INTRODUCTION
The two main approaches to consider spatial patterns of the distribution of organisms in natural systems are landscape ecology (for example Tischendorf & Fahrig Reference Tischendorf and Fahrig2000) and metapopulation theory (for example Hanski & Gilpin Reference Hanski and Gilpin1991; Hanski Reference Hanski1998). In their classical form, both approaches consider clearly delimitable patches to predict distribution and spatial dynamic of organisms (Fischer et al. Reference Fischer, Lindenmayer and Fazey2004). However, patch-based models may be overly simplistic in their prediction of occurrence patterns (Fischer et al. Reference Fischer, Lindenmayer and Fazey2004; Price et al. Reference Price, McAlpine, Kutt, Phinn, Pullar and Ludwig2009) and ignoring matrix conditions between patches may lead to serious error (see Marsh & Trenham Reference Marsh and Trenham2001; Prugh et al. Reference Prugh, Hodges, Sinclair and Brashares2008; Price et al. Reference Price, McAlpine, Kutt, Phinn, Pullar and Ludwig2009).
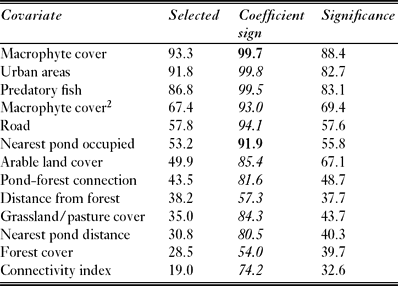
Pond-breeding amphibians represent a challenging group for patch-based analytical approaches of distribution. By their very nature ponds are landscape elements with a particular fit to the metapopulation or ‘patch model’ approach (Marsh & Trenham Reference Marsh and Trenham2001). Population size, population turnover and spatial dynamics are often linked to pond quality, primarily through reproductive success (for example Gill Reference Gill1978; Sjögren-Gulve Reference Sjögren-Gulve1994; Tryjanowski et al. 2008). Pond connectivity metrics are often applied in amphibian spatial ecology studies to evaluate the impact of potential source populations and alternative breeding sites on the use of the focal ponds. Four such metrics have been used in 42 European and North American studies (Table 1).
Table 1 Survey of pond-based studies (n = 42) exploring the distribution of amphibian species with one or more concentric buffers to characterize the surrounding landscape, using wetland area, nearest neighbour distance or metapopulation isolation parameters as covariates. Xj = pond area (Aj), population size (Nj) or occupancy data (Oj) (see details in Materials and Methods). *Connectivity index Ci = i=jexp(−αdij)Xj. Numbers in the Table represent the number of studies.

While the distribution and dynamics of some amphibian population systems can be appreciated from the metapopulation perspective (see Gill Reference Gill1978), elsewhere the landscape effect will mask metapopulation effects (Pope et al. Reference Pope, Fahrig and Merriam2000). In most of these studies, ‘landscape’ is represented by the various terrestrial areas (land covers) around the potential breeding pond. Pond-breeding amphibians require terrestrial areas to complete their life history and to replenish their energy reserves by foraging (for example Gibbons Reference Gibbons2003). These areas are often partitioned in habitat and non-habitat (or ‘matrix’; see Ray et al. Reference Ray, Lehmann and Joly2002). The habitat composition of an area, together with the source-sink character of the local population will often be complex and the delimitation of the habitat-population borders is therefore difficult (Thomas & Kunin Reference Thomas and Kunin1999). The range at which the landscape may affect the status of a local amphibian population is most commonly studied with concentric circles of varying size over which the landscape composition is described. The area does not normally exceed a radius of 4000 m, in line with direct observations on the dispersal range of individuals (Semlitsch Reference Semlitsch2008). This approach is attractive because it allows the survey of a large number of water bodies (potential breeding habitats) (for example Arntzen & Wallis Reference Arntzen and Wallis1991; Houlahan & Findlay Reference Houlahan and Findlay2003; Ficetola & De Bernardi Reference Ficetola and De Bernardi2004; Pellet et al. Reference Pellet, Guisan and Perrin2004; Van Buskirk Reference Van Buskirk2005; see Table 1).
Here we combine the classic metapopulation approach (Hanski & Gilpin Reference Hanski and Gilpin1991) with a niche modelling approach (sensu Hutchinson Reference Hutchinson1957; see also Schmidt & Pellet Reference Schmidt and Pellet2005) to explore the occurrence of the northern crested newt (Triturus cristatus) in a rural landscape from Transylvania (Romania). The study area is still largely under traditional management, with small patchy areas of subsistence agriculture, little heavy machinery, low pesticide use and use of natural fertilizers (Hartel et al. Reference Hartel, Öllerer, Cogălniceanu, Nemes, Moga and Demeter2010a). This type of landscape is complex, with high biodiversity in many places, frequently including species that are listed as endangered (Cogălniceanu & Cogălniceanu Reference Cogălniceanu and Cogălniceanu2010; see also Tscharntke et al. Reference Tscharntke, Klein, Steffan-Dewenter and Thies2005 for the characterization of this type of landscape). In these conditions, the habitat versus non-habitat (namely the matrix) character of ecological space may not be obvious for amphibians, owing to the intergradations from habitat to matrix (Hartel et al. Reference Hartel, Öllerer, Cogălniceanu, Nemes, Moga and Demeter2010a).
Triturus cristatus is declining in parts of its distributional range, mostly due to pond destruction (see Beebee Reference Beebee1997), and is protected in the European Union (Annex II of the Habitats Directive 92/43/EEC); 850 km2 of our study area is a Natura 2000 Site of Community Interest (ROSCI0227 Sighişoara-Târnava Mare).
MATERIALS AND METHODS
Study area and surveys
The study area covers c. 4000 km2, and is situated in the middle section of the Târnava Mare River. The dominant land use across this hilly area consists of meadows and pastures (c. 40%), deciduous forests (30%) and traditionally managed arable lands (15%) (Hartel et al. Reference Hartel, Schweiger, Öllerer, Cogălniceanu and Arntzen2010b). Ponds are mostly man-made, created following the regularization of the Târnava Mare in the 1970s and 1980s and by the damming of tributary streams and springs along the adjacent valleys (Hartel Reference Hartel2008). We studied 96 ponds during 2000–2008 and eliminated data on seven ponds that turned out to be of recent origin (created <10 years ago). We assumed that these newly-created ponds might give an erroneous picture of the effect of connectivity variables. The ponds were inspected three to four times in one particular year, from late February until May for adults and eggs and until August for larvae, by dip netting and by visual inspection both during the day and at night time. Based on repeated surveys we selected egg searching and night visits with torch use as the most efficient method of species recording for most species (T. Hartel et al., personal observations). The survey methodology and effort was detailed in Hartel et al. (Reference Hartel, Schweiger, Öllerer, Cogălniceanu and Arntzen2010b).
Environmental covariates and connectivity measures
We recorded several pond variables, including the percentage cover of emergent vegetation (Typha sp. and Phragmites sp.) and the occurrence of the predatory fish Perca fluviatilis, Squalius cephalus, Silurus glanis, Esox lucius, Stizosteidon lucioperca, Lepomis gibbosus, Pseudorasbora parva and Clarias gariepinus. The last three species are invasive; Clarias gariepinus has not been reported previously in this area (Hartel et al. Reference Hartel, Nemes, Cogălniceanu, Öllerer, Schweiger, Moga and Demeter2007).
Landscape variables recorded were the percentage cover of meadows and pastures, forests, arable land and urban areas, and the presence/absence of roads with up to 300 cars per hour at night time. These variables were recorded for a radius up to 800 m around each pond. We also noted the distance from each pond to the nearest forest area (in metres) and whether this connection was continuous or interrupted by the presence of presumed barriers, such as arable land, roads and urbanization.
Connectivity was measured as (1) the distance (in metres) to the nearest pond, (2) the occupied state of nearest pond and (3) a species specific connectivity index Ci=∑j≠iexp (−αd i,j)Oj (modified after Moilanen & Nieminen Reference Moilanen and Nieminen2002; see also Pellet et al. Reference Pellet, Fleishman, Dobkin, Gander and Murphy2007). Here Oj is an indicator variable representing the occupancy state of the pond j (namely Oj = 1 if the jth pond is occupied and Oj = 0 if not). Moilanen and Nieminen (Reference Moilanen and Nieminen2002) used the parameter Abj for the area of patch j, where b is usually set to 1. Pellet et al. (Reference Pellet, Fleishman, Dobkin, Gander and Murphy2007) substituted Abj with Nj, representing the population size in the jth patch. We used Oj because it was impossible for us to quantify the population size of the newts and the permanent pond area does not affect the occupancy by newts (Hartel Reference Hartel2008). dij is the distance between ponds i and j, and α is the inverse of the mean movement distance. Following Joly et al. (Reference Joly, Miaud, Lehmann and Grolet2001), we selected a movement distance of 400 m for T. cristatus, therefore α = 1/400.
Data analysis
We used logistic regression to model the presence of Triturus cristatus as a function of the recorded covariates. Prior to the modelling, we examined the data structure for possible non-linearity with the help of additive methods and smoothing functions. We also examined the occurrence of multicollinearity. We based our model selection procedure on the guidelines of Sauerbrei and Schumacher (Reference Sauerbrei and Schumacher1992) and Austin and Tu (Reference Austin and Tu2004). The presence of higher order terms and interactions was conditioned on the presence of main effects in the model. From the original data set we drew 1000 non-parametric bootstrap resamples. For each resample we ran a stepwise regression automated model selection procedure. This was a combination of both backward and forward selection, testing at each stage if a variable should be included or excluded from the model. As selection criteria, we chose the Akaike information criterion (AIC), as this is the most common all-purpose model selection tool. AIC is based on the likelihood function, and it seeks the model that best approximates the unknown truth. Information theoretic variable selection differs from that based on significance testing in the sense that non-significant variables can be retained in the model. For each bootstrap iteration, we noted whether a variable was kept in the model, the sign of the regression coefficient and its p-value. Data were summarized at 1000 iterations. True predictors were expected to be selected in a high proportion (>50% of iterations), to have a stabile sign and to be significant most of the time. After a final model was selected we further examined the effect of removing individual variables. We used receiver operating characteristic curve and the associated area under the curve (AUC) values to assess the predictive powers of the models.
RESULTS
Five predictors were identified as possible explanatory factors of the newt presence/absence (Table 2): macrophyte cover, urban areas, predatory fish, road and the nearest pond occupied. The effect of roads was marginally significant and the reduced model (namely without roads) had similar goodness of fit (ΔAIC = 1.228, χ2 = 3.228, p = 0.07) and greater predictive power (AUC = 0.90 with roads and AUC = 0.89 without roads). The removal of roads was, however, beneficial from a modelling perspective because it reduced the stratification of the data and offered more stable and better estimates with lower uncertainty.
Table 2 Sensitivity analysis based upon non-parametric bootstrapping for the stepwise model selection method incorporating the Akaike information criterion. All values are percentages. Positive values are shown in bold and negative values in italic.
No other predictor variables could be removed from the model without compromising its goodness of fit (Table 3). Of the remaining predictors, macrophyte coverage and the nearest occupied pond increased the likelihood of newt presence (Table 4). The effect of macrophyte coverage turned negative after reaching a threshold (c. 60%; Fig. 1). We did not find any interaction between the macrophyte cover and predatory fish in determining the pond occupancy by newts. Moreover, no interaction was found between the distance of the closest pond and the occupied state of the closest pond, in determining the occurrence of the newts in our study area. The scenarios suggest that the effect of urban areas on the probability of newt presence was stronger when there were predatory fish in the focal pond and when the neighbouring pond was unoccupied than in the reverse situation (Fig. 1).
Table 3 Influence of variable removal from the whole model on the information content (AIC) and predictive power (AUC). Likelihood ratio test (LRT) against the removal is also provided. When the macrophyte coverage was removed automatically, the second order term was also removed.
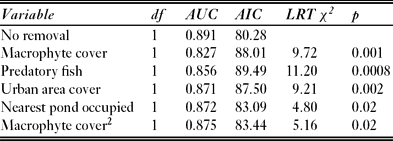
Table 4 Contribution of five selected environmental factors to the model that explores the occurrence of the northern crested newt (Triturus cristatus) in 89 ponds in central Romania.
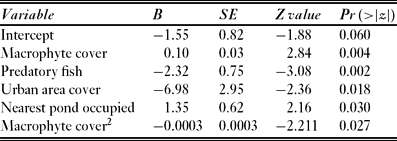
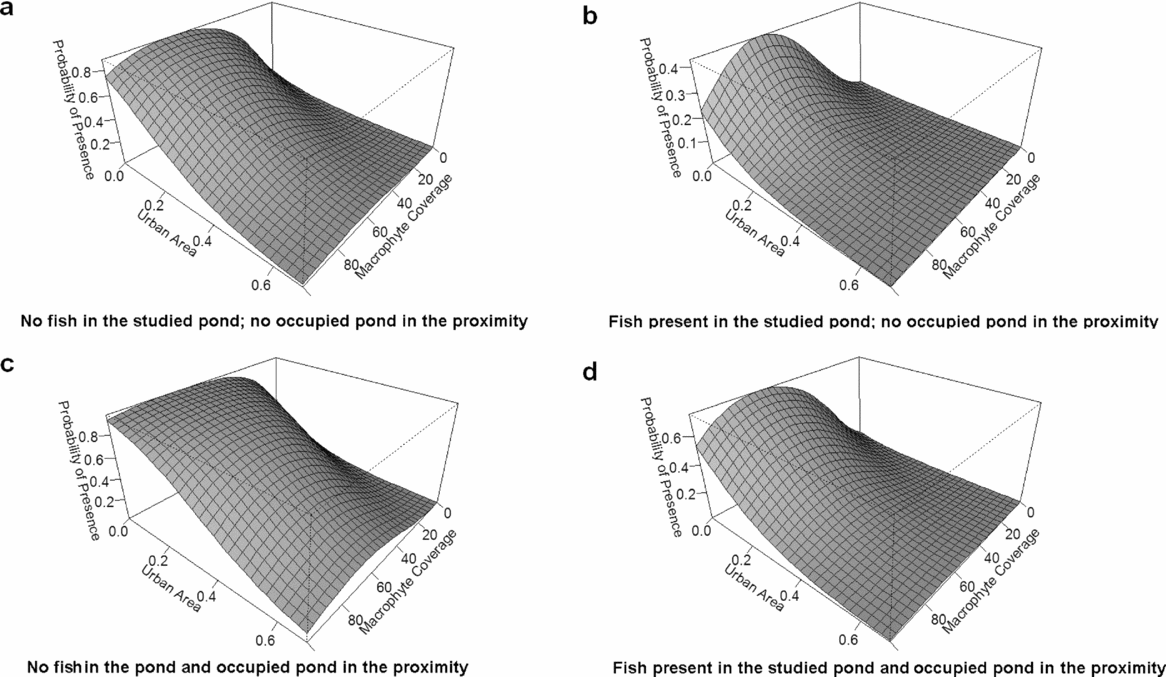
Figure 1 Probability of the presence of the northern crested newt (Triturus cristatus) as a function of pond macrophyte coverage and the degree of urbanization within an 800 m radius of the pond, in ponds with (b, d) and without fish (a, c), and the nearest unoccupied pond (a, b) versus those occupied by T. cristatus (c, d) in 89 ponds in central Romania.
DISCUSSION
Both metapopulation and niche models contributed to understanding the distribution of northern crested newt in the rural landscape of central Romania. We found that pond-related variables (predatory fish and macrophyte cover) were the most important determinants of T. cristatus distribution in the study area, followed by one landscape-related variable (the urban area cover), one spatial metric (the nearest occupied pond) and the quadratic effect of macrophyte cover. Six out of seven landscape covariates and two out of three connectivity variables were not found to be important for T. cristatus. Because human settlements and ponds, through their rapid change, represent the two most significant impacts in this rural area over the last decades, these results were not unexpected.
Increasing private ownership of ponds has resulted in increased pond stocking with both greater numbers of fish species and especially more predatory fish species. Triturus cristatus is sensitive to fish presence (for example Joly et al. Reference Joly, Miaud, Lehmann and Grolet2001; Van Buskirk Reference Van Buskirk2005; Skei et al. Reference Skei, Dolmen, Rønning and Ringsby2006; Gustafson et al. Reference Gustafson, Andersen, Mikusinski and Malmgren2009; Kloskowski Reference Kloskowski2010). Features that expose T. cristatus to fish predation include a long aquatic period both as adults (breeding and feeding) and larvae (Malmgren Reference Malmgren2001) and pelagic larvae that are easily spotted by predators, with no behavioural avoidance in the presence of aquatic predators. Also, the ability of northern crested newt larvae to respond to predators by accelerating larval development is very limited (Schmidt & Van Buskirk Reference Schmidt and Van Buskirk2001). This inability to reduce predation risk by morphological plasticity may further expose T. cristatus larvae to fish predation.
The emergent vegetation cover influenced the probability of newts occurring up to c. 60% coverage. A similar threshold of macrophyte cover on pond occupancy was found by Oldham et al. (Reference Oldham, Keeble, Swan and Jeffcote2000) for T. cristatus and on newt abundance in general by Joly et al. (Reference Joly, Miaud, Lehmann and Grolet2001). The rationale for the effect is probably the lack of available space for newt breeding and foraging activity (Oldham et al. Reference Oldham, Keeble, Swan and Jeffcote2000). Similarly, Gustafson et al. (Reference Gustafson, Andersen, Mikusinski and Malmgren2009) found that ponds in late succession stages had become inhospitable for T. cristatus and suggested management and restoration activities to keep ponds open. Late succession stages also negatively influenced pond use by Rana lessonae and R. dalmatina (Sjögren Gulve Reference Sjögren-Gulve1994; Hartel et al. Reference Hartel and Öllerer2009). We found no significant interaction between the macrophyte coverage and presence of predatory fish, suggesting that emergent vegetation will not buffer the effect of predators. This may be because small predatory fish (such as P. parva, L. gibbosus, P. fluviatilis) use the macrophyte as breeding area and fish fry may use it as a refuge area.
The distance to the next nearest occupied pond was the only spatial predictor of newt presence, confirming that simple linear connectivity measures can be useful for predicting animal distribution and may even outperform more complex metrics (Prugh Reference Prugh2009). The independent effect of newt presence in the next closest pond from the distance to the closest pond suggests that isolation by distance is not a problem in these landscapes (but see Halley et al. Reference Halley, Oldham and Arntzen1996). The connectivity index Ci had no effect on newt presence in the focal ponds, possibly because of the dichotomous character of Oj, as opposed to a variable that denotes area and/or population size of the potential sources (see Moilanen & Nieminen Reference Moilanen and Nieminen2002; Pellet et al. Reference Pellet, Fleishman, Dobkin, Gander and Murphy2007). This may lead to the loss of important information, because both population size and distance contribute to the persistence of a newt metapopulation network. Halley et al. (Reference Halley, Oldham and Arntzen1996) found that the likelihood of the persistence of newt ponds increased if ponds supported >40 females, or were <0.5 km from a typical source pond. The likelihood of persistence of small newt ponds (<10 females) depended on the existence of a source pond < 0.75 km away, whereas for large newt ponds a source pond could be <1.5 km away (Halley et al. Reference Halley, Oldham and Arntzen1996).
As expected, the landscape composition had little effect on newt occurrence, suggesting that unsuitable terrestrial habitats (other than urban area cover) are not currently a limiting factor for the newt (see also Hartel et al. Reference Hartel, Öllerer, Cogălniceanu, Nemes, Moga and Demeter2010a for other amphibians). Terrestrial habitat degradation may result in increased pond to pond isolation. In our study area, newly-created permanent ponds were colonized by T. cristatus within one or two years (Hartel & Öllerer Reference Hartel, Nemes, Cogălniceanu, Öllerer, Moga, Lesbarreres and Demeter2009). This may reflect the unaffected nature of the rural landscape because, in intensively-managed landscapes, the colonization capability of T. cristatus was lower than that of most other amphibian species (for example Laan & Verboom Reference Laan and Verboom1990; Beebee Reference Beebee1997).
CONCLUSIONS
We have shown that the occurrence of the northern crested newt can be understood using the combination of metapopulation and niche modelling approaches; occurrence in a traditionally managed rural area is primary related to pond variables. Avoidance of predatory fish introduction and management of aquatic vegetation density should be priority activities for northern crested newt conservation in this area. Fish removal from stocked ponds may also be an appropriate management implication, and such an activity may be encouraged especially in the area covered by the Natura 2000 regulations. No unequivocal results were found regarding the importance of terrestrial habitats around ponds, other than a negative effect of urbanization. Hence, a dense metapopulation network for T. cristatus would be composed of many permanent ponds distributed over a non-urban extensively-managed landscape. In order to better understand the connectivity between ponds in relation to pond management and land management in a cultural landscape, more studies are needed on the neighbourhood size and dispersal distance of the northern crested newt and other amphibians. We also suggest the fragment-based spatial models as employed in this study be complemented with alternative models that consider gradients between landscape elements (Fischer et al. Reference Fischer, Lindenmayer and Fazey2004), because these may be especially useful in complex traditionally-managed landscapes such as found in Romania and other parts of Eastern Europe.
ACKNOWLEDGEMENTS
This study was conducted with the financial support from the Declining Amphibian Populations Task Force and the Swedish Biodiversity Centre. We thank Dr. Jerome Pellet for his advice in calculating the connectivity metric and three anonymous reviewers for their constructive comments on the manuscript.







