INTRODUCTION
More than half the world's human population lives in cities, and this proportion is projected to grow in the coming years (United Nations 2014). Urbanization is therefore considered one of the most important threats to biological conservation worldwide (McDonald et al. Reference McDonald, Marcotullio, Güneralp, Elmqvist, Fragkias, Goodness, Güneralp, Marcotullio, McDonald, Parnell, Schewenius, Sendstad, Seto and Wilkinson2013). On the other hand, the urban green infrastructure may host a high variety of biotopes and species, sometimes greater than those of the surrounding rural areas (Angold et al. Reference Angold, Sadler, Hill, Pullin, Rushton, Austin, Small, Wood, Wadsworth, Sanderson and Thompson2006; Jones & Leather Reference Jones and Leather2012). Thus, urban green spaces may represent important biodiversity reservoirs, and there is increasing interest in their inclusion in urban planning and global biodiversity conservation actions (Secretariat of the Convention on Biological Diversity 2012). Because urban green spaces are generally small they may be of less value for large animals, but they can be important for organisms that can survive in small areas or that form meta-populations, such as small-sized mammals, reptiles and most invertebrates (Angold et al. Reference Angold, Sadler, Hill, Pullin, Rushton, Austin, Small, Wood, Wadsworth, Sanderson and Thompson2006; Hunter & Hunter Reference Hunter and Hunter2008; Fattorini Reference Fattorini2011a, Reference Fattorini2011b; Jones & Leather Reference Jones and Leather2012).
From the perspective of the organisms that live in urban green spaces, the urban environment can look like a set of habitat islands (the green patches where they live) separated by more or less inhospitable environments (the matrix represented by the built-up areas). Thus, biotic communities of urban green spaces are expected to have population dynamics that are similar to those of islands.
Important insights regarding the interplay of processes and patterns in evolution, ecology and biogeography have come from insular studies (Lomolino et al. Reference Lomolino, Riddle, Whittaker and Brown2010). In particular, the equilibrium theory of island biogeography (ETIB), proposed by MacArthur and Wilson (Reference MacArthur and Wilson1963, Reference MacArthur and Wilson1967) to explain variations in species number on islands, turned into one of the most productive research programmes in ecology (Lomolino et al. Reference Lomolino, Riddle, Whittaker and Brown2010). According to the ETIB, species richness on islands is the result of immigration/colonization and extinction processes, which in turn are regulated by physical features of the islands, such as area and isolation (Lomolino et al. Reference Lomolino, Riddle, Whittaker and Brown2010).
Providing a simple mechanistic model of variation in species richness based on extinction and colonization rates, the ETIB had impact far beyond its original scope and has influenced basic biogeographical thinking. If urban green spaces are islands in a ‘sea of concrete’, the principles of the ETIB should also apply to the urban environment.
The ETIB has in fact inspired biodiversity research in urban areas since the inception of urban ecology (Faeth & Kane Reference Faeth and Kane1978), and research in urban habitats has been frequently addressed to investigating how species richness is influenced by characteristics of green spaces (e.g. Clarke et al. Reference Clarke, Fisher and LeBuhn2008; Sattler et al. Reference Sattler, Obristb, Duellib and Moretti2011; McDonnell & Hahs Reference McDonnell and Hahs2013). From a more formal point of view, several ETIB predictions can be applied to urban green spaces as islands (Fattorini Reference Fattorini2016). These ETIB predictions are reformulated here and involve the influence of green space characteristics on patterns of species richness (Predictions 1–4) and processes of extinction/colonization (Predictions 5–8) (Fig. 1).
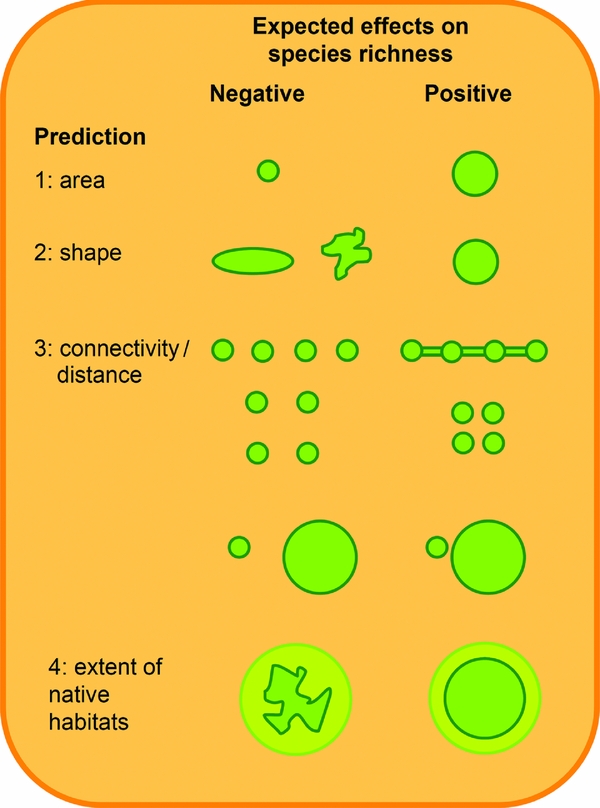
Figure 1 Conceptual representation of predictions regarding variation in the insect species richness of urban green spaces. Species richness is expected to increase with increasing area, circularization, connectivity (i.e. the presence of corridors or proximity to other green spaces or rural areas) and the extent of natural habitats.
Prediction 1: species richness should increase with island area (the so-called species–area relationship [SAR]) because larger islands will tend: (1) to support larger populations; (2) to have higher habitat diversity or heterogeneity (the so-called ‘habitat diversity hypothesis’); and/or (3) to be larger targets for potential colonists (the so-called ‘passive sampling hypothesis’). Distinguishing between these alternative explanations for the SAR is usually difficult (see Fattorini et al. Reference Fattorini, Dapporto, Strona and Borges2015). However, at least under certain circumstances, it is possible to statistically separate the contribution of area per se from that of habitat diversity. Habitat diversity is an elusive concept and may largely depend on the concerned group. In the few studies that considered habitat diversity of insects in urban green spaces, this was expressed as the number of vegetation types (Clarke et al. Reference Clarke, Fisher and LeBuhn2008), the Shannon index of land use types (Matteson & Langellotto Reference Matteson and Langellotto2010), biotopes (Shwartz et al. Reference Shwartz, Muratet, Simon and Julliard2013) or plant diversity (Fortel et al. Reference Fortel, Henry, Guilbaud, Guirao, Kuhlmann, Mouret, Rollin and Vaissière2014). The effect of area per se may be due to both the passive sampling hypothesis and the persistence of larger populations. These two explanations might be distinguished by using data on species abundance and immigration rates.
Prediction 2: a typical assumption of reserve design is that a circular shape of reserve is better than an elongated or indented one because a more rounded shape should reduce edge effects, and hence should allow for the persistence of a larger number of interior species (Yamaura et al. Reference Yamaura, Kawahara, Lida and Ozaki2008; Kotze et al. Reference Kotze, Lehvävirta, Koivula, O'Hara and Spence2012; Soga et al. Reference Soga, Kanno, Yamaura and Koike2013). This assumption is not strictly derived from the ETIB, but it is usually reported as one of its implications (e.g. Triantis & Bhagwat Reference Triantis, Bhagwat, Ladle and Whittaker2011). Because not all of the area of a green space can be suitable for insects, it is the area of the native habitats within it that may be important for insect conservation. Thus, species richness is expected to increase with circularization of fragments of native habitats within green spaces, if not with the shape of the entire green space (Sisk et al. Reference Sisk, Haddad and Ehrlich1997; Davies et al. Reference Davies, Gascon, Margules, Soulé and Orians2001; Yamaura et al. Reference Yamaura, Kawahara, Lida and Ozaki2008).
Prediction 3: species richness should be inversely correlated with the green space distance from source areas. In the ETIB framework, source areas may be either the mainland or other nearby islands. In the case of urban green spaces, the mainland is represented by the rural areas surrounding the cities, whereas other green spaces may act as nearby islands. For most organisms, including many insects, species richness tends to decline from the periphery (where more natural habitats occur) to the most densely built-up areas of the urban core (the so-called urban–rural gradient) (Adler & Tanner Reference Adler and Tanner2013; New Reference New2015). With reference to the ETIB, the landscapes at the periphery of a city should act as a ‘mainland’, and hence species richness should decrease from peripheral to city centre green spaces (McIntyre Reference McIntyre2000). Since less isolated sites are expected to be more easily colonized (i.e. to receive more immigrants), species richness in an urban green space should also increase with its proximity (or connection) to other green spaces that can act as a mainland or a stepping stone island (Magura et al. Reference Magura, Tóthmérész and Molnár2001).
Prediction 4: species richness should increase with the extent of native habitats within each green space. The species richness of insects that are strictly associated with humans (e.g. parasites) may increase with urbanization. However, most insect species tend to decline with the reduction of native habitats (New Reference New2015). Therefore, fragments that include remnants of native habitats should host more native species that depend on these habitats and, hence, more species in general (Donnelly & Marzluff Reference Donnelly and Marzluff2004).
Prediction 5: under ETIB assumptions, species richness in urban green spaces should result from a balance between local extinctions and continuous immigration. If the cities were in a steady state, immigration and extinction would be in equilibrium. However, because urbanization tends to make green spaces progressively smaller, more isolated and less hospitable, species immigration is probably insufficient to compensate for species loss (see Fattorini Reference Fattorini2011b, Reference Fattorini2013). Thus, although most urban green spaces benefit from continuous immigration, their increasing isolation progressively reduces the immigration rate of most species. The species that occur in urban green spaces are nested subsets of the fauna inhabiting the rural areas surrounding the city. Of course, immigration processes may lead a green space to gain a new species from the rural areas (i.e. the mainland), but because extinction rates likely exceed new arrivals of individuals belonging to new species or to species already occurring in a given green space, an increasing excess of extinction is expected over long periods. Under this assumption, extinction levels should be correlated negatively with green space size, because larger areas support larger populations that are less vulnerable to demographic oscillations, genetic drift, inbreeding and reduced heterozygosity (Lomolino et al. Reference Lomolino, Riddle, Whittaker and Brown2010).
Prediction 6: extinction levels should correlate negatively with circularization of the green spaces or of their native habitats because more rounded shapes should reduce the negative impact of edge effects (Davies et al. Reference Davies, Gascon, Margules, Soulé and Orians2001). In addition, more circular shapes should promote conspecific interactions and positively affect dispersal rates (Diamond Reference Diamond1975). By contrast, elongated shapes are expected to intercept fewer immigrants, and hence colonization rates should decline with decreasing circularization.
Prediction 7: extinction levels should be negatively correlated with proximity to areas that can serve as sources of immigrants, such as other green spaces and rural areas. This prediction follows from the basic assumptions that: (1) where there is more urban green space, there is more likely to be enough of a suitable environment to sustain a meta-population (Davis Reference Davis1979); (2) higher connectivity reduces the effects of genetic isolation (Davis et al. Reference Davies, Gascon, Margules, Soulé and Orians2001) and facilitates immigration; (3) small green spaces, even if unable to sustain a stable population of a given species, may sustain individuals that are dispersing towards more suitable areas (Thomas et al. Reference Thomas, Baguette, Lewis, Gosling and Sutherland2000); and (4) extinction is negatively correlated with distance from the city centre because the multiple negative effects of urbanization tend to diminish from the city centre to the most peripheral sectors (McKinney Reference McKinney2008), and more peripheral green spaces are closer to the areas that are sources of immigrants (Dias Reference Dias1996) and so can benefit from rescue effects (Gosselin Reference Gosselin1996) and increased colonization rates.
Prediction 8: extinction levels should be negatively correlated with the extent of native habitats, because larger habitats increase the long-term viability of populations (Andrén Reference Andrén1994; Fahrig Reference Fahrig1997; Donnelly & Marzluff Reference Donnelly and Marzluff2004).
These predictions can be retrospectively used to investigate whether the ETIB can be applied to urban insects. Despite the importance of island biogeography in urban ecology, there has been no review of how the principles of the ETIB can be applied to the urban environment. The aim of the present paper is to establish whether these predictions are confirmed by the current literature on urban insects. This is important not only in the context of urban ecology and conservation, but also from a general biogeographical perspective. Urban green spaces are much easier to reach and to sample than oceanic islands, thus providing intriguing opportunities for testing and developing island biogeography models. Thus, our review can also stimulate biogeographers to use urban green spaces as testbeds for their research.
METHODS
The literature was searched using the Web of Science (WOS) databases from Thomson Reuters (Institute for Scientific Information; ISI) at first using the following retrieve terms: ‘island biogeography urban’ or ‘island biogeography city’ or ‘island biogeography cities’. A second search used keywords that are commonly used to refer to insects in urban green spaces. In order to guarantee extensive coverage, keywords were used that resulted in relatively low search specificity, as recommended by Pullin and Stewart (Reference Pullin and Stewart2006). Thus, the search was conducted by using the following combinations of keywords: ‘urban park*’ or ‘city park*’ or ‘green space*’ or ‘green area*’ and ‘insect*’ (* indicating any ending possible). Our searches were performed up to 1 October 2016 and included no filtering related to the year of publication, type or language. Titles and abstracts that were identified by the searches were scrutinized and relevant publications were manually selected. We searched for references reported in the retrieved documents in order to avoid excluding important information not considered in WOS. In addition, all papers, book chapters and books that might include references to the application of ETIB principles to urban insects were searched. In particular, the references in two recent reviews of urban insects (Jones & Leather Reference Jones and Leather2012; New Reference New2015) were searched. The presence of recurrent patterns – the detection of which is not strongly influenced by the small methodological differences that can be found among different papers – was concentrated on. However, because methods varied among studies, the final results presented in each paper were given greater consideration than the specific statistical tests that had been applied in each case study.
RESULTS
We retrieved 69 records with the term ‘island biogeography urban’ and 25 with the term ‘island biogeography city’ (or cities) for a total of 77 papers using the WOS (17 papers were in common between the two searches). However, most of these papers did not concern urban green spaces, and only nine papers were potentially relevant. For example, Baz and Monserrat (Reference Baz and Monserrat1999) dealt with apartments, Hamerlik and Brodersen (Reference Hamerlik and Brodersen2010) dealt with fountains and Clark et al. (Reference Clark, Rykken and Farrell2011) dealt with true islands. The relatively small number of papers retrieved by the WOS indicated that few authors of urban ecological research had placed their results in a biogeographical context, even if dealing with issues that are typical of island biogeography. The second WOS search retrieved 70 publications, 14 of which were relevant to the present analysis, despite the authors not placing their results in a biogeographical context.
The number of case studies dealing with each prediction varied considerably (Fig. 2 and Table 1). There were 31 case studies that could be related to Prediction 1, which was therefore the most frequently investigated. Predictions 2–4 were investigated in 10 or fewer studies. Predictions 5–8 were explicitly dealt with in only one study (see below).
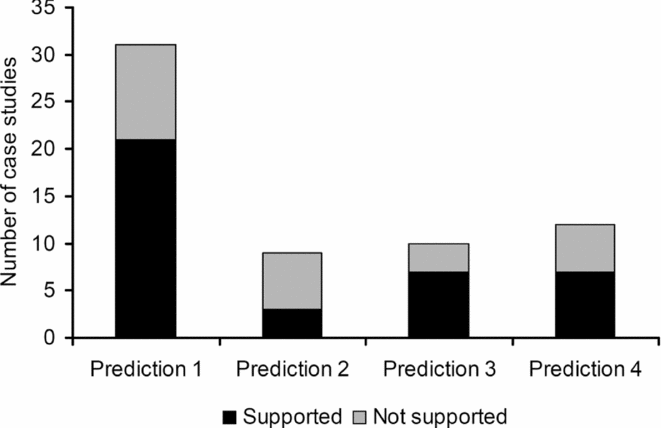
Figure 2 Number of case studies supporting or rejecting hypotheses regarding the influence of the geographical characteristics of green spaces on insect species richness. Prediction 1: species richness is positively related to green space size; Prediction 2: a more circular shape of green space increases species richness; Prediction 3: increasing isolation of green spaces negatively affects species richness; Prediction 4: increasing extent of native habitats increases species richness.
Table 1 Studies addressing predictions regarding insect species richness in urban green spaces.
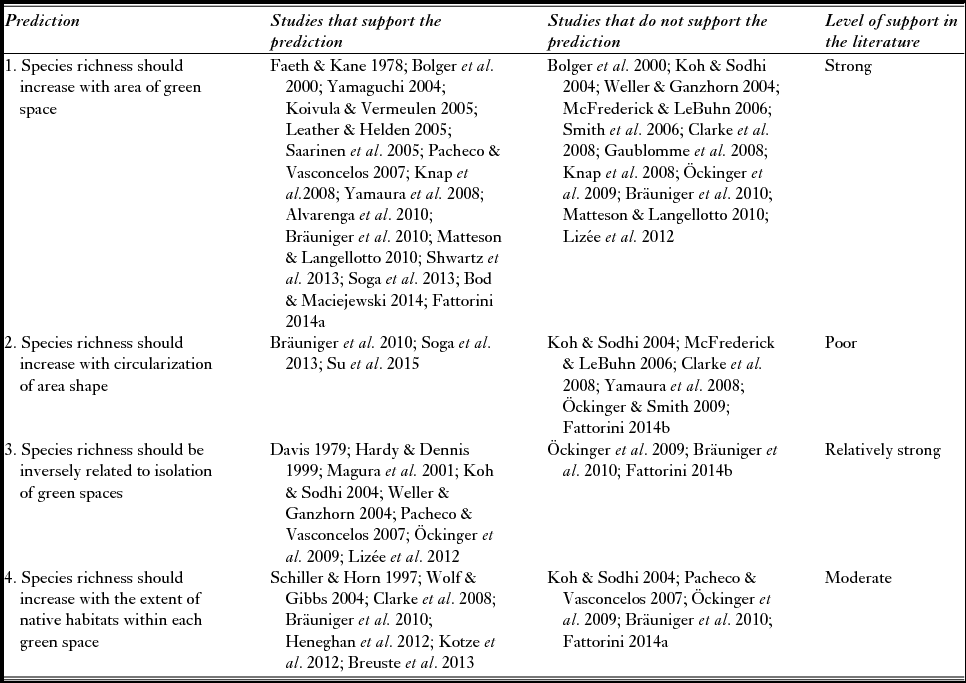
The most frequently observed pattern consistent with ETIB predictions is an increase in species richness (or other measures of diversity) with the area of green spaces (Prediction 1). Studies reporting this positive relationship include species richness of various insect orders (Coleoptera, Diptera, Homoptera, Hemiptera and Hymenoptera) in green spaces in California (Bolger et al. Reference Bolger, Suarez, Crooks, Morrison and Case2000), Shannon–Wiener diversity of butterflies on roadside verges in south east Finland (Saarinen et al. Reference Saarinen, Valtonen, Jantunen and Saarnio2005), species richness of Diptera and Coleoptera in city parks in Cincinnati (Faeth & Kane Reference Faeth and Kane1978), number of colonies of social wasps in urban gardens in Brazil (Alvarenga et al. Reference Alvarenga, De Castro, Santos-Prezoto and Prezoto2010), carabid species richness in urban green spaces in Bracknell (Leather & Helden Reference Leather and Helden2005), carabid species richness in road-enclosed forest patches in Helsinki (Koivula & Vermeulen Reference Koivula and Vermeulen2005), ant species richness in the Brazilian Cerrado (Pacheco & Vasconcelos Reference Pacheco and Vasconcelos2007) and in Tokyo and Chiba City (Yamaguchi Reference Yamaguchi2004), bee species richness in New York (Matteson & Langellotto Reference Matteson and Langellotto2010), butterfly species richness of green spaces in Halle and Saalekreis (Knap et al. Reference Knapp, Kuhn, Mosbrugger and Klotz2008; Bräuniger et al. Reference Bräuniger, Knapp, Kuhn and Klotz2010) and Hokkaido (Yamaura et al. Reference Yamaura, Kawahara, Lida and Ozaki2008), butterfly and pollinator species richness in Paris (Shwartz et al. Reference Shwartz, Muratet, Simon and Julliard2013), insect herbivores in abandoned city lots in Buffalo (Bod & Maciejewski Reference Bode and Maciejewski2014) and tenebrionid species richness of green spaces in Rome (Fattorini Reference Fattorini2014a). In this latter study in Rome, larger urban green spaces also had lower extinction rates, thus supporting Prediction 5 (Fattorini Reference Fattorini2014a). In general, area was identified as an important factor influencing not only insect diversity, but also community composition (Davies & Margules Reference Davies and Margules1998; Gibb & Hochuli Reference Gibb and Hochuli2002; Sadler et al. Reference Sadler, Small, Fiszpan, Telfer and Niemelä2006; Fujita et al. Reference Fujita, Maetro, Kagawa and Ito2008). However, no area effect was found for carabids (Weller & Ganzhorn Reference Weller and Ganzhorn2004; Gaublomme et al. Reference Gaublomme, Hendrickx, Dhuyvetter and Desender2008; Knap et al. Reference Knapp, Kuhn, Mosbrugger and Klotz2008; Bräuniger et al. Reference Bräuniger, Knapp, Kuhn and Klotz2010), bees (McFrederick & LeBuhn Reference McFrederick and LeBuhn2006), ants (Clarke et al. Reference Clarke, Fisher and LeBuhn2008) or butterflies (Bolger et al. Reference Bolger, Suarez, Crooks, Morrison and Case2000; Koh & Sodhi Reference Koh and Sodhi2004; Öckinger et al. Reference Öckinger, Dannestam and Smith2009; Matteson & Langellotto Reference Matteson and Langellotto2010; Lizée et al. Reference Lizée, Mane, Mauffrey, Tatoni and Deschamps-Cottin2012). Smith et al. (Reference Smith, Chapman and Eggleton2006) also found no area effect for ants of London's green spaces, but they used species density as a measure of diversity, not richness. Similarly, Su et al. (Reference Su, Li, Zhou and Ouyang2015) found a negative effect of green space area on overall insect density. This unexpected pattern can be explained by assuming that individual insects disperse more widely in larger urban green patches, causing a decrease in individual number per unit area (Su et al. Reference Su, Li, Zhou and Ouyang2015). Excluding studies dealing with density, out of a total of 31 case studies considered in our analysis, 21 (68%) provided support for Prediction 1 and 10 (32%) did not.
Although area is considered a strong predictor of species richness, its effect may be a consequence of the fact that larger areas have a greater habitat heterogeneity (see Fattorini et al. Reference Fattorini, Dapporto, Strona and Borges2015). For example, butterfly species richness in New York increases with park area and herbaceous plant species richness, suggesting that larger parks host more butterflies because their vegetation provides them with more food, greater habitat cover and potential niches (Giuliano et al. Reference Giuliano, Accamando and Mcadams2004). However, neither area nor habitat diversity explained the species richness of bumble bees and butterflies, respectively (Clarke et al. Reference Clarke, Fisher and LeBuhn2008; Matteson & Langellotto Reference Matteson and Langellotto2010). Similarly, plant diversity was not a predictor of wild bee species richness in the green spaces of Lyon (Fortel et al. Reference Fortel, Henry, Guilbaud, Guirao, Kuhlmann, Mouret, Rollin and Vaissière2014), and habitat diversity was not important for butterflies and pollinators in the green spaces of Paris (Shwartz et al. Reference Shwartz, Muratet, Simon and Julliard2013).
As regards the influence of habitat quality and extent, studies conducted in central and northern Europe and in North America in cities that had grown into agricultural and forested landscapes highlighted the importance of forest habitats (Schiller & Horn Reference Schiller and Horn1997; Clarke et al. Reference Clarke, Fisher and LeBuhn2008; Bräuniger et al. Reference Bräuniger, Knapp, Kuhn and Klotz2010; Heneghan et al. Reference Heneghan, Mulvaney, Ross, Umek, Watkins, Westphal and Wise2012; Kotze et al. Reference Kotze, Lehvävirta, Koivula, O'Hara and Spence2012; Breuste et al. Reference Breuste, Haase, Elmqvist, Wratten, Sandhu, Cullen and Costanza2013). By contrast, for tenebrionids in urban Rome, the percentage of forested area had a negative effect on species richness and species persistence (which contrasts with Predictions 4 and 8) (Fattorini Reference Fattorini2014a). This is attributable to the low number of truly forest interior species found among tenebrionids, which may also explain the lack of green space shape effects on species richness and extinction (in contrast with Prediction 2), as well as the lack of forest shape effects on species extinction (in contrast with Prediction 6). Similarly, in Halle and Saalekreis (Bräuniger et al. Reference Bräuniger, Knapp, Kuhn and Klotz2010), there was a negative relationship between carabid richness and forests, probably because most urban carabids are associated with open habitats. Other studies that failed to find a positive relationship between the extent of pristine habitats and species richness included those of Pacheco and Vasconcelos (Reference Pacheco and Vasconcelos2007), Öckinger et al. (Reference Öckinger, Dannestam and Smith2009) and Koh and Sodhi (Reference Koh and Sodhi2004). Thus, out of 12 studies that dealt with this subject, seven (58%) showed the importance of the extent of pristine habitats (typically forests), thus supporting Prediction 4, but five (42%) did not. The importance of a circular shape was detected for overall insect density (Su et al. Reference Su, Li, Zhou and Ouyang2015) and butterfly richness (Bräuniger et al. Reference Bräuniger, Knapp, Kuhn and Klotz2010). In addition, Soga et al. (Reference Soga, Kanno, Yamaura and Koike2013) found a negative effect of edge extent on forest carabids, thus supporting the importance of a circular shape. However, a negative effect of circularization was found for open-land butterflies in Hokkaido (Yamaura et al. Reference Yamaura, Kawahara, Lida and Ozaki2008) and tenebrionids in Rome (Fattorini Reference Fattorini2014b), which is in contrast to Prediction 2. No effect of green space shape was found for butterflies (Koh & Sodhi Reference Koh and Sodhi2004; Öckinger & Smith Reference Öckinger, Dannestam and Smith2009), bumble bees (McFrederick & LeBuhn Reference McFrederick and LeBuhn2006) and ants (Clarke et al. Reference Clarke, Fisher and LeBuhn2008). Thus, out of nine studies that dealt with green space shape, only three (33%) supported Prediction 2.
Examples of isolation/connectivity effects on insect diversity (Prediction 3) include: (1) the positive influence of the proportion of surrounding land occupied by green spaces within 1 km of study sites for arthropod species richness in London urban gardens (Davis Reference Davis1979) and butterfly species richness in the green spaces of Malmö (Öckinger et al. Reference Öckinger, Dannestam and Smith2009); (2) the negative influence of distance from natural areas (butterflies in Marseille) (Lizée et al. Reference Lizée, Mane, Mauffrey, Tatoni and Deschamps-Cottin2012); (3) the positive effect of increasing distance from the city centre (ant density in the Brazilian Cerrado) (Pacheco & Vasconcelos Reference Pacheco and Vasconcelos2007); (4) the total area of forests (i.e. reserves and fragments) within 2 km (butterflies in the green spaces of Singapore) (Koh & Sodhi Reference Koh and Sodhi2004); (5) the negative influence of increasing urban cover for butterfly richness in southwest Manchester and Mersey Valley (Hardy & Dennis Reference Hardy and Dennis1999); and (6) the negative impacts of the extent of surrounding buildings and roads, traffic density and distance to suitable environments for carabid richness in Hamburg (Weller & Ganzhorn Reference Weller and Ganzhorn2004). Distance between green spaces did not affect carabid and butterfly species richness in Halle and Saalekreis (Bräuniger et al. Reference Bräuniger, Knapp, Kuhn and Klotz2010), and distance from the city border was not correlated with butterfly species richness in Malmö (Öckinger et al. Reference Öckinger, Dannestam and Smith2009). Green space connectivity in Rome did not enhance tenebrionid richness, probably because these insects form relict communities of species with low dispersal ability and hence with few chances of moving from patch to patch (Fattorini Reference Fattorini2014b).
Insect density in Beijing's green spaces was positively influenced by the percentage of vegetated land and negatively influenced by impervious surfaces (Su et al. Reference Su, Li, Zhou and Ouyang2015). However, connectivity (measured as the number of functional links between green patches, where each pair of patches was connected by ≤5 m of cleared land) negatively influenced insect density, possibly because increasing connectivity may strengthen emigration from local patches (Su et al. Reference Su, Li, Zhou and Ouyang2015). Thus, out of 11 studies that dealt with the effect of isolation, seven (64%) supported Prediction 3 and three (36%) did not. Isolation also affects arthropod community structures in urban green spaces, as for butterflies in Palo Alto (Blair & Launer Reference Blair and Launer1997) and southeast Finland (Saarinen et al. Reference Saarinen, Valtonen, Jantunen and Saarnio2005; Valtonen et al. Reference Valtonen, Saarinen and Jantunen2007), for orthopterans and cicadellids in Bremen and Berlin (Strauss & Biedermann Reference Strauss and Biedermann2006) and for forest carabids in Brussels (Gaublomme et al. Reference Gaublomme, Hendrickx, Dhuyvetter and Desender2008). However, the influence of the habitat quality and urban matrix is complex, and a study on bumble bees in San Francisco parks indicated that park area, distance to the nearest source population and the perimeter:area ratio did not predict bumble bee community structures, whereas the openness of the surrounding matrix and the abundance of the dominant competitor did (McFrederick & LeBuhn Reference McFrederick and LeBuhn2006).
For tenebrionids in Rome, in accordance with Prediction 7, early (pre-1960) extinctions were promoted by increasing mean distance between green spaces, which suggests that increasing isolation had a detrimental effect on species persistence (Fattorini Reference Fattorini2014b). Isolation can also have a reduced impact for very mobile species, but for opposite reasons. In this case, even green spaces that are relatively far from each other can be easily reached. For example, carabids and butterflies in Birmingham are able to disperse even across inhospitable environments to reach isolated habitat islands and are therefore more affected by habitat quality than isolation (Angold et al. Reference Angold, Sadler, Hill, Pullin, Rushton, Austin, Small, Wood, Wadsworth, Sanderson and Thompson2006). Small urban gardens in the heart of New York City host c. 13% of the bee fauna of the entire New York State area (Matteson et al. Reference Matteson, Ascher and Langellotto2008). In Westchester County (a suburban area immediately north of New York City), residential gardens host c. 30% of the species recorded for New York State (Fetridge et al. Reference Fetridge, Ascher and Langellotto2008).
As regards the urban–rural gradient, many studies identified a negative trend (for reviews, see Sadler et al. Reference Sadler, Small, Fiszpan, Telfer and Niemelä2006; Jones & Leather Reference Jones and Leather2012; New Reference New2015), which is consistent with Predictions 3 and 7. However, other studies found peaks of insect species richness at the gradient mid-point (for reviews, see Blair & Launer Reference Blair and Launer1997; Marzluff Reference Marzluff2005; Jones & Leather Reference Jones and Leather2012; New Reference New2015). Most studies regarding insects have dealt with carabid beetles, butterflies and hymenopterans, with patterns varying according to the ecology of the concerned group (New Reference New2015). No urban–rural gradient effect was found for Rome tenebrionids (Fattorini Reference Fattorini2014a), possibly because these insects include both species associated with arid and sandy soils (which can be considered to be urban avoiders) and species associated with ruderal and archaeological sites (which can be considered to be urban adapters). In addition, there is an indication that biodiversity patterns along the urban–rural gradient are scale dependent because of the irregular distributions of key resources or other biotope elements along gradients (Hogsden & Hutchinson Reference Hogsden and Hutchinson2004).
DISCUSSION
The SAR is one of the best-documented patterns in island biogeography (e.g. Lomolino et al. Reference Lomolino, Riddle, Whittaker and Brown2010). Although many exceptions exist, the majority of the urban studies that we have reviewed report patterns that are consistent with the SAR (see also Nielsen et al. Reference Nielsen, van den Bosch, Maruthaveeran and van den Bosch2014), possibly suggesting that larger green spaces should be preferred to smaller spaces (everything else being equal). This may be an important point, because there is a debate as to whether a single, larger reserve should be preferred to several small areas (e.g. Fattorini Reference Fattorini2010). However, if the final goal is to preserve as many species as possible and the total area that can be preserved is a fixed amount, the idea that a single, larger reserve should be preferred to several small reserves may be questioned. For example, giving preference to larger reserves might lead to faster disease spread, or may expose species to local extinctions in cases of catastrophic events. In fact, when even the largest areas are small (as is the case for most urban green spaces) and species distribution within the city is highly fragmented, a network of many small spaces may be preferable to a few larger spaces. For example, the tenebrionid beetles of Rome (Fattorini Reference Fattorini2014b) revealed that, except for distance to other sites, no significant correlations were found between the conservation values of the tenebrionid communities of green spaces and site characteristics, thus suggesting that the conservation importance of urban green spaces cannot be predicted on the basis of their geographical characteristics, but rather must be established on the basis of the species that they actually host.
In the case of urban green spaces, much attention has been focused on the importance of the extent and quality of forests as native habitats for animal conservation in urban areas (Andrén Reference Andrén1994; Fahrig Reference Fahrig1997; Donnelly & Marzluff Reference Donnelly and Marzluff2004). However, the present analysis shows little support for this as being important. This result warns against the risk of generalizations regarding the conservation importance of particular ‘key habitats’ and calls for studies that take into account local ecological settings and species characteristics.
In general, urban green spaces tend to deviate substantially from a circular shape, being either elongated or indented. Because of constraints imposed by the architecture of built-up areas, it is virtually impossible to modify the shape of already existing urban green spaces. Thus, very limited support for the importance of circular shapes sounds like good news. Because circularization is inversely related to ecotonal development, this result may be explained by assuming that ecotonal development may have negative effects on interior forest species, but positive effects on open habitat and forest generalist species (Magura et al. Reference Magura, Tóthmérész and Molnár2001).
There is relatively strong support for the importance of connectivity. Most studies of insect conservation in urban areas assumed that the matrix is wholly hostile and inhospitable, at least for most of the native, non-synanthropic species. However, the urban matrix is not uniform (e.g. because of the different intensities of development), and many insects can use resources from regions beyond their immediate habitat patch (Dennis Reference Dennis2010). In general, the urban matrix isolating green spaces is expected to be less hostile (i.e. easier to be crossed) than water for oceanic island colonization. First, oceanic islands are typically very far from the mainland (hundreds or thousands of kilometres), whereas urban green spaces are isolated from source areas by only a few kilometres, or even meters. Second, sea water is a strong barrier to most land animals (Cox & Moore Reference Cox and Moore2010), especially those that disperse by walking and for which island colonization can occur only by passive dispersal. Although crossing the urban matrix may be very hazardous, terrestrial, flightless insects may move relatively easily among urban green spaces by active dispersal. In addition, matrix permeability is a function of species ecology, and the response of arthropod species to isolation depends on their ability to cross this matrix. Thus, promoting connectivity among urban green spaces via corridors or a network of even very small spaces that may act as stepping stones may be a useful strategy for insect conservation (New Reference New2015).
In general, a variable number of case studies provided valuable insights into evaluating the support for Predictions 1–4. These predictions deal with patterns that are consistent with the ETIB, but not with the underlying processes. For each of these patterns, we have identified the possible underlying processes on the basis of the ETIB (Predictions 5–8), but we found a virtually complete lack of studies that contained results that are useful to evaluating these postulated processes. In fact, despite the immense literature on the ETIB, very few studies explicitly test these mechanisms by recording extinction and colonization rates and the associated species turnover, especially over long periods (Wilson & Simberloff Reference Wilson and Simberloff1968; Rey Reference Rey1981; Robinson et al. Reference Robinson, Yurlina and Handel1994; Burns & Neufeld Reference Burns and Neufeld2009; Morrison Reference Morrison2010), probably because of the lack of reliable biological data. Even in the paradigmatic case of the Krakatau islands, Whittaker et al. (Reference Whittaker, Field and Partomihardjo2000) suggested great caution in interpreting colonization and extinction rates because the use of a limited dataset led to an overestimation of the extinction rates. Progress in urban ecology will promote the use of urban green spaces not only to test patterns of island biogeography, but also to explicitly test their underlying mechanisms and to better understand their implications for environmental conservation.
CONCLUSION
Island biogeography may continue to offer an important framework for urban ecology studies by providing explicit and testable hypotheses. When applied to oceanic islands, the ETIB is too simplistic to adequately capture the diversity of patterns and processes that involve species with different ecologies and island systems that vary in their geographical, historical and environmental characteristics (Lomolino et al. Reference Lomolino, Riddle, Whittaker and Brown2010). Yet, the ETIB still provides and will continue to provide insights for understanding biogeographical phenomena in island biology, and it may represent a useful framework for urban ecology research as well.
Recent developments in island biogeography have been prompted by studies in species co-occurrence and nestedness (Lomolino et al. Reference Lomolino, Riddle, Whittaker and Brown2010). Quite surprisingly, there is virtually no research dealing with co-occurrence and nestedness patterns in urban areas. We think that future co-occurrence and nestedness analyses would provide important insights not only into urban ecology, but also into island biogeography.
ACKNOWLEDGEMENTS
We are grateful to D. Bergamaschi and A. Di Giulio for useful discussions and N. Polunin and two anonymous referees for their comments on early versions of the manuscript.





