Introduction
Pregnancy represents a particularly vulnerable period for the onset, recurrence and exacerbation of major mental health conditions, including depression, anxiety and mood disorders (Howard et al. Reference Howard, Molyneaux, Dennis, Rochat, Stein and Milgrom2014). It has been reported that approximately 7–15% of women during pregnancy are affected by mental disorders (Gelaye et al. Reference Gelaye, Rondon, Araya and Williams2016; Van den Bergh et al. Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017), whose common symptoms, such as disordered appetite, sleep disturbances and mood swings are often difficult to distinguish from physiological changes occurring during pregnancy, and thus, the reported prevalence is likely underestimated. Sleep disturbances, for example, are among the major symptoms associated with depression, and during pregnancy are considered as both a result of stress and as a stressor per se that may contribute to adverse pregnancy outcomes (Palagini et al. Reference Palagini, Gemignani, Banti, Manconi, Mauri and Riemann2014). Moreover, mental disorders often coexist (Fried et al. Reference Fried, van Borkulo, Cramer, Boschloo, Schoevers and Borsboom2017) increasing the burden of adverse effects on the mother and her child.
A number of studies reported associations of prenatal maternal depression and anxiety with offspring health outcomes, including low birth weight, preterm birth (Grote et al. Reference Grote, Bridge, Gavin, Melville, Iyengar and Katon2010) and respiratory morbidity (van de Loo et al. Reference van de Loo, van Gelder, Roukema, Roeleveld, Merkus and Verhaak2016). Also, sleep disorders, such as obstructive sleep apnoea and insomnia have been shown to be associated with pregnancy complications and adverse perinatal outcomes (Bin et al. Reference Bin, Cistulli and Ford2016; Felder et al. Reference Felder, Baer, Rand, Jelliffe-Pawlowski and Prather2017). Furthermore, maternal mental disorders during pregnancy influence the child cognitive, emotional, social and behavioural development increasing the risk of emotional (internalising) and behavioural (externalising) difficulties, such as attention-deficit/hyperactivity disorder (ADHD) (Glover, Reference Glover2011; Stein et al. Reference Stein, Pearson, Goodman, Rapa, Rahman, McCallum, Howard and Pariante2014).
ADHD is a childhood-onset neurodevelopmental disorder characterised by symptoms of inattention, hyperactivity and impulsivity. Its aetiology is multifactorial (Thapar & Cooper, Reference Thapar and Cooper2016), including an important heritability component (heritability estimates ranging from 75 to 90%) (Goodman & Stevenson, Reference Goodman and Stevenson1989; Thapar et al. Reference Thapar, Holmes, Poulton and Harrington1999; Thapar et al. Reference Thapar, Harrington, Ross and McGuffin2000; Faraone et al. Reference Faraone, Perlis, Doyle, Smoller, Goralnick, Holmgren and Sklar2005), several environmental risk factors (Thapar & Cooper, Reference Thapar and Cooper2016) and gene–environment interactions (Nigg et al. Reference Nigg, Nikolas and Burt2010; Harold et al. Reference Harold, Leve, Barrett, Elam, Neiderhiser, Natsuaki, Shaw, Reiss and Thapar2013). Parental expectations of the child's behaviour play an important role in the definition of ADHD and are known to differ among populations (Zwirs et al. Reference Zwirs, Burger, Buitelaar and Schulpen2006).
Several studies reported an association of maternal anxiety and depression during pregnancy with an increased risk of ADHD in preschool children, but only a few of them had prospectively collected exposure data (Clavarino et al. Reference Clavarino, Mamun, O'Callaghan, Aird, Bor, O'Callaghan, Williams, Marrington, Najman and Alati2010; Van Batenburg-Eddes et al. Reference Van Batenburg-Eddes, Brion, Henrichs, Jaddoe, Hofman, Verhulst, Lawlor, Davey Smith and Tiemeier2013; Bendiksen et al. Reference Bendiksen, Aase, Diep, Svensson, Friis and Zeiner2015; Wolford et al. Reference Wolford, Lahti, Tuovinen, Lahti, Lipsanen, Savolainen, Heinonen, Hamalainen, Kajantie, Pesonen, Villa, Laivuori, Reynolds and Raikkonen2017). Much of the research was focused on maternal depression and anxiety during pregnancy and less attention has been paid to depression and anxiety occurring before pregnancy.
To take into account the parents’ cultural context and different exposure time windows, we aimed at examining maternal diagnoses of anxiety and depression before and during pregnancy in association with inattentive and hyperactivity-impulsivity ADHD traits in 4-year-old offspring in a large mother-child cohort carried out in South Europe (Italy). We also analysed doctor-diagnosed maternal sleep disorders before and during pregnancy, which can both contribute to mental health conditions and be a symptom of other mental disorders (Fried et al. Reference Fried, van Borkulo, Cramer, Boschloo, Schoevers and Borsboom2017). Maternal sleep disorders, to our knowledge, have not been studied before in association with child ADHD.
Methods
Study population
Data were collected from the study ‘Nascita e INFanzia: gli Effetti dell'Ambiente’ (NINFEA), whose protocol was approved by the Ethical Committee of the San Giovanni
Battista Hospital and CTO/CRF/Maria Adelaide Hospital of Turin. The NINFEA cohort study is an internet-based birth cohort with the aim of investigating prenatal and early life exposures in relation to childhood health and development from a life-course perspective (www.progettoninfea.it) (Richiardi et al. Reference Richiardi, Baussano, Vizzini, Douwes, Pearce, Merletti and Cohort2007).
Approximately 7500 pregnant women who had access to the Internet and enough knowledge of the Italian language to complete online questionnaires were recruited from 2005 until 2016. The women completed the first baseline questionnaire at any time during pregnancy, and the children have been followed-up with additional five questionnaires completed by their mothers 6 months after delivery and when the children turn 1½, 4, 7 and 10 years of age.
For this study, we used the NINFEA database version 11.2017. The outcome was assessed at the age of 4 years where the response rate of the questionnaire is 77% (Pizzi, Reference Pizzi2016). A total of 3634 singletons who at the time of the data download had completed the assessment at age 4 years, were included in the study.
Explanatory variables
Maternal mental disorder data were collected with a questionnaire completed during pregnancy (mean gestational age at completion 26.3 weeks, standard deviation (SD) 9.5) in which women were asked to answer a checklist of chronic conditions ever diagnosed by a doctor. The full checklist consisting of 30 different maternal chronic conditions is available online at the study website (Progetto Ninfea, 2005). We selected from the checklist the following maternal mental disorders: (i) diagnosis of depression, (ii) diagnosis of anxiety and (iii) diagnosis of sleep disorders. For each reported condition, participants were further asked to report whether the condition was present only before pregnancy, only during pregnancy or in both periods. Information on the third-trimester exposures was retrieved from the questionnaire completed 6 months after delivery.
We defined three exposure time windows: (i) lifetime diagnosis – a disorder ever diagnosed by a doctor, (ii) pre-pregnancy exposure – a previous diagnosis of a disorder that was not active during the index pregnancy and (iii) during pregnancy exposure – a disorder active during the index pregnancy.
The definitions of sleep disorders were based on any doctor-diagnosed a sleep disorder, as information on specific Diagnostic and Statistical Manual of Mental Disorders (DSM V) (American Psychiatric Association, 2013) subcategories was not available in the NINFEA cohort. In addition, for sleep disorders during pregnancy, we did not consider the third trimester of pregnancy in order to avoid exposure misclassification due to deterioration in sleep quality across pregnancy (Polo-Kantola et al. Reference Polo-Kantola, Aukia, Karlsson, Karlsson and Paavonen2017).
Potential confounders were chosen a priori and included maternal age at delivery (<30; 30–34; 35+ years), maternal educational level (university degree vs. lower level), maternal smoking during pregnancy (ever vs. never smoking), maternal alcohol consumption during the first trimester of pregnancy (at least 1 drink/day vs. < = 6 drinks/week), gender of the child and first-born status.
Outcome variables
When the child turned 4 years, mothers were asked to respond to a list of questions regarding the child's behaviour (mean age at questionnaire completion 4.1 years; SD, 0.2 years). This list is based on the criteria for ADHD diagnosis of the Diagnostic and Statistical Manual of Mental Disorders (DSM IV) (American Psychiatric Association, 1994) that provides a standard assessment of inattentive and hyperactive–impulsive symptoms prior to 7 years of age (Tandon et al. Reference Tandon, Si, Belden and Luby2009). The DSM IV questionnaire consists of 18 dichotomous (yes/no) items that are used to define two behavioural subscales: (i) inattentive score (ADHD-I) and (ii) hyperactive–impulsive score (ADHD-H).
For a clinical diagnosis, the two traits would have to be confirmed in two settings, e.g. at home and at school, showing evidence of interference on social and academic functioning, but for research purposes, we based our outcome definition only on the mothers’ report.
As from a population perspective, ADHD can be seen as a continuously distributed risk dimension (Larsson et al. Reference Larsson, Anckarsater, Rastam, Chang and Lichtenstein2012; Thapar & Cooper, Reference Thapar and Cooper2016), we analysed ADHD symptoms as continuous scores. One of the nine items of the inattentive sub-scale (‘Often has trouble keeping attention on tasks or play activities’) was not included in the NINFEA questionnaire until a later update of the follow-up questionnaires, and, therefore, we considered only eight items for the ADHD-I score.
Given the association of ADHD with Intelligence Quotient (IQ), intellectual disability (Dykens, Reference Dykens2000) and low long-term academic outcomes (Polderman et al. Reference Polderman, Boomsma, Bartels, Verhulst and Huizink2010; Washbrook et al. Reference Washbrook, Propper and Sayal2013), we used data from the NINFEA assessment at age 7 years (mean age 7.1 years; SD 0.2 years) in which mothers were asked to indicate their children's final grades in mathematics and reading/writing in the first year of the primary school. We considered that a positive association between ADHD scores reported at age 4-years and lower academic performance at school age would indicate that maternally reported ADHD scores are reliable and valid measures of children's cognitive impairments related to ADHD. Information from the assessment at age 7 years was available for 1392 children who were born before November 2010 and thus met the age criterion for the assessment at age 7 years. The primary school in Italy uses a grading system that ranges from 1 (impossible to assess) to 10 (excellent). We coded the child's academic achievement in mathematics and reading/writing as low (equal or less of 7) and high (8–10).
Statistical methods
The total ADHD, ADHD-H and ADHD-I scores were treated as continuous variables and analysed using linear regression models. The number of symptoms was log-transformed [log (y + 1)] to satisfy the assumption of normality. After the transformation, visual inspection and tests based on kurtosis and skewness indicated a normal distribution. Model estimates are reported as percentage differences in the number of symptoms (Törnqvist et al. Reference Törnqvist, Vartia and Vartia1985). We specified two adjustment models: (i) adjustment for child's gender, first-born status, mother's age and educational level, and (ii) additional adjustment for maternal smoking and alcohol use during pregnancy. Maternal anxiety, depression and sleep disorders were analysed separately and in the following time windows: (i) lifetime diagnosis, (ii) pre-pregnancy only and (iii) during pregnancy.
To take into account comorbidities between the three disorders, we additionally analysed the total number of disorders experienced during pregnancy. We categorised the exposed subjects in the following groups: (i) mothers who never had a diagnosis of any of the three disorders (reference), (ii) mothers with a history of at least one of the disorders before pregnancy but not during pregnancy, (iii) mothers with only one of the disorders during pregnancy, (iv) mothers with the two disorders during pregnancy and (v) mothers with all the three disorders during pregnancy. Finally, to explore the relative importance and contribution of each of the disorders to ADHD symptoms we specified a model where all the three disorders were mutually adjusted (i.e. all variables included in the same model).
Associations of the number of symptoms on the two ADHD subscales with the academic outcomes in mathematics and reading/writing were estimated using logistic regression models adjusted for maternal depression, anxiety and sleep disorders, maternal age and education, child's gender and first-born status. As information on academic outcomes was missing for 9.2% of our sample, we performed multivariate multiple imputations using chained equations (20 imputed data sets) to replace missing values of both outcomes and all confounding factors (Buuren & Groothuis-Oudshoorn, Reference Buuren and Groothuis-Oudshoorn2011). Statistical analyses were performed using R software version 3.3.1 (R Core Team, 2016).
Results
The study included 3634 children with the completed assessment at 4 years of age. Children lost to follow-up at age 4 were not significantly different from those included in the study in all the baseline characteristics, including being first-born, maternal age, maternal education and smoking during pregnancy (all p-values > 0.05). The percentage of missing data for maternal and child characteristics was <2.6%.
Maternal characteristics are reported in Table 1, while Table 2 summarises the main child characteristics. Mothers were mostly Italian born (96.5%), highly educated (63.5%) and were aged on average 33.6 (SD 4.2) years at delivery. In our sample, 3.8% of mothers reported a diagnosis of depression, 8.9% anxiety and 1.7% sleep disorders. In total, 402 (11.1%) mothers had at least one of the analysed mental disorders. At 4 years of age, children had a mean total ADHD score of 3.6 (SD 3.0), a mean ADHD-H score of 2.4 (SD 2.1) and a mean ADHD-I score of 1.2 (SD 1.5). The associations of the confounding variables with ADHD-H and ADHD-I are reported in Table S1.
Table 1. Maternal characteristics (n = 3634)
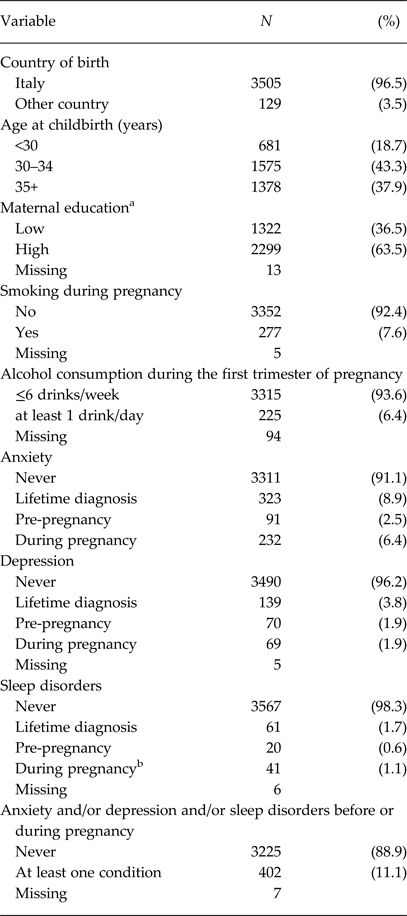
a High – University degree, Low – other.
b Sleep disorders during pregnancy do not include the third trimester exposures.
Table 2. Child characteristics
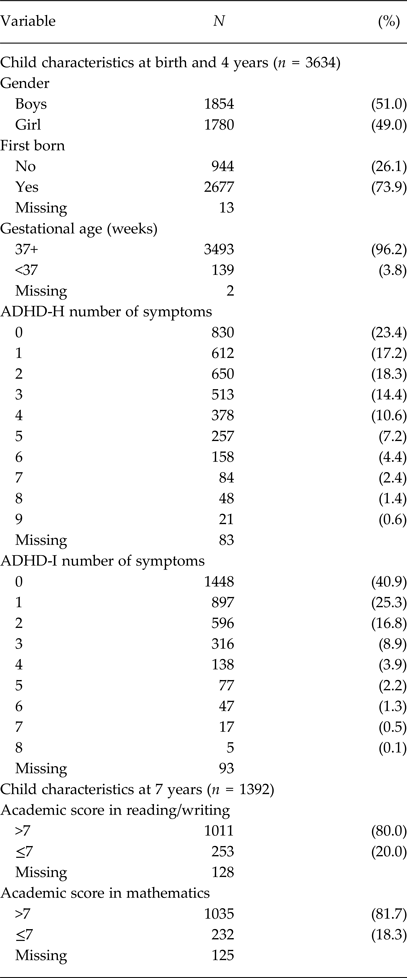
ADHD, Attention-deficit/hyperactivity disorder; ADHD-H, = ADHD hyperactive–impulsive score; ADHD-I, ADHD inattentive score, >7 means good academic performance.
The total ADHD score was associated with maternal lifetime diagnosis of anxiety (ever vs. never: 17.1%; 95% CI 7.3–27.9%), sleep disorders (35.7%; 95% CI 10.7–66.5%), and depression (17.5%; 95% CI 3.2–33.8%).
The associations between maternal mental disorders and child ADHD-H and ADHD-I scores at 4 years of age are reported in Table 3. Both maternal anxiety and sleep disorders were associated with an increase in ADHD-H score. A positive association, though weaker in magnitude, was observed also between maternal depression and ADHD-H score. The direction of the effects was similar also for ADHD-I, although the association of maternal sleep disorders with ADHD-I was somewhat weaker. All the estimates were higher when the disorders were active during pregnancy, for both ADHD traits, and were diminished or annulled for disorders active only during the pre-pregnancy period.
Table 3. Associations between maternal mental disorders and children's ADHD-H and ADHD-I scores at 4 years of age (n = 3634)
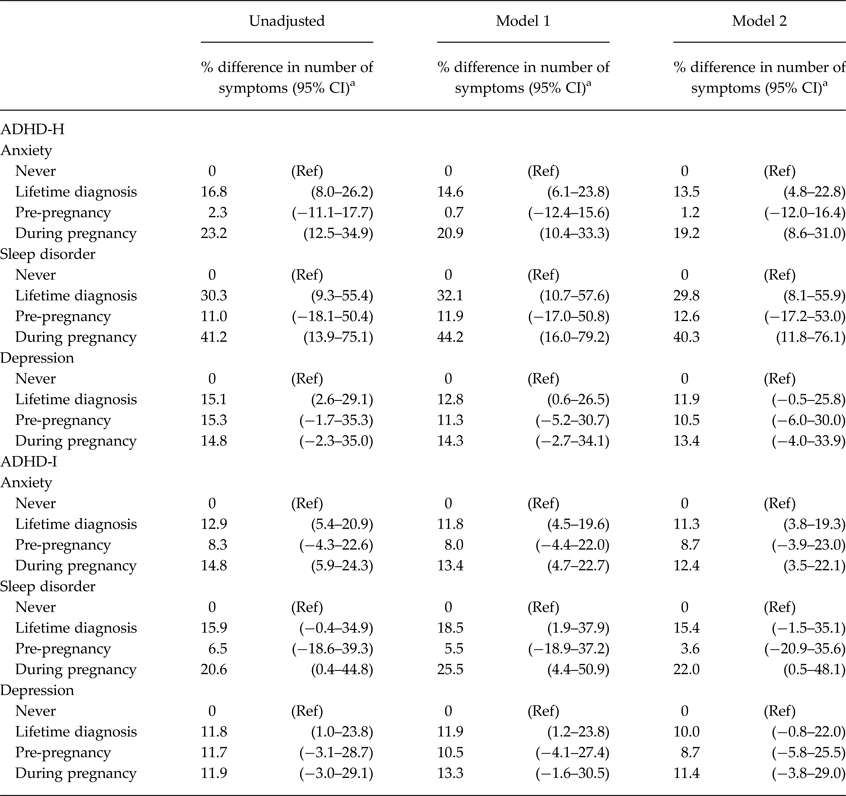
CI, confidence interval, Model 1: Adjusted for maternal age and education, child gender and first-born status, Model 2: Adjusted as Model 1 and additionally adjusted for maternal smoking and alcohol use during pregnancy; ADHD-H, ADHD hyperactive–impulsive score; ADHD-I, ADHD inattentive score.
a Negative values indicate a relative decrease in the number of ADHD sub-scale symptoms.
Of the 135 (3.7%) mothers with a history of at least one disorder before but not during pregnancy, 84 (62.2%) had anxiety, 12 (8.9%) sleep disorders and 39 (28.9%) depression. Of the 212 (5.8%) mothers with only one disorder active during pregnancy, 172 (81.1%) had anxiety, 19 (9.0%) sleep disorders and 21 (9.9%) depression. Among the 42 (1.2%) mothers with two disorders active during pregnancy, 33 (76.7%) had depression and anxiety without sleep disorders, nine (20.9%) had anxiety and sleep disorders without depression, and only one (2.3%) mother had sleep disorders and depression without anxiety. Twelve mothers (0.3%) had all three disorders during pregnancy. Depression more likely co-occurs with anxiety and sleep disorders and there is also a large overlap between anxiety and sleep disorders (all chi-square test p-values < 0.05).
The associations between the number of maternal mental disorders during pregnancy and child ADHD-H and ADHD-I scores at 4 years of age are presented in Table 4. Both ADHD-H score and, to a lesser extent, ADHD-I score showed a relative increase with increasing the number of disorders active during pregnancy. When all the three conditions were included in the same model (i.e. mutually adjusted) lifetime anxiety (11.2%; 95% CI 2.1–21.2%) and sleep disorders (22.4%; 95% CI 1.3–48.1%), but not depression (2.5%; 95% CI −9.7 to 16.4%), remained associated with ADHD-H, while only maternal anxiety was associated with offspring ADHD-I (anxiety: 8.6%; 95% CI 0.7–17.1%; depression: 3.4%; 95% CI −7.6 to 15.6%; sleep disorders 9.5%; 95% CI −7.1 to 29.1%).
Table 4. Associations of the Number of Comorbid maternal mental Disorders with children's ADHD-H and ADHD-I scores at 4 years of age (n = 3634)
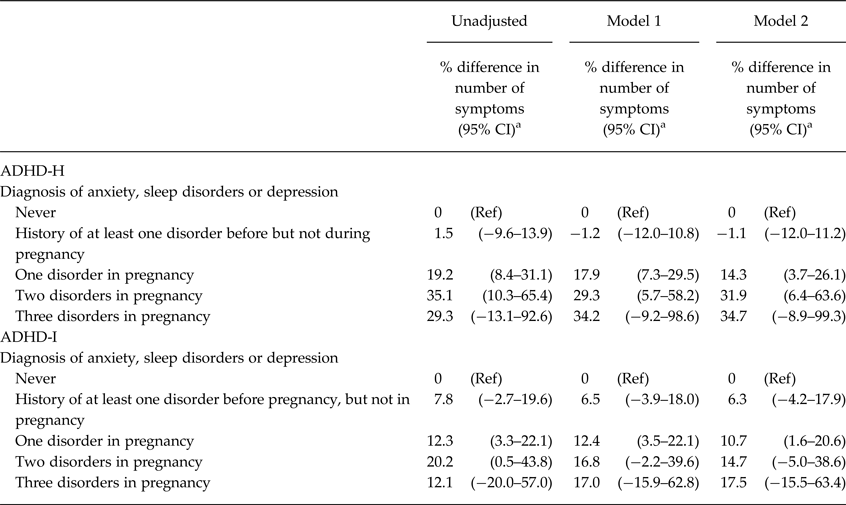
CI, confidence interval, Model 1: Adjusted for maternal age and education, child gender and first-born status, Model 2: Adjusted as Model 1 and additionally adjusted for maternal smoking and alcohol use during pregnancy; ADHD-H, ADHD hyperactive–impulsive score; ADHD-I, ADHD inattentive score.
a Negative values indicate a relative decrease in the number of ADHD sub-scale symptoms.
Associations between child's ADHD at age of 4 years and their academic achievement at the end of the first year of primary school are reported in Table 5. ADHD-I score was negatively associated with academic performance at age 7 years, while no association was found with the ADHD-H score.
Table 5. Associations between ADHD scores at age 4 and poor academic performance in reading/writing and mathematics at age 7 (n = 1392)
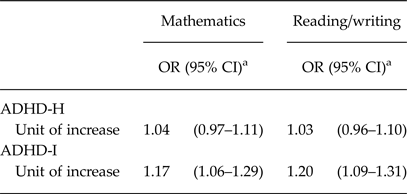
OR, odds ratio; CI, confidence interval; ADHD-H, ADHD hyperactive–impulsive score; ADHD-I, ADHD inattentive score.
a Results from logistic regression analyses adjusted for maternal anxiety, depression or sleep disorders before and during pregnancy, maternal age and education, child gender and first-born status.
Discussion
Our study found positive associations of maternal lifetime anxiety, depression and sleep disorders with offspring ADHD symptoms at 4 years of age. Although the magnitude of the effects and the width of the confidence intervals varied, the associations were quite consistent for both inattentive and hyperactive–impulsive ADHD subscales. Notably, all the associations were stronger when the disorders were actively symptomatic during pregnancy, and there was an evident increase in the number of ADHD symptoms with increasing the number of disorders active during pregnancy. All the associations were largely attenuated if the disorders were present only during the pre-pregnancy period. Anxiety and sleep disorders contributed uniquely to the ADHD-H symptoms in the mutually adjusted model, while only maternal anxiety contributed to the ADHD-I symptoms. Finally, the ADHD-I score, but not the ADHD-H score, at 4 years of age was associated with lower scores in reading/writing and mathematics.
Our findings are generally consistent with those reported by previous longitudinal birth cohort studies, but with slightly stronger effects of maternal mental disorders during pregnancy on offspring ADHD. In the Norwegian MoBa cohort, an increase in maternal prenatal distress score was associated with an increase in the number of ADHD-H, but not with ADHD-I symptoms (Bendiksen et al. Reference Bendiksen, Aase, Diep, Svensson, Friis and Zeiner2015). The authors explained that the lack of the association with ADHD-I may be due to lack of power, as only a few children had a clinically significant ADHD-I. Consistently, the PREDO cohort study found an increase in ADHD symptoms in 3–6-year-old children born to mothers with depressive symptoms during pregnancy (Wolford et al. Reference Wolford, Lahti, Tuovinen, Lahti, Lipsanen, Savolainen, Heinonen, Hamalainen, Kajantie, Pesonen, Villa, Laivuori, Reynolds and Raikkonen2017). Furthermore, a positive association between maternal anxiety during pregnancy and persistent attention problems in children was found in the Australian MUSP cohort (Clavarino et al. Reference Clavarino, Mamun, O'Callaghan, Aird, Bor, O'Callaghan, Williams, Marrington, Najman and Alati2010), and antenatal maternal anxiety and depression were associated with an increased risk of child inattention at 3 years of age in the UK ALSPAC and Dutch Generation R cohorts (Van Batenburg-Eddes et al. Reference Van Batenburg-Eddes, Brion, Henrichs, Jaddoe, Hofman, Verhulst, Lawlor, Davey Smith and Tiemeier2013).
To our knowledge, this is the first study reporting an association between maternal sleep disorders and offspring ADHD. We observed that doctor-diagnosed maternal sleep disorders, especially if active during pregnancy, are strongly associated with offspring ADHD. These associations were evident particularly for the ADHD-H trait, where the observed difference was independent of maternal comorbid depression and anxiety. Maternal insomnia and sleep apnoea have been associated with preterm birth (Felder et al. Reference Felder, Baer, Rand, Jelliffe-Pawlowski and Prather2017) and pregnancy complications, including gestational diabetes and hypertension (Bazalakova, Reference Bazalakova2017; Bourjeily et al. Reference Bourjeily, Danilack, Bublitz, Lipkind, Muri, Caldwell, Tong and Rosene-Montella2017). Chronic sleep deprivation is also known to be related to stress system activation that may influence adverse pregnancy outcomes (Palagini et al. Reference Palagini, Gemignani, Banti, Manconi, Mauri and Riemann2014). It should be noted that we assessed only doctor-diagnosed disorders and, therefore, the effect of less severe sleep disturbances, which have much higher prevalence in general population and among pregnant women, requires future research. However, our findings suggest the importance of the sleep disorders assessment in women of reproductive age.
In our analyses, we took into account several important confounding factors, and the associations we found between these confounders and ADHD-H and ADHD-I were consistent with previous research (Sayal et al. Reference Sayal, Heron, Draper, Alati, Lewis, Fraser, Barrow, Golding, Emond, Davey Smith and Gray2014; Arnett et al. Reference Arnett, Pennington, Willcutt, DeFries and Olson2015; Obel et al. Reference Obel, Zhu, Olsen, Breining, Li, Gronborg, Gissler and Rutter2016), providing indirect support to the validity of our research setting. Preterm birth is a potential mediator of the association between maternal mental health and neurodevelopmental problems (McCoy et al. Reference McCoy, Rickert, Class, Larsson, Lichtenstein and D'Onofrio2014), and was thus not considered as a potential confounder in our study. However, further controlling for gestational age as a continuous variable or restricting analysis to children born at term did not change the results more than marginally (data not shown).
Although the specific mechanism involved in the associations between maternal mental disorders and offspring attention and/or hyperactivity/impulsivity problems are still unclear, several possible explanations have been suggested. First, maternal mental disorders could act by activating the HPA (hypothalamic–pituitary–adrenal) axis, which, through an excessive increase in cortisol levels, might compromise fetal brain development (Van den Bergh et al. Reference Van den Bergh, Mulder, Mennes and Glover2005; Beijers et al. Reference Beijers, Buitelaar and de Weerth2014; Glover, Reference Glover2015). In addition, the observed relationship could also be due to confounding by shared familial characteristics, such as genetics (Thapar & Cooper, Reference Thapar and Cooper2016), as well as residual confounding by socioeconomic status (Foulon et al. Reference Foulon, Pingault, Larroque, Melchior, Falissard and Cote2015) and/or lifestyle (Sayal et al. Reference Sayal, Heron, Draper, Alati, Lewis, Fraser, Barrow, Golding, Emond, Davey Smith and Gray2014; Rijlaarsdam et al. Reference Rijlaarsdam, Cecil, Walton, Mesirow, Relton, Gaunt, McArdle and Barker2017). Finally, mental disorders are generally persistent and could affect parenting style and mother–child attachment during postnatal period (Harold et al. Reference Harold, Leve, Barrett, Elam, Neiderhiser, Natsuaki, Shaw, Reiss and Thapar2013; Webb & Ayers, Reference Webb and Ayers2015; Thapar & Cooper, Reference Thapar and Cooper2016) – factors that are known to be associated with later ADHD symptoms (Storebo et al. Reference Storebo, Rasmussen and Simonsen2016).
The main strength of the NINFEA study is that the exposure information was collected prospectively during pregnancy. To the best of our knowledge, this is the first study on prenatal risk factors for ADHD in the Italian population, and thus, serves as a replication of findings from other populations (Zwirs et al. Reference Zwirs, Burger, Buitelaar and Schulpen2006). Our findings provide further evidence that maternal anxiety and depression contribute to the onset of offspring ADHD symptoms and extend the existing evidence also to maternal sleep disorders. We were able to evaluate two distinct ADHD subscales and most of the observed associations were evident both for inattentive and for hyperactive–impulsive trait. Finally, the follow-up at 7 years of age on the academic performance supports the clinical significance of the ADHD-I phenotype.
Our study has some limitations that should be considered when interpreting the results. First, the assessment of child's behavioural problems was entirely based on maternal report, and mothers with mental disorders at the time of the completion of the questionnaire might have overreported child ADHD symptoms (Najman et al. Reference Najman, Williams, Nikles, Spence, Bor, O'Callaghan, Le Brocque and Andersen2000). However, the observed associations were qualitatively similar for depression, anxiety and sleep disorders, and it is unlikely that the misreporting of child symptoms would have been driven in the same direction by these three disorders. Moreover, empirical evidence suggests a weak association between maternal mental health and differential reporting of offspring ADHD symptoms. In particular, a study on ADHD children showed that parental ADHD status does not affect maternal reporting of ADHD symptoms in their children (Faraone et al. Reference Faraone, Monuteaux, Biederman, Cohan and Mick2003).
Considering that in the NINFEA cohort ADHD score and the academic achievement were assessed prospectively 3 years apart, and that the reported grades at school are not likely to be affected by maternal perception of her own child, our finding of a lower academic achievement among children with ADHD-I further supports the validity of the ADHD assessment in our cohort. Similarly, a previous study reported a lower academic achievement among children with an inattentive trait, but not among those with hyperactive behaviour (Polderman et al. Reference Polderman, Huizink, Verhulst, van Beijsterveldt, Boomsma and Bartels2011). These associations have been consistently replicated in large sample size studies with information on several potential confounding factors, including intelligence, family income and comorbidities (Polderman et al. Reference Polderman, Boomsma, Bartels, Verhulst and Huizink2010).
As different functions and structures of the brain develop in different periods of gestation, it has been hypothesised that the effects of prenatal stress on specific offspring neurodevelopmental outcomes may differ according to the pregnancy trimester (Van den Bergh et al. Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017). We did not analyse single trimester exposures as the prevalence of these disorders during pregnancy is rather low (e.g. depression prevalence is 2%), and the stratified analyses would have limited power. However, in this study, we used doctor-diagnosed mental disorders capturing, therefore, more serious and chronic conditions that generally do not pass in short time periods, such as pregnancy trimester.
Another limitation of our study is the lack of information on maternal ADHD diagnosis that potentially could act as a confounding factor. It should be noted that ADHD was unrecognised and rarely diagnosed in Italy before the 1990s (Gallucci et al. Reference Gallucci, Bird, Berardi, Gallai, Pfanner and Weinberg1993), and therefore, difficult to be assessed in most of the mothers participating in the NINFEA cohort. However, given the relatively low ADHD prevalence in general population (Simon et al. Reference Simon, Czobor, Balint, Meszaros and Bitter2009) compared with anxiety, depression and sleep disorders, and the relatively strong associations that we found, it is unlikely that confounding by maternal ADHD could entirely explain the findings of our study.
Finally, participants of the NINFEA cohort, like those of many other cohort studies, are a selected population with relatively high education and socioeconomic status. However, it has been extensively shown that, although this selective participation might affect prevalence estimates, it does not imply distorted estimates of association in cohort studies (Pizzi et al. Reference Pizzi, De Stavola, Pearce, Lazzarato, Ghiotti, Merletti and Richiardi2012; Rothman et al. Reference Rothman, Gallacher and Hatch2013).
Conclusions
Our findings indicate that antenatal maternal mental disorders, in particular depression, anxiety and sleep disorders, are associated with higher scores of inattentive and hyperactive–impulsive symptoms in their children at age 4 years and that these associations are stronger if the disorders are active during pregnancy. Antenatal preventive strategies focused on identification and reduction of mental disorders may be important for improving child psychological development.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796018000185.
Acknowledgement
The authors are grateful to all the participants of the NINFEA study.
Financial support
The NINFEA cohort has been partially funded by the Compagnia SanPaolo Foundation and the Piedmont Region.
Conflict of interest
The authors declare no competing interests.
Availability of data and materials
Anonymised data are available upon request to qualified researchers who meet the criteria for access to confidential data for the purpose of academic, non-commercial research, as required by the authors’ IRB. Data on exposure and outcome variables are available upon request by contacting lorenzo.richiardi@unito.it







