Introduction
Worldwide, people living near forests are more food secure than those living farther from them (Rasolofoson et al. Reference Rasolofoson, Hanauer, Pappinen, Fisher and Ricketts2018). Although several mechanisms could explain this, one of the most plausible is that people rely on wildlife for food (Ingram et al. in press) and such direct provision increases the food security of forest-adjacent households. Yet such provision is not without consequences, as wildlife populations are declining (Barnosky et al. Reference Barnosky, Matzke, Tomiya, Wogan, Swartz and Quental2011, WWF 2020), largely due to hunting and habitat destruction (Ripple et al. Reference Ripple, Abernethy, Betts, Chapron, Dirzo and Galetti2016).
In an effort to curb hunting and habitat destruction, conservation practitioners have developed various governance approaches to protecting wildlife and wild places. There has been significant research on the ability of protected areas to reduce the negative impacts of human activity (Geldmann et al. Reference Geldmann, Manica, Burgess, Coad and Balmford2019). Protected areas can take multiple forms, ranging from strict protection to highly decentralized forest management approaches (or community-based forest management; CBFM). In Madagascar, protected areas span the spectrum of management types, and CBFM is widely used (Chatelperron Reference Chatelperron, Montagne, Razanamaharo and Cooke2007).
Throughout Madagascar, many forms of forest and wildlife governance are managed according to guidelines set out by Secured Local Management, locally known as GELOSE (Gestion Locale Sécurisée; Law No 96-025), which outlines decentralized natural resources management and the Management Code for Protected Areas, locally known as COAP (Code de gestion des Aires Protégées; Law No 2001-05/2015-05). Protected areas administered through GELOSE are a type of CBFM. CBFM has had a long history in Madagascar, dating back to the reign of Andrianampoinimerina (1745–1810; Jones et al. in press). CBFM was designed to support orphans, widows and vulnerable people so that they could have access to natural resources to survive (Rahajarizafy Reference Rahajarizafy1954). When Madagascar was colonized by the French in 1896, the colonial administration seized exclusive control of the forests and structured strict regulation of the use of natural resources. In 1996, following decolonization in 1960 and decades of grassroots community organizing to return control of their land and natural resources, the Malagasy government passed GELOSE, allowing local communities to manage their natural resources if certain contractualized agreements between the Ministry of the Environment and Sustainable Development (MEDD) and the local communities were mutually made (Bertrand et al. Reference Bertrand, Horning, Andriankova, Ratsimbarison, Andriatahina, Montagne, Razanamaharo and Cooke2007, Raik Reference Raik2007).
The GELOSE/GCF (Gestion Contractualisée des Forêts; Decree 2001-2002) was launched in 2001 as a contractual agreement between the MEDD and the representatives of COBA (Communauté de Base) or the local community, enabling a legal management transfer of the forest to the local community (Randrianasolo Reference Randrianasolo2000, Montagne Reference Montagne2004). Although intermediaries are not technically required by law, non-governmental organizations (NGOs) are often made part of the process by including their own environmental agenda in addition to that of the communities (Hockley & Andriamarovololona Reference Hockley and Andriamarovololona2007, Pollini & Lassoie Reference Pollini and Lassoie2011, Cullman Reference Cullman2015). Following the signing of a GCF contract, the state retains full ownership of the forest for 3 years and the right to revoke or modify the components of the contract. Following the 3-year period, the contracts can be renewed for a 10-year period if community management during the initial period is found to be satisfactory (Hockley & Andriamarovololona Reference Hockley and Andriamarovololona2007). Typically, under the technical advisory role of the MEDD and the project implementer, the community implements, monitors and evaluates the following activities: zoning for conservation, extraction of natural resources, reforestation and overseeing the implementation of management plans for each of these zones (Raik Reference Raik2007, Raik & Decker Reference Raik and Decker2007).
Recent empirical research has assessed the impact of CBFM on forest cover and broad metrics of human well-being and found that CBFM improves the living standards of the communities living in and around forest ecosystems and somewhat reduces deforestation in Madagascar (Rasolofoson et al. Reference Rasolofoson, Ferraro, Jenkins and Jones2015, Reference Rasolofoson, Ferraro, Ruta, Rasamoelina, Randriankolona, Larsen and Jones2017). Yet the impacts of conservation policy and forest governance on the behaviour of people hunting wildlife still remain under-researched. In Madagascar, wildlife is a critical source of nutrition, serving as a safety net for people living in rural poverty (Reuter et al. Reference Reuter, Randell, Wills and Sewall2016, Borgerson et al. Reference Borgerson, Razafindrapaoly, Rajaona, Rasolofoniaina and Golden2019, Golden et al. Reference Golden, Vaitla, Ravaoliny, Vonona, Anjaranirina and Randriamady2019, Merson et al. Reference Merson, Dollar, Johnson and Macdonald2019). Yet unsustainable hunting, climate change and deforestation are driving wildlife population declines and risks of local extinction (Golden Reference Golden2009, Brook et al. Reference Brook, Herrera, Borgerson, Fuller, Andriamahazoarivosoa and Rasolofoniaina2018, Morelli et al. Reference Morelli, Smith, Mancini, Balko, Borgerson and Dolch2019, Annapragada et al. Reference Annapragada, Brook, Luskin, Rahariniaina, Helin and Razafinarivo2021). While it is presumed that by expanding the global protected area network such population declines can be reduced or mediated, the actual effects of management on longitudinal changes in human behaviour are rarely quantitatively tested. Recognizing the critical role of wildlife to local culture and health and the ongoing threats to wildlife, our analysis seeks to empirically test the potential for conservation to mitigate the damaging effects of human hunting behaviour through conservation policy.
Our research aimed to understand the impact of CBFM conservation policies on wildlife hunting and consumption in the Makira protected area. These policies include: (1) traditional management (TM) with no external assistance implemented in the buffer zones of the park; (2) CBFM or GCF with non-governmental support and externally developed policies adopted in the buffer zones of the park; and (3) strict external management policies in ZOC (Zone d’Occupation Controlée), meaning a zone of controlled residence for communities inside the core protected area. In addition to the governance policies, we also examine the role of public education associated with CBFM (1 year prior to policy implementation and adoption) in influencing natural resource extraction behaviours in local communities. To our knowledge, this study provides the first empirical evidence of the effects CBFM policies on hunting in Madagascar.
Methods
Study site
Makira Natural Park (Makira) is the largest remaining contiguous rainforest (374 083 ha) in Madagascar (Fig. 1). The area is characterized by a dense, moist evergreen forest with a humid climate. The average daily temperature ranges between 16.5°C and 25.4°C and precipitation averages 2291 mm of rainfall each year (Goodman et al. Reference Goodman, Raherilalao and Wohlhausser2020). All endemic carnivorans in the north-east are found in Makira, and it has the highest diversity of lemurs (Goodman et al. Reference Goodman, Raherilalao and Wohlhausser2020). There are two primary ethnolinguistic groups living in the area – Betsimisaraka and Tsimihety (Golden et al. Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014) – and communities are primarily agriculturalists focused on the production of rice, vanilla and cloves (Cullman Reference Cullman2015). Most hunting in Madagascar is driven by subsistence consumption needs (Brashares et al. Reference Brashares, Golden, Weinbaum, Barrett and Okello2011, Golden et al. in press), and local communities in this region are heavily reliant on wildlife for nutrition and livelihoods (Golden et al. Reference Golden, Fernald, Brashares, Rasolofoniaina and Kremen2011, Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014, Reference Golden, Vaitla, Ravaoliny, Vonona, Anjaranirina and Randriamady2019). Unsustainable hunting in this region is a major threat to the conservation of lemurs (Brook et al. Reference Brook, Herrera, Borgerson, Fuller, Andriamahazoarivosoa and Rasolofoniaina2018), carnivorans (Farris et al. Reference Farris, Golden, Karpanty, Murphy, Stauffer and Ratelolahy2015), birds (Murphy et al. Reference Murphy, Farris, Karpanty, Kelly, Miles and Ratelolahy2017) and tenrecs (Annapragada et al. Reference Annapragada, Brook, Luskin, Rahariniaina, Helin and Razafinarivo2021).

Fig. 1. Makira Natural Park and study sites. GCF = Gestion Contractualisée des Forêts; N = north; TM = Traditional Management; ZOC = Zone d’Occupation Controlée.
Policy options
Although the region that would become the Makira protected area was state-owned, local people had managed this land without external influence for millennia. In 2005, the Wildlife Conservation Society (WCS), in coordination with the MEDD, began a process of decentralizing governance and developing the co-management of Makira. Although local people generally felt as though their land was being taken from them (Cullman Reference Cullman2015), the co-management process was designed, in theory, to offer both de facto and de jure ownership and management rights of the land (Urech et al. Reference Urech, Rabenilalana, Sorg, Felber, Colfer and Pfund2010). In the Makira region, forests are now managed under three distinct policies: GCF, ZOC and TM without external assistance.
GCF policy is characterized by: (1) a 3-year contract that formally gives forest resource management authority to the COBA; (2) a list of dina or local conventions that identifies all of the rules pertaining to forest resource management and corresponding penalties in case of infraction; (3) a contractual conditions list that details all allowable resource extraction practices within the GCF site; and (4) a site development plan that defines zonation and practices inside each zone within a GCF site. All four components are agreed upon and signed by government authorities as well as by the community.
ZOCs include those communities within the boundaries of a protected area and are unique from GCFs in two primary ways: (1) they have stricter rules regulating natural resource use; and (2) no immigration is allowed, effectively limiting population growth and the intergenerational future of the community. Activities in ZOCs are limited and controlled by the MEDD to minimize infractions within the protected area. For example, within a ZOC, no commercial logging is allowed, but subsistence-related extraction is permitted. ZOC communities are the most affected by the creation of the Makira protected area (MEDD et al. 2013). Therefore, ZOCs are more similar to models of centralized and restrictive management, and extraction rules are stricter in ZOCs compared to GCFs.
TM is characterized by the administration of state-owned forests by a local community. Its management, though traditional, is regulated by the Forest Law (Law No 1997-17); natural forest resource extraction such as of timber products needs authorization from MEDD local administration. However, local communities are entitled to exercise their customary resource rights. Forests that are traditionally managed are held by kin groups who have been using the state-owned forests for many generations. Thus, they are de facto owners of the forests (Urech et al. Reference Urech, Rabenilalana, Sorg, Felber, Colfer and Pfund2010, Cullman Reference Cullman2015).
Communities were offered the opportunity for co-management after resource inventories were conducted by the WCS and the MEDD. If contractually accepted, they began the process of becoming a GCF community. If the contract was not accepted, they maintained TM practices. Communities living within the boundary of the protected area did not have to negotiate these policies and were mandated the ZOC policy.
Natural resource extraction data
In this study, we collected longitudinal data from 2004 to 2012 on household-level natural resource extraction in 36 communities (1260 households) near and within the Makira (Fig. 1; Golden et al. Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014, Reference Golden, Anjaranirina, Fernald, Hartl, Kremen and Milner2017). This sample included 11 GCF communities (GCFs), 5 ZOC communities (ZOCs) and 20 TM communities (TMs) with no external policy influence. Beginning in 2004, annual household surveys were conducted to understand household interactions with the forest and to estimate the volume and frequency of natural resource extraction (Golden Reference Golden2009, Golden et al. Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014, Reference Golden, Anjaranirina, Fernald, Hartl, Kremen and Milner2017). Surveyed individuals self-classified themselves as predominantly Betsimisaraka (52%) or Tsimihety (27%), and the majority of households (85%) had a male head. Only 36% of the household heads were born in the community where they currently reside. Farming was the dominant occupation (84%), with most farmers cultivating rice, tubers, fruits and cash crops such as vanilla, cloves and coffee (Golden et al. Reference Golden, Anjaranirina, Fernald, Hartl, Kremen and Milner2017).
In these surveys, we asked the heads of household about the number of various wildlife species consumed across 23 different species. We grouped these animals into five taxonomic categories (all endemic except as noted): (1) bushpigs (Potamochoerus larvatus (introduced)), (2) tenrecs (Setifer setosus, Tenrec ecaudatus), (3) carnivorans (Cryptoprocta ferox, Eupleres goudotii, Felis catus, Fossa fossana, Galidia elegans, Viverricula indica (introduced)), (4) bats (Miniopterus spp., Pteropus rufus, Rousettus madagascariensis) and (5) lemurs (Daubentonia madagascariensis, Eulemur albifrons, Eulemur rubriventer, Hapalemur occidentalis, Indri indri, Lepilemur seali, Microcebus spp., Phaner cf. furcifer, Propithecus candidus, Varecia variegata).
Hunters use various methods to harvest wildlife in Makira (Golden Reference Golden2009). The surveys explicitly asked whether wildlife was obtained through (1) active hunting with the use of slingshots, machetes and spears, (2) passive hunting with the use of snares and traps, (3) opportunistic hunting, (4) purchase, (5) gifting or (6) consumption at a friend’s house.
Empirical strategy
To evaluate the effectiveness of the GCF and ZOC policies, we used a difference-in-differences approach, comparing the changes in outcomes (i.e., hunting harvest and consumption) in the GCF and ZOC communities before and after the policy adoption to the change in outcomes in the TM communities, which serve as our control. First, we estimated a baseline difference-in-differences model that estimated the basic treatment effects of the GCF and ZOC policies, which can be specified as a two-way fixed-effect linear regression model:
where
![]() ${Y_{ict}}$
is the number of animals consumed and hunted by household i in community c in year t. In other words,
${Y_{ict}}$
is the number of animals consumed and hunted by household i in community c in year t. In other words,
![]() ${Y_{ict}}$
is the sum of animals consumed and hunted obtained from different types of methods such as actively hunted, passively hunted, opportunistically hunted, purchased, received as a gift and consumed at a friend’s house. Approximately 98% of wildlife consumed was hunted by the household and 2% was purchased primarily in household-to-household transactions (Golden et al. Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014). The outcomes include both overall numbers and numbers by taxonomic categories and hunting methods.
${Y_{ict}}$
is the sum of animals consumed and hunted obtained from different types of methods such as actively hunted, passively hunted, opportunistically hunted, purchased, received as a gift and consumed at a friend’s house. Approximately 98% of wildlife consumed was hunted by the household and 2% was purchased primarily in household-to-household transactions (Golden et al. Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014). The outcomes include both overall numbers and numbers by taxonomic categories and hunting methods.
![]() $GC{F_{ct}}$
is a dummy variable that equals 1 if community c is a community-based management system under the GCF contract in year t.
$GC{F_{ct}}$
is a dummy variable that equals 1 if community c is a community-based management system under the GCF contract in year t.
![]() $ZO{C_{ct}}$
is a dummy variable that equals 1 if community c has a ZOC status in year t.
$ZO{C_{ct}}$
is a dummy variable that equals 1 if community c has a ZOC status in year t.
![]() ${X_{ict}}$
represents the household-level covariates, including the household head’s age, level of education, gender, place of birth and tribe. The community fixed effect
${X_{ict}}$
represents the household-level covariates, including the household head’s age, level of education, gender, place of birth and tribe. The community fixed effect
![]() ${\omega _c}$
controls for time-invariant characteristics and is common to all households in the same community. The time fixed effect
${\omega _c}$
controls for time-invariant characteristics and is common to all households in the same community. The time fixed effect
![]() ${\sigma _t}$
controls for factors that are common to households surveyed in a given year, and
${\sigma _t}$
controls for factors that are common to households surveyed in a given year, and
![]() ${\varepsilon _{ict}}$
is the error term. The coefficients on
${\varepsilon _{ict}}$
is the error term. The coefficients on
![]() $GC{F_{ct}}$
and
$GC{F_{ct}}$
and
![]() $ZO{C_{ct}}$
(
$ZO{C_{ct}}$
(
![]() ${\delta _1}$
and
${\delta _1}$
and
![]() ${\delta _2}$
, respectively) are the estimates of the impacts of the GCF and ZOC policies. This model is intended to provide consistent estimates as long as there are no other concurrent shocks such as other policies or climate events that happened at the same time only in GCFs and ZOCs, but not in TMs, which affect wildlife hunting and consumption.
${\delta _2}$
, respectively) are the estimates of the impacts of the GCF and ZOC policies. This model is intended to provide consistent estimates as long as there are no other concurrent shocks such as other policies or climate events that happened at the same time only in GCFs and ZOCs, but not in TMs, which affect wildlife hunting and consumption.
In addition to the basic treatment effects, we explored whether the GCF and ZOC policies had an anticipatory effect – having an impact 1 year before policy adoption with the public education campaigns. Anticipatory effects may exist when individuals are forward-looking, get access to information on future treatments and there is a benefit to taking actions ahead of a treatment (Malani & Reif Reference Malani and Reif2015). Following Hockley and Andriamarovololona (Reference Hockley and Andriamarovololona2007), a public education campaign is a combination of education and training on CBFM in addition to a persuasive approach to the community regarding the importance of CBFM’s worth. Public education messages included: (1) the necessity of signing a contract in order not to lose access to the forest and to protect the forest; (2) the promise of a development project when becoming a COBA; and (3) the source of revenue generated by the forest use. Failing to account for anticipatory behaviour could bias the real impact of a policy (Malani & Reif Reference Malani and Reif2015, Alpert Reference Alpert2016). This is particularly relevant for the GCF and ZOC policies, as 1 year before the adoption the MEDD and the project implementer would conduct public education campaigns in the implementing communities to raise awareness regarding the benefits of adopting the GCF or ZOC policies. Usually, this process takes 6–8 months before the community formally requests to become a COBA (Blanc-Pamard & Ramiarantsoa Reference Blanc-Pamard and Ramiarantsoa2008). The anticipatory effect due to public education campaigns is an empirical question. Households may reduce their wildlife hunting and consumption ahead of the policy adoption because they were persuaded by the public education campaigns. Alternatively, households may increase their wildlife hunting and consumption 1 year prior to the policy adoption in anticipation of a future ban.
We estimated the anticipatory effects using the following difference-in-differences equation that includes public education and implementation as separate indicator variables:
where
![]() $Public\,Educatio{n_{ct}}$
is a dummy variable that equals 1 if public education campaigns on the GCF or ZOC policies were made in community c in year t – that is, at least 1 year before the policy adoption in GCFs or ZOCs. Thus, the anticipatory effects are measured by the coefficients on the interaction terms (
$Public\,Educatio{n_{ct}}$
is a dummy variable that equals 1 if public education campaigns on the GCF or ZOC policies were made in community c in year t – that is, at least 1 year before the policy adoption in GCFs or ZOCs. Thus, the anticipatory effects are measured by the coefficients on the interaction terms (
![]() ${\alpha _1}$
and
${\alpha _1}$
and
![]() ${\alpha _2}$
). We used RStudio 1.3.959 (RStudio Team 2020) for the statistical analysis.
${\alpha _2}$
). We used RStudio 1.3.959 (RStudio Team 2020) for the statistical analysis.
Validity of the empirical strategy
The key identifying assumption in our models is that the change in wildlife hunting and consumption in control communities is an unbiased estimate of the changes in the GCF and ZOC communities had they not been treated. While this cannot be directly tested, we can test whether the control and treatment communities had parallel trends in wildlife hunting and consumption in pre-intervention periods. We used a logarithmic weighted average to represent the trend (Fig. 2) of the average annual household wildlife hunting and consumption from 2004 to 2012. The GCF and ZOC policies were adopted in different years depending on the community. The GCF policy started being implemented between 2004 and 2008, with each community having its own timeline. All of the communities in our sample became ZOCs in 2007. While the policy start year varied across communities, there was a parallel trend between ZOCs, GCFs and TMs before 2007 (Fig. 2), which was when most communities adopted the policy.
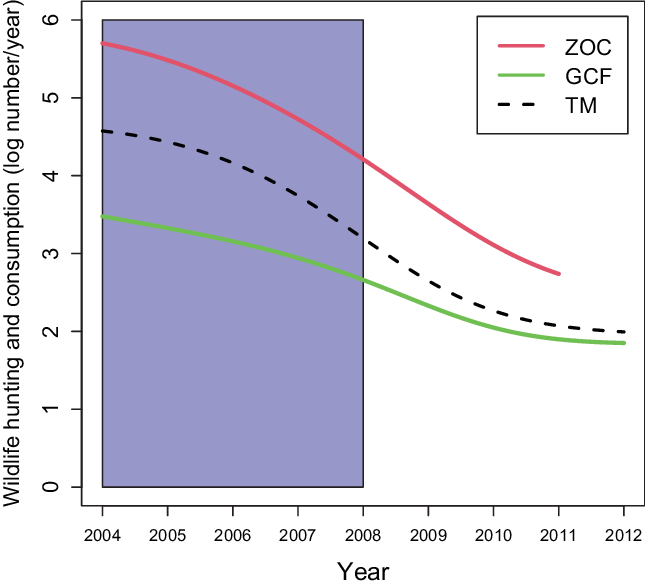
Fig. 2. Trend of household annual average wildlife hunting and consumption. The blue rectangular shaded area, from 2004 to 2008, represents the timeline when GCF and ZOC policies were adopted. GCF = Gestion Contractualisée des Forêts; TM = Traditional Management; ZOC = Zone d’Occupation Controlée.
Results
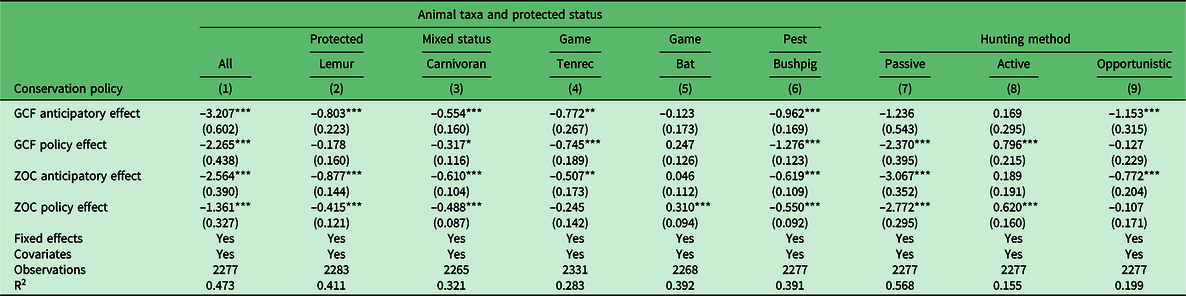
Overall, we found that the GCF and ZOC policies often decreased wildlife hunting and consumption, though the direction and magnitude of the impact varied by policy and type of wildlife (Table 1). For overall wildlife hunting and consumption (column 1 of Table 1), the treatment effect was only significant for GCFs, where reported hunting and consumption decreased by 126.9%. Specific policy impacts on lemur hunting and consumption did not have discernible trends and were not statistically significant. Both the GCF and ZOC policies led to reduced carnivoran, bushpig and tenrec hunting and consumption, with both policies significantly reducing bushpig hunting and consumption (–98.1% in GCFs and –30.6% in ZOCs). Only the impact of GCFs significantly reduced the hunting and consumption of tenrecs (–50.8%), while the impact of ZOCs significantly reduced the hunting and consumption of carnivorans (–24.8%). Both the GCF (28.2%) and ZOC (29.3%) policies led to significant increases in bat hunting and consumption.
Table 1. The effects of Gestion Contractualisée des Forêts (GCF) and Zone d’Occupation Controlée (ZOC) policies on the amount of wildlife hunted and consumed (log) by taxa and by hunting method (Makira Natural Park, 2004–2012). The effects of the GCF and ZOC policies on wildlife hunting and consumption are organized by the overall effects of the policies for all animals (column 1), by taxa (columns 2–6) and by hunting method (columns 7–9). We adjusted our standard significance level (*p < 0.10, **p < 0.05, ***p < 0.01) with a Bonferroni correction: *p < 0.011, **p < 0.006, ***p < 0.001. The numbers in the parentheses represent the standard error. Covariates are gender, age, born in the community, occupation, level of education and ethnicity. It is important to note that each species has a different protection status based on the Malagasy hunting regulations, which might slightly differ from the International Union for Conservation of Nature (IUCN) status; for instance, Viverricula indica is a pest, though we grouped it with other carnivorans having a protected status (Decree No 2006-400).

We found a large and significant decrease in hunting via passive hunting methods (e.g., traps and snares) in both GCFs (–193.6%) and ZOCs (–157.2%) and an increase in hunting via active hunting methods (e.g., slingshots, machetes, spears, etc.) in both GCFs (74.2%) and ZOCs (54.5%). Opportunistic hunting also appeared to increase, though not significantly.
We found large, significant declines in wildlife hunting and consumption relative to the control group after public education campaigns in GCFs and ZOCs (Table 2), highlighting the anticipatory effects of policy implementation driven by public education campaigns related to conservation. These conservation education campaigns prior to policy adoption and implementation led to a 320.7% decrease in wildlife hunting and consumption in GCFs and a 256.4% decrease in ZOCs. These large declines indicate the presence of strong anticipatory effects.
Table 2. The effects of Gestion Contractualisée des Forêts (GCF) and Zone d’Occupation Controlée (ZOC) policies on the amount of wildlife hunted and consumed (log) by taxa and by hunting method with anticipatory effects (Makira Natural Park, 2004–2012). The effects of the GCF and ZOC policies on wildlife hunting and consumption are organized by the overall effects of the policies for all animals (column 1), by taxa (columns 2–6) and by hunting method (columns 7–9). We adjusted our standard significance level (*p < 0.10, **p < 0.05, ***p < 0.01) with a Bonferroni correction: *p < 0.011, **p < 0.006, ***p < 0.001. The numbers in the parentheses represent the standard error. Covariates are gender, age, born in the community, occupation, level of education and ethnicity. It is important to note that each species has a different protection status based on the Malagasy hunting regulations, which might slightly differ from the International Union for Conservation of Nature (IUCN) status; for instance, Viverricula indica is a pest, though we grouped it with other carnivorans having a protected status (Decree No 2006-400).
When accounting for anticipatory effects, we found considerable changes in policy impacts, suggesting a treatment effect bias when ignoring anticipatory effects. Thus, integrating the anticipatory effects into our model dramatically increased the magnitude of the policy impact (column 1 of Table 2) compared to the baseline model (column 1 of Table 1). When assuming no anticipatory effects, we found declines in overall wildlife hunting and consumption of 126.9% and 34.8% in GCFs and ZOCs, respectively, with only the former being statistically significant (column 1 of Table 2). However, after adding the anticipatory effects, we found greater declines in overall wildlife hunting and consumption of 226.5% and 136.1% for GCFs and ZOCs, respectively, with both being statistically significant (p < 0.001; Table 2). The stronger impact of the GCF and ZOC policies in reducing wildlife hunting and consumption when accounting for anticipatory effects is consistent across different wildlife categories, except for bat hunting and consumption. Thus, while we observed some implementation effects of the GCF and ZOC policies on wildlife and hunting consumption in the baseline model, accounting for the anticipatory effect almost doubled the magnitude of the impact for GCFs and more than tripled the impact for ZOCs. This suggests that omitting anticipatory effects would provide a biased estimate of the GCF and ZOC policies, underestimating the magnitude of the effects.
Across different hunting methods, we also found a stronger treatment effect on most hunting methods when accounting for anticipatory effects. We found a decline in passive hunting techniques after implementation in both GCFs and ZOCs (column 7 of Table 2), with only ZOCs experiencing impacts (–306.7%) deterring passive hunting through anticipatory effects (Table 2). However, active hunting (column 8 of Table 2) techniques (although lower in volume than passive hunting) significantly increased, even after accounting for anticipatory effects. We also found that the GCF and ZOC policies were associated with reduced opportunistic hunting after accounting for anticipatory effects (column 9 of Table 2), with significant reductions 1 year before adoption in both GCFs (–115.3%) and ZOCs (–77.2%).
Discussion
Conservation policy treatment effects in both GCFs and ZOCs led to a general decrease of wildlife consumption in Makira. By analysing trends in hunting techniques for obtaining wildlife for consumption, we found that passive hunting decreased and active and opportunistic hunting increased in response to conservation policies. As the vast majority of hunting in the region uses passive techniques (Golden et al. Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014), this led to an overall decrease in consumption of wildlife among GCF and ZOC communities in line with policy objectives. Yet this may also illustrate behaviour modification to escape policy repercussions; by reducing passive hunting and increasing active and opportunistic hunting, local people are shifting away from more visible hunting activities (e.g., clearing forest areas and installing traps and snares). This type of behaviour has also been observed in Tsitongabarika Protected Area, a rainforest similar to Makira in south-east Madagascar where the dominant ethnolingustic group is Antanosy (Campera et al. Reference Campera, Phelps, Besnard, Balestri, Eppley, Nijman and Donati2017), and this could explain the trends we observed.
By examining the results according to taxonomic categories, we found that, in Makira, GCF and ZOC communities seem to have understood the conservation rules (Ratsimbazafy et al. Reference Ratsimbazafy, Harada and Yamamura2012) – catching less of protected species and more of species that are legal to hunt. These impacts are encouraging as declines in lemur and carnivoran hunting are needed because both taxa are unsustainably hunted in the region (Farris et al. Reference Farris, Golden, Karpanty, Murphy, Stauffer and Ratelolahy2015, Brook et al. Reference Brook, Ranaivoson, Broder, Cunningham, Héraud and Peel2019b). As bats could be legally hunted in a specific season of a year, local people may be increasing bat consumption to buffer against losses in protected wildlife consumption; for instance, one needs to eat roughly four adult Pteropus rufus (540 g) to get the same amount of protein as from one adult Eulemur albifrons (2000 g). This trend may not be sustainable for the long term and may also expose communities to potential zoonotic diseases (Brook et al. Reference Brook, Ranaivoson, Andriafidison, Ralisata, Razafimanahaka and Héraud2019a, Reference Brook, Ranaivoson, Broder, Cunningham, Héraud and Peel2019b), leading to a potentially unexpected consequence of conservation policy.
We found strong anticipatory effects of public conservation education campaigns in both GCFs and ZOCs 1 year prior to policy enactment. These anticipatory effects were in the same direction as the treatment, indicating that both education and policy work together to reduce hunting behaviours. This result also highlights the importance of environmental education in conservation efforts (Schüßler et al. Reference Schüßler, Richter and Mantilla-Contreras2019), as we find that public education 1 year before adoption was largely effective at decreasing wildlife hunting and consumption. This suggests a potential underestimation of the policy treatment effect if anticipatory effects are not taken into account, especially for conservation policies that have a public education component prior to implementation.
Yet there are several alternative explanations for the results that we found. The presence of strong anticipatory effects could highlight that these public conservation education campaigns may create fear of wrongdoing, acting as instruments of coercion rather than empowering local communities (Hockley & Andriamarovololona Reference Hockley and Andriamarovololona2007, Ratsimbazafy et al. Reference Ratsimbazafy, Harada and Yamamura2012, Cullman Reference Cullman2015, Jones et al. in press). If this is the case, it is possible that our estimates are susceptible to social desirability bias, with local people in GCFs or ZOCs lying during their interviews and overstating their reliance on unprotected species rather than protected species. However, this is not supported by the increase in active hunting, as we would expect participants to report no hunting activities if fear was truly the motivator. It seems most likely that hunters knew which species could be legally hunted and shifted their behaviours to (1) reduce overall hunting activity, (2) reduce the visibility of illegal hunting and (3) increase reliance on legal hunting in accordance with the Malagasy hunting regulations that allow certain species (e.g., bats, tenrecs and carnivorans) to be hunted in a specific period of the year (Decree No 2006-400).
In addition to education and conservation, there are other confounding factors that may change either the availability of wildlife (e.g., deforestation, climate change, etc.) or the behaviour of hunters (e.g., transitions in income or greater understanding of disease risk). For instance, recent outbreaks of Ebola and COVID-19 have reshaped global narratives about the risk of wildlife hunting and consumption and have led to reductions in hunting efforts in certain parts of the world (Akem & Pemunta Reference Akem and Pemunta2020). These broad regional factors (whether macro-scale environmental processes or behavioural impacts) were not accounted for in our modelling approach, as they are more likely to affect all communities consistently rather than produce targeted impacts in only GCFs and ZOCs. Therefore, our modelling approach would not be influenced by these types of dynamics.
The strong anticipatory effects and muted impacts of the actual policy implementation demonstrate that the legal infrastructure of conservation must be paired with education, monitoring, enforcement and development incentives to curb local people’s appetite for wildlife. Increasing the number of patrols and having an independent committee external to the community could reduce wildlife hunting and consumption. Indeed, the local enforcement committee might face a conflict of interest, whereby the enforcement of dina or local conventions may jeopardize the welfare of their kin and neighbours (Campera et al. Reference Campera, Phelps, Besnard, Balestri, Eppley, Nijman and Donati2017, Reuter et al. Reference Reuter, Sewall and Di Minin2017). These efforts could be paired with development interventions, such as poultry vaccination programmes (Annapragada et al. Reference Annapragada, Borgerson, Iams, Ando, Crawford and Helin2019) or insect farming (Filou Reference Filou2019), which have been shown to be successful in the region. If these programs were appropriately scaled, they could simultaneously provide much-needed nutrition in the region and limit pressure on local wildlife, creating synergies with the goals of decentralized conservation.
Acknowledgements
We would like to thank the entire Madagascar Health and Environmental Research (MAHERY) team that contributed to the data collection from 2004 to 2012 and all of the communities that have participated in the survey. We would like also to thank an anonymous Wildlife Conservation Society staff member for describing the process of community-based forest management in Makira. We are grateful to Tohera Iarinaly Razafitsiarovana from Catholic Relief Services Madagascar for helping us produce the Makira Natural Park map.
Financial support
We acknowledge the National Geographic Society Conservation Trust (grant C135-08), the Margot Marsh Biodiversity Fund (grant 023815) and the National Science Foundation (Doctoral Dissertation Improvement grant 1011714 and Coupled Human Natural Systems grant NSF-GEO1115057).
Conflict of interest
None.
Ethical standards
This study was conducted according to the guidelines of the Declaration of Helsinki and all procedures involving human subjects were approved by the Harvard T.H. Chan School of Public Health Institutional Review Board (protocol #22826) and the UC Berkeley Institutional Review Board (protocol #2010-01-608). Verbal informed consent was obtained from all subjects. Verbal consent was witnessed and formally recorded.






