Students of dinosaurs generally know that the group was named by Richard Owen in Reference Owen1842, and that Cuvier and several other French savants might have come close some years earlier, but identified their own collected remains as those of crocodiles (Desmond Reference Desmond1976; Torrens Reference Torrens1992; Rüpke Reference Rüpke1994; Dean Reference Dean1999). Owen himself did not include what we now recognise as the British dinosaurs Thecodontosaurus and Cetiosaurus, as well as several continental European forms that he had not seen personally, when he named Dinosauria.
Owen (Reference Owen1842) famously translated the term “Dinosauria” as “fearfully great reptiles.” A synonym for “fearfully great” might be “awe-inspiring,” or in more contemporary terms, “awesome.” If he were naming them today, he might have translated his term as “awesome reptiles,” which, in modern parlance, they were. Owen seemed to recognise this when he diagnosed Dinosauria (on three taxa: Megalosaurus, Iguanodon, and Hylaeosaurus) on the basis of three main features: large size but terrestrial habits (distinct from plesiosaurs and ichthyosaurs, and what he thought to be the whale-like cetiosaurs); upright posture (different from the marine reptiles and the largest crocodiles); and a sacrum with five vertebrae (because his avatars were all Late Jurassic and Cretaceous forms, he could not have known that the first dinosaurs had three or fewer sacrals). These characteristics were simply not reptilian. In fact, they were more mammalian.
Desmond (Reference Desmond1979) argued that Owen named the Dinosauria, perching the great beasts functionally a bit closer to mammals and birds than to traditional reptiles, yet using tortuous arguments about metabolism and its hard-part correlates, to proclaim that the advances of dinosaurs were “purely adaptive,” because after all, they were unquestionably categorised as reptiles. In the calculus of Owen's day, the typology of classification trumped whatever apparent adaptive and physiological features may have characterised a bizarre, extinct group. So he could deny the “progressivism” that suggested that through time, groups of animals could become more sophisticated in their structures and adaptations, and he could also deny transmutation (a concept that preceded Darwin: see Desmond Reference Desmond1982).
This paper traces the development of ideas about the origin of dinosaurs in three senses: phylogenetic, functional-ecological, and chronological. Although not all workers have been interested in all problems, without studying and integrating all three approaches it has been easy to draw naïve inferences about the rise of dinosaurs in the Late Triassic and their dominance for the next 135 million years (exclusive of birds). The present author wishes to emphasise that until dinosaurs were recognised as a natural group (which today we call monophyletic), it was difficult to ask questions about their phylogenetic origins, although those questions were nonetheless attempted at times, as were questions about functional and ecological origins. It is further argued that, even with the acceptance of dinosaur monophyly, the absence of critical basal representatives of major lineages has frustrated progress on both phylogenetic and functional-ecological fronts. The algorithms of cladistics, despite a tremendous record of success and progress in discerning relationships, have sometimes facilitated the erection of false groupings. This paper documents how new approaches to biostratigraphy, life history and palaeoecology, measured against repeated independent phylogenetic analyses, have provided a series of new paradigms for the origin and timing of the rise of dinosaurs, and suggests a way of reframing questions of dinosaurian origins in order to stimulate new approaches to perpetual questions.
1. Early assessments of dinosaur interrelationships
1.1. Harry Govier Seeley and the division of Dinosauria
Many traditional sources recount that Harry Govier Seeley was the first to subdivide dinosaurs into Saurischia and Ornithischia. However, as he summarised in his 1887 paper, Cope, Huxley and Marsh had already subdivided them into various schemes of orders and suborders (e.g. Huxley Reference Huxley1870). The difference was that Seeley thought that the distinctions among them implied that the animals called dinosaurs did not form a natural group. That is, they had some general features in common, but features of the pelvis, pneumaticity of the vertebrae, and braincase implied that they could not have had a monophyletic origin. This is why Seeley erected Ornithischia and Saurischia and denied the monophyly of dinosaurs.
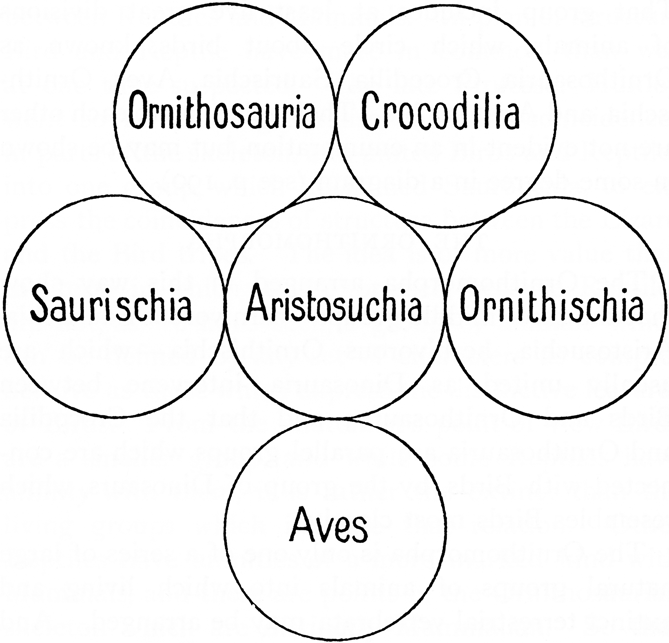
Seeley, who often commented on presentations at scientific meetings and was consistently a taxonomic splitter, was something of a maverick. He had studied law and, like many of his contemporaries, came to palaeontology as an avocation after hearing Richard Owen's lectures (Seeley Reference Seeley1901). He became an expert on pterosaurs (which he called ornithosaurs) and dicynodonts, but he was generally interested in issues of vertebrate paleontology and the philosophy behind natural science. Critical to the analysis here is that, although Seeley accepted evolution, when it came to classification he was what I have called a casual Quinarian (Padian Reference Padian and Sarjeant1995). Quinarianism was the practice of classifying organisms in groups of five, arranged in a circle of adjacent groups that shared certain features with their neighbors. This system was developed by the English (later Australian émigré) entomologist William Sharpe MacLeay, who popularised it in his tome Horae Entomologicae (Reference MacLeay1821). It was widely popular in England, largely among entomologists, until Darwin's explanation of “affinities” through common descent (Thomson Reference Thomson2009). The system mostly failed in the end because Darwin's (Reference Darwin1859) recasting of classification based on ancestry explained so much (Padian Reference Padian1999), and there was no conceivable empirical reason why the Quinarian system should work. So it is strange to find Seeley (Reference Seeley1892, p. 368, and later in his great popular book on pterosaurs Dragons of the Air, 1901), using Quinarian diagrams to postulate relationships among his “ornithosaurs” and other reptile groups (Fig. 1, from Seeley Reference Seeley1901, p. 190).

Figure 1 Seeley's (Reference Seeley1901) Quinarian diagram of the relationships of Archosauria. “Ornithosauria” was what Seeley called Pterosauria. “Aristosuchia” was an old term of Cope's for what we would loosely call basal Archosauromorpha and other things. Note that Seeley has not only separated Ornithischia and Saurischia; they are not even “osculating” (connecting) groups.
The importance of Seeley's use of Quinarianism speaks to why he was so ready to do away with the concept of Dinosauria. In his Reference Seeley1887 paper (p. 170, my italics), he writes: “the Dinosauria has no existence as a natural group of animals, but includes two distinct types of animal structure with technical characters in common which show their descent from a common ancestry rather than their close affinity.”
What could Seeley have meant by having common ancestry but not close affinity? The term “affinity” was used in the 19th Century in a non-evolutionary (non-transmutationist) sense, that is to say, not implying a genealogical (evolutionary) relationship (e.g. Owen Reference Owen1870). Seeley was saying that what he called Ornithischia and Saurischia shared common ancestry, but were not necessarily each other's closest relatives. This can be seen in the Quinarian diagram reproduced in Figure 1: the two dinosaurian orders that he erected are not placed next to each other. Seeley (Reference Seeley1887) supported this reasoning by analysing characters that he thought gave more weight to relationships than others. For him, the differences in pelvic (pubic) structure, presence or absence of pneumatisation, and configuration of the braincase were more reliable as characters than other structures of the “teeth, mandible, ilium, femur, and the absence or presence of dermal armour” (Seeley Reference Seeley1887, p. 166).
Seeley (Reference Seeley1887, p. 167) felt that, “The characters on which these animals should be classified are, I submit, those which pervade the several parts of the skeleton, and exhibit some diversity among the associated animal types” So his reasons for dividing dinosaurs were based on two major convictions: a Quinarian perspective on classification that was not tree-like but built on a non-evolutionary scheme of groupings into five, and a presumption (common to most systematists until the advent of numerical taxonomy in the 1950s and cladistics in the 1970s) that some characters should be weighted more heavily than others in establishing relationships.
Seeley was silent on the functional and ecological circumstances involved in the rise of dinosaurs and other groups, as indeed were most later 19th Century authors, because there was very little evidence of possible precursors.
1.2. Dinosaur origins: archosaurs, thecodontians, and pseudosuchians in the 19th–20th centuries
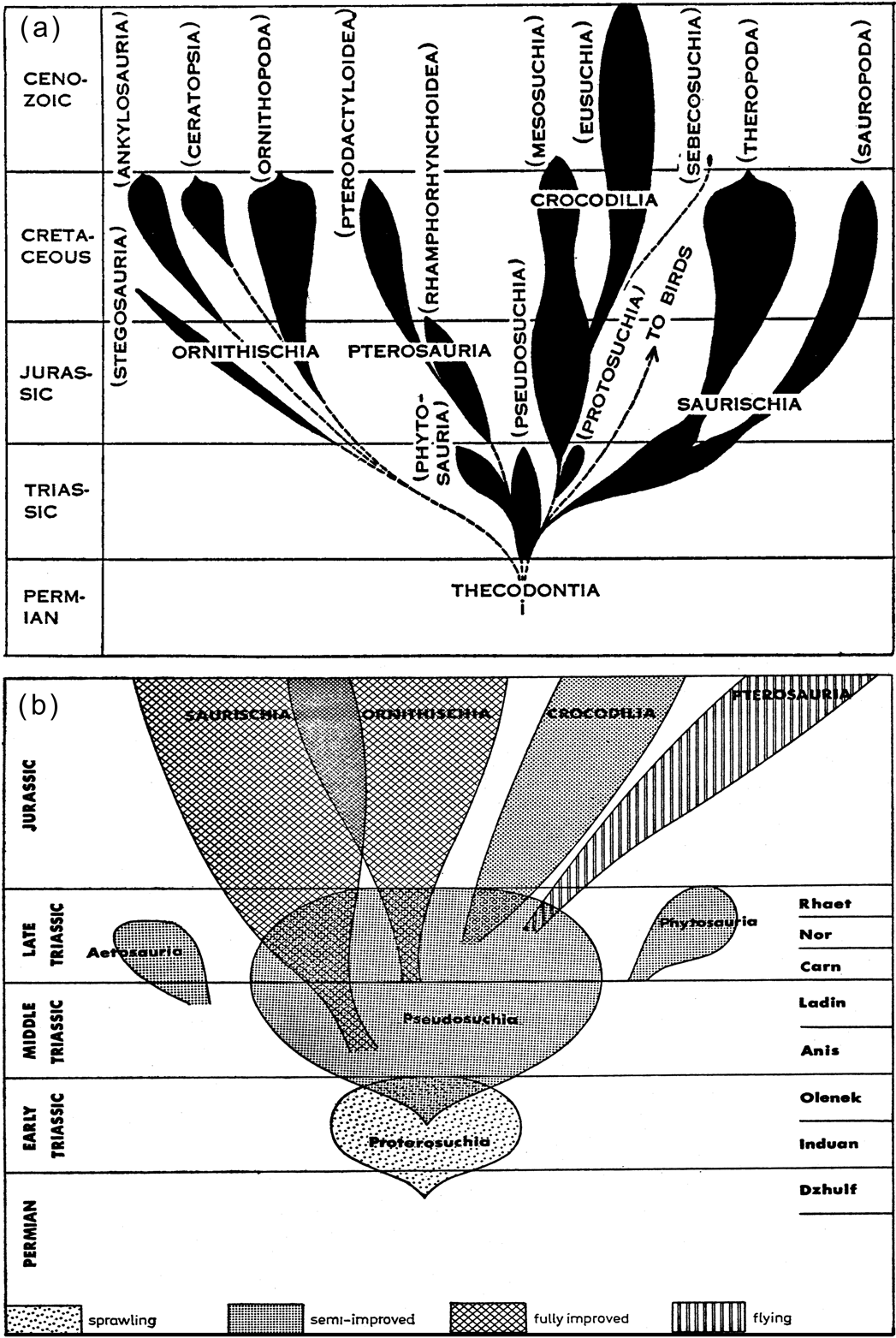
As a result of Seeley's (Reference Seeley1887) analysis, the monophyly of Dinosauria was placed in considerable doubt. Although “Dinosauria” was used by scientists and commonly by the public afterward, it was largely disregarded as a formal category for another century (see e.g. Huene Reference Huene1914, Reference Huene1956; Romer Reference Romer1945, Reference Romer1968). This taxonomic limbo is instantiated in illustrations that accompanied some of the most influential works on dinosaurs; namely, the “bubblegrams” typical of the 1940s through comparatively recent times (e.g. Romer Reference Romer1945; Carroll Reference Carroll1990; Fig. 2). Bubblegrams allowed “stem groups,” which were explicitly paraphyletic, to “give rise” to a series of different, more derived groups, without specifying their relationships to each other. So, for example, a bubble of “thecodonts” (sic: “thecodont” is a type of socketed dentition; a “thecodontian” was a type of archosaur [sensu lato] that had this dental configuration but was not a dinosaur, pterosaur, or crocodilian) was broadly viewed as “ancestral” to other archosaurian groups.

Figure 2 Sample bubblegrams to show postulated relationships of some archosaurian groups in the middle decades of the 20th Century: (a) from Romer (Reference Romer1945); (b) from Charig (Reference Charig and Kuhn1976a)
But where did the terms “archosaur” and “thecodont” and even “pseudosuchian” come from? Scholars today are brought up with late 20th-Century versions of these terms, which are mostly based on phylogenetic systematics and, therefore, rooted in diagnostic synapomorphies. But it is not possible to understand the literature of the earlier 20th century on dinosaur origins without knowing something of the historical genesis of these terms, and remembering that synapomorphies were not explicitly recognised or required for diagnosing taxa.
1.2.1. Archosauria
Cope erected the name Archosauria (usually translated as “ruling reptiles”; a better description might be “elite” or “advanced”) in Reference Cope1869, and in it he included the crocodiles, both “orders” of dinosaurs, the “thecodontians” (see below), and also the Sauropterygia, the anomodont dicynodonts, and the rhynchocephalians (comprising rhynchosaurs and sphenodontians in those days). However, he did not include the pterosaurs, which he regarded as a separate taxon of equivalent rank with the archosaurs. Nothing unites these groups except that they are not amniotes with overall primitive character states, and the constitution of Cope's Archosauria soon began to unravel (Charig Reference Charig and Kuhn1976a).
1.2.2. Thecodontia
For the history of this group I largely paraphrase Charig's (Reference Charig and Kuhn1976b) superb summary. Owen named Thecodontia in Reference Owen1842, as a subgroup of Lacertilia, because they had thecodont rather than acrodont or pleurodont teeth. He did not think that these were “lacertilians” in the sense of lizards, only that they were of a similar grade of organisation and with vaguely similar features (divergent fifth toes, etc.). At first, he included in Thecodontia only Thecodontosaurus, Palaeosaurus, and Cladeiodon, the first of which is now accepted as a basal sauropodomorph, the second (sensu stricto) probably teeth of some kind of pseudosuchian (sensu Gauthier, Reference Gauthier1986), the third simply a large tooth; but at least they all represented types of thecodont dentition. In Reference Owen1859, Owen added to Thecodontia: Protorosaurus (surprisingly); the phytosaur Belodon; and, oddly, the “pelycosaur” Bathygnathus. This seems surprising to specialists today, but Protorosaurus is a basal archosauromorph, and “pelycosaurs” were then unknown as a group related to mammals, and at least Bathygnathus had thecodont teeth. But again, Owen was looking more at grade of organisation than at group membership through synapomorphies. After the incorrect and dubious taxa are removed, only the phytosaur is left and so phytosaurs embodied the main concept of “thecodont(ian)” for some time, because they were relatively well known.
As pseudosuchians (see below) became better known, they were grouped with the phytosaurs by von Huene (Reference Huene1902), although under Parasuchia, an old name for the aetosaurs and phytosaurs (“Parasuchia” were “alongside crocodiles”). Huxley (Reference Huxley1877) used Parasuchia as the most basal taxon within Crocodilia, along with Stagonolepis and Belodon. Boulenger (Reference Boulenger1903) resurrected Owen's Thecodontia for a largely overlapping constellation of taxa. For a while, there was much confusion about whether to use the word as a taxonomic term or a dental condition, but D. M. S. Watson (Reference Watson1917) eventually clarified it to refer to “archosaurians with clavicles and an interclavicle. Pelvis platelike.” Watson added to the group some “eosuchians,” or basal diapsids, and Howesiidae, a basal group of rhynchosaurs. Von Huene's later work (e.g. Reference Huene1936, Reference Huene1956) gradually helped to develop the concept of Thecodontia that reigned in the mid- to late-1900s (Charig Reference Charig and Kuhn1976b).
1.2.3. Pseudosuchia
This term was erected by Karl von Zittel in the third part of his Handbuch der Palaeontologie (Reference Zittel1887–1890), to receive three genera of aetosaurs: Aetosaurus, Typothorax, and Dyoplax (the last relatively indeterminate). The first discovery of aetosaur remains was only an impression of dermal armour; it was described by Louis Agassiz (Reference Agassiz1844) as the remains of a ganoid fish, and for this reason he named it Stagonolepis (the “-lepis” suffix was frequently given to fishes; it means “scale”). Huxley studied new material in Reference Huxley1859 and realised that it was a reptile. In Reference Huxley1875 and Reference Huxley1877, he grouped it with the phytosaur Belodon and a few other forms into Parasuchia, regarding this group as an early offshoot (or “para-”) of crocodilian stock.
On the basis of new material of Aetosaurus in the Stuttgart museum, Zittel separated aetosaurs from phytosaurs (which were still recognised as Parasuchia, related to crocodilians) and gave the aetosaurs the name Pseudosuchia (“false crocodiles”), as a separate suborder within Crocodylia. Therefore, from the outset crocodilians, phytosaurs, and aetosaurs were recognised as closely related to each other: “rauisuchians” were not yet known, but otherwise this mostly corresponds to present-day thinking. Aetosaurs were the original pseudosuchians, although the term and its membership were considerably expanded in the century since Zittel. The term Parasuchia was used more loosely, corresponding to some degree with Thecodontia by some authors, but after von Huene's influential reclassification (Reference Huene1911, Reference Huene1936) Parasuchia became an equivalent term to Phytosauria alone. Indeed, it is generally argued that these two terms are synonymous and that Parasuchia should have precedence because it is an older term. Von Huene recognised Phytosauria and Pseudosuchia as the two groups of Thecodontia: for him, the Pseudosuchia meant all “primitive” archosaurs that were not phytosaurs. Eventually the Proterosuchia (including the Erythrosuchia) were also separated from the Pseudosuchia.
1.2.4. Taxonomic fluidity and evolution
This brief narrative is hoped to convey some of the ambiguity and incomplete knowledge that invested the use of the names “archosaur,” “thecodont(ian),” and “pseudosuchian” in the early to mid 20th century. However, by mid-century there was at least a general hierarchical stabilisation. Archosauria included Thecodontia, Crocodylia, Pterosauria, and both orders of dinosaurs, which were not recognised as a natural group. Thecodontia included Parasuchia and Pseudosuchia, although Romer (Reference Romer1966) also included Proterosuchia and Erythrosuchia and most later authors followed suit; e.g. Charig in the Handbuch der Palaeoherpetologie (1976). Pseudosuchia included a variety of “families,” depending on the author.
From even before the 1950s until Gauthier's phylogenetic classification of 1984–1986, the whole “bubble” of Thecodontia comprised a group of “family-level” taxa, some monotypic (Scleromochlidae, Erpetosuchidae, Lagosuchidae) and others with many genera and species (Phytosauria, Aetosauria). The family-level designation was applied more to show distinction from other groups, and never to unite groups with each other. If anything, Pseudosuchia was used in its more or less original sense of phytosaurs and aetosaurs, plus anything that generally resembled them, such as the animals that were seen as the precursors of crocodiles. “Thecodontians” represented a grade of organisation, a level of archosaurian evolution (remembering that in those days “archosaur” was more like what we would today call Archosauromorpha [Gauthier Reference Gauthier1984, Reference Gauthier1986], broader than the bird-crocodile crown group) from which both orders of dinosaurs, the crocodiles, and the pterosaurs had evolved.
How did this classification scheme influence mid-century ideas of the origins of dinosaurs and their features? Colbert (Reference Colbert1955, pp. 148–150) saw the Early Triassic South African archosauriform Euparkeria as a sort of archetypal model “thecodont” from which various archosaurian types evolved, including early theropods such as Coelophysis (which became more gracile and agile) and ornithosuchians and aetosaurs (which became larger, more ponderous, and more heavily armoured). Romer (Reference Romer1968, p. 131) was more explicit in his Notes and Comments on Vertebrate Paleontology, emphasising that Euparkeria showed that early thecodontians were bipedal despite later tendencies in some subgroups to quadrupedality. Romer held this view for a long time, and he was largely influenced by von Huene's various earlier works on archosaurs. In his Reference Romer1945 edition of Vertebrate Paleontology, for example, Romer speaks of archosaurs being characterised by a tendency toward bipedal gait, although he admits that some archosaurs never seem to have had it and some reverted to quadrupedality. The possession of relatively short forelimbs was commonly all that was required to attribute bipedality, facultative bipedality, or its tendency to an extinct archosaur. And, as Romer (Reference Romer1945) detailed, a cascade of features such as more sacral vertebrae, a more robust pelvis, an inturned femoral head, longer hindlimbs, a mesotarsal ankle, and a parasagittal gait often followed. It is curious today to think of being able to speak of “evolutionary tendencies” and convergences without an independently derived phylogeny: Romer (Reference Romer1945, p. 212) remarked that “in forms as far apart as carnivorous dinosaurs and birds the feet are almost identical in structure and in phalangeal formula”. But the subtext of Romer's views will become clearer in discussing Alan Charig's work.
2. Mid-latter 20th century scenarios for the origin of dinosaurs
How dinosaurs evolved their body plan and the functional and ecological features that differentiated them from contemporary archosaurs was a difficult question to answer at mid-century, given that there were no good candidates for “ancestors,” “precursors,” or any other term that signified phyletic origin. Also, it was generally acceptable to search for direct ancestors in the fossil record. Given that the monophyly of dinosaurs was generally doubted, it was possible to imagine different scenarios for the origin of different groups. To make matters more complicated, most of the “thecodontians” described from the Middle and Late Triassic were large forms, in the order of 3–4 metres in length or longer, whereas Triassic dinosaurs were half that size (except the large plateosaurs and some other basal sauropodomorphs), and had the peculiar distinction of a mesotarsal ankle and more gracile pelvic and limb proportions. Moreover, the potential “thecodontian” ancestor candidates were often contemporaneous with the first known dinosaurs, and so they were considered “too late” to be directly ancestral. The footprint faunules of the Late Triassic and Early Jurassic provided no clue about functional transitions, but merely a stratigraphic succession of quite distinct types of footprints (e.g. Olsen & Galton Reference Olsen and Galton1984; Haubold Reference Haubold and Padian1986).
2.1. Alan Charig and the mid-century problem of dinosaur origins
The work of Alan Charig and his colleagues was considerably influential in the thinking of the early 1960s through the 1970s on the problems of dinosaur origins. Charig was Curator of Fossil Amphibians, Reptiles and Birds at the British Museum (Natural History), now the Natural History Museum, in London, from 1961 to 1987 (see Moody & Naish Reference Moody, Naish, Moody, Buffetaut, Naish and Martill2010). Although he published relatively few descriptions of taxa, Charig was a stimulating and critical thinker, and he had decisive views on the origins of dinosaurs, based largely on some field work and anatomical analyses that he had performed on Triassic archosauromorphs from eastern and South Africa in the course of his dissertation work.
However, before citing Charig's views here, several particulars should be recalled about palaeontological consensus views at mid-century: (1) Dinosauria was not regarded as monophyletic; (2) almost no Triassic ornithischians were recognised, even though, at the time, many formations now considered Early Jurassic were considered Late Triassic (Olsen & Galton Reference Olsen and Galton1977); (3) as a result, and because ornithischians were considered so derived morphologically, they were not usually considered part of the “origin of dinosaurs” problem; (4) saurischians were usually divided into Sauropoda, Theropoda and Prosauropoda, the last of which were often considered part of Theropoda (but also as having “given rise” to Sauropoda or classified within Sauropodomorpha: e.g. Romer Reference Romer1945, Colbert Reference Colbert1955); (5) so the problem of the “origin of dinosaurs” usually reduced to the problem of the “origin of Saurischia,” because theropods (which were seen as the least derived saurischian group) were regarded as the most primitive saurischians. To be precise, the origin of dinosaurs was focused on what was then understood to be Coelurosauria: specifically, in the sense of von Huene (Reference Huene1932), who had essentially regarded the large ones as Carnosauria and the small ones as Coelurosauria (in contrast to how Gauthier parsed them cladistically in 1986, although von Huene recognised tyrannosaurs as coelurosaurs). It must be said, however, that this description simplifies a complex picture. Some “prosauropods” were classified as theropods or Sauropodomorpha by some anothers. It was not out of the question to classify the same animal within theropods but to accept that it could have been close to the origin of sauropods, for example.
Clearly, the methods by which phylogenetic relationships were assessed were less prescriptive than those of today. Nevertheless, it seems clear that for most mid-century paleontologists, the origin of dinosaurs was essentially a problem of getting from a generalised “pseudosuchian” (read basal archosaur) to a generalised, small “coelurosaur” (read basal theropod). At the time, Triassic theropods were known from forms such as Coelophysis, which Colbert had described briefly in Reference Colbert1947; Podokeosaurus, known only by that time from a cast of an impression of a partial skeleton on a rock of Triassic (now considered Jurassic) sandstone (the original had been destroyed in a fire; Talbot Reference Talbot1911); some fragmentary taxa from Europe that von Huene (Reference Huene1932) had summarised; and Segisaurus, which Charles Camp (Reference Camp1936) had described from the Navajo Sandstone of Arizona (then thought to be Triassic, but now considered Jurassic), which was often overlooked. The difficulty, again, was that the “pseudosuchians” were generally larger than the “coelurosaurs,” which meant that they were usually quadrupedal, often armoured, and tended to approach crocodilian habits more than dinosaurian ones.
Ornithosuchus, from the Late Triassic of Scotland, was an exception because it seemed to have bipedal tendencies and had short arms, which partly accounted for inferences about its bipedal tendencies. Alick Walker (Reference Walker1964), whose main research focus was the study of the fossil reptiles from the Triassic Lossiemouth Sandstone Formation of northeastern Scotland (often called the “Elgin Sandstones,” which included both Permian and Triassic rocks), produced a painstaking and anatomically thorough monograph on this animal which T.H. Huxley had originally described. Walker, however, thought that Ornithosuchus prefigured not the ancestry of all the dinosaurs, but specifically the Carnosauria, which (in von Huene's (Reference Huene1932) sense) were known only from the Late Jurassic (Allosaurus) through the Cretaceous (Tyrannosaurus). This created a huge complication: how could Carnosauria and Coelurosauria have separate origins if they are all theropods, and how could a Late Triassic animal like Ornithosuchus be the ancestor of a group of large theropods that did not show up until 70 million years later (and where could carnosaurs have hidden for that long)?
Alfred S. Romer followed Walker's (Reference Walker1964) assignment of Ornithosuchus to Saurischia in his third edition of Vertebrate Paleontology (1966), but not without misgivings (Romer Reference Romer1968). This situation requires some context. Romer was the dean of vertebrate paleontology until his sudden death in late 1973. His was the only American textbook on the market except E.C. Olson's Vertebrate Paleozoology (Reference Olson1971), influential in a different way but without Romer's broad success. As a result, Romer was constantly besieged by colleagues to include their findings in his next printing, and he was often berated for not adequately considering their discoveries or points of view (his legendary reply was “we'll get it in the next edition”). It was important for him to be diplomatic, especially in the absence of decisive evidence. In Notes and Comments (1968, pp. 131–132, 136–137), he says that he accepted Ornithosuchus as a saurischian rather than a pseudosuchian “with considerable qualms,” and that it might rather be “an advanced, near carnosaur thecodont.” The flavour of phylogenetic thinking of those days might be had from Romer's statement that “Very probably the saurischians arose in mildly polyphyletic fashion from two or several pseudosuchian forms.” (Reference Romer1968, p. 132) So perhaps it was not out of the question in those days for Carnosauria and Coelurosauria to have had separate origins but to be included in Theropoda.
However, not everyone agreed, and one strong voice to the contrary was Alan Charig's. Charig did not think that the first dinosaurs evolved from small, lightly built, bipedal “pseudosuchians.” He thought that the first dinosaurs were quadrupedal, not bipedal, and he based this on the kinds of animals that he and his colleagues found in the earlier Triassic localities of eastern and South Africa. The South African animals were not well known. Sometimes, there were isolated bones, and sometimes footprints. The bones in eastern Africa were not of dinosaurs, but of animals that shared some features with dinosaurs (although, as currently understood, not to the exclusion of other Triassic archosaurian groups). For his thesis at Cambridge (1956), Charig described some Middle Triassic archosauromorphs from Tanzania, which he informally named “Mandasuchus,” “Teleocrater,” and “Nyasasaurus” (see Appleby et al. Reference Appleby, Charig, Cox, Kermack, Tarlo, Harland, Holland, House, Hughes, Reynolds, Rudwick, Satterthwaite, Tarlo and Willey1967 for some details). Regrettably, he never actually published formal descriptions of these and other animals, although he referred to them in various abstracts and commentaries for some years to come. Charig frequently alluded to studies in preparation as manuscripts in press and this caused substantial confusion.
Moody & Naish (Reference Moody, Naish, Moody, Buffetaut, Naish and Martill2010) provided a nuanced picture of Alan Charig's Weltanschaaung and his contributions to the field over the years. The judgment of his colleagues suggests that Charig, for all his abilities and insights, was a better rhetorician than an empirical scientist, and intensely interested in the rhetoric of scientific arguments. More than one of his colleagues remarked that he should have been a barrister. As Moody and Naish noted, Charig often published papers that were styled a “commentary” or “critical review” or “reasoned approach” or “point of view,” and so on, of the works of others, which seldom introduced new empirical information. But Charig was more than this; in his capacity as Curator at the Natural History Museum and the only dinosaur worker in the UK, he commanded a substantial pulpit and had considerable influence in the field, as well as among prestigious journals for which he was a frequent reviewer.
Given that dinosaurian palaeontology at mid-century was something of a Wild West, it may be understood how Charig could have imputed to his African fossils a critical role in dinosaurian evolution, even though there was little evidence that they were particularly close to dinosaurs. Most of Charig's Middle Triassic Tanzanian archosauromorphs were indeed quadrupedal, but recent research has shown that most of them may not even be crown-group archosaurs (reviewed by Moody & Naish Reference Moody, Naish, Moody, Buffetaut, Naish and Martill2010). In the late 1950s, Charig collaborated with John Attridge and A.W. (“Fuzz”) Crompton in South Africa, who had come across some deposits (considered Triassic at the time) that preserved the bones and footprints of large and early quadrupedal dinosaurs. The three collaborated on an influential paper (Charig et al. Reference Charig, Attridge and Crompton1965), in which they argued that dinosaurs or effectively saurischians (because ornithischians were considered an afterthought, having almost no Triassic representatives) originated from large quadrupedal forms that only secondarily attained bipedal gait in some lineages. Complications arose, however, when Charig concluded that his eastern African Manda archosaurians were related to the South African prosauropods.
Charig et al. (Reference Charig, Attridge and Crompton1965) began with a re-assessment of melanorosaurid (“prosauropod”) material in the South African Museum. They observed that most inferences of previous authors had been based on isolated bones or on collections of bones that had been mistakenly put together and so were useless for taxonomic or proportional studies. They pointed instead to newer studies of material from southern Africa in the “Passage Beds” between the Molteno and “typical Red Beds” (now mostly considered of Jurassic age), saying: “This [material] comprises several incomplete skeletons of a large saurischian (the ‘Maphutseng dinosaur’), the articulated hind-limb of a second large saurischian (the ‘Blikana dinosaur’), footprints ascribed to both of these, and the footprints of yet a third large saurischian (the ‘Soebeng trackways’).” These remains largely comprised the basis of their hypothesis of dinosaurian origins.
Charig and his colleagues never actually described any of this material. The “Maphutseng dinosaur” was later informally given the name Thotobolosaurus by Ellenberger (Reference Ellenberger1970); it has never been described scientifically and appears to be based on a collection of poorly preserved bones found in association with a trash heap in Lesotho, probably pertaining to a prosauropod. The “Blikana dinosaur” was eventually named Blikanosaurus by Galton & Van Heerden (Reference Galton and Van Heerden1985), and it is a basal sauropod. The “Soebeng trackways” have never been described, although Thulborn (Reference Thulborn2006) and others have regarded them as prosauropod tracks. So the argument of Charig et al. (Reference Charig, Attridge and Crompton1965) reduces to the contention that, although these early sauropodomorphs are variously quadrupedal or bipedal (or both facultatively), their original condition was quadrupedal, based on the Manda archosaurians and other proterosuchians; yet there was no real evidence for this at the time, and it is generally understood today that the first sauropodomorphs were bipedal. Romer (Reference Romer1968, p. 137) remarked that this paper influenced him in writing his 1966 textbook, concluding that none of the large, mainly bipedal dinosaurs of the Late Triassic have any relationship to later theropods but are related to sauropod ancestry. In contrast, the more quadrupedal melanorosaurids of the later Triassic seem to be closest to the great quadrupedal sauropods. In this sense Charig et al. (Reference Charig, Attridge and Crompton1965) did the profession a service in removing Prosauropoda from Theropoda and reaffirming that it should be united with Sauropoda as von Huene (Reference Huene1932) had proposed. However, it was never broadly accepted that dinosaurs evolved from large quadrupeds, nor that bipedality was secondary in the group. To the contrary, the idea persisted that dinosaurs must have evolved from small, bipedal, lightly built “pseudosuchians.” But just how this must have happened remained a mystery.
Charig (Reference Charig, Joysey and Kemp1972), in a second very influential paper, complicated the issue further, based largely on his studies (that were never published) of the Middle Triassic archosauromorphs from eastern Africa that he studied for his dissertation (Charig Reference Charig1956). He was convinced that forms such as “Mandasuchus” were related to dinosaurs, but that they were neither sprawling in their posture nor erect and columnar. He essentially invented a posture intermediate between a sprawling and upright gait that he called “semi-improved.” Others had used the terms “semi-improved” or “semi-erect” (see e.g. Bakker Reference Bakker and Hallam1977, Reference Bakker, Thomas and Olson1980), but Charig's deliberate use of it as an intermediate stage in locomotor evolution was original. His choice of terms effectively assumed an evolutionary progression of locomotor function among grades of Triassic archosaurs.
In his 1972 paper, Charig maintained that “Thecodontia were certainly incapable of adopting a ‘vertical’ limb posture,” but that beyond the Chasmatosaurus (Proterosuchian) grade of organisation, the “thecodontians” (i.e. archosauriforms and basal archosaurs) were “semi-improved” and could modify their stance to a limited extent. In this respect, Charig regarded them as comparable to crocodylians. However, Charig interpreted crocodylian stance and gait in an unusual way. Rather than adopting a “semi-erect” stance, crocodylians today tend either to sprawl or to walk with a virtually erect stance and parasagittal gait in their hindlimbs (Charig quoted Bobb Schaeffer (Reference Schaeffer1941), accurately on this point), although the forelimbs and the hindlimbs have different arcs (see e.g. Brinkman Reference Brinkman1980; Padian & Olsen Reference Padian and Olsen1984). There was at the time (and still is) no evidence from living crocodiles of a stance and gait that is evolutionarily intermediate between an all-out “sprawling” condition of most lizards and a fully “upright” condition seen in today's birds: Bakker (Reference Bakker and Hallam1977, Reference Bakker, Thomas and Olson1980) appears to have followed Charig in this scenario. It is important to distinguish between terms used to describe stance and those used to describe gait, because conflicting combinations of stances and gaits are possible (Padian et al. Reference Padian, Li and Pchelnikova2010). What an animal is doing with its limbs (in a neuromuscular sense) to actuate its muscles to adopt a particular limb posture or gait is again a wholly different question, experimentally outside the realm of paleontology in almost all cases. Padian et al. (Reference Padian, Li and Pchelnikova2010) also showed that, if the Triassic trackway Apatopus is indeed attributable to a phytosaur as historically asserted, the ability to draw the limbs under the body and walk parasagittally was common to crown-group Archosauria at least, and perhaps even more basal, depending on whether Phytosauria are basal Pseudosuchia or outside Archosauria.
Charig based his view that Thecodontia could not adopt a vertical limb posture on the structure of the pelvis and hindlimb, compared to those of crocodiles and birds. This was a reasonable view at the time, but it was soon contested. Re-analyses of extinct archosaurian hindlimb morphology, plus the ichnological evidence of narrow-gauge trackways, suggested that “Thecodontia” could indeed adopt a “vertical” limb posture in the sense of an upright stance and parasagittal gait (see Parrish Reference Parrish1986). As Bonaparte (Reference Bonaparte1984) showed, they did this in two different ways: the “crocodile-normal” forms by producing a lateral overhang of the ilium beneath which the femur could swing laterally (abduct and adduct) to adopt different poses; and the “crocodile-reversed” forms by evolving a well-offset femoral head that fit snugly into the (usually perforated) acetabulum but could not adopt any other gait than parasagittal.
2.2. Ankles and ankles
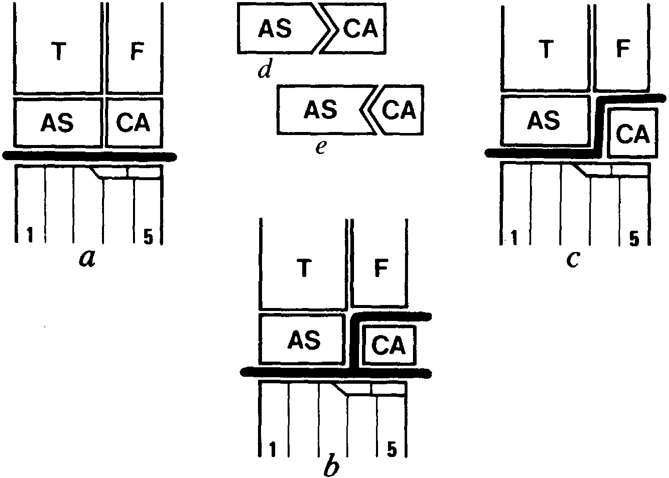
The change in flexion of the ankle from crurotarsal to mesotarsal was critical to explaining the origin of dinosaurian stance and gait in the later 20th century, but the transition had long stymied palaeontologists. The problem was that, in the absence of an independently derived phylogeny for dinosaurs and other archosaurs, no pattern of ankle evolution was independently supported, and so process explanations of functional shifts were the only other available approach. The Swiss palaeontologist Bernard Krebs approached this problem in the early 1960s, with important new evidence. Krebs, a student of the influential European diapsid palaeontologist Emil Kuhn-Schnyder, was describing an animal called Ticinosuchus, from the Middle Triassic of the San Giorgio region of the foothills of the Alps (Krebs Reference Krebs1963, Reference Krebs, Kuhn-Schnyder and Peyer1965). He referred to it, as was customary in those times, as a “rauisuchian,” although the term was coming to be considered something of a waste basket. Krebs realised that his animal was crurotarsal; that is, it flexed its ankle between the astragalus and calcaneum, so that the astragalus was linked to the tibia and the calcaneum to the distal tarsals and metatarsus, whereas dinosaurs flexed their ankles mesotarsally: the tibia, astragalus, fibula, and calcaneum were bound in a functional unit, and the distal tarsals and metatarsus flexed in a straight plane against these (Fig. 3).

Figure 3 A latter 20th-Century view of archosaurian ankle structure, from Thulborn (Reference Thulborn1982). The views in (a–c) are anterior of the left ankle, with functional configurations according to Thulborn: (a) mesotarsal ankle, in which the proximal tarsals (AS+CA) flex with the tibia and fibula against the distal tarsals and foot (dinosaurs, pterosaur, and other ornithodirans); (b) the “duplex” ankle (generally thought primitive), in which the calcaneum can move with either the upper leg or foot (proterosuchians and perhaps other archosauromorphs); (c) a crurotarsal ankle, in which the calcaneum and the foot rotate against the astragalus and upper leg (crocodiles and other pseudosuchians); (d) diagram of the “crocodile-normal” ankle (crocodiles and other pseudosuchians), in which the “peg” of the archetypal “peg-and-socket” joint is on the astragalus and the “socket” is on the calcaneum; (e) the “crocodile-reversed” ankle (ornithodirans) in which the peg is on the calcaneum. Abbreviations: AS=astragalus; CA=calcaneum; F=fibula; T=tibia; 1–5=numbered metatarsals.
How could this transition from crurotarsal to mesotarsal ankle have been accomplished? Krebs (Reference Krebs, Kuhn-Schnyder and Peyer1965) thought that it was not possible. He reasoned that dinosaurs could not have evolved from any known crurotarsal “pseudosuchians.” Their ancestry would have to be sought farther back, in the earliest Triassic or even the latest Permian, among archosaurs with more generalised ankle types, such as proterosuchians or erythrosuchians (e.g. Euparkeria). This idea was generally accepted in those years for a variety of reasons: stratigraphically, dinosaurs and pseudosuchians were considered contemporaneous, so one could not have “given rise” to the other by the reasoning of those times; moreover, each types was specialised. In this sense, the perceived inability for a functional change to occur between two configurations found in two different groups was allowed to force back common ancestry to a more remote time.
Ironically, it may have been Charig (Reference Charig, Joysey and Kemp1972, pp. 150–151) who first suggested an “alternative solution” to the evolution of the dinosaurian ankle other than his favoured idea of a direct evolution of large quadrupedal saurischian dinosaurs from large quadrupedal “thecodontians.” Charig envisioned (almost as a thought experiment) the evolution of digitigrady in the context of erect posture and parasagittal gait, as a way to make locomotion more efficient. Digitigrady would obviate the need for the calcaneal tuber, which actuated the lateral flexion of the crocodile-normal ankle through the astragalo–calcaneal joint; the tuber could therefore be reduced. The elongation of the metatarsus could have followed, to extend the stride. Charig effectively advanced the possibility (which he did not accept) that, to evolve the dinosaurian condition, first the ankle joint would have had to be “stiffened” – that is, the flexion between the astragalus and calcaneum would have constricted, and parasagittal motion would have become largely limited to the interphalangeal and metatarso–phalangeal joints. Then, after the digitigrade stance and parasagittal gait were fully evolved, flexion could have returned between the proximal and distal tarsals, thus creating a mesotarsal ankle.
Charig's “alternative scenario” was not picked up as the most likely hypothesis to solve the problem of dinosaurian origins in the functional-ecological sense, although elements of what he described were already commonly accepted, such as the fact that dinosaurs had upright posture and parasagittal gait, and that their ankle type must have evolved from some kind of more basal ankle type.
However, at nearly the same time that Charig's paper appeared, the dinosaurian world was rocked by the publication of Romer's preliminary descriptions (Reference Romer1971, Reference Romer1972) of the South American Triassic “pseudosuchians” Lagosuchus and Lagerpeton. These small, lightly built animals were not dinosaurs, but were very close to them, as José Bonaparte showed in his Reference Bonaparte1975 monograph on Lagosuchus. And they had mesotarsal ankles. These discoveries showed, as Bonaparte realised, that dinosaurs did not evolve their stance and gait at large size, but at very small size. The problem of the origin of the dinosaurian ankle, with its erect stance and parasagittal gait, was effectively solved. Bernard Krebs appeared to be vindicated. The importance of the discovery of these animals for the understanding of dinosaurian origins cannot be overestimated.
And yet, controversy continued over the details. Because “pseudosuchians” were such a mixed group of animals with no clear phylogenetic lines, arguments about ankle evolution persisted. It was still not clear from which “pseudosuchians” dinosaurs and lagosuchids had evolved, and so the origin of the mesotarsal ankle was still in doubt. Arthur Cruickshank (Reference Cruickshank1979) first laid out the contrast between the “crocodile-normal” and “crocodile-reversed” ankle, the first common to most “pseudosuchians” and the latter common to most dinosaurs, ornithosuchids, and other forms such as Euparkeria. In the former configuration the “peg-and-socket” joint had the peg on the astragalus and the socket on the calcaneum, such that the calcaneum, its heel, the distal tarsals, and the foot rotated as a functional unit through this joint against this astragalus and the upper leg. In the latter configuration, the peg was on the calcaneum but there was relatively little rotation in the ankle. Cruickshank's view was that both ankle types arose from a more generalised (and more mesotarsal) proterosuchian-type ankle, probably in the earliest Triassic or earlier. For Cruickshank, the “prosauropod” ankle type was derived (to the exclusion of the ankles of other dinosaurs) from a crocodile-normal configuration, a view that never gained much currency. He also accepted (following Walker Reference Walker1964) that “carnosaurs” evolved from ornithosuchians, and so he saw a polyphyletic origin of the “advanced mesotarsal” ankle of various dinosaurian groups. Most people agreed with Krebs (Reference Krebs1963) that the mesotarsal ankle must have evolved from something much older and more generalised, such as a proterosuchian or erythrosuchian ankle (e.g. Euparkeria).
Chatterjee (Reference Chatterjee1982) followed Cruickshank in some respects of deriving various archosaurian ankle types from others, including suggesting a phylogenetic arrangement of groups based on ankle structure. He recognised that all dinosaurs shared the same type of “advanced mesotarsal” joint (as compared to the “primitive mesotarsal” joint of proterosuchians, which is too generalised to be so named). He also recognised the importance of Lagosuchus, a “pseudosuchian,” sharing the same dinosaurian configuration and to acknowledge this properly, he proposed the erection of a suborder Lagosuchia within the Order Thecodontia. Although it was still not clear how these types could have transmuted functionally, the pattern of evolution was becoming clearer. There were still quibbles about “advanced mesotarsal-normal” and “advanced mesotarsal-reversed” dinosaurian configurations, and about just which configurations were present in certain ambiguous fossils, but the general outlines of ankle evolution were established. As far as they went, they showed that the mesotarsal ankle was correlated with longer and more gracile hindlimbs, an offset femoral head, a tibia longer than the fibula, elongated metatarsals, upright stance, and parasagittal gait (e.g. Charig Reference Charig, Joysey and Kemp1972).
Oddly enough, even long after descriptions by Romer (Reference Romer1971, Reference Romer1972) and Bonaparte (Reference Bonaparte1975) of the “rabbit thecodonts” Lagosuchus and Lagerpeton, and Bonaparte's demonstration of their importance to dinosaurian origins, Charig (Reference Charig1980) continued to prefer scenarios based on only large quadrupedal forms, with only secondary reversions to bipedality, and he persisted in his progressivist view of “sprawling” to “semi-improved” to “fully improved” archosaurs. But it was generally felt that there was little obstacle to deriving a “crocodile-normal” ankle from any other kind; after all, it had clearly happened. This vindicated what the late Don Baird used to call “The Harvard Law of the Improbable: it happened, therefore it was possible.”
3. The “Dinosaur Renaissance” of the 1970s and the problem of dinosaur origins
3.1. Dinosaurs join the cladistic revolution
The “Dinosaur Renaissance” of the 1970s started innocuously enough with John Ostrom's Reference Ostrom1970 paper, “Mesozoic vertebrates as indicators of terrestrial climates.” He argued that it was difficult enough to predict climates today by the distribution of reptiles, supposed to be “cold-blooded”, which range almost between polar circles but with no particular predictable distributions. The palaeodistributions of dinosaurs, ranging from the equator to well within the Arctic Circle, made little sense in the context of typological views of “reptilian” metabolism and ecology, given, for example, the differences between how Sphenodon and horned toads live (Ostrom Reference Ostrom1969).
But Ostrom had more in mind, because his studies of the coelurosaur Deinonychus (Reference Ostrom1969) and Archaeopteryx had convinced him of a strong connection between dinosaurs and birds; in fact, he concluded on the basis of some strong derived similarities that birds had evolved from theropod dinosaurs such as Deinonychus (Ostrom Reference Ostrom1973, Reference Ostrom1975). For him, dinosaurs were likely more metabolically active than the slow, sluggish reptiles pictured in mid-century publications, although Ostrom was always cautious about going too far. In his works, however, the seeds of Jurassic Park were clearly sown.
In Reference Bakker and Galton1974 Robert Bakker and Peter Galton instantiated Ostrom's vision in systematic terms: they proposed, for perhaps the first time since 1842, that Dinosauria was indeed a monophyletic group, and that it should be separated (along with birds) from other reptiles as a distinct “Class.” Bakker, first an undergraduate student of Ostrom's at Yale and then a graduate student at Harvard, and Galton, a research associate at the Yale Peabody Museum, detailed how different dinosaurs were from “thecodontians” and how ornithischians and saurischians, despite their differences, shared some features not present in non-dinosaurians. The features were not only structural but functional and physiological: dinosaurs were metabolically more active than other reptiles, and were likely endothermic (Bakker Reference Bakker1972, Reference Bakker1975).
The firestorm that greeted this proposal, and Ostrom's more modest argumentation about the differences between dinosaurs and other reptiles, comprised a series of arguments from various fields, most of which were summarised in Thomas & Olson (Reference Thomas and Olson1980). However, that book considered only functional, ecological, and physiological arguments about the origin of dinosaurs and their differences (or lack thereof) from other reptiles. The other half of the form-function problem – the relationships of dinosaurs to other taxa – was again the “yin” to the functionalist “yang.” The person who wound up tackling it never intended to do so.
Jacques Gauthier was a PhD student at Berkeley in the late 1970s, who had done his Masters' degree work at San Diego State University with Richard Estes and Richard Etheridge. His interest was primarily in lizards and, having been steeped in the waters of phylogenetic systematics at SDSU, he wanted to devise a phylogeny of the major lizard groups for his PhD dissertation. For this reason, it was quite appropriate for him to come to Berkeley, because although his initial mentor J. T. Gregory had worked little on lizards per se, Gregory had succeeded Charles Camp, whose Reference Camp1923Classification of the Lizards (based on his PhD work at the American Museum of Natural History) was still the standard in the field over 50 years later.
Gauthier initially did not accept Ostrom's hypothesis that birds were descended from theropod dinosaurs; he had heard arguments by Larry Martin and others (Reference Martin, Stewart and Whetstone1980) that these similarities could be convergences, and besides, Ostrom had not framed his arguments in cladistic terminology. However, to establish character polarities for Lepidosauria and Squamata in the course of his PhD dissertation, Gauthier found that he had to revert to the level of Amniota, and he had to explore the character distributions of Archosauria and their diapsid relatives as well. So, almost as a by-product of his thorough phylogenetic analysis of the sister taxon of archosaurs, Archosauria received its first rigorous cladistic analysis.
The results clarified and simplified things. In his Thesis (1984) and a greatly pared-down summary (1986), Gauthier showed that Dinosauria was cladistically monophyletic (i.e. the members of the group shared apomorphies), and that birds were hierarchically included in Saurischia and Theropoda. He dispensed with “Thecodontia” as a redundant paraphyletic group (a grade rather than a clade) and formalised Archosauria as its two living members (birds and crocodilians) and all the descendants of their most recent common ancestor. He was, thus, able to show that crocodiles were related to a variety of “thecodontians” that had the crocodile-normal ankle joint, and that dinosaurs were related to other “thecodontians” such as Lagosuchus and Lagerpeton that had a mesotarsal ankle (“crocodile-reversed” in many cases). He called the crocodile-line archosaurs Pseudosuchia, even though the “false crocodiles” would thereby include true crocodiles, because it was a more inclusive name. He called the bird-line archosaurs Ornithosuchia (“bird-crocodiles”) because they included birds and because his analysis showed that Ornithosuchidae also belonged there. (Later phylogenetic analyses showed that Ornithosuchidae did not belong among bird-line archosaurs, but that has no bearing on the legitimacy of the definition of the name Ornithosuchia, even though ICZN rules do not apply to phylogenetic definitions.) Pterosaurs were the major sister group to dinosaurs, as Padian (Reference Padian1983b, Reference Padian, Reif and Westphal1984) had suggested. Obviously their adaptations for flight modified much of their pectoral appendicular skeletons, but they still shared apomorphies with dinosaurs, particularly in their less extremely modified vertebrae and hindlimbs. And, although they were highly derived in other parts of their skeletons, they still formed a phylogenetic nexus with dinosaurs, Lagosuchus, and Lagerpeton (Gauthier Reference Gauthier1984, Reference Gauthier1986).
This was not the last word on ankle evolution, however, and the picture is still not entirely clear. As Cruikshank (1979), Chatterjee (Reference Chatterjee1982) and Parrish (Reference Parrish1986), among others, showed, the variation among archosaur tarsals was not simple or easy to characterise in broad patterns. With the shift of Ornithosuchidae from Ornithosuchia to Pseudosuchia (Sereno Reference Sereno1991), the picture became more consistent, and a new phylogeny by Nesbitt (Reference Nesbitt2011) places phytosaurs outside Archosauria.
The credit for the modern argument that Dinosauria is monophyletic goes to Bakker and Galton because most of the features they described do indeed make Dinosauria a monophyletic group, even though they did not perform a strict phylogenetic analysis to demonstrate it. On the other hand, many of the features that they described were known at the time to apply also to pterosaurs and to Lagosuchus and Lagerpeton, and others have been discovered since then. Gauthier (Reference Gauthier1984, Reference Gauthier1986) established the necessary cladistic analysis and also formally linked the last three taxa to dinosaurs within the new taxon Ornithodira.
4. An evolving revision of the timing of dinosaur origins and early evolution
4.1. Stratigraphic realignment and the discovery of an Early Jurassic terrestrial record
The role of biostratigraphy in assessing the timing of faunal succession and the origin of dinosaurs has a long and important history. “Romer's Gap” is the name commonly given to the absence of terrestrial vertebrate fossils between the advent onto land in the Devonian and the radiation of tetrapods in the Carboniferous. But Romer (Reference Romer1968) bemoaned a second gap, the absence of fossils between the first dinosaurs of the Late Triassic and the fully developed dinosaurian ecosystems of the Late Jurassic (complete with allosaurs, coelurosaurs, stegosaurs, huge sauropods, and various ornithopods). Briefly, a contributing factor was that the geological timescale was established on European deposits. In the Early Jurassic, epeiric seas covered most of Europe, so there was little idea of what an Early Jurassic European terrestrial vertebrate fauna should look like. Triassic vertebrate faunas of phytosaurs, aetosaurs, prosauropods, and some theropod remains were known from Europe, and these plus associated footprint faunas created a diverse picture of life in the Triassic. These faunas were correlated worldwide with formations in North America, South America, Africa, China and India.
However, studies of European deposits suggested that “thecodontians” did not survive the Triassic. As a result, horizons worldwide were correlated by a simple metric: if “thecodontians” were present, it was Triassic; if they were absent, it was Jurassic. Absence of “thecodontians” was not necessarily definitive, and it was possible, for example, for Welles (Reference Welles1954) to make the argument that the Kayenta Formation of Arizona was of Jurassic age because the hindlimb of the theropod Dilophosaurus was of “Jurassic grade.” To this argument, Colbert (Reference Colbert1981) countered that aetosaurs were known from the Kayenta, and therefore it was of Triassic age. Padian (Reference Padian1989) showed that the scutes on which the aetosaur identification was based actually belonged to Scelidosaurus, a thyreophoran dinosaur known from the Sinemurian–Pliensbachian (Early Jurassic) of England. This removed evidence that the Kayenta Formation was of Triassic age, and tended to lend support to a Jurassic age. But this was only a minor piece of the whole puzzle.
In the 1970s, a group of researchers independently studying the palaeontology of the Newark Group of the eastern seaboard of North America came across a conundrum. Exposures of the Newark Group extended from Nova Scotia to North Carolina and represented a series of rift lake basins formed by the split of North America and Africa beginning in the Middle Triassic or even earlier. Among many others, Nick McDonald and Bobb Schaeffer were studying the fishes; Bruce Cornet tackled the palynology; Paul Olsen, Don Baird, and Peter Galton worked on the skeletal and ichnological fossil tetrapods. After most of a decade of work, the results were clear. Although the Newark Group had been traditionally considered entirely Triassic, approximately half of it was situated firmly in the Jurassic. The basis of this work was lithostratigraphic correlation, but the fossils told an impressive story. Not all basins contained the same fossil floras and faunas; and stratigraphically, within basins, the associations of plants and animals correlated well with each other. According to Cornet's studies, the Jurassic was marked by: the loss of striate Classipollis pollen; the loss of “thecodontians”; the evolution of larger theropods and sauropodomorphs; and the advent of ornithischians.
Through a series of papers, Paul Olsen and his colleagues (e.g. Olsen & Galton Reference Olsen and Galton1977; Olsen et al. Reference Olsen, Shubin and Anders1987; Olsen & Sues Reference Olsen, Sues and Padian1986) characterised a distinct set of terrestrial faunas and floras across the Triassic-Jurassic boundary in the Newark Group (now renamed the Newark Supergroup), and revised the dating of geological formations worldwide to reflect these biostratigraphic correlations. Following this revision, about half of the horizons previously considered Late Triassic were placed in the Early Jurassic. Romer's second gap was filled – at least through the Early Jurassic. To some observers, the loss of “thecodontians” in the latest Triassic was both close to the Triassic–Jurassic boundary and relatively abrupt (e.g. Olsen et al. Reference Olsen, Shubin and Anders1987), even though the faunal evidence was limited both in specimens and in geographic extent.
What did these advances mean for the question of dinosaur origins? At the very least, the stratigraphic realignment appeared to both order and constrain the first and last appearances of dinosaurs and their relatives during the Late Triassic and Early Jurassic. “Thecodontians” did not persist into the Jurassic; almost no ornithischians were known from the Triassic (with almost the sole exception of the poorly preserved and questionable Pisanosaurus from Argentina, and a couple of fragments from elsewhere); and Triassic theropods and basal sauropodomorphs were unevenly represented among the continents (“prosauropods” were absent in North America, theropods rare in Europe and southern Africa, for example). By the Early Jurassic, all three major lineages of dinosaurs were found worldwide.
The Triassic picture of faunal succession became clearer. Research by Romer's Harvard field crews and later by José Bonaparte and his colleagues in Argentina had discovered faunas of terrestrial tetrapods ranging from what was reckoned the Middle Triassic up through the latest Triassic (Bonaparte Reference Bonaparte1982). The earliest of these, from the Middle Triassic, were dominated by various therapsids but (among other reptiles) Lagosuchus and Lagerpeton were also present (Chañares Formation). The last two dinosaur relatives were absent from the early Late Triassic assemblage (Ischigualasto Formation), but the basal dinosaurs (or very close thereto) Herrerasaurus and Ischisaurus were present, as was Pisanosaurus, and Staurikosaurus, usually considered close to Herrerasaurus, is present in the roughly coeval deposits of Brazil. By the latest Triassic various basal sauropodomorphs were found, but no ornithischians and no theropods. Because South America was the only continent on which dinosaur relatives such as Lagosuchus (later named Marasuchus by Sereno & Arcucci [Reference Sereno and Arcucci1994]) and Lagerpeton (and herrerasaurids) had been found, it was presumed that these animals were quickly replaced by true dinosaurs in the latest Triassic. However, the South American fossil record, did not exactly say that because true dinosaurs were not well represented in the latest Triassic, apart from sauropodomorphs.
4.2. Recent discoveries and yet another paradigm shift
Since the 1970s and 1980s, when the paradigm shift just described provided us with our Early Jurassic record, palaeontological exploration of Triassic–Jurassic sediments has not been idle (Fraser & Sues Reference Fraser and Sues1994; Fraser Reference Fraser2006; Sues & Fraser Reference Sues and Fraser2010). And some very important new works have re-analysed and synthesised our knowledge of the Triassic–Jurassic record of dinosaurs and their relatives (e.g. Upchurch et al. Reference Upchurch, Hunn and Norman2002; Irmis et al. Reference Irmis, Nesbitt, Padian, Smith, Turner, Woody and Downs2007a, Reference Irmis, Parker and Nesbittb; Nesbitt et al. Reference Nesbitt, Irmis and Parker2007; Brusatte et al. Reference Brusatte, Nesbitt, Irmis, Butler, Benton and Norell2010; Langer et al. Reference Langer, Ezcurra, Bittencourt and Novas2010).
From South America came a variety of new dinosaurs, including the basal sauropodomorphs Saturnalia, Panphagia, Chromogisaurus, and the theropods Guibasaurus and Zupaysaurus (reviewed in Irmis Reference Irmis2011), but no ornithischians except a possible heterodontosaurid jaw fragment from Patagonia (Báez & Marsicano Reference Báez and Marsicano2001). More sauropodomorphs have been found in the South African Late Triassic, but no theropods, and the ornithischian Eocursor (Butler et al. Reference Butler, Smith and Norman2007) comes from what is recognised as the latest Triassic beds (although there are suggestions that it could equally be from the Early Jurassic). Ornithischians and theropods begin to radiate in the Early Jurassic. In western North America, there are no ornithischians or sauropodomorphs in the Late Triassic, but the pattern differs in eastern North America (where footprint records suggest their presence, somewhat questionably) and Europe (Nesbitt et al. Reference Nesbitt, Irmis and Parker2007). These patterns appear in the time of Pangaea, when all the continents are together and physical barriers to dispersal are more difficult to establish. As Irmis (Reference Irmis2011) noted, no single hypothesis appears to explain the distribution of the various dinosaur groups worldwide in the Late Triassic; sometimes they are there, and sometimes they aren't. But as a general pattern, ornithischians are virtually absent from the Late Triassic record. Revueltosaurus, known only from teeth, was widely considered a Triassic ornithischian for many years, but Parker et al. (Reference Parker, Irmis, Nesbitt, Martz and Browne2005) showed that these teeth actually belonged to a pseudosuchian. And, given the poor preservation and incompleteness of Pisanosaurus and the heterodontosaurid jaw described by Báez and Marsicano (Reference Báez and Marsicano2001), and the absence of certified Triassic ornithischian footprints, Eocursor may be the earliest valid ornithischian.
So, stratigraphically, we are left with almost no ornithischians worldwide in the Triassic, and a disjunct distribution of sauropodomorphs or theropods (occasionally both) among various continents at the same time. This stratigraphic disjunction would be odd if ornithischians and saurischians are sister groups, because we would expect them to be equally ancient in their representation in the fossil record, absent circumstances of preservation such as the often-invoked but untested “upland vs. lowland” differences among taxa. But theropods and sauropodomorphs are also sister taxa, and they do not often co-occur in the Late Triassic.
In a recent paper, Rowe et al. (Reference Rowe, Sues and Reisz2011) proposed that Early Jurassic “prosauropods” of North America have been inappropriately lumped taxonomically, and that the three currently known North American forms evidently do not form a monophyletic group to the exclusion of other basal sauropodomorphs on other continents. These authors conclude that the North American “prosauropod” fauna evolved from three or four independent sources on other continents. If this pattern is confirmed (and it obtains even using two quite different phylogenetic analyses of other authors), it will be interesting to see whether other taxa follow a similar pattern.
4.2.1. A modest proposal: reconsidering ornithischian origins
Given the global lack of ornithischians in the Triassic and despite the intensity of sampling of thousands of specimens across many continental regions, the hypothesis has to be entertained whether ornithischians evolved from another saurischian group such as prosauropods or silesaurids, as Galton (Reference Galton1970) and Langer & Benton (Reference Langer and Benton2006) proposed, respectively. This would remove the supposition that ornithischians are so rare in Late Triassic deposits because they were restricted to environments that are not preserved in the rock record. The phylogenetic hypothesis that ornithischians may have evolved from saurischians cannot be regarded as most likely at present, because many cladistic analyses have accepted the unity of Saurischia. However, the composition of Saurischia (and even Theropoda), whether or not it includes forms such as Herrerasaurus and Eoraptor, has been variable. And, at some level in Dinosauria, the characterisation of Saurischia is based to some extent on plesiomorphies, because even the most basal Ornithischia that we know are highly derived in comparison to basal Saurischia. It is possible, and it should be investigated openly, that Ornithischia is regarded as the sister taxon of a (relatively mutable) Saurischia (that may or may not include Herrerasaurus, Eoraptor, and similar forms) largely because the first ornithischians are so derived and basal saurischians are not.
Pisanosaurus, the isolated jaw described by Báez & Marsicano (Reference Báez and Marsicano2001), and Eocursor are all generally assigned to basal ornithischian taxa. Pisanosaurus has been considered a heterodontosaurid, but is usually regarded as outside all other ornithischians (review in Butler et al. Reference Butler, Upchurch and Norman2008); the isolated jaw was assigned to Heterodontosauridae; and Eocursor is regarded as crownward of Pisanosaurus and Heterodontosauridae but outside other ornithischians (Butler et al. Reference Butler, Smith and Norman2007). If these assignments are valid, then Heterodontosauridae, but not “fabrosaurs” Thyreophora or other groups, were present by the end of the Triassic.
If we accept these records and the hypothesis that Ornithischia and Saurischia diverged at least by the early mid-Triassic, then we have to ask where the ornithischians hid out until the Jurassic, because they are so rare. Rarity per se is not unusual in the Triassic: turtles, pterosaurs, non-sphenodontid squamates, and other groups have relatively poor Triassic records, given the distinctness of their body plans and their estimated dates of first appearance. We can accept ornithischian rarity for what it is; we can recur to the conventional and poorly testable hypothesis that they must have lived in upland or other environments not conducive to preservation (this does not work for Triassic sauropodomorphs, which have an uneven geographic distribution). Or we can pursue a different possible path.
It is unconventional to question phylogenetic results based on stratigraphic disjunctions, and I do not propose that stratigraphy should trump phylogeny; rather, they should strive for mutual illumination when they pose inconsistencies. The correspondence between phylogeny and stratigraphy has always been variable (e.g. Norell & Novacek Reference Norell and Novacek1992), and the approach cannot be discarded philosophically. The correct use of parsimony (methodological parsimony) tells us that the simplest hypothesis should be selected first for further testing, not that it is likely to be the correct hypothesis (ontological parsimony). It would not be the first time that a major cladistic division would have proven incorrect or highly questionable, and that some of the first clues to these problems have come from stratigraphic disjunctions. For example, Sereno's (Reference Sereno1986) early cladistic analysis found that that Marginocephalia (Ceratopsia and Pachycephalosauria) were the sister taxon to all ornithopods (sensu lato), which would have meant their divergence and first appearance as far back as the latest Triassic, some 70 million years before there is evidence of the first marginocephalians. This hypothesis has not been sustained by further research.
Rowe & Gauthier (Reference Rowe, Gauthier, Weishampel, Dodson and Osmólska1990) likewise proposed the erection of a monophyletic Ceratosauria, which appeared to include all Late Triassic and Early to Middle Jurassic theropods known at the time, leaving its proposed sister group Tetanurae (true Carnosauria and Coelurosauria) with no fossil representatives until the Late Jurassic. In both cases, and in the case of Ornithischia, it was possible to be influenced by the fact that one group appeared to share a series of synapomorphies that separated it so distinctly from other groups that it seemed difficult to imagine that it was not monophyletic. We still have a substantial morphological gap between the first ornithischians and other dinosaurs: we appear to lack forms with only one or a few ornithischian synapomorphies that separate them from other dinosauriforms. Such differences can make symplesiomorphies of related groups seem like synapomorphies. And so, as we continue to emphasise the cladistic distinctness of Ornithischia, we risk incorrectly polarising characters and separating taxa by asking the simple tool that we call a cladogram to do more than it is really capable of doing.
I want to be clear that I am not proposing that ornithischians must have evolved from dinosaurs that we now call saurischians; I am proposing that the question be reconsidered in future studies of the anatomy and phylogenetic analysis of ornithodirans, because the stratigraphic gap in the Late Triassic is unquestionably significant, and it may be telling us something. It may be that ornithischian “ancestors” – taxa that can link known ornithischians more closely to other dinosauriforms – are already known to science but are not recognised as such. New fossil discoveries may help to answer this question; for example, Panphagia (Martinez & Alcober Reference Martinez and Alcober2009) showed the carnivorous or omnivorous origins of Sauropodomorpha (to no one's surprise, because its outgroups are all carnivorous). It could also be that character codings are unintentionally obscuring patterns of relationship. Sorting out problems such as these comprise some of the challenges that remain, and ought to come from an integration of several independent lines of evidence. After all, in the Late Triassic we have theropods from every region of the world where land tetrapods are found. We have basal sauropodomorphs at many of them but not others. But we have unquestioned ornithischians from almost none of them over a period of 25–30 million years, depending on whether Pisanosaurus is accepted as an ornithischian (Parker et al. Reference Parker, Irmis, Nesbitt, Martz and Browne2005; Irmis et al. Reference Irmis, Parker and Nesbitt2007b; Butler et al. Reference Butler, Upchurch and Norman2008).
5. Disjunction between the skeletal and ichnological records of early dinosaurs
A final line of evidence that must be broached in the question of dinosaur origins is ichnological. Hypotheses about the timing of origin and the early diversification of dinosaurs have historically faced a nagging problem: the bones and the footprints do not match very well. In fact, the world of dinosaurs looks quite different when alternately perceived through osteological or ichnological lenses (e.g. Irmis Reference Irmis2011, pp. 407–408). When distinct, diagnostic footprints and trackways of dinosaur groups are first identified in the Late Triassic, they fall morphologically into the classic three taxonomic divisions of dinosaurs: theropod, sauropodomorph, and ornithischian. The basal sauropodomorph (or “prosauropod”) morphology is distinguished by a broad track with four robust pedal digits and a broad, crescentic manus print. Theropod and ornithischian footprints are tridactyl, and can be distinguished because ornithischian pedal digit impressions are generally more robust, the angle formed by the second and fourth digits is closer to 90° than 30–45° (as in theropods), the third digit in theropods is generally longer than in ornithischians (though this can vary with size), and the claw impressions are generally more acute in theropods (Thulborn Reference Thulborn2006; see Fig. 4). The morphological features preserved in typical footprint faunas would suggest no relationship between theropods and sauropodomorphs; in fact, the tridactyl tracks of theropods and ornithischians are more similar to each other. Sauropodomorph trackways are commonly quadrupedal, especially in larger forms, but it is uncommon for manus prints to be preserved in ornithischian and theropod (and sometimes basal sauropodomorph) trackways. When they are present, ornithischian manus prints are usually pentadactyl and the digits are divergent over 180° or more, whereas the theropod manus print (presuming that Atreipus is a theropod track, following Thulborn Reference Thulborn2006; it was named as an ornithischian track by Olsen & Baird Reference Olsen, Baird and Padian1986) has four or three digits that point anteriorly.
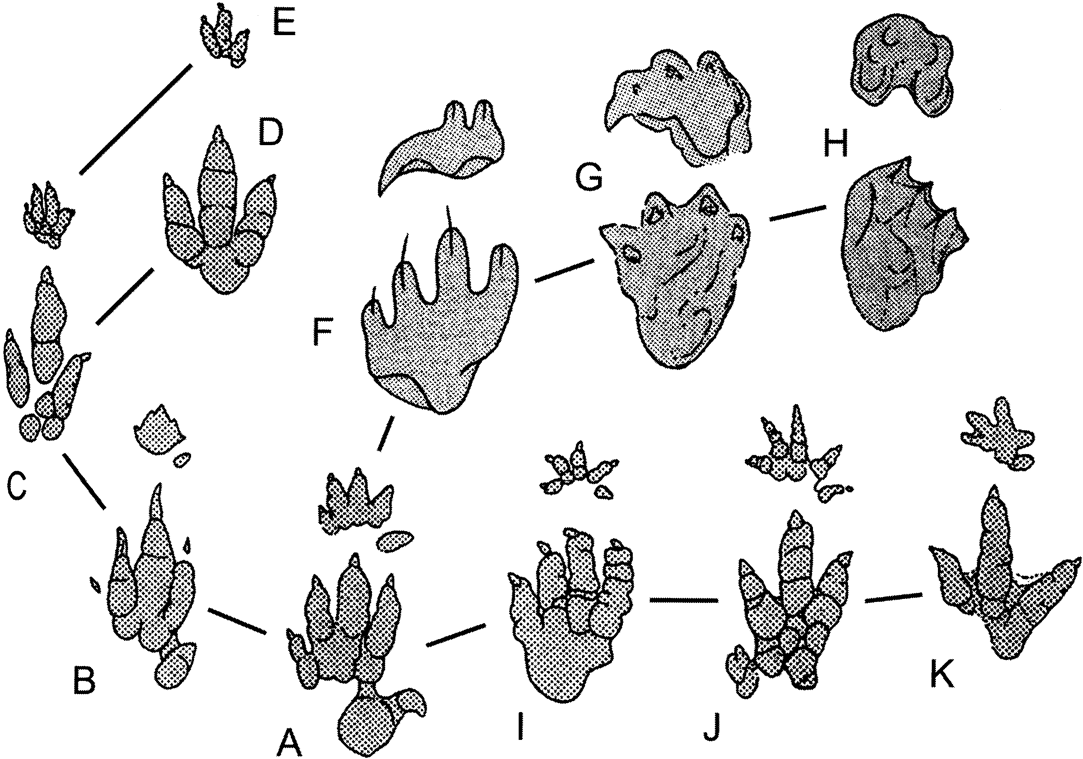
Figure 4 Emergence of three dinosaurian track morphotypes from a common chirotherioid pattern, from Thulborn Reference Thulborn2006, with his following interpretation: each diagram shows general form of a right pes and manus (A) derived (mesaxonic) chirotherioid pattern, based principally on Chirotherium barthi. Emergence of theropod (dinosauroid) morphotype (A–E) entailed reduction and loss of digits V and I, both in pes and manus, exemplified by: (B) Sphingopus ferox; (C) Atreipus acadianus; (D) pes of Eubrontes giganteus; (E) manus of Atreipus milfordensis. Emergence of sauropodomorph morphotype (A, F–H) entailed retention of flat-footed posture for the pes, but development of an erect digitigrades posture for manus; all five digits persisted in both manus and pes, but in abbreviated form; the pollex carried a large claw, but this was retractable and frequently failed to leave a recognisable impression in prints of the manus. Sauropodomorph characteristics exemplified by: (F) Navahopus falcipollex; (G) Pseudotetrasauropus jaquesi; (H) Brontopodus birdi. Emergence of the ornithischian morphotype (A, I–K) entailed progressive reduction and loss of pedal digits V and l, but persistence of all five well-developed digits in the small mesaxonic manus (often modified in highly derived ornithischians); in bipedal gait the hindfoot was functionally tridactyl, but the short digit I often left an impression when the track-maker adopted quadrupedal gait. Ornithischian characteristics exemplified by: (I) Otozoum moodii; (J) Anomoepus crassus; (K) Moyenisauropus natatalis.
Recent assessments of the correspondence (or lack thereof) between body and track fossil records find little to agree upon. Carrano & Wilson (Reference Carrano and Wilson2001) advocated a synapomorphy-based approach to taxonomic assignments of footprints, following Olsen (Reference Olsen1995; see also Padian Reference Padian2003). According to their assessment of body fossils, Ornithischia, Theropoda, and “Prosauropoda” (these authors are unusual in accepting monophyly of this group apart from Sauropoda) are known as far back as the Ladinian–Carnian boundary (ca. 227 Ma), and putative theropod trackways from France and Argentina could bring the dinosaurian track record back to the latest Anisian (ca. 234 Ma). Thulborn (Reference Thulborn2006, fig. 1) disagreed, accepting the record of both body fossils and tracks no farther back than the mid-Carnian (over 230 Ma) for theropods, and later for sauropodomorphs (near the Carnian-Norian boundary, some 228 Ma) and ornithischians (Triassic–Jurassic boundary with questionable earlier occurrences), while acknowledging a possible “dinosauroid” track record as far back as the Ladinian-Carnian boundary (ca. 235 Ma). Most current evidence places the earliest dinosaurs in the middle Carnian, at least 230 Ma, whereas the earliest South American ornithodirans (Lagerpeton, Marasuchus, Lewisuchus) are from the Anisian–Ladinian boundary (ca. 240 Ma) and the east African Asilisaurus is known even earlier in the Anisian (ca. 245 Ma). The new report of Nyasasaurus (Nesbitt et al. Reference Nesbitt, Barrett, Werning, Sidor and Charig2012) places the first dinosaur or its closest known sister group in the Anisian stage of the Late Triassic, in the Lifua Member of Tanzania in which Asilisaurus is found.
Thulborn was emphatic that in reconstructing the timing of the rise of dinosaurs, the track record should not be held hostage to the body fossil record nor to ideas of dinosaurian relationships or monophyly (which he does not accept) based on body fossils alone. In his view, trackway evidence suggests that these three lineages of dinosaurs (a grade rather than a monophyletic group) evolved independently from the makers of chirotherioid trackways, which extend through the Triassic and into the Early Jurassic (Thulborn Reference Thulborn2006). However, here the body fossils would strongly contradict phylogenetic inferences based solely on trackways. Nearly all workers regard chirotherian trackways, with their plantigrade feet and divergent fifth pedal digits, as those of pseudosuchians (crocodile-line archosaurs) or of non-archosaurian archosauromorphs not closely related to dinosauromorphs. Ornithosuchian (bird-line archosaurs) trackways apart from those of dinosaurs or derived pterosaurs are so far unknown or unrecognised.
This last statement was challenged by a recent paper from Brusatte et al. (Reference Brusatte, Niedźwiedzki and Butler2011a), who also attempted to use a synapomorphy-based approach to identify dinosauromorph footprints from Poland from the early Olenekian (ca. 249–251 Ma). These earliest tracks are quadrupedal, although the authors recognise bipedal dinosauromorph tracks by the early Anisian (ca. 246 Ma). However, the evidence that these tracks are dinosauromorphan or even archosaurian is not convincing. Brusatte et al. (Reference Brusatte, Niedźwiedzki and Butler2011a) attempted to link the Olenekian ichnogenus Rotodactylus (and the similar Prorotodactylus) to the late Ladinian dinosauromorph Lagerpeton (Romer Reference Romer1971, Reference Romer1972; Sereno & Arcucci Reference Sereno and Arcucci1994), but the comparison is very difficult, as Figure 5 shows: the pes of Lagerpeton could not have laid down three pedal digits at once, and even if the impressions of three of its digits were sequentially forced into a substrate, their relative lengths and positions would have been significantly more disparate than the digit impressions of Rotodactylus. Moreover, the first pedal digit of Lagerpeton could not have been impressed at all unless the animal was plantigrade, which neither Brusatte et al. nor other authors have argued. Rotodactylus, which is confined to the Early Triassic, has digits of increasing length from I–IV, and a divergent, almost thumb-like digit V. This morphology is widespread among Triassic trackways, notably in the entire group called Chirotheriidae (and its many assigned ichnogenera). Pedes with such characters are found in most basal archosauromorphs of the Triassic, as Haubold showed (Reference Haubold1984). They are also characteristic of living and fossil lepidosauromorphs, but not of ornithodirans.
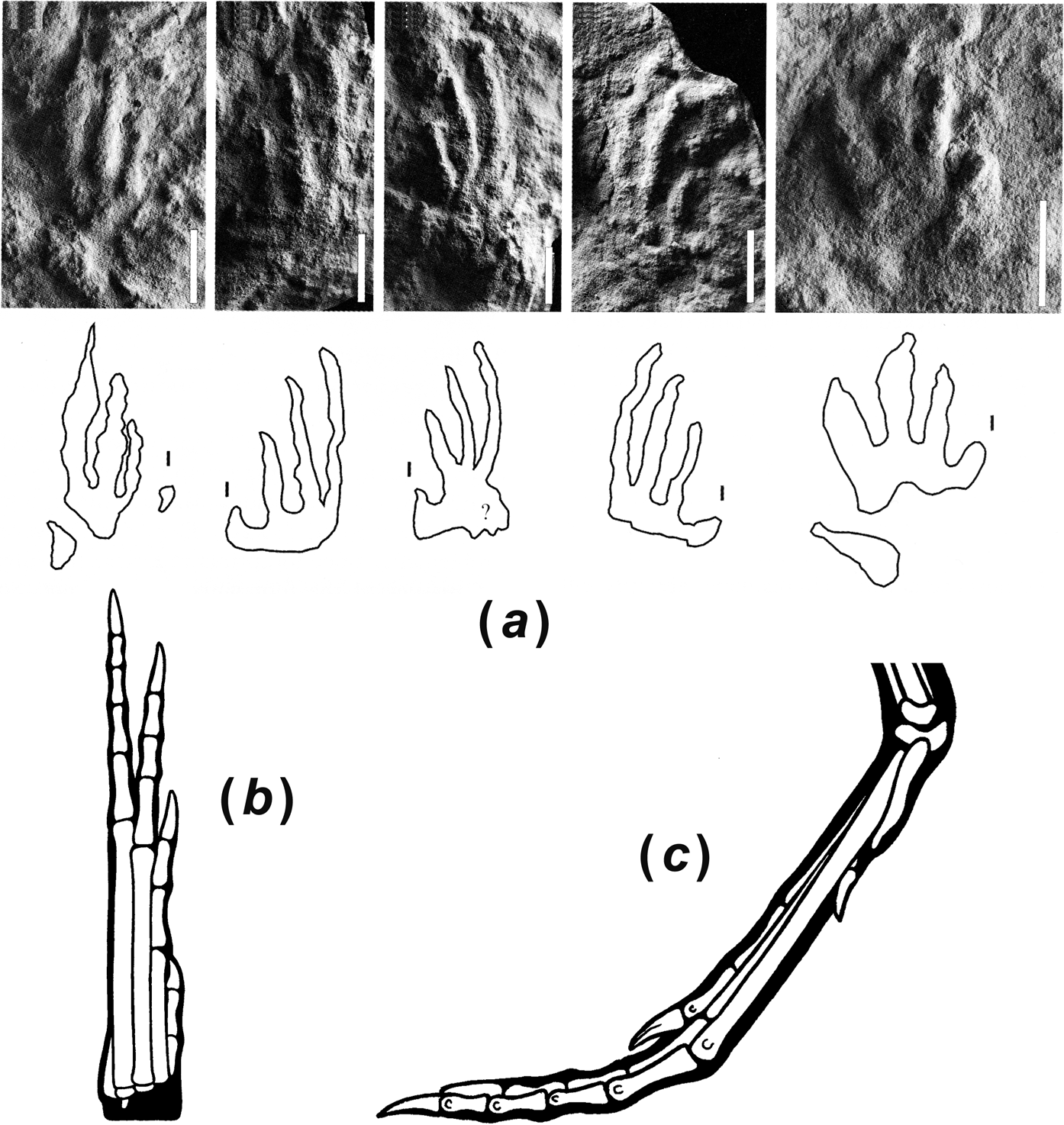
Figure 5 Comparison of trackways from the Lower Triassic of Poland, attributed to lagerpetids by Brusatte et al. (Reference Brusatte, Niedźwiedzki and Butler2011a), compared to the morphology of the foot of the early Late Triassic Lagerpeton from Argentina: (a) five examples of Prorotodactylus isp. from the Early Olenekian of Stryczowice – reprinted from Proceedings of the Royal Society, London B 278, 1107–1113, by permission of The Royal Society (CCC License No. 3176530364952); (b) left pes of Lagerpeton in anterior view and (c) right pes in lateral view, both from Sereno & Arcucci (Reference Sereno and Arcucci1994) – reprinted by permission of Taylor & Francis (http://tandfonline.com) from Sereno, P. C. & Arcucci, A. B. Reference Sereno and Arcucci1994. Dinosaurian precursors from the Middle Triassic of Argentina: Lagerpeton chanarensis. Journal of Vertebrate Paleontology 13, 385–99. It can be easily seen that the foot in (b) could not have produced the footprints immediately above it.
The split between the crocodile- and bird-line archosaurs must have occurred by the very end of the Permian or the beginning of the Triassic. On the crocodile line, relatives of Arizonasaurus are known from the Early Triassic (Olenekian) of China and Germany, and on the bird line, Asilisaurus (a silesaurid) is known from the Anisian of Africa, as is the dinosauriform Nyasasaurus (Nesbitt et al. Reference Nesbitt, Barrett, Werning, Sidor and Charig2012). Therefore, the Polish footprints would not effectively extend these osteological records back in time, even if they were correctly assigned (Irmis Reference Irmis2011; Nesbitt Reference Nesbitt2011, fig. 58B).
6. Competition, lucky break, or superiority of dinosaurs?
Various hypotheses have been proposed through time to explain the success of dinosaurs in the Late Triassic and Early Jurassic. These questions are important not only to macroevolution but to biostratigraphy and biogeography. “Why” questions (e.g. “why” dinosaurs replaced other taxa) are difficult to answer in evolutionary biology, but it is sometimes possible to parse them into hypotheses of “how” groups were replaced, and test these. Two approaches are clade dynamics, which chart the temporal succession of individual lineages through time and allow direct comparison of patterns (e.g. Brusatte et al. Reference Brusatte, Benton, Ruta and Lloyd2008a, Reference Brusatte, Benton, Ruta and Lloydb, Reference Brusatte, Benton, Lloyd, Ruta and Wang2011b) and palaeobiology, which assesses the structural, functional, and physiological differences among groups, and looks for patterns that may explain differential success (e.g. Kubo & Benton Reference Kubo and Benton2007). Both approaches test biotic and abiotic hypotheses in useful ways.
6.1. Abiotic factors in extinction
The loss of taxa at or near the Triassic–Jurassic boundary (Olsen & Sues Reference Olsen, Sues and Padian1986) has been considered relatively rapid (Olsen et al. Reference Olsen, Kent, Sues, Koeberl, Huber, Montanari, Rainforth, Fowell, Szajna and Hartline2002, Reference Olsen, Kent and Whiteside2011), and perhaps associated with both volcanic activity and the Manicouagan impact in eastern Canada, although the latter hypothesis has been generally discounted because the events are not temporally coincident. Moreover the hypothesis that the groups that became extinct by the end of the Triassic persisted until very close to the Triassic–Jurassic boundary needs further substantiation. The Cretaceous–Tertiary boundary is the only major extinction event known to be associated with a bolide impact, but it is also associated with large-scale volcanism; in fact three of the five largest extinctions are (Archibald et al. Reference Archibald, Clemens, Padian, Rowe, Macleod, Barrett, Gale, Holroyd, Sues, Arens, Horner, Wilson, Goodwin, Brochu, Lofgren, Hurlbert, Hartman, Eberth, Wignall, Currie, Weil, Prasad, Dingus, Courtillot, Milner, Milner, Baipai, Ward and Sahni2010), but there are many instances of large impacts with no evident biotic effect (Shoemaker Reference Shoemaker1983). Furthermore, it is difficult to explain why the effects of a bolide impact would have favoured dinosaurs at the Triassic–Jurassic boundary but disfavoured them at the Cretaceous–Tertiary boundary (Archibald Reference Archibald2011).
6.2. Competition
Competition among amniote groups has long been a popular hypothesis to explain the Triassic–Jurassic land vertebrate extinctions. But who is competing with whom: is it (1) archosaurs with therapsids throughout the Triassic (e.g. Bakker Reference Bakker and Hallam1977); (2) dinosaurs with therapsids in the Middle to Late Triassic (e.g. Bonaparte Reference Bonaparte1982; Charig Reference Charig and Ferguson1984); or (3) dinosaurs and their closest relatives with pseudosuchians and other reptiles in the Late Triassic (e.g. Charig Reference Charig and Ferguson1984)? Only the latter two questions involve dinosaurs. The second question is problematic because, compared to their Late Permian and Early Triassic diversity, therapsids had waned considerably before the dinosaurs diversified extensively (Bonaparte Reference Bonaparte1982; Charig Reference Charig and Ferguson1984). The third question was initially answered by Bonaparte's (Reference Bonaparte1982) stratigraphic scheme showing that dinosaurs (notably prosauropods and the dinosauriform herrerasaurids) replaced lagosuchids and related forms in South America without taxonomic overlap; however, as noted above, this pattern did not hold in lower-latitude faunas (Irmis et al. Reference Irmis, Nesbitt, Padian, Smith, Turner, Woody and Downs2007a).
In any event, it is difficult to substantiate the hypothesis that extinct groups were directly competing. Benton (Reference Benton, Jablonski, Erwin and Lipps1996) surveyed several hundred cases in which competition in the fossil record had been hypothesised or presumed, and could find plausibility or even possibility in only a handful of them. It is difficult to establish whether hypotheses of competition are even testable in the fossil record.
6.3. Felicity
The “lucky break” hypothesis – the idea that other organisms vacated ecological space in becoming extinct, and the dinosaurs eventually just moved in – is based on an observation of non-overlapping geological ranges and the presumption that dinosaurs occupied the same resource zones or adaptive zones (e.g. Brusatte et al. Reference Brusatte, Benton, Ruta and Lloyd2008a, Reference Brusatte, Benton, Ruta and Lloydb). Ideas about resource zones or adaptive zones can only be examined very generally, because trophic webs are difficult to establish from direct evidence. And, the observation that one group of organisms became extinct and another radiated in its wake is not really a hypothesis. If the idea is that there is “nothing to explain” in terms of competition or differential success under environmental stress, then it is a classic “null hypothesis” in historical biology (e.g. Raup Reference Raup1991). The hypothesis that there is “nothing to explain” because there is nothing unusual, connected, non-random, or requiring causal explanation in the macroevolutionary pattern is viable, and it can only be rejected by the demonstration of a correlative pattern of differences among the groups affected, which suggests a causal explanation that then can be tested independently.
Because early dinosaurs co-existed with their close relatives for so long at lower latitudes, it is difficult to accept that they got a “lucky break” when other taxa became extinct, particularly because dinosaurs did not demonstrably radiate into many adaptive zones of their erstwhile contemporaries. During the Late Triassic dinosaurs were a minor component of the ecosystem, functioning as small carnivores (theropods) and omnivores to generalised herbivores (sauropodomorphs). Early Jurassic theropods and sauropods included larger forms (Dilophosaurus, Vulcanodon) that do not show departures from the habits of their Late Triassic relatives. Early Jurassic ornithischians (lesothosaurids and thyreophorans such as Scutellosaurus and Scelidosaurus), virtually unknown in the Late Triassic, show leaf-shaped teeth quite similar to those of basal sauropodomorphs (and the Triassic crocodile-line revueltosaurs); the only new invention comes in the molariform battery of cheek teeth in heterodontosaurids. However, the specialised feeding structures of trilophosaurs, rhynchosaurs, phytosaurs, and many other groups were not duplicated by Early Jurassic dinosaurs, so it is difficult to argue that it was necessary for other groups to vacate the territory in order that dinosaurs could move in.
6.4. Superiority
In a non-competitive sense, this means that dinosaurs evolved adaptations that are not found in their contemporaries and that enabled them to thrive differentially in the world of the Late Triassic and beyond. Romer (Reference Romer1968) noted many of these, such as elongated hindlimb elements, light build, erect stance, parasagittal gait, offset femur, “cursorial” proportions (e.g. tibia longer than femur, and elongated metatarsals), mesotarsal ankle, and so on. Bakker (Reference Bakker and Hallam1977, Reference Bakker, Thomas and Olson1980) provided a litany of arguments to support the superiority of dinosaurs. The features that Romer and Bakker listed indeed differentiate dinosaurs (and other ornithodirans) from their contemporaries. Bakker, perhaps more than Ostrom, was convinced that dinosaurs were endothermic, although terms such as endothermic, “warm-blooded,” homeothermic, and tachymetabolic were often conflated (Bennett & Ruben Reference Bennett and Ruben1979).
Eventually, comprehensive surveys of the ontogeny and phylogeny of dinosaur long bone histology established that dinosaurs and pterosaurs grew considerably more quickly than other reptiles (Horner et al. Reference Horner, de Ricqlès and Padian1999, Reference Horner, de Ricqlès and Padian2000; Sander Reference Sander1999; Ricqlès et al. Reference Ricqlès, Padian, Horner and Francillon-Viellot2000, Reference Ricqlès, Padian and Horner2003, Reference Ricqlès, Padian, Knoll and Horner2008; Padian et al. Reference Padian, de Ricqlès and Horner2001, Reference Padian, Horner and de Ricqlès2004). Their bones rarely showed the periodic “annuli” of poorly vascularised bone that characterised many other reptiles; in fact, Mesozoic dinosaur bone is as well vascularised as the bones of most large birds and mammals, suggesting comparable growth rates (Köhler et al. Reference Köhler, Marín-Moratalla, Jordana and Aanes2012; Padian Reference Padian2012). It is difficult to explain these sustained growth rates without underlying high metabolic rates, and so it is difficult to escape the conclusion that dinosaurs and pterosaurs were relatively tachymetabolic (Padian & Horner Reference Padian and Horner2002). High growth and metabolic rates imply a more active feeding style, faster growth to a size that escapes smaller predators, and likely the earlier onset of reproductive maturity, all of which are related to size. Unlike virtually all other Triassic reptiles, ornithodirans (dinosaurs, pterosaurs, and their common relatives) shared a suite of characters related to habitual upright posture, parasagittal gait, bipedality, and cursorial proportions, as well as high growth and metabolic rates (Romer Reference Romer1968; Padian et al. Reference Padian, de Ricqlès and Horner2001; Padian Reference Padian2008). The two groups of tetrapods today that share these features are birds and mammals (although most mammals are not bipedal), and they are also the most numerically diverse groups of tetrapods.
Superiority can only be inferred as a post hoc metric of success. In that sense, post- Triassic dinosaurs did better than their Triassic contemporaries, and they were successful in a variety of forms and roles for another 135 million years (and longer in the case of birds). In the post-Triassic world they radiated into a variety of forms that matched or exceeded previous reptilian groups in diversity and disparity. However, in the Late Triassic they were less conspicuous. In any event, although the features of growth, posture, and physiology that distinguish dinosaurs and their ornithodiran relatives from their Late Triassic contemporaries, and perhaps contributed substantially to their later success, became much more obvious after the Triassic, those features evolved during the Triassic.
7. Beyond “Dinosauria” to a new paradigm
The previous section highlighted the rise of Dinosauria as a major phenomenon that has traditionally begged explanation. But perhaps our focus on dinosaurs has obscured what is really happening in the Triassic. What happens if we try to rearrange slightly some of the information that has been the subject of this review?
The conventional literature puts the “beginning of the Age of Dinosaurs” in the Late Triassic. But the principal event that happened then was the differentiation of Saurischia. There are apparently almost no ornithischian groups who did their radiating before the Jurassic (even accepting the records of Pisanosaurus, the fragmentary Los Colorados jaw, and Eocursor: Butler et al. Reference Butler, Upchurch and Norman2008), and significant size changes and feeding diversity took place in both Saurischia and Ornithischia only after the Triassic (despite a couple of large Triassic sauropodomorphs such as Ohmdenosaurus). In the 1980s it was easy to separate dinosaurs from other archosaurs by reference to a list of discrete synapomorphies (e.g. Gauthier Reference Gauthier1984, Reference Gauthier1986). But it is no longer easy to separate dinosaurs in this way, because new discoveries and phylogenetic analyses of Late Triassic ornithodirans have blurred the distinction (e.g. Irmis Reference Irmis2011; Nesbitt et al. Reference Nesbitt, Barrett, Werning, Sidor and Charig2012). There may be as few as three synapomorphies that set apart Owen's defined group from other ornithodirans (Nesbitt Reference Nesbitt2011), and if research persists, possibly one or more of these features may prove to be convergent.
What we have learned from this is that the origin of Dinosauria was not an immediate success or even a minor inflection point. It is not accompanied by a sudden burst of speciation or a proliferation of highly disparate ecological types – particularly if compared to the radiation of Triassic pseudosuchians. Whether Herrerasaurus, Eoraptor, Guibasaurus, Silesaurus, or a variety of other taxa are technically dinosaurs does not much matter to our perception of what evolutionary changes were most conspicuous in the Late Triassic tetrapod fauna. What once seemed like a great innovative revolution in the evolution of reptiles – upright posture, bipedality, parasagittal gait, large size – now has largely been eclipsed by the discovery that the characters related to these features evolved considerably earlier in the ornithodiran line, and did not provide their bearers with immediate evolutionary dominance.
7.1. Filling the evolutionary gaps
It is clear, then, that we have to re-think our conception of what really comprises the “beginning of the Age of Dinosaurs.” Several other discoveries of the past decades have changed our view of this.
(a) The first piece of evidence to shift thinking was the discovery of Lagosuchus and Lagerpeton in the Middle Triassic of Argentina (Romer Reference Romer1971, Reference Romer1972; Bonaparte Reference Bonaparte1975). In his monograph on Lagosuchus, Bonaparte took pains to show what parts of the skull and skeleton were “dinosaurian,” “thecodontian,” or a mixture of the two. The distinction was beginning to blur and it was because the characters were truly intermediate. The iliac blade was short as in “thecodontians” but the pubis had a curved, flattened blade that looked dinosaurian; the acetabulum might have been open (or incipiently open, or just damaged). The femoral head was offset but not as much as in most dinosaurs. The astragalus had an ascending process but not exactly like those in dinosaurs. And so on.
(b) Another realisation was that pterosaurs had many of the characters that appeared to distinguish dinosaurs from other reptiles (Gauthier Reference Gauthier1984; Padian Reference Padian, Reif and Westphal1984), particularly in the hindlimbs, although much of the rest of the skeleton was highly derived even in basal forms (Padian Reference Padian1983a, Reference Padianb). This nexus of pterosaur-dinosaur forms, which turned out to include Lagosuchus, Lagerpeton, and some of their relatives, comprised a group that Gauthier (Reference Gauthier1984, Reference Gauthier1986) called Ornithodira, and they were very distinct from other archosaurs. It seemed that the features of posture and locomotion that were thought to characterise dinosaurs were shared by a larger group on the “bird-line archosaur” side of the ledger. These anatomical features also conveyed functional changes in the whole lineage, including upright posture, parasagittal gait, and bipedality (Padian Reference Padian1983b, Reference Padian2008).
(c) New discoveries of basal dinosaurs and dinosauriforms such as Panphagia, Saturnalia, Pantydraco, and the silesaurs further blurred the distinction between traditionally known non-dinosaurs (pterosaurs, pseudosuchians) and traditionally known basal dinosaurs such as Coelophysis and Plateosaurus, both saurischians (Irmis Reference Irmis2011).
(d) Studies of the bone histology of basal dinosaurs and their archosaurian relatives (e.g. de Ricqlès et al. Reference Ricqlès, Padian, Horner and Francillon-Viellot2000, Reference Ricqlès, Padian and Horner2003, Reference Ricqlès, Padian, Knoll and Horner2008; Padian et al. Reference Padian, de Ricqlès and Horner2001; Knoll et al. Reference Knoll, Padian and de Ricqlès2010; Werning et al. Reference Werning, Irmis, Smith, Turner and Padian2011) revealed that many microanatomical features linked to rapid growth and elevated metabolic rates evolved before dinosaurs, were shared by pterosaurs, and may have first appeared much earlier in archosaurs.
7.2. The Triassic–Jurassic boundary marks the evolutionary inflection point
One insight that emerges from all the recent work on Triassic–Jurassic terrestrial vertebrate paleontology is that the “Age of Dinosaurs” really does not begin until the Early Jurassic.
(a) Regardless of whether Pisanosaurus is an ornithischian or something else, there are virtually no ornithischians known from the Triassic; nearly all their records have been reassigned for one reason or another (Parker et al. Reference Parker, Irmis, Nesbitt, Martz and Browne2005; Irmis et al. Reference Irmis, Parker and Nesbitt2007b; Nesbitt et al. Reference Nesbitt, Irmis and Parker2007). In the Early Jurassic the ornithischians quickly radiated into fabrosaurids, heterodontosaurids, thyreophorans, and other forms and by the Sinemurian they reached lengths of two meters or more (e.g. Scelidosaurus).
(b) Saurischian dinosaurs were smaller before the Triassic-Jurassic boundary than soon afterwards, which is to say that, notably in theropods, their maximum known size increased from one or two metres (e.g. Coelophysis) to four metres (Dilophosaurus). Less clear is the maximum size difference between Late Triassic and Early Jurassic sauropodomorphs. The largest basal sauropodomorphs (“prosauropods”) of the Late Triassic are the size of Plateosaurus (about 6 m on average, although one morph apparently reached 10 m: Sander & Klein Reference Sander and Klein2005). Late Triassic sauropods such as Isanosaurus were similarly about 6·5 m long, and most Early Jurassic sauropods are also in the range of 6·5 m (e.g. Vulcanodon).
(c) All non-crocodylomorph pseudosuchians and all other “indigenous” Triassic forms (Padian Reference Padian and Padian1986) became extinct by the end of the Triassic. Most of these forms had growth rates and attendant metabolic rates that were lower than in ornithodirans. Exceptions that survived include crocodiles, turtles, lepidosaurs, and champsosaurs; it was a great winnowing, and the causes are still not well understood.
7.3. The need for a new paradigm to open the “Age of Dinosaurs”
As for the previous section, we have to reconsider what we have previously considered “the beginning of the Age of Dinosaurs” – namely, the Late Triassic.
(a) The origin of Dinosauria per se was not much of an evolutionary event, now that we have filled in so many of the gaps in the Late Triassic that bridge dinosaurs with their closest relatives. The “Age of Dinosaurs” does not properly begin in the Late Triassic, although dinosaurs are present and reasonably diverse (but not much more so than several other groups). The “Age of Dinosaurs” properly begins in the Early Jurassic, with the diversification of ornithischians and the radiation of saurischians (which soon attain larger mean size than in the Triassic). Also, by the Early Jurassic a variety of large and diverse pseudosuchians and other terrestrial reptiles were out of the way.
(b) The event of note in the Triassic in this regard is the origin of Ornithodira, with their upright posture, bipedality, parasagittal gait and elevated growth rates and metabolic regimes. This had certainly happened by the early Middle Triassic. The “Age of Ornithodira,” though perhaps not as catchy as “The Age of Dinosaurs,” is the more important macroevolutionary event.
8. Conclusions
“One of the difficulties with evaluating hypotheses for the rise of dinosaurs is that authors are not always explicit about cause and effect. For example, some authors have proposed that increasing aridity caused the extinction of synapsid groups and the rise of dinosaurs. But these authors do not explicitly explain how aridity had this effect. In these cases, the first step is to test the correlation, but it is difficult to evaluate causation when hypotheses are incompletely explained.”
(Irmis Reference Irmis2011, p. 409)This review began by parsing the question of the origin of dinosaurs into three kinds of problems: dinosaur monophyly and relationships; dinosaurian functional-ecological advances; and the timing and pacing of dinosaur origins and diversification. None of these can fruitfully be studied in isolation and, as Irmis (Reference Irmis2011) pointed out, a hypothesis that invokes one approach does not invalidate others, nor does elimination of several alternative hypotheses mean that the remaining one(s) must be correct.
Because it is generally accepted that dinosaurs are monophyletic, it is possible to ask about their phyletic origins as a group. However, stratigraphic and biogeographic information has complicated this picture because some dinosaur groups appear later than others, some appear later in different places and also independently (e.g. Rowe et al. Reference Rowe, Sues and Reisz2011), and some geographic regions appear to show longer co-occurrence of dinosaurs with their dinosauromorph relatives (e.g. Irmis et al. Reference Irmis, Nesbitt, Padian, Smith, Turner, Woody and Downs2007a). New and ever-desirable fossils that “bridge gaps” between apparently distinct taxa will help to clarify this conundrum, but greater attention to character codings and polarities of known taxa will also help.
It was not only dinosaurs but also their closest relatives – lagosuchids, lagerpetids, silesaurids and pterosaurs – that shared a suite of structural, functional and metabolic features that differentiated them considerably from other reptiles before the Late Triassic onwards. Dinosaurs, compared to their immediate dinosauromorph outgroups (all of the above except pterosaurs), were tremendously successful, as were pterosaurs (although less diverse through the rest of the Mesozoic Era). They were also more successful than any other tetrapod groups through the rest of the Mesozoic. It is difficult to regard this as coincidental.
It is not clear whether environmental factors can account for the differential representation and spread of early dinosaur groups in time and space during the Late Triassic. Whereas the three major Triassic lineages (theropods, sauropodomorphs and ornithischians) are relatively cosmopolitan by the Early Jurassic, this is not so in the Late Triassic, where paleoenvironments represent lake shores, ephemeral marshes, flood plains and arid regions. Twenty years ago, one might have reasonably hypothesised that South America could have been the centre of origin of Dinosauria because varied groups such as lagosuchids, lagerpetids, herrerasaurids (seen as either non-dinosaurs or theropods), ornithischians, and sauropodomorphs were well represented in successive Late Triassic faunas. Now this is not so certain. As Triassic–Jurassic formations become progressively realigned and dated by methods independent of palaeontology (e.g. Olsen et al. Reference Olsen, Kent and Whiteside2011), the picture may become clearer, or our picture may remain as geographically and stratigraphically heterogeneous as ever (e.g. Irmis et al. Reference Irmis, Nesbitt, Padian, Smith, Turner, Woody and Downs2007a).
Regardless of the foregoing uncertainties, it appears that a paradigm shift is needed. Current evidence indicates that the origin of dinosaurs was initially trivial in its evolutionary effects compared to the origin of Ornithodira, and that the “Age of Dinosaurs” proper did not begin until the Jurassic. Repositioning our scholarly inquiry toward these problems, regardless of popular fascination with the origin of dinosaurs as a group, should yield more fruitful future research results.
9. Acknowledgments
Because this is a Langston Festschrift, it is fitting to pay tribute to Marietta Langston, beloved both to her esteemed spouse and to generations of his colleagues and students. Many of us, going back decades, will never forget the kindnesses that she showed us, which we owe in turn to the generations of students that follow us.
Wann himself was long regarded as one of the greatest field palaeontologists, “bone people,” and all-around savants that the profession has ever had. In a field where people respect the ability to find and identify fossils, Wann was primus inter pares. He once remarked to me in his understated way that over the years he had acquired something of a reputation for being able to identify fossil bones. (This is like saying that James Bond was a rather good hand with women, weapons and fast cars.) Wann confided that when confronted with a “mystery bone,” his secret was to state the first thing that came into his head; because if you thought about it too long, you might come up with other possibilities, but the first one was usually right. It would not have occurred to him, of course, that the only reason that this approach could work at all would be to have the vast experience of a Wann Langston.
There are few people you meet in your profession about whom you can say that you remember almost every word they ever uttered to you. The reason is that what they said contained so much experience and wisdom. Such colleagues are not just great palaeontologists but vessels of our common history. In honouring Wann we honour his tradition, which is being continued by so many great successors.
I thank Michael Benton, Nicholas Fraser, Sterling Nesbitt, David Norman, William Parker, Andrew Smith, Hans Sues, Corwin Sullivan, Keith Thomson and David Weishampel for their comments on the manuscript, without implying their agreement with any and all ideas that it raises, and Randy Irmis, Paul Olsen, Michael Parrish and Sarah Werning for many productive discussions. This is UCMP Contribution No. 2032.