INTRODUCTION
The agricultural transformation of forested watersheds in the tropics has significant consequences for soil and water management, biodiversity conservation, and timber and non-timber forest resource availability (Bruijnzeel Reference Bruijnzeel1991; Bonell & Bruijnzeel Reference Bonell and Bruijnzeel2004; Pattanayak Reference Pattanayak2004; Trancoso et al. Reference Trancoso, Filho, Tomasella, Schietti, Forsberg and Miller2010). Agriculturally-impacted habitats now so dominate many tropical landscapes that environmental scientists devote increasing attention to their study (Pimentel et al. Reference Pimentel, Stachow, Takacs, Brubaker, Dumas, Meaney, O'Neil, Onsi and Corzilius1992; Parrotta & Turnbull, Reference Parrotta and Turnbull1997; Scherr & McNeely Reference Scherr and McNeely2008; Jose Reference Jose2009; Gardner et al. Reference Gardner, Barlow, Sodhi and Peres2010). Tropical environmental conservation is likewise increasingly framed in the context of multi-use landscapes, watersheds or forest-agriculture habitat mosaics (see for example Naughton-Treves Reference Naughton-Treves2002; Schroth et al. Reference Schroth, da Fonseca, Harvey, Gascon, Vasconcelos and Izac2004; Faria et al. Reference Faria, Laps, Baumgarten and Cetra2006; Cassano et al. Reference Cassano, Schroth, Faria, Delabie and Bede2009; Norris et al. Reference Norris, Asase, Collen, Gockowski, Mason, Phalan and Wade2010; Peres et al. Reference Peres, Gardner, Barlow, Zuanon, Michalski, Lees, Vieira, Moreira and Feeley2010; Trancoso et al. Reference Trancoso, Filho, Tomasella, Schietti, Forsberg and Miller2010; Dewi et al. Reference Dewi, van Noordwijk, Ekadinata and Pfund2013). Agriculture impacts are typically framed in negative terms (such as causing deforestation, soil erosion or biodiversity loss), but there is growing appreciation that such impacts vary in space, change over time, and may not be lasting in their effects.
First, there is now a sizable body of research that documents extensive afforestation, usually natural re-growth of secondary forest on abandoned farm land, following earlier deforestation of the same lands (Brown & Lugo Reference Brown and Lugo1990; Corlett Reference Corlett1995; Finegan Reference Finegan1996; Guariguata & Ostertag Reference Guariguata and Ostertag2001; de Jong et al. Reference de Jong, Chokkalingam and Smith2001; Chazdon Reference Chazdon2003). These so-called forest transitions have resulted from a variety of causal events, including declining on-farm productivity, rising farm input costs, reduced commodity prices, rural out-migration and policies that redirect incentives away from agricultural development and towards promotion of forest conservation and tree planting (Foster & Rosenzweig Reference Foster and Rosenzweig2003; Rudel et al. Reference Rudel, Coomes, Moran, Achard, Angelsen, Xu and Lambin2005). Post-agriculture, secondary forests typically differ in composition and structure from pre-existing forests, but may still be rich in native species and can quickly re-establish a complex vegetation structure that protects soil and water resources (Corlett Reference Corlett1995; Grau et al. Reference Grau, Aide, Zimmerman, Thomlinson, Helmer and Zou2003; Chazdon Reference Chazdon2003 Junqueira et al. Reference Junqueira, Shepard and Clement2010). Such forests may also serve as buffers between protected areas and surrounding human populations (Laurance et al. Reference Laurance2012; Dewi et al. Reference Dewi, van Noordwijk, Ekadinata and Pfund2013).
A second line of research has sought to understand the ecological characteristics and interactions between different habitats within agricultural landscapes. Some farmed habitats serve as reservoirs of plant and animal species and otherwise sustain many benefits in their own right, including soil and watershed protection (Naughton-Treves Reference Naughton-Treves2002; Norris Reference Norris2008; Jose Reference Jose2009; da Silva Moco et al. Reference da Silva Moco, da Gama-Rodrigues, da Gama-Rodrigues, Machado and Baligar2009). For example, traditional ‘home gardens’ often include dozens of plant species cultivated for food, fuel, construction materials, medicines and aesthetics (Brierley Reference Brierley1991; Padoch & de Jong Reference Padoch and de Jong1991; Hylander & Nemomissa Reference Hylander and Nemomissa2008). Another common agricultural land use, agroforestry, entails the mixed planting of tree crops often with native tree species and/or non-tree crops (Denevan & Padoch Reference Denevan and Padoch1987; Schroth et al. Reference Schroth, da Fonseca, Harvey, Gascon, Vasconcelos and Izac2004; Bhagwat et al. Reference Bhagwat, Willis, Birks and Whittaker2008; Asase & Tetteh Reference Asase and Tetteh2010). These often structurally and compositionally diverse habitats are commonly found, not only within indigenous and smallholder peasant communities, but also on large estates (Perfecto et al. Reference Perfecto, Rice, Greenberg and Van der Voort1996; Sambuichi & Haridasan Reference Sambuichi and Haridasan2007; Walters Reference Walters2012a). They stand in stark contrast to the near monospecific habitats of annual crops that characterize many contemporary farmlands. Like secondary forest, agroforests can buffer the impacts of human activity on protected areas while providing livelihood opportunities for local people (Schroth et al. Reference Schroth, da Fonseca, Harvey, Gascon, Vasconcelos and Izac2004; Dewi et al. Reference Dewi, van Noordwijk, Ekadinata and Pfund2013).
Caribbean forests and watersheds are especially vulnerable to human disturbance given the rugged geography and historical confluence of high population densities, intensive agriculture and highly restricted habitat distributions that result from steep topographic and climate gradients and island insularity (Lugo et al. Reference Lugo, Schmidt and Brown1981). With the exception of Puerto Rico, there has been little research on post-agricultural forests and agroforests within the Caribbean, and almost none on the smaller islands of the Eastern Caribbean (Kimber Reference Kimber1988; Gonzales & Zak Reference Gonzales and Zak1996; Foster et al. Reference Foster, Fluet and Boose1999; Grau et al. Reference Grau, Aide, Zimmerman, Thomlinson, Helmer and Zou2003; Aide & Grau Reference Aide and Grau2004; Lugo & Helmer Reference Lugo and Helmer2004; Chazdon et al. Reference Chazdon, Letcher, van Breugel, Martinez-Ramos, Bongers and Finegan2007; Chai & Tanner Reference Chai and Tanner2011; Levesque et al. Reference Levesque, McLaren and McDonald2011).
This paper reports on the changing interactions between people, trees and forests in two watersheds in St Lucia, West Indies (Sargent Reference Sargent2007; Hansen Reference Hansen2009; Walters Reference Walters2012a, Reference Waltersb). The watershed was used as the unit of study because it enabled sampling of a wide variation in topography and the full range of forested habitats, from coastal mangrove and dry scrub to wet mountain rain forest, and situates these within a wider landscape of changing land use and land-use planning. Watersheds have long constrained the settlement and agricultural development of St Lucia's rugged mountainous terrain. For example, most rural roads ascend from the coast inland along valley bottoms, roughly parallel to river courses; few cross watershed boundaries laterally. Watersheds are also increasingly used as the basis for land-use and conservation planning, particularly in the designation of critical water source and watershed protection areas. We selected one watershed from the east side and one watershed from the west side of the island to account for geographic differences in agricultural history.
In this study, we first compared the vegetation structure between the four most common habitat types found within the study watersheds: annual crop agriculture, perennial agroforest, post-agriculture secondary forest and mature forest. We then compared the species richness between these habitats. Finally, we assessed the land use history and, in particular, the influence of selected topographic and socioeconomic variables on the distribution of these four habitat types within the study watersheds. For St Lucia as elsewhere, understanding the changing distribution of forest and agricultural habitats is critical for long-term and landscape-level conservation planning.
Study area
Saint Lucia is a small country in the Windward Islands of the Eastern Caribbean (Fig. 1). It experiences a wet tropical climate and is characterized by a mostly rugged, mountainous topography with fertile soils of relatively recent volcanic origin (Stark et al. Reference Stark, Lajoie and Green1966). The foundation of the island's economy has for over two centuries rested on export-oriented agriculture (sugar, coconuts, cocoa, citrus, bananas and mixed fruit), much of this concentrated in large estates located along the flat bottom lands and lower slopes of the island's river valleys. Smallholder farms dominate nearby hillsides and ridge tops and, in many watersheds, penetrate deeply into the mountains.
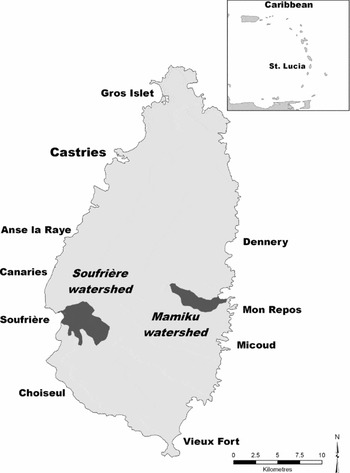
Figure 1 Map of Saint Lucia and study watersheds.
Field work for this study was focused on the Soufriere (1570 ha) and Mamiku (790 ha) watersheds (Fig. 1). Lying leeward of the tallest mountains in St Lucia, Soufriere receives the highest rainfall on the island and is also significantly protected from easterly-prevailing winds that often damage crops during storm events. While ideal climatically for agriculture, Soufriere's especially rugged terrain placed it at a disadvantage when bananas rose to ascendency in the 1960s because these fruit are easily bruised in transport. This discouraged farmers in Soufriere from converting their existing tree crops (such as cocoa, lime or coconut) wholesale to banana production.
By contrast, the relatively drier conditions, gentler slopes and accessibility of lands within the Mamiku watershed made it amenable to growing less water-demanding crops, especially sugar, coconuts, livestock and, more recently, bananas. The ‘banana boom’ saw St Lucian exports grow annually from 5000 tonnes in 1955 to 130 000 tonnes in 1990, and back down to 20 000 tonnes in 2010 (GOSL [Government of Saint Lucia] 2011). Over this period, extensive areas of already farmed land were converted to bananas and smallholders cleared forests from progressively steeper and more remote sites to expand banana cultivation. The subsequent ‘bust’ in bananas led many of these farmers to abandon farming, triggering a wave of upland afforestation (Walters Reference Walters2012a).
METHODS
We undertook vegetation survey work between February and April 2006, and conducted interviews and most archival investigations between November 2005 and August 2007. To assess vegetation characteristics, we employed the quadrat-census plot method (Walters Reference Walters2005). Each census plot was 10 m × 10 m, with corners and boundaries marked, and vertical and horizontal axes running through the centre of each plot using measuring tapes. We used a relatively small plot size for this study because, first, trees in most of the stands surveyed were typically small and densely crowded as a result of the relatively young stand ages and, second, we viewed sampling a relatively large number of different sites as advantageous for capturing the huge variation in landscape and vegetation characteristics. In light of this concern and to minimize site selection bias, we employed a systematic stratified sampling protocol by using pre-determined GPS grid coordinates to identify specific sampling sites. Plots were thus located in a large grid across each watershed, with individual plots 500 m apart north-to-south and 1000 m apart east-to-west. We pre-programmed specific plot site coordinates into a GPS, which was then used to guide researchers to sampling locations. Using this approach, a total of 56 plots were sampled: 34 in Soufriere and 22 in Mamiku (numbers reflective of differences in watershed size). Severe terrain compelled small deviations from the grid-designated locations in a few cases.
Terrain in both watersheds, but especially Soufriere, was highly varied and often rugged. Vegetation plots were sampled at 0–646 m altitude (X = 239.8, SD = 159.3) and on slopes of 0–47° (X = 18.9, SD = 14.2). Mean altitude of plots was significantly higher in Soufriere (X = 286.4 m, SD = 166.3) than Mamiku (X = 167.6 m, SD = 118.3; p < 0.005, F = 8.44, df = 1,54), but slope and distance to the nearest road were similar between watersheds. We documented four types of land tenure: large private estates (12 plots); small private holdings (10 plots); communal ‘family’ holdings where tenure claims are shared amongst multiple family heirs (23 plots); and government lands (11 plots). The relative frequency of land tenure types was similar between watersheds.
This sampling protocol captured a wide variety of ecosystem and land use types, including coastal mangrove forest, coastal dry scrub forest, mixed wet-dry tropical forest, wet tropical forest, agroforest, annual crop agriculture, pasture land, residential property, a grassy school yard, and the rock caldera and grassy emission plume field of a semi-active volcano. To enable a robust comparative analysis, we clustered the plots into four habitat types: annual crop agriculture (six plots), agroforest (mixed perennial tree crops 19 plots), secondary forest (13 plots) and mature forest (14 plots). We excluded the four plots that did not fit readily into these categories (the school yard, residential yard and two volcano plots) from the comparative habitat-vegetation analysis, but included them in the analysis of general watershed characteristics.
The distinctions between the four main habitat types were with almost no exception easy to make in the field. In short, where annuals and perennial tree crops co-mingled, we based the habitat distinction on the dominant crop form. Similarly, while no forest within the study areas would qualify as totally undisturbed by human activity, we could identify stands composed of post-agricultural regrowth using site visual assessments, farmer interviews and archival records. Forest stands classified as ‘mature’ may have been impacted by selective logging before 1980 (when there was an active timber industry), but there was no apparent evidence of such disturbance; such sites may also have been impacted long ago by agriculture, but there was no existing evidence of this. By contrast, all sites classified as ‘secondary forest’ were clearly of post-agricultural origin, although in a couple cases, where secondary forests were regrowing amidst formerly managed agroforest, we based the habitat distinction on the dominant plant form (for example, wild or cultivated tree crops).
We recorded altitude, slope and aspect measures, and other plot characteristics including evidence of human or natural disturbance, on-site at each sample plot location. We obtained information about land tenure, land holding size and land-use history of each site through interviews with local farmers and by consulting official land registry maps. The distance from the site to the nearest drivable road was estimated using air photos and maps.
Within each of the 56 sample plots, we numbered, mapped and measured every tree >2.9cm dbh, for a total of 1699 trees. Local guides identified tree species on site in either the local Patoi dialect or Latin nomenclature (when known). Appropriate samples of leaves, flowers and fruit were collected where possible, along with photographs. Post identification was assisted by Graveson (Reference Graveson2005) and verifications were later made at the St Lucia Herbarium based on collections and Howard (Reference Howard1974). Species were classified as ‘rare’ or ‘endangered’ based on Graveson (Reference Graveson2005).
We classified trees as being planted or natural, where planted referred to trees which were known to be traditionally cultivated by St Lucians and natural referred to uncultivated trees. All trees within the plot, whether dead or alive, were surveyed and classed accordingly as live trees, snags (standing dead trees taller than 1.37 m), cut stems (cut by man and <1.37 m), uncut stumps (<1.37 m) or deadwood (dead fallen trees). We recorded the presence of ‘cut branches’, ‘slashed bark’ and ‘broken stems’ on live trees.
We derived canopy height for each sample plot by taking the mean height (n = 4) of the largest (dbh) individual trees within each of the plots’ four quadrats, where individual tree heights were measured using a clinometer. To classify canopy structure, we walked through the centre, along the length of the horizontal and vertical axes of each plot, stopping every metre and observing the canopy vertically above. Canopy structure was thus derived from 20 measures that were subsequently summarized and converted to plot percentages. We classified canopy structure as either ‘gap’ (fully open), ‘expanded gap’ (under tree foliage but proximate to a gap) or ‘closed canopy’ (completely covered by tree foliage) following the criteria employed by Walters (Reference Walters2005). A further class, ‘man-made structure’, was included to account for the occasional presence of built structures within sample plots.
We took ground cover measures every metre along the length of the horizontal and vertical axes running through the centre of each plot, for a total of 20 measures in each plot, subsequently converted to plot percentages for each measure. At each measuring point, the presence or absence of a vegetative shrub layer (> 30 cm height) and herb layer (< 30 cm) was noted, and the surface character of the ground was classified as either ‘leaf litter’, ‘moss’, ‘exposed root’, ‘exposed soil’, ‘dead wood’, ‘exposed rock’, ‘concrete’ or ‘other’.
We analysed quantitative data statistically using SPSS. Plot measures were log transformed for statistical analysis when they did not meet the test for homogeneity of variances (Zar Reference Zar1984).
RESULTS
Vegetation structure
Based on plot sampling frequency, agroforest was overall the most common habitat type (19 of 56 plots), but significantly more common in Soufriere (17 of 34 plots) than in Mamiku watershed (2 of 22 plots; χ2 = 6.63, p < 0.01). Mature and secondary forests were equally common overall (14 of 56 and 13 of 56 plots, respectively), but Mamiku had significantly more combined mature and secondary forest than Soufriere (16 of 22 versus 11 of 34 plots, respectively; χ2 = 4.52, p < 0.05). All measures of vegetation structure were similar between the two watersheds, with the exception that planted tree density and basal area were significantly higher in Soufriere (Appendix 1, Table S1, see supplementary material at Journals.cambridge.org/ENC). In particular, planted trees represented 41.5% of total tree basal area for Soufriere, but only 6.9% in Mamiku.
The four habitat types displayed highly significant differences by almost every vegetation measure (Table 1). Densities of total trees, natural trees, snags, deadwood and stumps were comparable and much higher in secondary and mature forests compared with either agroforest or annual crop habitats. By contrast, density of planted trees was ten-fold higher in agroforests than either secondary forests or agricultural habitats. Secondary forests had the lowest mean DBH values for all trees combined and canopy trees only, with the other habitats sharing comparable, but higher values for these measures (Table 1). Mean DBH measures for planted trees were higher than for natural trees across all three habitats where they occur (annual crop, agroforest and secondary forest). Total live tree basal area in mature forest was twice that in secondary forest or agroforest, which in turn was seven-fold higher than in annual crop habitats. Natural trees constituted 100% and 95% of basal area of mature forest and secondary forest, respectively, but only 14% of basal area in agroforest.
Table 1 Summary of select ecological characteristics of vegetation census plots, comparing mean values (± standard deviation) between different categories of primary habitat. *p < 0.05; **p < 0.01; ***p < 0.005; ****p < 0.001.
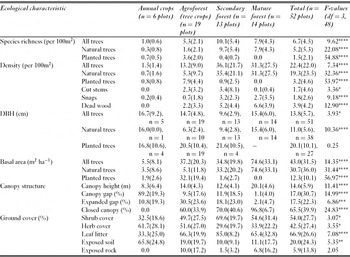
Mature forest had both a taller (mean = 20m) and more closed canopy than the other habitats (Table 1). Only 1% of mature forest canopy was open gap, whereas 97% was closed. By contrast, tree canopies of annual crops were 89% open gap, 11% expanded gap and 0% closed canopy. Canopy measures for agroforests and secondary forests were similar both in height (14.0 m and 12.6 m, respectively) and openness (60% and 70% closed canopy, respectively).
Shrub cover and leaf litter were highest in secondary forest and lowest in annual crops (Table 1), with mature forest and agroforest in between. Exposed soil was detected in 65% of measures in annual crops, 19% in agroforests, 11% in mature forest and 10% in secondary forest. Deadwood was highest in mature forest (5.7%), followed by agroforest (3.7%), secondary forest (1.2%) and agriculture habitats (0%). Cut stems were significantly more abundant in agroforest than the other habitats. Secondary forests revealed intermediate levels of both natural and human-caused disturbance. Little cutting disturbance was detected in mature forest, but stumps and snags were commonplace (Table 1).
Plant species richness
Overall tree richness was constituted by 80% natural and 20% planted species. Secondary forests had the highest total species richness: 10 times more than that of annual crops, twice that of agroforests and 20% more than mature forests. Planted species were non-existent in mature forest, comprised only 4% of tree richness in secondary forest, and 68% of all tree species in agroforest (Table 1).
Cumulative species counts were consistent with mean richness measures (Figs 2–4; Appendix 1, Table S2, see supplementary material at Journals.cambridge.org/ENC). A total of 26 planted species and 99 natural species were recorded. After 13 plots, secondary forests had the highest overall number of species (n = 72), followed by mature forest (n = 57) and agroforest (n = 37). There were no planted species in mature forest and only three (of 72) in secondary forest (Cocos nucifera, Gliricidia sepium and Pimenta racemosa). Planted and natural species made roughly equal contributions to the cumulative richness of agroforests (25 and 19, respectively). One-third of all natural tree species (32 of 99) were recorded in both mature and secondary forest plots (Appendix 1, Table S2, see supplementary material at Journals.cambridge.org/ENC). Eleven tree species were found in both agroforest and secondary forests, and 11 species were found in both agroforest and mature forests. Five rare or endangered species were found in secondary forests (Eugenia duchassaingiana, E. pseudopsidium, E. tapacumensis, Pisonia suborbiculata and Turpinia occidentalis), three in mature forest (Acrocomia aculeate, Croton niveus and E. duchassaingiana), one in agroforest (A. aculeata) and none in annual crop habitats (Appendix 1, Table S2, see supplementary material at Journals.cambridge.org/ENC).

Figure 2 Cumulative species richness of natural trees from randomized vegetation plots by habitat.
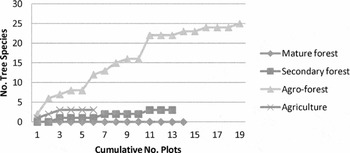
Figure 3 Cumulative species richness of planted trees from randomized vegetation plots by habitat.

Figure 4 Cumulative species richness of all trees from randomized vegetation plots by habitat.
Watershed topography and land use
Mean altitude of habitats varied from 144 m for annual crops to 300 m for mature forest, but differences were only significant when lumped by farmed (annual crops + agroforest) or forest habitat (secondary + mature) (p < 0.05; Table 2). Forest habitats tended to be on steeper slopes than farmed habitats, but differences were not statistically significant (Table 3).
Table 2 Comparing mean altitude of vegetation plots by habitat type. *p < 0.05.

Table 3 Comparing mean slope of vegetation plots by habitat type.

Mean distance to the nearest road varied highly significantly between habitats, with mature forest four times and secondary forests three times more distant than either annual crops or agroforests (p < 0.005; Table 4). When habitat types were lumped, secondary + mature forests were found to be on average 3.4 times further from roads than agriculture + agroforests (p < 0.005).
Table 4 Comparing mean distance to road of vegetation plots by habitat type. *p < 0.005.

There was a strong relationship between habitat and land use (Table 5). Annual crops (χ2 = 9.64, p < 0.01, df = 1) and agroforests (χ2 = 9.99, p < 0.01, df = 1) were both concentrated on lands being actively farmed, although four of 19 agroforest habitats were on lands that been recently abandoned and one site had come under intentional protection for environmental conservation purposes. With only one exception, secondary forest were found on abandoned/idle land (χ2 = 15.93, p < 0.001, df = 1). Ten of 14 mature forests were on lands now under intentional protection (χ2 = 18.45, p < 0.001, df = 1). The four mature forest sites not under intentional environmental protection all fell on family-owned lands where, specifically, a combination of inaccessibility and ambiguous ownership claims have kept them from being cut or cleared.
Table 5 Summary of vegetation plots comparing land use by habitat. *p < 0.01, **p < 0.001.

DISCUSSION
Vegetation structure
The watersheds under study have been heavily impacted by agriculture for over two centuries, yet the arboreal vegetation today is structurally complex and diverse across the landscape. Fifty-two of 56 plots (93%) contained at least one tree, but usually trees occurred in dense natural or planted stands. This reflected an abundance of mature and secondary forests, as well as the structural complexity that characterized many agroforest habitats. These findings bode well for conservation and watershed management given the importance of trees and the vegetation structure they provide.
Secondary forests were structurally less well developed than mature forests (Table 1). Findings from elsewhere suggest that post-agriculture second growth forests often achieve important structural, but typically not compositional, similarities with mature tropical forest in 30–40 years (Marcano-Vega et al. Reference Marcano-Vega, Aide and Baez2002; Chazdon Reference Chazdon2003; Grau et al. Reference Grau, Aide, Zimmerman, Thomlinson, Helmer and Zou2003; Toniato & Oliveira-Filho Reference Toniato and de Oliveira-Filho2004; Piotto et al. Reference Piotto, Montagnini, Thomas, Ashton and Oliver2009). Consistent with this, the oldest secondary forest sites assessed in this study (estimated as 20–30 years old) had tree basal area and canopy height measures just below the average measures for mature forest.
Agroforest and secondary forests are similar across a range of structural measures (Table 1), a finding consistent with studies elsewhere (see for example Asase & Tetteh Reference Asase and Tetteh2010). Planted trees dominated the basal area of agroforests (86%), formed a minor component of secondary forests (5%) and were absent altogether from mature forests. This had structural as well as compositional consequences because mean DBH of planted trees across all habitats was twice that of natural trees (20.1 cm versus 11.0 cm; Table 1). In short, there were relatively few planted trees in young size classes. There are two plausible explanations for this. First, in contrast to natural tree populations, where large numbers of young are established only to be thinned-out as the stand matures, tree crops would typically be planted in smaller numbers, but cultivated and thus subject to low rates of mortality. A second explanation is that the agroforests of St Lucia are maturing and, in many cases, not being actively regenerated by their owners. The relatively longer lifespan and lower ongoing maintenance associated with tree crops makes farmers reluctant to either abandon them completely or cut them down to make space for other crops. But, given the challenges facing the agriculture industry, it is not surprising to find agroforests suffering from neglect, including low rates of replanting (personal observation).
Annual crops commonly cultivated in the study areas included banana, plantain, root crops (dasheen, yam and sweet potato), mixed vegetables and livestock pasturage. The few scattered trees that were recorded in such habitats were typically planted and/or protected by farmers who wanted localized shade, windbreak or property boundary demarcation, or they were preserved because of cultural prohibitions against cutting trees on family lands (Walters Reference Walters2012a). The conservation significance of isolated trees in agricultural landscapes has not been well studied (for example Manning et al. Reference Manning, Fischer and Lindenmayer2006). Annual crop habitats are likely at greater risk from erosion because they lack trees, have low shrub and herb cover, and more exposed soil (Table 1), the varied effects of animal grazing and trampling, ploughing, weeding and erosion.
Plant species richness
The 99 natural species identified in this study represent 41% of the total 241 tree species recorded in St Lucia (GOSL 1998). The 26 planted tree species identified are likewise indicative of the high diversity of tree crops cultivated in St Lucia, and this number does not account for the varietal diversity that exists within species like cocoa, mango and citrus (GOSL 1998).
Post-agriculture, secondary forests contained the highest natural and overall tree species richness, both in terms of plot means (Table 1) and cumulative counts (Figs 2–4), although mature forests were a close second. Studies elsewhere have showed that secondary forests can range widely in tree species composition depending on stand age and location, as well as founder effects (Brown & Lugo Reference Brown and Lugo1990; Grau et al. Reference Grau, Aide, Zimmerman, Thomlinson, Helmer and Zou2003; Toniato & Oliveira-Filho Reference Toniato and de Oliveira-Filho2004; Chazdon et al. Reference Chazdon, Letcher, van Breugel, Martinez-Ramos, Bongers and Finegan2007). The relatively higher richness measured in secondary forests in this study in part reflects the diversity of sampled stand ages, from three to 30 years, and microhabitats, which ranged from dry coastal scrub to interior rainforest. By contrast, mature forest plots were concentrated in the higher altitude rainforest zone, the only sites historically spared from agriculture.
Agroforests had far fewer natural tree species than either secondary or mature forests, but their cumulative 25 planted and 19 natural species revealed them to be significant reservoirs of diversity, further supporting the fact that agroforests around the world contribute to floral and faunal diversity (Perfecto et al. Reference Perfecto, Rice, Greenberg and Van der Voort1996; Schroth et al. Reference Schroth, da Fonseca, Harvey, Gascon, Vasconcelos and Izac2004; Faria et al. Reference Faria, Laps, Baumgarten and Cetra2006; Bhagwat et al. Reference Bhagwat, Willis, Birks and Whittaker2008; Cassano et al. Reference Cassano, Schroth, Faria, Delabie and Bede2009; Jose Reference Jose2009; Mendez et al. Reference Mendez, Bacon, Olson, Morris and Shattuck2010).
But generalizations about the habitat similarity and conservation value of agroforest as compared to natural forest need to be made cautiously for three reasons. First, it is unclear whether many forest species can establish within agroforests and, once there, persist independent of replenishment from nearby forest patches (Rolim & Chiarello Reference Rolim and Chiarello2004; Franzen & Mulder Reference Franzen and Mulder2007). At least some of the native trees recorded in agroforests in this study were remnants that had never been cut, while others had more recently colonized. Given we did not analyse the relationships between the new recruits and proximity to seed sources, we cannot speculate on their origins, but this topic clearly warrants further study.
Second, site-specific management practices have a big influence on the structure and biodiversity of agroforests (Schroth et al. Reference Schroth, da Fonseca, Harvey, Gascon, Vasconcelos and Izac2004; Herve & Vidal Reference Herve and Vidal2008). While it was beyond the scope of this study to rigorously evaluate such factors, the effects of selective planting and harvesting, along with practices such as understorey weeding, were apparent and exemplified in the highly varied composition and structure of agroforest stands.
Finally, perennial agroforests may succumb to economic pressures that lead to their clearing and replacement or gradual conversion to more intensive crop production. These kinds of changes have been documented, for example, in cocoa agroforests in West Africa (Ruf Reference Ruf2011) and mixed fruit and timber agroforests in Indonesia (Feintrenie et al. Reference Feintrenie, Schwarze and Levang2010). St Lucian banana farmers, in particular, were known to have converted extensive agroforest habitats to monoculture banana production during the banana boom.
Watershed topography and land use
Agroforestry is widely practised in St Lucia, but especially so within the wider Soufriere area, which has for decades been the island's centre of lime (Citrus aurantifolia), coconut (C. nucifera) and cocoa (Theobroma cacao) production. St Lucia's only remaining factory for copra, the main commercial product of coconut, is in Soufriere, and limes were actively promoted through the mid-20th century by an influential member of the estate farming community in Soufriere.
Most important was cocoa, a tree crop that thrives under the partially-shaded and wind-protected conditions afforded by larger trees. Cocoa became a mainstay of the agricultural estates around Soufriere following the decline of sugar in the early 20th century, although smallholders often grow cocoa as well. Cocoa is usually interplanted with other trees, including tall-statured fruit trees like coconut (C. nucifera), breadfruit (Artocarpus altilis) or mango (Mangifera indica), timber trees like mahogany (Swietenia macrophylla), or broad-canopied ornamental trees like flamboyant (Delonix regia) and immortal (Erythrina fusca). Residual forest trees are also sometimes preserved as shade trees or to protect steep slopes from erosion and landslides. Perennial tree crops are better suited to the rugged terrain that is so commonplace in Soufriere.
The extensive forest habitats in Mamiku watershed reflect the penetration of the Government Forest Reserve into the upper watershed reaches there, plus the prevalence of abandoned former banana lands. Few regions of the island remained untouched by cultivation during the banana boom of the 1960s–1990s, but the rugged less accessible lands on the west side of the island, including Soufriere, often precluded extensive clearing and cultivation of bananas. By contrast, the suitability of land in Mamiku for bananas and the ensuing boom-bust of the industry created a striking multi-decade cycle of deforestation and subsequent afforestation across much of the watershed. Many pre-existing tree crops in Mamiku were likewise cut to clear space for the sun-loving banana.
Yet, agricultural abandonment has not been limited to just former banana lands. Other crops, including coconut and cocoa, have been subject to increasing price pressures and, more generally, the agricultural sector in St Lucia is struggling with an aging farmer population and growing farm labour scarcity (Walters Reference Walters2012a). In combination, these factors have driven declines in cultivation on many farms, but especially geographically marginal ones. In this respect, post-agricultural secondary forests are not restricted to former banana lands, but are common across St Lucia, as well as other islands in the Caribbean (Lugo & Helmer Reference Lugo and Helmer2004; Rudel Reference Rudel2005).
Importantly, this study has shown that mature and recovering forests are not randomly distributed in the watershed landscape. These forests were typically found on lands at higher altitude, on steeper slopes and more distant from roads (Tables 2–4). In light of recent challenges facing the agricultural sector in St Lucia, farmers have downsized production and abandoned cultivation of at least some portion of their lands. In such cases, they selectively abandoned those sites most difficult to farm because of steepness or inaccessibility (Walters Reference Walters2012a). These findings are consistent with others that have documented post-agriculture secondary forests occurring more often on marginal agricultural lands (Rudel et al. Reference Rudel, Bates and Machinguiashi2002, Reference Rudel, Coomes, Moran, Achard, Angelsen, Xu and Lambin2005; Aide & Grau Reference Aide and Grau2004; Lugo & Helmer Reference Lugo and Helmer2004).
St Lucia's recent agricultural decline has created much economic hardship, particularly within the farming sector. But the concomitant return of forests to former agricultural lands is a positive trend and presents strategic opportunities for environmental conservation. For example, government efforts to secure private lands for expansion of the system of forest and watershed protection areas are made easier because land owners will more likely negotiate exchange or sale of lands already abandoned and otherwise deemed marginal for farming. Alternatively, farmers with underused land may be amenable to collaborative initiatives that promote conservation-oriented practices on these properties, mixed-species agroforestry being an obvious model (Geoghegan et al. Reference Geoghegan, Krishnaryan, Pantin and Bass2003). Either way, the opportunity for outcomes positive for all parties is high because lands deemed least valuable for farming because they are too steep or remote are often the most valuable for conservation given their higher risk of soil erosion and relative proximity to existing protected areas.
ACKNOWLEDGEMENTS
Funding for this research was provided by a grant from the Social Sciences and Humanities Research Council of Canada. For assistance with field work, we are grateful to Melvin Smith, Marshall Symons, Jennifer Sargent, Nigel Selig, Frances Ross and Shannon White. Roger Graveson's generous sharing of his knowledge and data base of St Lucian plants was invaluable. For the various ways in which they assisted our efforts, we thank Kai Wulf and the Soufriere Marine Management Authority; Michael Bobb, Michael Andrew and Anias Vernai of the Saint Lucia Forestry Department; and Peter Jackson, Gregor Williams, Chris Alicindor and Yves Renard. Finally, we thank Kevin Flesher, Carolyn Dubois and anonymous reviewers for their helpful comments on earlier drafts of this manuscript, and Christina Tardif for aid with the map.











