Arthropods are the most diverse group of animals in terms of species number, as well as, together with molluscs and annelids, body plan disparity. Zhang (Reference Zhang and Zhang2011) reported 1,214,295 extant species, comprising 1,023,559 hexapods, 111,937 chelicerates (arachnids and horseshoe crabs), 66,914 crustaceans (all pancrustacean lineages excluding hexapods) and 11,885 myriapods. Although the total number of fossil arthropods is impossible to estimate, they have probably been a major component of the Earth's fauna since the Cambrian (541–485.4 Ma), in which they diversified greatly (e.g. Edgecombe & Legg Reference Edgecombe and Legg2014; Lee et al. Reference Lee, Soubrier and Edgecombe2013). Arthropods are also the first animals to venture on land, by at least the Silurian (443.8–419.2 Ma) with myriapods and arachnids (acarids, spiders and scorpions among others; see Dunlop et al. Reference Dunlop, Scholtz, Selden, Minelli, Boxshall and Fusco2013 for a review; Waddington et al. Reference Waddington, Rudkin and Dunlop2015).
But terrestrial arthropods may have existed earlier, as suggested by trackways in coastal dune strata from the Middle Cambrian of New York (c.500 Ma; Hagadorn et al. Reference Hagadorn, Collette and Belt2011) and the Late Cambrian–Early Ordovician of Ontario (MacNaughton et al. Reference MacNaughton, Cole, Dalrymple, Braddy, Briggs and Lukie2002; Braddy Reference Braddy2004; Collette & Hagadorn Reference Collette and Hagadorn2010; Collette et al. Reference Collette, Gass and Hagadorn2012), and by molecular time trees (Rota-Stabelli et al. Reference Rota-Stabelli, Daley and Pisani2013). The earliest hexapods are documented from the Early Devonian Rhynie locality (Scotland, c.410 Ma), with the springtail (Collembola) Rhyniella praecursor Hirst & Maulik, Reference Hirst and Maulik1926 (Scourfield Reference Scourfield1940), the possible bristletail (Zygentoma or Archaeognatha) Leverhulmia maria Anderson & Trewin, Reference Anderson and Trewin2003 (Fayers & Trewin Reference Fayers and Trewin2005) and Rhyniognatha hirsti Tillyard, Reference Tillyard1928 interpreted as the oldest true insect (Engel & Grimaldi Reference Engel and Grimaldi2004). Modern phylogenies (e.g., Regier et al. Reference Regier, Shultz, Zwick, Hussey, Ball, Wetzer, Martin and Cunningham2010) suggest that all these land-living arthropod clades evolved independently, and at least seven separate terrestrialisation events may have occurred within arthropods: in myriapods, arachnids, and at least five times in pancrustaceans. Amongst the latter, hexapods and at least four groups of crustaceans (namely isopods, amphipods, ostracods and decapods), colonised terrestrial environments independently (Dunlop et al. Reference Dunlop, Scholtz, Selden, Minelli, Boxshall and Fusco2013).
Unfortunately, the morphological evolution of these lineages in the Late Silurian to the Late Devonian is very poorly known, because the fossil record for early continental arthropods is extremely scarce; mainly due to the very few continental sedimentary localities at this time. The complex and diverse continental ecosystem from the Late Devonian of Strud, which yields an abundant and diverse flora (including early cupulate seed plants (Prestianni et al. Reference Prestianni, Steel, Thorez, Gerrienne, Steemans and Javaux2007), vertebrate fauna including early tetrapods (Clément et al. Reference Clément, Ahlberg, Blieck, Blom, Clack, Poty, Thorez and Janvier2004; Blieck et al. Reference Blieck, Clément, Blom, Lelièvre, Luksevics, Streel, Thorez, Young, Becker and Kirchgasser2007, Reference Blieck, Clément, Streel, Vecoli, Clément and Meyer-Berthaud2010), various placoderms (Olive Reference Olive2015; Olive et al. Reference Olive, Clément, Daeschler and Dupret2015a), actinopterygian, acanthodian and sarcopterygian fishes (Clément & Boisvert Reference Clément and Boisvert2006), and well-preserved aquatic crustacean fauna), appears as a unique snapshot of the terrestrialisation processes. Here, we review and update this crustacean fauna. This article also describes a newly discovered species of spinicaudatan.
Institutional abbreviations. IRSNB, Royal Institute of Natural Sciences, Brussels, Belgium; MNHN, Muséum national d'Histoire naturelle, Paris, France.
1. Geological setting
1.1. History and depositional environments
Strud is a village in the Belgian municipality of Gesves (Wallon Region, Namur Province). The outcrop that yields the fossils (50°26′, 43″N, 5°03′, 24″E) results from the extraction of sandstone layers for local building stone during the second half of the 19th Century, and corresponds today to a small quarry of a few dozen square metres exposing oblique and vertical deposits. Since the first paleontological investigation by the palaeobotanist Hock in 1878, who mentioned the finding of well-preserved Rhacophyton condrusorum Crépin, Reference Crépin1875 (Hock Reference Hock1879), this quarry has yielded various other fossil remains of plants (Stockmans Reference Stockmans1948) as well as ‘fishes' (Lohest Reference Lohest1888; Leriche Reference Leriche1931) from the Late Devonian. Amongst this vertebrate material, Clément et al. (Reference Clément, Ahlberg, Blieck, Blom, Clack, Poty, Thorez and Janvier2004) recognised a tetrapod ichthyostegid jaw fragment, misidentified as a fish by Lohest. Clément and collaborators then started looking for this new early tetrapod-bearing locality, in order to find additional material. The quarry was finally rediscovered in October 2004, after having been abandoned for over a century (Clément & Boisvert Reference Clément and Boisvert2006).
The fossil-bearing quarry of Strud is only the younger part of a longer section, beginning in the Lower Famennian greyish to greenish shales with one or more layers of oolitic ironstone deposited as tempestites in a muddy shallow marine environment. The beds yielding the fossils are precisely located in the upper part of the overturned section, dipping at 80° to the south. The depositional environment is characterised by a 1.4 m-thick fining-upward channel-fill that preserves distinct but synchronous, continental fossil assemblages (Fig. 1). The succession begins with yellow to brown arkosic sandstones, deposited in a flood channel, that yielded isolated remains of vertebrates, including early tetrapods (Clément et al. Reference Clément, Ahlberg, Blieck, Blom, Clack, Poty, Thorez and Janvier2004; Clément & Boisvert Reference Clément and Boisvert2006; Olive Reference Olive2015; Olive et al. Reference Olive, Clément, Daeschler and Dupret2015a, Reference Olive, Ahlberg, Pernègre, Poty, Steurbaut and Clément2016), and plant macrofossils (Prestianni et al. Reference Prestianni, Steel, Thorez, Gerrienne, Steemans and Javaux2007). Arthropods have been found in fine shales corresponding to the last channel-fill phases. Complete eumalacostracans (Gueriau et al. Reference Gueriau, Charbonnier and Clément2014a, Reference Gueriau, Charbonnier and Clémentb), a putative insect (Garrouste et al. Reference Garrouste, Clément, Nel, Engel, Grandcolas, D'Haese, Lagebro, Denayer, Gueriau, Lafaite, Olive, Prestianni and Nel2012, Reference Garrouste, Clément, Nel, Engel, Grandcolas, D'Haese, Lagebro, Denayer, Gueriau, Lafaite, Olive, Prestianni and Nel2013; Hörnschemeyer et al. Reference Hörnschemeyer, Haug, Béthoux, Beutel, Charbonnier, Hegna, Koch, Rust, Wedmann, Bradler and Willmann2013), eurypterid remains and plant microfossils, including the first seeds (Prestianni et al. Reference Prestianni, Steel, Thorez, Gerrienne, Steemans and Javaux2007), are found in black to green shales, indicating a low energy, restricted floodplain habitat. Well-preserved branchiopods (notostracans, spinicaudatans and anostracans; Lagebro et al. Reference Lagebro, Gueriau, Hegna, Rabet, Butler and Budd2015; Gueriau et al. Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016) were recovered, together with plant microfossils from small (a few tens of centimetres in width) lenses of fine, dark grey shale lying on a dark siltstone exhibiting millimetre-thick sandy laminae, interpreted as fresh to brackish, shallow-water pool deposits which periodically dried out.

Figure 1 Geography, palaeogeography (modified from the Late Famennian to early Viséan (359–338 Ma) (map from Golonka Reference Golonka2000) and stratigraphy of the Strud channel-filling deposits, showing their main fossiliferous content (Late Devonian, Belgium). Modified from Gueriau et al. Reference Gueriau, Charbonnier and Clément2014a and Denayer et al. Reference Denayer, Prestianni, Gueriau, Olive and Clément2016.
Denayer et al. (Reference Denayer, Prestianni, Gueriau, Olive and Clément2016) precisely described the stratigraphy and palaeoenvironments of the locality. According to regional stratigraphic correlations, these deposits belong to the Upper Famennian Bois des Mouches Formation. A miospore assemblage (VCo Oppel Rugospora radiata interval biozone) confirms a late Famennian age (Denayer et al. Reference Denayer, Prestianni, Gueriau, Olive and Clément2016).
1.2. Taphonomy of the well-preserved arthropods
The preservation of soft-bodied or lightly skeletised organisms such as arthropods in the fossil record requires exceptional physical and chemical conditions (Seilacher et al. Reference Seilacher, Reif and Westphal1985). The occurrence of well-preserved crustaceans (eumalacostracans, spinicaudatans, notostracans and anostracans) in the Upper Famennian sedimentary rocks of the Strud locality is thus remarkable and attests to exceptional depositional and taphonomic processes. They are preserved as a pale to dark brown carbonaceous film consisting of “shadows” of organic origin and tending to flake-off in patches, as revealed by scanning electron microscopy and energy-dispersive X-ray (SEM-EDX) spectroscopy (Fig. 2).
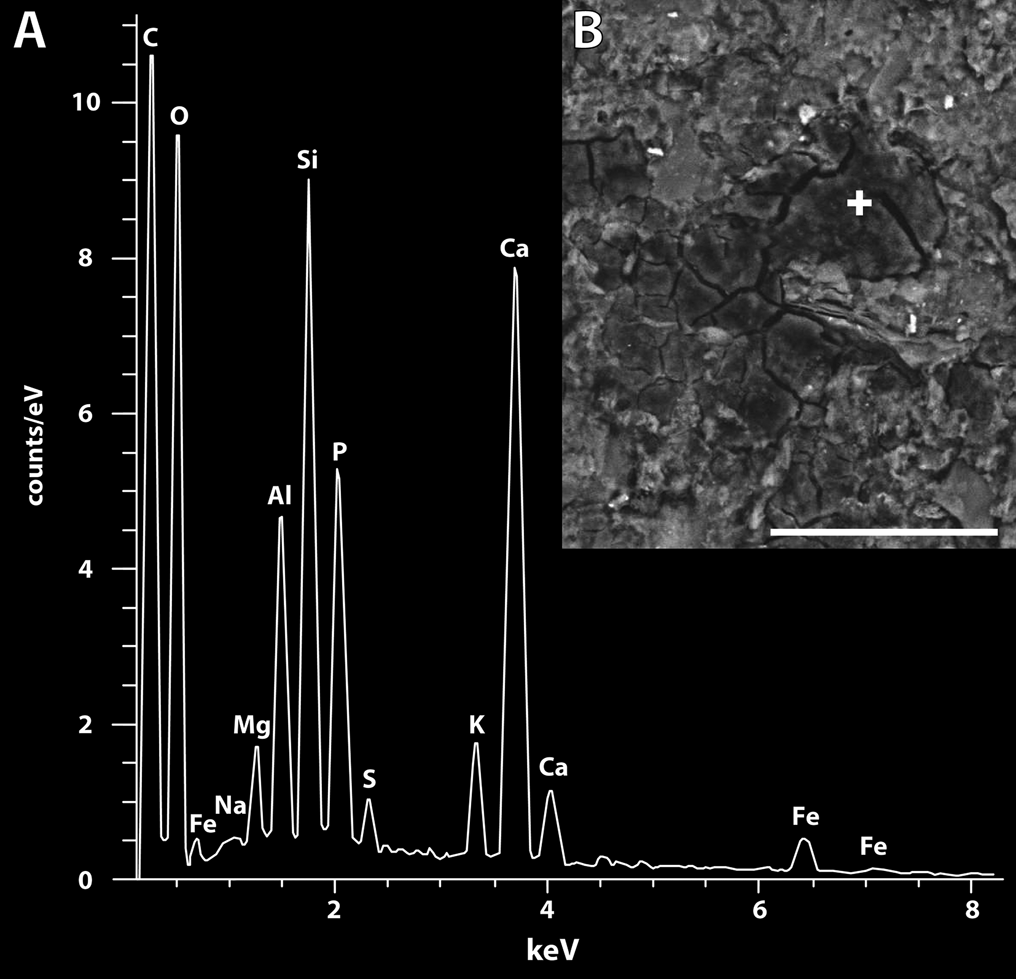
Figure 2 SEM-EDX point spectroscopy from the head of Strudiella devonica Garrouste et al., 2012: (A) EDX spectra showing presence of carbon, oxygen and phosphorous within the fossil (LV 30 Pa, 20 kV); (B) SEM photograph of the precise area investigated; white cross indicates exact location of chemical analysis. See Fig. 3G for a view of the complete specimen. Scale bar=50 µm.
A large majority of specimens presents a dorso-ventral compression, with only a few preserved in lateral aspect. They are mostly disarticulated, but many were found nearly complete in the finer green and grey shales (carapace, pleon and telson articulated; Fig. 3), which is evidence of the absence of – or very limited – transport in the calm and confined environment of the floodplain. Such conditions allowed the minute preservation of delicate anatomical structures, such as the cephalic and thoracic appendages. The best-preserved legs, antennae and mandibles have been found isolated within the sediment; whereas their morphologies and connections with the body are often hardly distinguishable in the articulated specimens, due to compacted superimposition of the different anatomical structures. Many specimens preserve traces of the gut, suggesting that they represent carcasses rather than moults (see Tetlie et al. Reference Tetlie, Brandt and Briggs2008).

Figure 3 Arthropod fossils from Strud, Belgium: (A) Tealliocaris walloniensis Gueriau, Charbonnier & Clément, Reference Gueriau, Charbonnier and Clément2014a, paratype IRSNB a 12869a, nearly complete specimen in ventral view; (B) Schramidontus labasensis Gueriau, Charbonnier & Clément, Reference Gueriau, Charbonnier and Clément2014b, paratype IRSNB a 12878a, anterior part in lateral view; (C) Strudops goldenbergi Lagebro et al., Reference Lagebro, Gueriau, Hegna, Rabet, Butler and Budd2015, paratype IRSNB a 12859, complete specimen in dorsal view. (D–E) Gesvesia pernegrei Gueriau et al., Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016: (D) holotype IRSNB a 12932, external morphology of the carapace; (E) paratype IRSNB a 12934a, juvenile specimen in lateral view. (F) Haltinnaias serrata Gueriau et al., Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016, paratype, IRSNB a 12930, male in dorsal view; (G) Strudiella devonica Garrouste et al., 2012, holotype IRSNB a12818a. Abbreviations: a2 = antenna; ab = abdomen; ac = anterior corner; car = carapace; e = pedunculate eye; fu = caudal furca; gl = growth line; h = head; hl = hinge line; hs = head shield; lv = left valve; mxp = maxillipeds; pc = posterior corner; rv = right valve; s = scaphocerite; T = thoracopods; th = thorax; um = umbo; vm = ventral margin. Scale bars = 5mm (A, B); 1 mm (C–G).
Only decay-resistant gut and cuticular structures fossilised here. This corresponds to the decay stages 4–5 proposed by Butler et al. (Reference Butler, Cunningham, Budd and Donoghue2015), with substantial autolysis and microbial decay of the labile soft-tissues, such as musculature and other internal organs, before fossilisation. The preservation of the arthropods is then interpreted as the result of rapid burial in fine-grained sediment before complete decay occurred.
2. Material and methods
Specimens were collected during the successive field campaigns organised each year since the rediscovery of the locality in 2004. The material is housed at the IRSNB (Brussels, Belgium). The fossils were observed under a binocular microscope with polarised light, both dry and covered in 95 % ethanol, to see cuticular remains with a low angle light to reveal relief. Drawings were produced using a camera lucida. Specimens were photographed covered in ethanol with cameras coupled with macro lens equipped with polarising filters. Anatomical measurements were performed using ImageJ software. Statistical analysis was performed using the ‘R' statistical environment (R Development Core Team 2011). Scanning electron microscopy (SEM) imaging and spectroscopy were carried out in back scattered electrons (BSE) mode using the Tescan SEM (VEGA II LSU) associated with a Bruker SD3 energy-dispersive spectrometer (EDS) detector of the microscopy platform of the MNHN, Paris (France).
3. Systematic palaeontology of the Strud crustacean fauna
Malacostraca Latreille, Reference Latreille1802
Eumalacostraca Grobben, Reference Grobben1892
?Eucarida Calman, Reference Calman1904
?Decapoda Latreille, Reference Latreille1802
Tealliocarididae Brooks, Reference Brooks1962
Tealliocaris Peach, Reference Peach1908
Tealliocaris walloniensis Gueriau, Charbonnier & Clément, Reference Gueriau, Charbonnier and Clément2014a
(Fig. 3A)
Holotype. IRSNB a 12866a–b, isolated carapace.
Paratypes. IRSNB a 12867a–b, isolated carapace; IRSNB a 12868a–b, IRSNB a 12869a–b, IRSNB a 12870a–b, nearly complete specimens; IRSNB a 12871a–b, IRSNB a 12872a–b, articulated pleons; IRSNB a 12873a–b, posterior part of the pleon showing telson and uropods; IRSNB a 12874a–b, isolated cephalic appendages (antennula, antenna and scaphocerite).
Discussion. The total material includes more than 50 specimens. Tealliocaris was previously only known from the Carboniferous. Clark (Reference Clark2013) ascribed it to Decapoda, based upon a suite of characters indicating a closer relationship to decapod crustaceans (in particular, Astacida, Homarida and Glypheoidea) than to any other eumalacostracan clade. Tealliocaris walloniensis then enlarges the stratigraphical distribution of the genus to the Late Devonian, and represents the third Devonian decapod known, after the rather derived lobster-like Palaeopalaemon newberryi Whitfield, Reference Whitfield1880 (Schram et al. Reference Schram, Feldmann and Copeland1978) and Aciculopoda mapesi Feldmann & Schweitzer, Reference Feldmann and Schweitzer2010 from Famennian marine deposits of the USA. T. walloniensis was, therefore, thought to document the earliest occurrence of continental decapod crustaceans and to indicate that decapods have been part of continental ecosystems at least since the Late Devonian. However, the phylogenetic affinities of Tealliocaris remain under debate, as its assignment to Decapoda has recently been contested by Jones et al. (Reference Jones, Feldmann, Schram, Schweitzer and Maguire2016), who transferred it back to Peracarida: Pygocephalomorpha, based particularly on the presence of an oostegite marsupium in females (interpreted by Clark (Reference Clark2013, fig. 6) as phyllobranchiate gills), a distinct terminal telson lobe and a pair of lateral telson lobes (shown by Clark (Reference Clark2013, fig. 12) to be an artefact of compaction of a larger single terminal lobe).
Eucarida Calman, Reference Calman1904
Angustidontida Gueriau, Charbonnier & Clément, Reference Gueriau, Charbonnier and Clément2014b
Angustidontidae Cooper, Reference Cooper1936
Schramidontus Gueriau, Charbonnier & Clément, Reference Gueriau, Charbonnier and Clément2014b
Schramidontus labasensis Gueriau, Charbonnier & Clément, Reference Gueriau, Charbonnier and Clément2014b
(Fig. 3B)
Holotype. IRSNB a 12880a–b, thoracic appendages in ventral view.
Paratypes. IRSNB a 12878a–b, nearly complete specimen; IRSNB a 12879a–b, isolated cephalic and first thoracic appendages.
Discussion. Besides the type material, only one additional fragment of maxilliped has been collected. Angustidontid remains have been known for more than 80 years, but their crustacean affinity was only recently established (Rolfe & Dzik Reference Rolfe and Dzik2006). These fossils, recognisable by their peculiar first pair(s) of thoracopods modified into long, slightly curved maxillipeds bearing elongated teeth of alternating sizes (Fig. 3B), have been recovered from Upper Devonian marine deposits through most of Euramerica (Rolfe & Dzik Reference Rolfe and Dzik2006; Shpinev Reference Shpinev2010) and are interpreted as pelagic predators. Gueriau et al. (Reference Gueriau, Charbonnier and Clément2014b) ascribed them to Eucarida, based upon their carapace being fused to thoracic segments 1–7, and erected the new order Angustidontida, based upon the absence of autapomorphies of each of Euphausiacea, Amphionidacea and Decapoda. Schramidontus labasensis documents the first occurrence of angustidontids in a continental environment. Unlike Angustidontus seriatus Cooper, Reference Cooper1936 (Rolfe & Dzik Reference Rolfe and Dzik2006), S. labasensis possess two pairs of maxillipeds, suggesting that angustidontid crustaceans fill the gap between Amphionidacea (Decapoda sensu lato; characterised by a unique pair of thoracopods modified into maxillipeds) and Decapoda sensu stricto (three maxillipeds) (Gueriau et al. Reference Gueriau, Charbonnier and Clément2014b). This implies that early decapods may have had autapomorphically at least one pair of maxillipeds that were further modified in Decapoda sensu stricto.
Branchiopoda Latreille, Reference Latreille1817
Notostraca Sars, Reference Sars1867
Strudops Lagebro et al., Reference Lagebro, Gueriau, Hegna, Rabet, Butler and Budd2015
Strudops goldenbergi Lagebro et al., Reference Lagebro, Gueriau, Hegna, Rabet, Butler and Budd2015
Holotype. IRSNB a 12858a–b, nearly complete specimen.
Paratypes. IRSNB a 12854, IRSNB a 12856, IRSNB a 12857, IRSNB a 12859, IRSNB a 12877, nearly complete specimens; IRSNB a 12855, IRSNB a 12860a–b, carapace with cephalic and thoracic appendages; IRSNB a 12861a–b, IRSNB a 12864, posterior part of the abdomen with telson; IRSNB a 12862a–b, IRSNB a 12863, carapace with mouth parts.
New material. IRSNB a 13055, nearly complete specimen; IRSNB a 13056a–b, incomplete carapace with thoracic appendages.
Discussion. Strudops goldenbergi is the earliest unequivocal member of total-group Notostraca (Lagebro et al. Reference Lagebro, Gueriau, Hegna, Rabet, Butler and Budd2015). It is morphologically very similar to modern notostracans, but differs from extant Triopsidae in the absence of dorsal carina, the spineless posterior notch and the larger size, but smaller number, of abdominal segments (Fig. 3C). More than 40 specimens have been collected and most preserve soft-tissues, such as the phyllopodous thoracic appendages, the mouthparts and even clusters of resting eggs. Since the original description, two newly excavated specimens show additional, fine anatomical details of the thoracopods (Fig. 4). One specimen preserves serrated, trapezoidal structures at the tip of the thoracopods (Fig. 4A, C–D) that strongly resemble the 4th and 5th endites and endopod of extant notostracans (see Reference LonghurstLonghurst 1955, fig. 10 for comparison with extant taxa). However, the morphology of the first thoracopod appears different, with more elongated, articulated “antenna-like” structures (Fig. 4A–B; and see Longhurst Reference Longhurst1955, fig. 10), suggesting limb differentiation. Specialisation of the first thoracopods in most extant notostracans consists of elongated 4th–5th endites (Longhurst Reference Longhurst1955). Such a type of thoracopods had never before been recorded in fossils (Hegna Reference Hegna2011), but our data suggest that differentiation of the first legs could be as ancient as the Late Devonian. Furthermore, another specimen displays “hockey-stick” setae in the thoracic region, in the same area as the endites and endopods (Fig. 4E–F). Very similar, but smaller, structures were extracted from shale of the early Cambrian Mount Cap Formation, Canada (Harvey & Butterfield Reference Harvey and Butterfield2008, fig. 1a–c). The latter authors suggest a scraping function, based on their proportions and spatial arrangement, and found strong similarities with scraping armatures that are located along the margins of the distal endites in many branchiopod crustaceans (T. H. Harvey, pers. comm. Reference Harvey and Butterfield2015), particularly in the laevicaudatan Lynceus (L. gracilicornis Packard, Reference Packard1871 and L. simiaefacies Harding, Reference Harding1941) (Fryer & Boxshall Reference Fryer and Boxshall2009).

Figure 4 Thoracopod morphology of Strudops goldenbergi Lagebro et al., Reference Lagebro, Gueriau, Hegna, Rabet, Butler and Budd2015: (A) close-up of the right thoracopds of IRSNB a 13055, complete specimen in dorsal view; (B–D) enlargements of the articulated 4th and 5th endites of the first thoracopod (B), and of endites and endopods (C, D) of following thoracopods, from boxed areas in (A); (E) IRSNB a 13056a, partially preserved anterior thoracic region; (F) enlargement on the “hockey-stick” setae. Scale bars=1mm (A, E); 500 µm (B–D, F).
Anostraca Sars, Reference Sars1867
Haltinnaias Gueriau et al., Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016
Haltinnaias serrata Gueriau et al., Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016
(Fig. 3F)
Holotype. IRSNB a 12928a–b, nearly complete specimen, female.
Paratypes. IRSNB a 12930, nearly complete specimen, male; IRSNB a 12929, IRSNB a 12931a–b, nearly complete specimens.
Discussion. Haltinnaias serrata is the earliest member of total-group Anostraca with a modern morphology, whilst the Early Devonian stem-group anostracan Lepidocaris rhyniensis Scourfield, Reference Scourfield1926 from Scotland is morphologically distinct in having locomotory biramous antennae. The fossil record for anostracans is extremely limited, due to their small size, delicate nature and lack of a carapace, and no other specimens have been excavated from this locality. Exceptionally, H. serrata preserved details of most cephalic and thoracic appendages (Fig. 3F), which bear diagnostic characters of the Anostraca (i.e., pedunculated eyes and phyllopodous thoracic legs). Although it differs from all extant anostracans, it is not possible to assign it to any extant family (Gueriau et al. Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016).
Spinicaudata Linder, Reference Linder1945
Gesvesia Gueriau et al., Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016.
Gesvesia pernegrei Gueriau et al., Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016.
Holotype. IRSNB a 12932, bivalved carapace.
Paratypes. IRSNB a 12933, carapace with preserved digestive system; IRSNB a 12934a, b, IRSNB a 12935a, b, nearly complete specimens with preserved internal anatomy; IRSNB a 12936, carapace with resting eggs.
Discussion. Gesvesia pernegrei is the main constituent of the branchiopod community, and hundreds of specimens have been found. The affinities of bivalved branchiopod fossils are usually difficult to assess precisely because their carapaces, which are most often the only remains preserved, display highly homoplastic features that provide little systematic information. Carapace morphology, the presence of growth lines on the whole carapace, and a small head with a relatively long trunk in G. pernegrei (Fig. 3D–E) are features similar to those in extant Spinicaudata, but clearly different from extant Laevicaudata (Gueriau et al. Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016). Further research has begun to determine the phylogenetic relationships between extant and fossil spinicaudatans (Astrop & Hegna Reference Astrop and Hegna2015), but many points still need clarification, and it is not possible to confidently assign G. pernegrei to any subclade of Spinicaudata.
Spinicaudata Linder, Reference Linder1945
Vertexioidea Kobayashi, Reference Kobayashi1954 sensu Zhang et al., Reference Zhang, Chen and Shen1976
Undulatesta gen. nov.
Etymology. The name is derived is from “undulata” (Latin for wavy) and “testa” (Latin for carapace), referring to the sinuous growth lines on the bivalved carapace.
Type species. Undulatesta bounonensis sp. nov. by monotypy.
Diagnosis. Bivalved branchiopod with limnadiform carapace, covered only in the anterior half by growth lines connecting the hinge line sinuously backward.
Undulatesta bounonensis sp. nov.
(Fig. 5)

Figure 5 Undulatesta bounonensis gen. et sp. nov.: (A) left: holotype, IRSNB a 13057a, right valve in lateral view, head oriented to the left; right: paratype IRSNB a 13059a, left valve in lateral view, head to the right; (B) close-up of the holotype from boxed area in (A); (C) line drawing of holotype, not to scale; (D) holotype, counterpart, IRSNB a 13057b, head to the right; (E) paratype, IRSNB a 13058a, left valve in lateral view, head oriented to the right. Abbreviations: ac = anterior corner; gl = growth line; hl = hinge line; pc = posterior corner; um = umbo; vm = ventral margin. Scale bars = 5mm.
Etymology. From Bounon, the name of the street just next to the Strud quarry.
Holotype. IRSNB a 13057a–b (Fig. 5A–D), carapace with growth lines.
Paratype. IRSNB a 13058a–b (Fig. 5E), IRSNB a 13059a–b (Fig. 5A, right), incomplete carapaces.
Type locality. Strud, Gesves municipality, Namur Province, Belgium (50°26′43″N, 5°03′24″E).
Stratigraphic age. Late Famennian (age based upon miospore assemblages and regional stratigraphic correlations; Denayer et al. Reference Denayer, Prestianni, Gueriau, Olive and Clément2016).
Occurrence. Known only from the type locality.
Description. Bivalved limnadiform carapace (length=10.66 mm and 11.02 mm, and width=5.26 mm and 5.29 mm, respectively, for IRSNB a 13057 and IRSNB a 13058, which are the only two specimens that could be confidently measured); mean length/height ratio 2.05±0.04; umbo slightly pronounced; first larval valve unornamented; numerous growth lines forming narrow concentric ribs on the anterior half surface of the valve, connecting dorsally to the hinge line sinuously backward, and converging ventrally at the anterior cardinal angle (Fig. 5A–C); growth lines more closely spaced further from this angle; straight hinge line about two thirds the total length of the carapace; anterior and posterior cardinal angles 140° and 120°, respectively; no internal anatomy is preserved.
Discussion. Undulatesta bounonensis gen. et sp. nov. is ascribed to Spinicaudata Linder, Reference Linder1945, based upon the presence of growth lines on the carapace. Indeed, previous external laminae remain during moulting in Spinicaudata, overlying the youngest (larger) plates, whereas the carapace is smooth in other bivalved branchiopods (Laevicaudata and most of Cladoceromorpha), because the oldest laminae are not retained during moulting (Roessler Reference Roessler1995). It differs from Gesvesia pernegrei in its larger size and higher mean length/height ratio (Fig. 6), but most importantly in its striation pattern, which involves growth lines that only cover the marginal part of the carapace, suggesting a different development, with complete valve moulting during the early developmental stages followed by conservation of external laminae later in the development. This type of development is characteristic of the extant family Limnadiidae (Rogers et al. Reference Rogers, Rabet and Weeks2012) and found in many fossils clustered with limnadiids in Vertexioidea Kobayashi, Reference Kobayashi1954 sensu Zhang et al., Reference Zhang, Chen and Shen1976 (See Astrop & Hegna Reference Astrop and Hegna2015). Recent molecular clock estimates suggest that limnadiids are no older than 200 Ma (Bellec & Rabet Reference Bellec and Rabet2016), which is not compatible with the assignation of Undulatesta to the Limnadiidae. Undulatesta is, therefore, here assigned to the more inclusive Vertexioidea.

Figure 6 Length/height distributions of the spinicaudatans Gevesia pernegrei (circles) and Undulatesta bounonensis gen. et sp. nov. (black dots) from Strud, Belgium. Mean L:H ratio in G. pernegrei = 1.629 ± 0.390 (n = 251), shown with linear model (intercept = 0.440; slope = 0.512; p-value<2.2e-16; R2 = 0.637). Mean L:H ratio in U. bounonensis gen. et sp. nov. = 2.055± 0.040 (n = 2).
Remark. Interestingly, the general morphology of U. bounonensis gen. et sp. nov. looks very similar to that of the Silurian marine bivalve Pteronitella retroflexa Wahlenberg, Reference Wahlenberg1821 (see Walmsley Reference Walmsley1962). However, the latter fossils are significantly bigger and bear growth lines covering the whole valves. Moreover, the organic preservation of U. bounonensis is not consistent with the calcareous shells of molluscs.
4. Discussion
4.1. A complex Late Devonian floodplain ecosystem
Terrestrialisation of land by plants and animals is the second most important event in the history of multicellular life, after the Cambrian and Ordovician radiations that led to the colonisation of the marine realm and to the first modern-looking trophic webs. The first complex continental ecosystems were settled by the Middle to Late Devonian, after plants, arthropods and tetrapods ventured onto land, respectively, in the Ordovician (c. 470 Ma) (Rubinstein et al. Reference Rubinstein, Gerrienne, de la Puente, Astini and Steemans2010; Wellman Reference Wellman2014), Late Silurian and Late Devonian (433.8–358.9 Ma) (Shear & Selden Reference Shear, Selden, Gensel and Edwards2001; Giribet & Edgecombe Reference Giribet, Edgecombe, Minelli, Boxshall and Fusco2013; Dunlop et al. Reference Dunlop, Scholtz, Selden, Minelli, Boxshall and Fusco2013; Clack Reference Clack2012). They included some high-diversity forest communities (although forests were not typical in the Mid to Late Devonian) (DiMichele et al. Reference DiMichele, Hook, Beerbower, Boy, Gastaldo, Hotton, Phillips, Scheckler, Shear, Sues, Behrensmeyer, Damuth, DiMichele, Potts, Sues and Wing1992; Stein et al. Reference Stein, Berry, Hernick and Mannolini2012) and irreversibly changed atmospheric pCO2 and pO2, climate and sedimentation patterns (Algeo & Scheckler Reference Algeo and Scheckler1998; Dahl et al. Reference Dahl, Hammarlund, Anbar, Bond, Gill, Gordon, Knoll, Nielsen, Schovsbo and Canfield2010; Godderis et al. Reference Godderis, Donnadieu, Le Hir, Lefebvre and Nardin2014).
The Late Devonian Strud locality, together with the Red Hill locality in Pennsylvania (see Cressler et al. Reference Cressler, Daeschler, Slingerland, Peterson, Vecoli, Clément and Meyer-Berthaud2010 for a review of this locality), constitute unique examples of early complex and diversified continental biocenoses, with ecological partitioning of the floodplain biotopes at a high taxonomic level. At Strud, vertebrates, including early tetrapods, placoderms, acanthodians and sarcopterygians, inhabited a deltaic flood channel. Eumalacostracan crustaceans, as well as acanthodian and actinopterygian fishes, were collected from fine shales witnessing a calm and confined floodplain habitat, but were probably more generally living both in floodwaters and in river branches. A community of branchiopod crustaceans (notostracans, anostracans and spinicaudatans) colonised calm, fresh to brackish temporary ponds that seasonally dried and flooded. They overcame constraints of the terrestrial environment, such as seasonal desiccation, by the production of resting (drought-resistant) eggs, which accumulate as an egg bank in sediment and are resistant for long periods of drought, allowing them to await favourable conditions (Gueriau et al. Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016). Eurypterid remains have been recovered from all depositional environments and were living in, or exploiting, all the aquatic biotopes. The only unequivocal terrestrial organisms were abundant and diversified plants, including early cupulate seed plants. Garrouste et al. (2012) suggested that the putative insect Strudiella devonica Garrouste et al., 2012 was a terrestrial species, but the affinities and lifestyle of this fossil remain under debate (Hörnschemeyer et al. Reference Hörnschemeyer, Haug, Béthoux, Beutel, Charbonnier, Hegna, Koch, Rust, Wedmann, Bradler and Willmann2013; Garrouste et al. 2013).
4.2. Freshwater bodies as key environments for terrestrialisation
It must be considered that the new continental niches available in the Middle to Late Devonian continental realm were harsher and less stable than the long-colonised fluvial and marine ones. This results in many morphological and physiological transformations in plants and animals. The most important palaeobotanical innovations were the development of arborescence (tree stature) and the production of seeds which, respectively, increased root penetration and rhizoturbation, resulting in intensified pedogenisis (and consequently in major changes in weathering processes), and allowed colonisation of drier upland and primary successional areas (see Algeo & Scheckler Reference Algeo and Scheckler1998).
When they managed to colonise the land, animals faced an identical series of challenges, namely gas exchange, reproduction, osmoregulation and exposure to ultraviolet radiation (Little Reference Little1990). Tracheae enable air breathing in insects, myriapods and some arachnids (whereas others use book lungs as respiratory organs for atmospheric gas exchange). The enlargement and thickening of the jointed legs present in aquatic arthropods living on the substrate probably gave arthropods a great advantage in ensuring their water-to-land transitions (Dunlop et al. Reference Dunlop, Scholtz, Selden, Minelli, Boxshall and Fusco2013; Waddington et al. Reference Waddington, Rudkin and Dunlop2015). Another advantageous adaptation of arthropods is their exoskeleton, the waxy epicuticle of which controls water loss and facilitates osmoregulation (Moussian Reference Moussian, Minelli, Boxshall and Fusco2013). Dunlop et al. (Reference Dunlop, Scholtz, Selden, Minelli, Boxshall and Fusco2013) further point out that osmoregulation is a central challenge that constrained arthropods' terrestrialisation; in particular, it may also indicate whether it occurred directly from the marine environment or via freshwater. In this regard, the colonisation of the ephemeral pool habitat by branchiopods during the Late Devonian, together with the presence of early tetrapods in surrounding deltaic rivers, suggests that ephemeral pools and more generally freshwater bodies may have been key environments in the terrestrialisation process.
Another central question that still remains to be settled is why some taxa rather than others risked venturing onto land, as the continental realm offers only limited environmental stability. Of the pressures that may have driven multiple lineages to exploit continental ecosystems, ecological pressure, and especially predation (avoidance of predators), has been proposed both for arthropods (Dunlop et al. Reference Dunlop, Scholtz, Selden, Minelli, Boxshall and Fusco2013) and for tetrapods (McNamara & Selden Reference McNamara and Selden1993).
4.3. Palaeogeographical considerations
The poor fossil record of soft-bodied fossils in the Late Devonian makes palaeogeographical considerations very difficult, since we need localities with exceptional preservation. It is easier for vertebrates because they have a much greater fossilisation potential, and do not require exceptional conditions to enter the fossil record. Most of the fossil vertebrates recovered from the Late Devonian worldwide consist mostly of isolated bones, bony plates, teeth or scales. Nevertheless, strong similarities have been highlighted between the vertebrate fauna from Belgium and that of the Red Hill site, Pennsylvania, USA (Cressler et al. Reference Cressler, Daeschler, Slingerland, Peterson, Vecoli, Clément and Meyer-Berthaud2010). By looking for faunal relationships and similarity between the different tetrapod localities worldwide, using cluster analyses based on taxonomic and anatomical datasets, Delgehier (Reference Delgehier2014) showed faunal similarities between both areas at the species level. Olive et al. (Reference Olive, Clément, Daeschler and Dupret2015a) also pointed out this pattern in their study of material of the placoderm Phyllolepis from Belgium, which they identified as Phyllolepis undulata Lohest, Reference Lohest1888, and is also known from Red Hill.
However, from comparing the Strud and Red Hill arthropod fauna, no links can be established between both sites. Strud has exclusively yielded aquatic crustaceans, whereas only the trigonotarbid Gigantocharinus szatmaryi Shear, Reference Shear2000 and the millipede Orsadesmus rubecollus Wilson, Daeschler & Desbiens, Reference Wilson, Daeschler and Desbiens2005 have been described from Red Hill; although greater diversity is evidenced by enigmatic body impressions, burrow traces and walking traces (Cressler et al. Reference Cressler, Daeschler, Slingerland, Peterson, Vecoli, Clément and Meyer-Berthaud2010).
At the regional levels, strong similarities exist between the Strud flora and fauna and other localities from Belgium; in particular, the Becco locality in Liège Province, eastern Belgium (Olive et al. Reference Olive, Clément, Denayer, Derycke, Dupret, Gerienne, Gueriau, Marion, Mottequin and Prestianni2015b). Moreover, several taxa found in Strud are also present in the Pont-de-Bonne Modave locality; in particular, the lungfishes Soederberghia cf. S. groenlandica Lehman, Reference Lehman1959 and Jarvikia Lehman, Reference Lehman1959 (Clément & Boisvert Reference Clément and Boisvert2006) and some placoderms (Olive Reference Olive2015). It is not surprising to find such similarities between the Strud, Becco and Modave assemblages, since the whole Condroz area consisted of a single deltaic unit during the Late Famennian, therefore suggesting that vertebrates and arthropods may have colonised all the river branches.
4.4. The fate of this early continental ecosystem
One of the “Big Five” mass extinctions (Raup & Sepkoski Reference Raup and Sepkoski1982) occurred within the Late Devonian, marked by two extinction pulses: the Kellwasser Event at the Frasnian–Famennian boundary (374 Ma); and the Hangenberg Event at the Devonian–Carboniferous boundary (359 Ma) (Raup & Sepkoski Reference Raup and Sepkoski1982; McGhee Reference McGhee1996; Caplan & Bustin Reference Caplan and Bustin1999; Bambach Reference Bambach2006). This end-Devonian extinction is associated with important losses in marine diversity, and has been particularly recognised as a bottleneck in the evolutionary history of vertebrates (Sallan & Coates Reference Sallan and Coates2010). According to the stratigraphical distribution of genera listed in Sepkoski's fossil marine animal database (Sepkoski Reference Sepkoski2002), these important biotic crises also strongly affected marine crustaceans. However, very few data exist with regard to the continental realm, due to the scarcity of continental fossil animals collected between the Late Devonian and the middle-to-late early Carboniferous, leading to the definition of two fossil gaps, known as “Romer's gap” for vertebrates (360–345 Ma), and the “arthropod gap” for continental arthropods, particularly myriapods, arachnids and hexapods (385–325 Ma). Both fossil-poor intervals have long been interpreted as resulting from global atmospheric change affecting early continental ecosystems (Ward et al. Reference Ward, Labandeira, Laurin and Berner2006), but a series of recent discoveries suggests that they rather derive from a preservational artefact (Garrouste et al. 2012; Smithson et al. Reference Smithson, Wood, Marshall and Clack2012).
Regarding the fate of the Strud crustacean taxa, Tealliocaris is widespread in Euramerica after the Devonian–Carboniferous boundary, and has been recorded from marginal marine, brackish, lagoonal, hypersaline and freshwater environments (see Gueriau et al. Reference Gueriau, Charbonnier and Clément2014a). No occurrence of angustidontids is known after the Late Devonian, nor of the branchiopod genera Strudops, Haltinnaias, Gesvesia or Undulatesta. Nevertheless, branchiopods survived the end–Devonian mass extinction, as well as the following ones, as demonstrated by their exceptional morphological and ecological stasis since the Late Devonian, with recurrent associations of anostracans, notostracans and spinicaudatans typically inhabiting temporary freshwater bodies worldwide today (Gueriau et al. Reference Gueriau, Rabet, Clément, Lagebro, Vannier, Briggs, Charbonnier, Olive and Béthoux2016). It is very likely that their reproductive strategy involving the production of drought-resistant eggs, like seeds in plants, made these lineages far less vulnerable and better equipped to survive crises and extinctions than other lineages lacking the adaptations to survive stressful periods.
5. Acknowledgements
We thank the Gesves local council staff and field workers of the Strud expeditions, as well as all the contributors to the original descriptions. We acknowledge O. Béthoux (MNHN) for help with photographs, S. Pont (MNHN) for the SEM imaging and spectroscopy, and A. Folie and A. Drèze (IRSNB) for our requests of catalogue numbers. T. Harvey (University of Leicester) is warmly acknowledged for discussion about the hockey-stick setae, and J. Denayer (Liège University) for information on the depositional environments at the Belgian localities. The authors also thank the editors, A. Ross and V. Hammond, and reviewers, N. Clark and G. Edgecombe, for their remarks and corrections. This work is a contribution to the French National Agency for Research TERRES project (ANR-2010-BLAN-607-03 grant), which supported PG and the Strud expeditions.








