Introduction
Pemphigus is a rare but life-threatening autoimmune bullous disease of the skin and mucous membranes, mediated by autoantibodies directed against desmosomal adhesion proteins (in particular, desmogleins 1 and 3) responsible for maintaining the integrity of the epidermis (Bystryn & Rudolph, Reference Bystryn and Rudolph2005).
Pemphigus typically presents with mucosal ulcerations and superficial blisters and erosions affecting the trunk, face, scalp and proximal limbs. Since the blister forms within the epidermis, it is fragile and breaks easily. Mucosal lesions mainly involve the oral cavity, but can also spread to affect the larynx, pharynx, oesophagus, eyes and genitalia (Groves, Reference Groves2009). Mortality due to pemphigus was dramatically reduced from 75% to 5–30% (Bystryn & Steinman, Reference Bystryn and Steinman1996; Uzun et al. Reference Uzun, Durdu, Akman, Gunasti, Uslular, Memisoglu and Alpsoy2006; Risser et al. Reference Risser, Lewis and Weinstock2009) since the introduction of corticosteroid treatment in the early 1950s. Morbidity as a consequence of therapy is now more common than mortality related to the underlying disease (Kridin et al. Reference Kridin, Sagi and Bergman2017).
Pemphigus is a relapsing, difficult-to-treat illness requiring long-term hospitalisation and immunosuppressive treatment. As it also affects the patient's appearance, it may inflict significant psychological trauma. Patients with pemphigus experience a major decrease in their quality of life, affecting physical, psychological and social aspects (Mayrshofer et al. Reference Mayrshofer, Hertl, Sinkgraven, Sticherling, Pfeiffer, Zillikens and Messer2005; Terrab et al. Reference Terrab, Benchikhi, Maaroufi, Hassoune, Amine and Lakhdar2005; Tabolli et al. Reference Tabolli, Mozzetta, Antinone, Alfani, Cianchini and Abeni2008; Paradisi et al. Reference Paradisi, Sampogna, Di Pietro, Cianchini, Didona, Ferri, Abeni and Tabolli2009; Sung et al. Reference Sung, Roh and Kim2015).
Although psychodermatological research on pemphigus is relatively sparse, anxiety and depression have been reported to correlate with disease activity (Tabolli et al. Reference Tabolli, Mozzetta, Antinone, Alfani, Cianchini and Abeni2008) and to persist during quiescent periods (Tabolli et al. Reference Tabolli, Pagliarello, Paradisi, Cianchini, Giannantoni and Abeni2014). A previous study performed by our group has shown an increased prevalence of comorbid depression among patients with pemphigus relative to matched control subjects (Wohl et al. Reference Wohl, Mashiah, Kutz, Hadj-Rabia and Cohen2015). In a recent Indian study of 50 pemphigus patients, psychiatric comorbidity was diagnosed in 26% of the patients (according to ICD-10) and in 40% of the patients (according to GHQ-12) (Kumar et al. Reference Kumar, Mattoo and Handa2013). A recent Iranian study using GHQ-28 bimodal scores reported a 73.7% prevalence of mental disorders among patients with pemphigus (Arbabi et al. Reference Arbabi, Ghodsi, Mahdanian, Noormohammadi, Shalileh, Darvish, Ashrafinia and Chams2011).
Immunological hypotheses have become increasingly prominent in psychiatric research (Müller & Schwarz, Reference Müller and Schwarz2010), suggesting that autoimmunity may be involved in the etiopathogenesis of some patients with symptoms of schizophrenia (Drexhage et al. Reference Drexhage, Weigelt, van Beveren, Cohen, Versnel, Nolen and Drexhage2011; Benrós & Mortensen, Reference Benrós and Mortensen2015). Large-scale population-based epidemiological studies have shown that individuals with schizophrenia have a nearly 50% higher lifetime prevalence of autoimmune disorders (Eaton et al. Reference Eaton, Byrne, Ewald, Mors, Chen, Agerbo and Mortensen2006; Benros et al. Reference Benros, Nielsen, Nordentoft, Eaton, Dalton and Mortensen2011). Moreover, schizophrenia was found to be associated with a wide range of autoimmune and immune-related diseases, including Celiac disease, Graves’ disease, psoriasis, pernicious anaemia, and hypersensitivity vasculitis (Chen et al. Reference Chen, Chao, Chen, Chang, Wu, Wu, Yeh, Chen and Tsai2012). However, the epidemiological association between pemphigus and schizophrenia has not yet been examined.
The aim of our study was to investigate the association between pemphigus and schizophrenia in a large-scale cross-sectional study, utilising one of the largest cohorts of patients with pemphigus in the literature.
Methods
Study design and database
The study was designed as a retrospective, population-based, cross-sectional study. Data-mining techniques were utilised to access information from the Clalit Health Services (CHS) database. CHS is the largest managed care organisation in Israel, serving a population of approximately 4 400 000 in 2016. CHS has a comprehensive computerised database with continuous real-time input from pharmaceutical, medical and administrative operating systems that facilitates gathering data for epidemiological studies. The validity of diagnoses in this registry, which are based on reports from hospital and primary care physicians and specialists, has been shown to be reliable (Rennert & Peterburg, Reference Rennert and Peterburg2001). The CHS database undergoes a continuous validation process by logistical checks (such as comparing the diagnoses from various sources) and by direct validation of the diagnoses by each patient's treating physician.
Study population and covariate factors
Patients were defined as having pemphigus when there was either a diagnosis of pemphigus documented at least twice in the medical records by a physician in the community or when pemphigus was listed in the diagnoses of a hospital discharge letter. Up to five control patients were randomly selected for each case patient. After excluding patients with pemphigus from the list of CHS members, the control group was randomly selected and matched to cases based on age, sex and ethnicity. Age matching was based on the exact year of birth (1-year strata). Controls were confirmed to be alive and to be contributing data to CHS on the date of the diagnosis of the matched case. This date of ‘pseudodiagnosis’ was assigned to each control subject and indicated the date of diagnosis of the matched case.
Data available from the CHS database included age, sex, socioeconomic status (SES), and diagnoses of chronic diseases. These diagnoses were extracted from the CHS registry of chronic diseases, which is based on data from hospital and primary care physicians’ reports and validated by primary physicians. A Charlson comorbidity score was calculated for each of the study participants (Charlson et al. Reference Charlson, Pompei, Ales and MacKenzie1987). Healthcare utilisation was determined by the number of total visits per individual in the year before the diagnosis of pemphigus in cases and before ‘pseudodiagnosis’ in controls.
Statistical analysis
The distribution of sociodemographic and clinical factors between cases and control subjects was compared using χ 2 test for sex and SES and using t-test for age. Logistic regression was then used to calculate odds ratio (OR) and 95% confidence interval (CI) to compare schizophrenia between cases and controls. Homogeneity of ORs across strata was tested using Breslow–Day and Tarone's tests. The exact age matching permitted the use of unconditional logistic regression (Pearce, Reference Pearce2016).
Outcome measures were adjusted for comorbidities as determined by Charlson scores. To ensure that observed associations were not merely due to increased ascertainment, outcome measures were also adjusted for overutilisation of health services.
All statistical analysis was performed using SPSS software, version 18 (SPSS, Chicago, IL, USA).
Results
The study consisted of 1985 patients with pemphigus and 9874 age-, gender- and ethnicity-matched control subjects. The mean (±s.d.) age at presentation of pemphigus was 72.1 ± 18.5, which is identical to the age of control subjects at the date of their enrolment. 797 (40.2%) cases were male, with a similar proportion seen in controls. The ethnic and socioeconomic structure of the two groups was similar. While the prevalence of drug and alcohol abuse was comparable in the two groups, there was a higher proportion of smokers among control subjects. Comorbidity rates as measured by the Charlson index were higher in cases, with 1059 (53.4%) having severe comorbidity compared with 4055 (41.1%) in controls. Healthcare utilisation rates were lower in cases than controls; 65% of cases and 71% of controls had more than 12 consultations in the year prior to pemphigus diagnosis (Table 1).
Table 1. Baseline characteristics of the study population
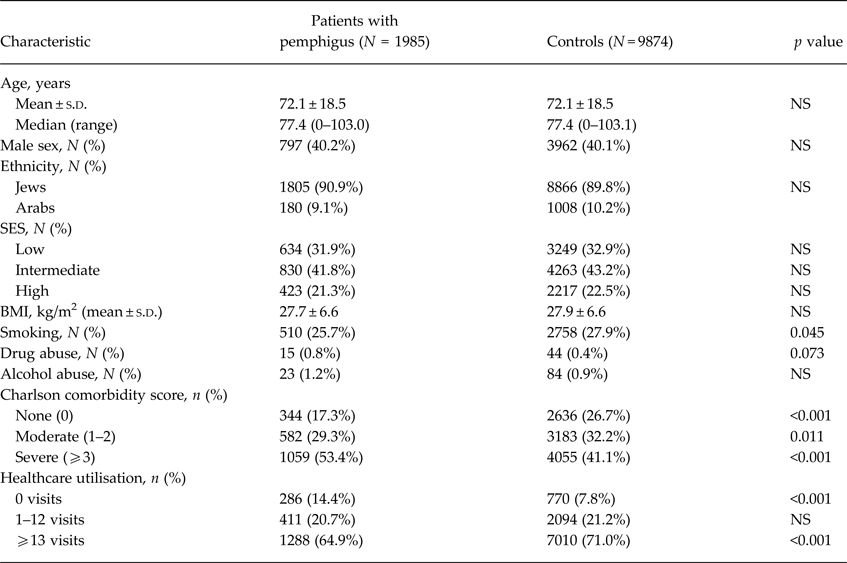
OR, odds ratio; n, number; SES, socioeconomic status; CI, confidence interval; NS, non-significant; s.d., standard deviation; BMI, body mass index.
The prevalence rate of schizophrenia was greater in patients with pemphigus than in control subjects (2.0% v. 1.3%, respectively; OR, 1.5; 95% CI, 1.1–2.2; p = 0.019). When stratified by age, the association was prominent for patients older than 60 years of age. A significant association was also seen among females and among patients of Jewish ancestry (Table 2).
Table 2. The association between pemphigus and schizophrenia stratified by age, gender, and ethnicity

OR, odds ratio; n, number; CI, confidence interval; NS, non-significant.
Bold: significant value.
No significant confounding by age, sex, or ethnic background was noted, as the Mantel–Haenszel ORs are within 10% of the crude ORs. No modification-significant effect of the association between pemphigus and schizophrenia was noted by any covariate (data not shown).
After controlling for confounders such as age, sex, ethnicity, SES, drug abuse, alcohol abuse, healthcare utilisation and comorbidities, pemphigus demonstrated a substantial independent association with schizophrenia in multivariable logistic regression analysis (OR, 1.6; 95% CI, 1.1–2.3; Table 3). Younger age, Jewish ethnicity, intermediate SES and drug abuse were also found to be associated with schizophrenia (Table 3).
Table 3. The association between pemphigus and schizophrenia after controlling for confounders by logistic regression model

CI, confidence interval; NS, non-significant; SES, socioeconomic status.
Bold: significant values.
Discussion
This is the first population-based study aiming to investigate the association between pemphigus and schizophrenia in a large cohort of pemphigus patients. Our findings revealed a significant association between pemphigus and schizophrenia with a multivariate OR of 1.6 (95% CI 1.1–2.3). The prevalence rate of schizophrenia in patients with pemphigus is remarkably higher than in age-, gender- and ethnicity-matched control subjects (2.0% v. 1.3%, respectively; p = 0.019).
Psychiatric comorbidity in pemphigus
Psychiatric and psychological morbidity, such as anxiety, depression, obsessive-compulsive disorder and psychosis, is common and is reported in up to 30% of dermatologic disorders in all age groups (Gupta & Gupta, Reference Gupta and Gupta2003; Wakkee & Nijsten, Reference Wakkee and Nijsten2009; Al Hawsawi & Pope, Reference Al Hawsawi and Pope2011). The few studies that have investigated the presence of psychiatric comorbidity among patients with pemphigus have reported even higher prevalence rates of psychiatric comorbidities (Arbabi et al. Reference Arbabi, Ghodsi, Mahdanian, Noormohammadi, Shalileh, Darvish, Ashrafinia and Chams2011; Kumar et al. Reference Kumar, Mattoo and Handa2013). In the study of Kumar et al. (Reference Kumar, Mattoo and Handa2013), 20 of 50 patients (40%) were screened GHQ-12 positive, including one patient who was diagnosed with acute and transient psychosis. Arbabi et al. (Reference Arbabi, Ghodsi, Mahdanian, Noormohammadi, Shalileh, Darvish, Ashrafinia and Chams2011) found that 157 of 212 pemphigus patients scored ⩾6 on GHQ-28 bimodal scoring, indicating a 73.7% prevalence of comorbid mental disorder.
Autoimmune comorbidity in schizophrenia
Increased prevalence of autoimmune diseases has been observed among patients with schizophrenia relative to control subjects (Benrós & Mortensen, Reference Benrós and Mortensen2015). In addition, increased autoantibody titres, as well as autoantibody reactivity, were identified even in patients without overt clinical manifestation of autoimmune disease (Tanaka et al. Reference Tanaka, Matsunaga, Kimura, Tatsumi, Hidaka, Takano, Uema, Takeda and Amino2003; Laske et al. Reference Laske, Zank, Klein, Stransky, Batra, Buchkremer and Schott2008). Danish population-based studies of up to 20 317 patients with schizophrenia and 39 076 patients with non-affective psychosis have demonstrated that individuals with schizophrenia had an approximately 50% higher prevalence of autoimmune conditions (Eaton et al. Reference Eaton, Byrne, Ewald, Mors, Chen, Agerbo and Mortensen2006; Benros et al. Reference Benros, Nielsen, Nordentoft, Eaton, Dalton and Mortensen2011).
Screening studies of individuals with schizophrenia have observed a 500% higher seropositivity to tissue transglutaminase as compared with matched control subjects (Reichelt & Landmark, Reference Reichelt and Landmark1995; Samaroo et al. Reference Samaroo, Dickerson, Kasarda, Green, Briani, Yolken and Alaedini2010; Cascella et al. Reference Cascella, Kryszak, Bhatti, Gregory, Kelly, Mc Evoy, Fasano and Eaton2011), suggesting a connection to the pathogenesis of celiac disease. Observational studies have estimated the prevalence of celiac disease to range between 2.1% and 2.6% among patients with schizophrenia, as compared with a prevalence between 0.3% and 1.0% seen in the general population (Kalaydjian et al. Reference Kalaydjian, Eaton, Cascella and Fasano2006; Cascella et al. Reference Cascella, Kryszak, Bhatti, Gregory, Kelly, Mc Evoy, Fasano and Eaton2011). In a cross-sectional national Taiwanese study, 3.4% of individuals with a hospital contact for autoimmune diseases also had a hospital contact with schizophrenia during the follow-up period (Chen et al. Reference Chen, Chao, Chen, Chang, Wu, Wu, Yeh, Chen and Tsai2012). According to Danish registry data, hospital contacts due to autoimmune diseases had occurred in 2.4% of patients before a schizophrenia diagnosis and in 3.6% of patients after the diagnosis, indicating that 6% of people with schizophrenia had a hospital contact with autoimmune diseases during the study duration (Benros et al. Reference Benros, Nielsen, Nordentoft, Eaton, Dalton and Mortensen2011, Reference Benros, Pedersen, Rasmussen, Eaton, Nordentoft and Mortensen2014).
Explanation for the observed association between pemphigus and schizophrenia
The pathophysiological mechanism-linking schizophrenia with autoimmune comorbidities has not yet been fully elucidated. It has been suggested that the psychiatric symptoms in patients with autoimmune comorbidities can be triggered by the direct effect of immune factors, such as brain-reactive autoantibodies and cytokines, on the central nervous system (CNS) or be secondary to systemic inflammation indirectly affecting the brain (Benrós & Mortensen, Reference Benrós and Mortensen2015).
With regard to the direct effect of immune components, experimental studies have found that neuropsychiatric syndromes can be induced after the influx of brain-reactive antibodies into the brain (Kowal et al. Reference Kowal, DeGiorgio, Nakaoka, Hetherington, Huerta, Diamond and Volpe2004). Brain-reactive autoantibodies have also been suspected to induce the high prevalence of neuropsychiatric symptoms encountered in some autoimmune disease (Margutti et al. Reference Margutti, Delunardo and Ortona2006; Ballok, Reference Ballok2007; Sundquist et al. Reference Sundquist, Li, Hemminki and Sundquist2008). This is of great relevance in a B-cell-mediated disease like pemphigus, where autoantibodies are necessary for the stimulation of the disease. In addition, one of the target autoantigens implicated in the pathogenesis of pemphigus, desmoglein-1, was recently found to be expressed in the corpus callosum of mouse models (Miyata et al. Reference Miyata, Yoshikawa, Taniguchi, Ishikawa, Tanaka, Shimizu and Tohyama2015). Given that desmoglein-1 is expressed both in the epithelial cell surface and in the CNS (Kljuic & Christiano, Reference Kljuic and Christiano2003; Miyata et al. Reference Miyata, Yoshikawa, Taniguchi, Ishikawa, Tanaka, Shimizu and Tohyama2015), the hypothesis of cross-reactivity between its epithelial and neuronal isoforms and the production of brain-reactive autoantibodies as a reason for the higher prevalence of schizophrenia in pemphigus patients cannot be thoroughly excluded. The most representative model for CNS symptoms associated with antibodies has been recognised in cancer patients with paraneoplastic symptoms that may in part be induced by the cross-reaction between antibodies against tumour antigens with elements of the nervous system (Darnell & Posner, Reference Darnell and Posner2003; Kayser et al. Reference Kayser, Kohler and Dalmau2010). The inflammatory state existing in autoimmune diseases, and in pemphigus in particular, might increase the permeability of the blood–brain barrier, making the brain more vulnerable to these autoantibodies and inflammatory cytokines (Irani & Lang, Reference Irani and Lang2008).
The indirect contribution of systemic inflammation accompanying autoimmune diseases in triggering neuropsychiatric symptoms may be mediated by the interaction with the neuroendocrine system, which is thought to play an important role in psychiatric disorders through multiple pathways (Dantzer et al. Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008; Rivest, Reference Rivest2010). An iatrogenic effect of corticosteroid treatment, which may hypothetically increase the risk of psychosis, seems unlikely to explain the major associations (Benrós & Mortensen, Reference Benrós and Mortensen2015). Separate research shows a reduced risk for psychosis observed with the use of corticosteroids(Laan et al. Reference Laan, Smeets, de Wit, Kahn, Grobbee and Burger2009) and newer biological treatment of autoimmune diseases has been shown to decrease the risk of mood disorders (Benrós & Mortensen, Reference Benrós and Mortensen2015). In addition, antipsychotic treatments were very rarely associated with the induction of pemphigus (Pérez España et al. Reference Pérez España, Hervella Garcés, Belén Piteiro and Casado Jiménez2003).
The associations in our population could also be caused by a shared genetic predisposition, as both pemphigus and schizophrenia are more frequent among patients of Ashkenazi Jewish ancestry (Lencz et al. Reference Lencz, Guha, Liu, Rosenfeld, Mukherjee, DeRosse, John, Cheng, Zhang, Badner, Ikeda, Iwata, Cichon, Rietschel, Nöthen, Cheng, Hodgkinson, Yuan, Kane, Lee, Pisanté, Gregersen, Pe'er, Malhotra, Goldman and Darvasi2013; Kridin et al. Reference Kridin, Zelber-Sagi, Khamaisi, Cohen and Bergman2016). Although the association seems biologically plausible, it remains widely unclear whether it is a causal association or an epiphenomenon due to, for instance, other environmental factors. The cross-sectional nature of the current study does not enable to address the temporal relationship between the two entities; hence, limited conclusions should be drawn regarding a causal association between the entities (Höfler, Reference Höfler2005).
Strengths and limitations
Our population-based study utilises a representative database, created over 11 years, of 4.4 million individuals, decreasing the susceptibility to selection and ascertainment biases. Data collection in pemphigus is difficult due to disease rarity and the limited number of patients available for study. This lack of large-scale clinical data is a significant impediment to a better understanding of the disease associations and comorbidities. Our study includes one of the largest cohorts of patients with pemphigus reported in the literature and is the first study aiming to examine the association between pemphigus and schizophrenia.
The study has several considerable limitations. Firstly, the study lacks clinical data concerning pemphigus subtype (vulgaris, foliaceus or paraneoplastic), clinical characteristics and severity. Secondly, since the data extracted from the database represents only a current situational analysis of co-existing variables without regard to chronology, the date of schizophrenia diagnosis was not available for the current study. Hence, we could not address temporal relationships between pemphigus and schizophrenia; limited conclusions should be drawn regarding a causal association between the two entities (Höfler, Reference Höfler2005). Additionally, the use of routinely-collected ‘real-life’ data did not enable direct validation of diagnoses nor did it allow for the elimination of cases of misclassification. However, if misclassification did occur, it would be non-differential and might lead to a null bias. Pemphigus in Israel is uncommonly encountered in general practice. Diagnosis relies on skin biopsies, as well as direct and indirect immunofluorescence (Kridin et al. Reference Kridin, Zelber-Sagi, Khamaisi, Cohen and Bergman2016) usually carried out in secondary and tertiary care facilities; therefore, it is very likely to be precise and validated. Furthermore, the diagnosis of schizophrenia is performed by trained psychiatrists, who are the only practitioners legally allowed in Israel to treat patients with psychiatric disorders. The existence of ascertainment bias was ruled out when our outcome measures were reproduced after adjusting for overutilisation of healthcare services.
Conclusions
In conclusion, our population-based study depicts a significant association between pemphigus and schizophrenia. It appears that the highest increase in schizophrenia burden among pemphigus patients occurs in older Jewish females. Patients with pemphigus should be carefully assessed for comorbid psychiatric disorders and be treated appropriately. Experimental research is needed to better understand the molecular mechanisms behind this novel observation.
Acknowledgements
Dr Kridin, Dr Comaneshter, and Prof. Cohen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Dr Kridin and Dr Zelber-Sagi. Acquisition, analysis, and interpretation of data: Dr Kridin and Dr Zelber-Sagi. Drafting of the manuscript: Dr Kridin and Prof. Cohen. Critical revision of the manuscript for important intellectual content: Dr Kridin and Prof. Cohen. Statistical analysis: Dr Zelber-Sagi. Obtained funding: None. Administrative, technical, or material support: Prof. Cohen and Dr Comaneshter. Study supervision: Dr Zelber-Sagi and Prof. Cohen.
Financial Support
This research received no specific grant from any funding agency, commercial or not for profit sectors.
Conflict of Interest
The authors declare no conflict of interests.
Ethical Standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Availability of Data and Materials
The full row data will not be shared as we have no ethical permission to perform this.





