed in the brain and causes anxiolytic-like and antidepressive-like behavioral effects.Reference Moussaieff62St. John's Wort has been used medicinally for over 5000 years. Relatively recently, one of its phloroglucinol derivatives, hyperforin, an antidepressive compound, has been identified as an effective activator of TRPC6.Reference Leuner63 Hyperforin has been shown to have cognitive enhancing, memory facilitating properties and has probably neuroprotective effects.Reference Griffith, Varela-Nallar and Dinamarcaand N. Inestrosa64 In particular TRPM2, which is highly expressed in the striatum (caudate nucleus and putamen), is supposed to play a key role in bipolar disorders.Reference Aita65–Reference Xu67 Recent case-control studies implicate TRPM2 conferring risk for bipolar disorder (BD) and genetic variants of TRPM2 have been identified to be coupled with BD supporting a role for this channel in the pathogenesis of this disorderReference Xu68 (see for a review Ref. Reference Chahl69).
TRPs Channels in Brain Injury and Stroke
Traumatic brain injury elicits a sequence of complex biochemical changes including oxidative stress, oedema, inflammation and excitotoxicity. TRPM2 channels are activated by such conditions, e.g. processes involving oxidative stress. Downregulation of this channel clearly causes a neuroprotective effect in the cerebral cortex and hippocampus after brain injury, oxidative stress, inflammation and neuronal death.Reference Cook, Vink, Helps, Manavis and van den Heuvel70
The Ca2+ activated TRP channel, TRPM4, is involved in the damaging secondary events that accompany traumatic brain injury (TBI). Changes in capillary permeability result in the extravasation of extracellular fluid, inflammatory cells, and blood, thereby producing cerebral edema, inflammation, and progressive secondary haemorrhage. Inhibition of TRPM4 channels provides beneficial effects in animal models of TBI- and ischemia-associated cerebral edema and secondary haemorrhage.Reference Simard, Kahle and Gerzanich71 Therefore, TRPM4 inhibition plays a neuroprotective role, reduces brain or spinal chord lesion volume and produces a substantial improvement in neurological function.Reference Gerzanich72 Also, TRPM7 is a potential target for neuroprotection after brain injury. Suppressing the expression of TRPM7 in hippocampal CA1 neurons conferred resistance to ischemic cell death, preserved cell function and prevented ischemia-induced deficits in memory.Reference Rempe, Takano and Nedergaard73, Reference Sun74 TBI confers a major burden to Western society and effective treatments are urgently required to improve the long-term deficits of survivors of TBI. Depletion of intracellular Mg2+, a symptom of TBI and a reduction of extracellular Ca2+ are both associated with poor neurological outcome and are both conditions that activated TRPM7, thereby possibly increasing the Ca2+ load of neuronal cells. This leads to secondary injury processes and to cell death following TBI.Reference Cook, Van Den Heuvel and Vink75 TRPM7 has been implicated in ischemic brain damage. TRPM7 gene variation might play a role in the risk of ischemic stroke.Reference Romero, Ridker and Zee76
Conclusion
TRP channels are relatively new membrane proteins that are involved in a plethora of cell functions and are mainly appreciated as sensory ion channels. This short review maps TRP channels as important players in the function of our brain including the forming of hard-wired connections in our developing brain by growth cone guidance, regulation of synaptogenesis, spine forming and modulation of synaptic plasticity. This new view on the function of TRP channels in our central nervous system has already identified some of these channels as potential pharmaceutical targets and has led to a new understanding of several brain diseases. However, we have just entered a new era of neurophysiology and we anxiously await exciting discoveries in a rapidly expanding field of brain research.
Bernd Nilius is Emeritus Professor at the Catholic University of Leuven (KU Leuven). After several positions in Germany, including a Group Leader position at the Max Planck Society, he was appointed as Full Professor of Physiology at the KU Leuven in 1993. He worked on many types of ion channels, discovered in 1985 the T-type Ca2+ channels in cardiac cells and later on focused on ion channels in vascular endothelium, especially Cl– channels. In Leuven, he initiated very successful research on TRP cation channels studying their molecular characterization, their function in native cells, and their impact on human diseases. He is a member of several Academies (e.g. Foreign Associated Member Royal Academy of Medical Science, Belgium), EMBO, has received some prestigious awards, is Editor-in-Chief of Pflügers Archiv, Reviews Physiol Biochem Pharmacol and serves as editor to many journals such as Journal of Physiology (London), Physiological Reviews, EMBO Journal, EMBO Reports, EMBO Molecular Mechanisms of Disease, Journal of Biological Chemistry, Journal of Neuroscience etc. He keeps the rank of seventh worldwide as for overall citation score Ion Channel Citation Ranking System (http://www.ionchannels.org/labs.php), has a Hirsch factor 68, more than 18,000 citations and more than 400 peer reviewed papers. Bernd Nilius is a member of Academia Euopaea.