The Cretaceous is one of the most important periods in Earth's history. Major changes of entomofauna started at the beginning of this period with the rapid radiation of various groups of insects, co-evolving with Angiospermae (Grimaldi Reference Grimaldi2010). In the Early Cretaceous (early Barremian (Granier et al. Reference Granier, Toland, Gèze, Azar and Maksoud2016; Maksoud et al. Reference Maksoud, Azar, Granier and Gèze2016) and Aptian (Menor-Salván et al. Reference Menor-Salván, Simoneit, Ruiz-Bermejo and Alonso2016)), species with a very elongated rostrum, such as Helius ewa Krzemiński, Kania & Azar, Reference Krzemiński, Kania and Azar2014, started appearing, which is probably connected with the early appearance of angiosperms (Krzemiński et al. Reference Krzemiński, Kania and Azar2014).
The Recent fauna of Limoniidae is diverse, with over 11,000 species (Oosterbroek Reference Oosterbroek2015), and these are associated mainly with moist, temperate environments, such as wooded areas, both in temperate and tropical regions, but also with open habitats, such as meadows, deserts and oases (Starý & Freidberg Reference Starý and Freidberg2007). The imago of the Recent representatives of the genus Helius occur in sunny meadows near streams, or in moist coppice habitats. It could be assumed that their ancestors probably lived in similar ecological niches. A number of Cretaceous amberiferous localities with insect inclusions are known, but only a few of them have yielded representatives of the genus Helius. Only five species are known from this period (Table 1) (Rayner & Waters Reference Rayner and Waters1990; Ribeiro Reference Ribeiro2002; Kania et al. Reference Kania, Krzemiński and Azar2013; Krzemiński et al. Reference Krzemiński, Kania and Azar2014), with only one from Álava, Spain (Kania et al. Reference Kania, Krzemiński and Arillo2016). Many more species are known from younger periods, such as the Eocene (Loew Reference Loew1850; Meunier Reference Meunier1906; Alexander Reference Alexander1931; Krzemiński Reference Krzemiński1985, Reference Krzemiński1993; Podenas Reference Podenas2002; Reference KaniaKania 2014), the Oligocene (Statz Reference Statz1934, Reference Statz1944; Krzemiński Reference Krzemiński1991) and the Miocene (Krzemiński Reference Krzemiński2002).
Table 1 List of fossils belonging to the genus Helius, known from the Cretaceous. Localities marked with asterisk (*) are amber outcrops (part after Kania et al. Reference Kania, Krzemiński and Azar2013); stage marked by double asterisk (**) – information about stage according to latest data (Maksoud et al. Reference Maksoud, Azar, Granier and Gèze2016).

Helius spiralensis sp. nov, described below, is one of the smallest fossil representatives of the genus. It has an elongated rostrum, with a peculiar morphology of the hypopygium and with an extremely elongated aedeagus. These features clearly differentiate it from other species of the genus Helius.
This is the second representative of the genus from Cretaceous Álava amber and the third limoniid described from this locality (Krzemiński & Arillo Reference Krzemiński and Arillo2007).
1. Material and methods
The study herein is based on specimen No. MCNA 15078 from the Upper Albian amber of Álava (Barrón et al. Reference Barrón, Peyrot, Rodriguez-López, Meléndez, López del Valle, Najarro, Rosales and Comas-Rengifo2015), Peñacerrada I outcrop (northern Spain), located on the northern slope of Sierra de Cantabria, within the Basque–Cantabrian Basin (Peñalver & Delclos Reference Peñalver, Delclòs and Penney2010) (Figs 1, 2). The material is deposited in the collection of the Museo de Ciencias Naturales de Álava, Vitoria, Spain (MCNA).
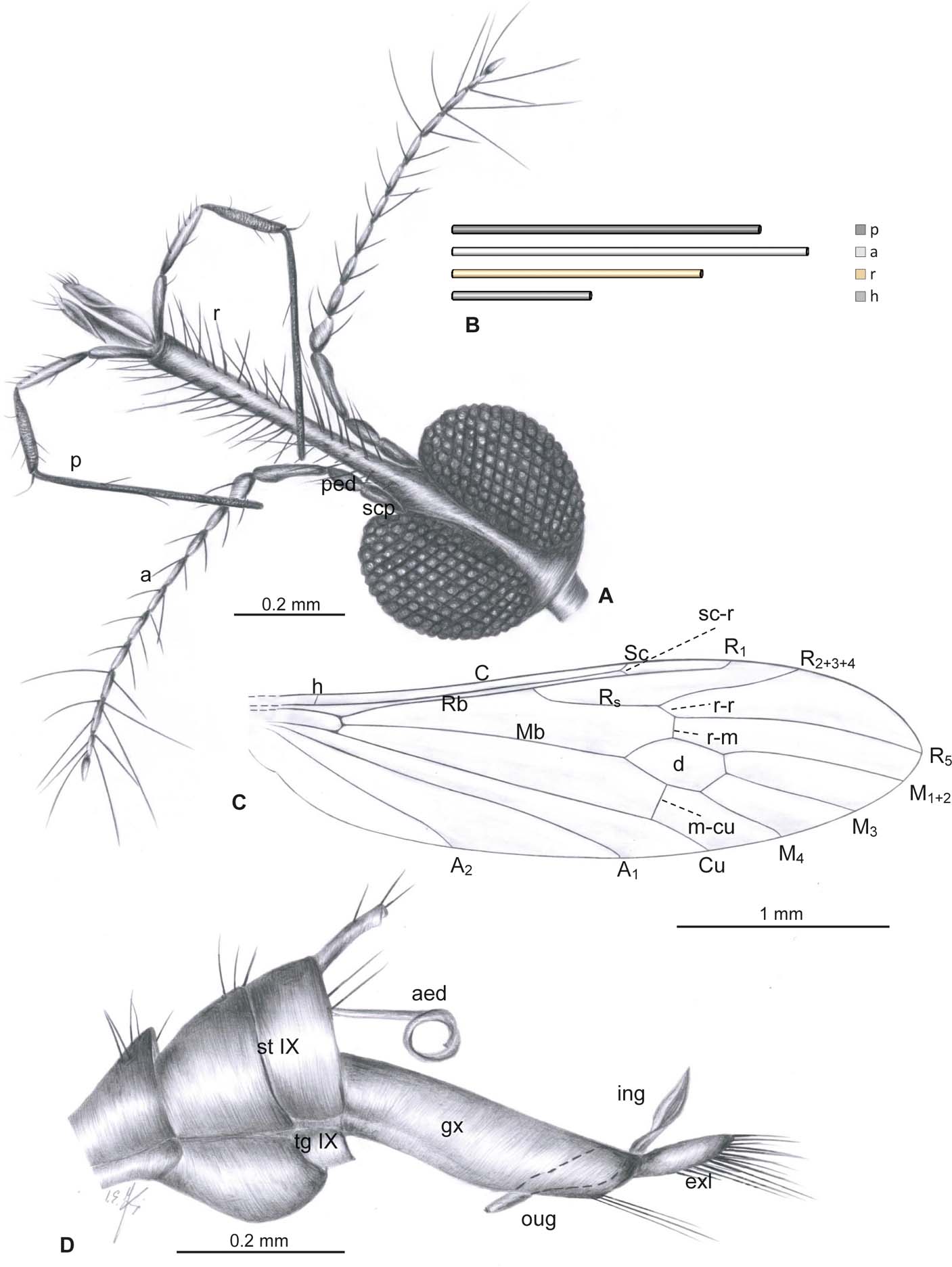
Figure 1 Helius spiralensis sp. nov., holotype, specimen MCNA 15078 (male): (A) drawing of the head (reconstructed), latero-dorsal view; (B) schematic representation of the relation between the length of the antenna (a), rostrum (r), palpus (p) and head (h); (C) drawing of the wing venation; (D) drawing of the last tergites, sternites and gonocoxite, gonostyles. Abbreviations: a = antennae; aed = aedeagus; exl = extra lobe; gx = gonocoxite; ing = inner gonostylus; oug = outer gonostylus; p = palpus; ped = pedicel; r = rostrum; scp = scape; st IX = sternite IX; tg IX = tergite IX.

Figure 2 Helius spiralensis sp. nov., holotype, specimen MCNA 15078 (male): (A) photograph of the antenna, dorsal view; (B) photograph of the head, latero-dorsal view; (C) photograph of the head, lateral view; (D) photograph of the palpus, dorsal view.
The specimen was studied using a Nikon SMZ 1500 stereomicroscope equipped with a Nikon DS-Fi1 camera; the measurements were taken with NIS-Elements D 3.0 software. The length of discal cells was measured from the hind edge of the discal cells to the connection of cross-vein m–m with vein M3. The drawings for the analysis were based on the specimen and photographs.
2. Systematic palaeontology
Order Diptera Linnaeus, Reference Linnaeus1758
Family Limoniidae Speiser, Reference Speiser1909
Subfamily Limoniinae Speiser, Reference Speiser1909
Genus Helius Lepeletier & Serville, Reference Lepeletier and Serville1828
Subgenus Helius Lepeletier & Serville, Reference Lepeletier and Serville1828
Type species. Helius longirostris (Meigen, Reference Meigen1818).
Helius spiralensis sp. nov.
(Figs 1–4)
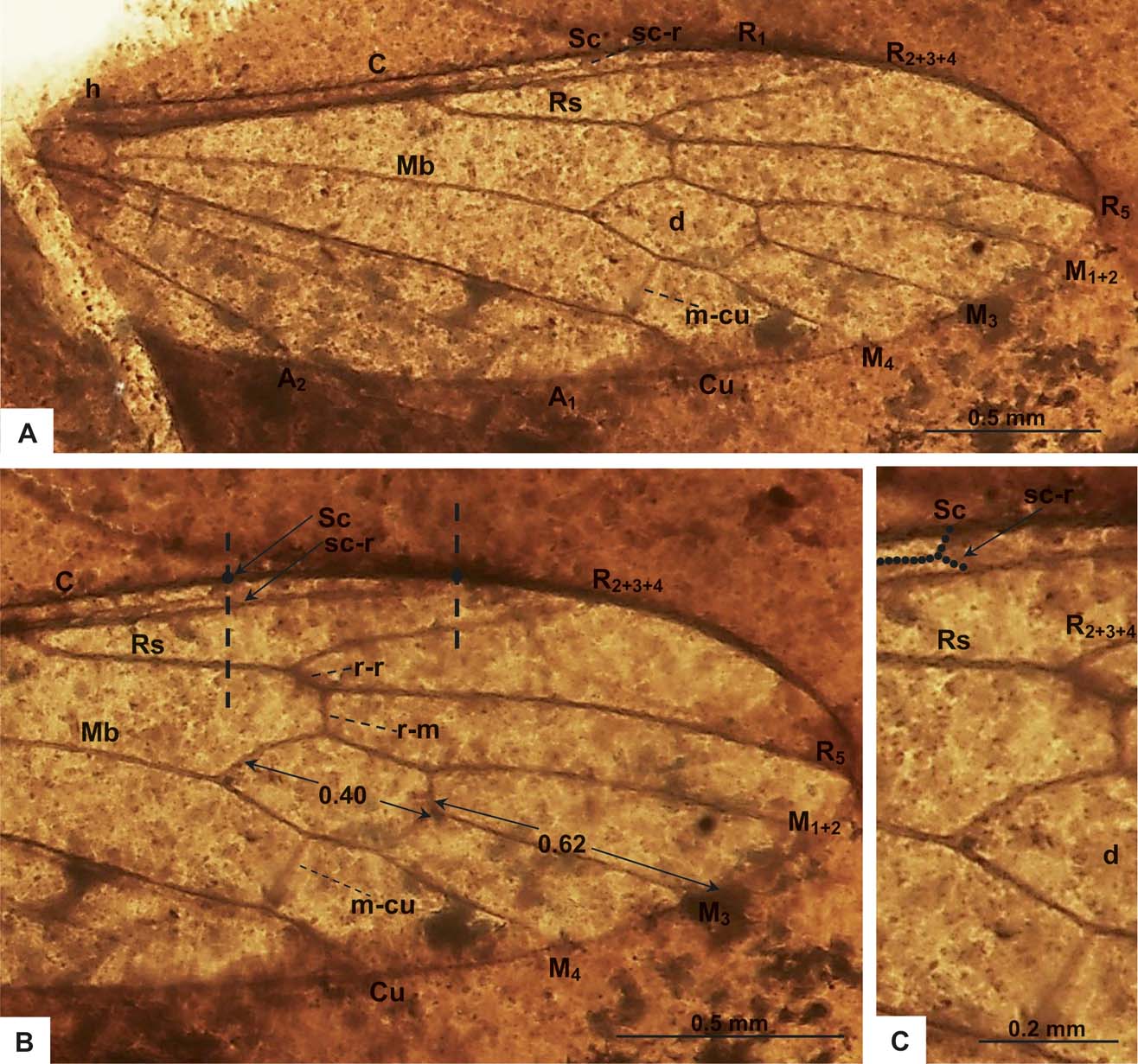
Figure 3 Helius spiralensis sp. nov., holotype, specimen MCNA 15078 (male): (A) photograph of the wing; (B) photograph of the wing venation, apical part of the wing; (C) photograph of the wing venation, with end of subcostal vein (Sc) and cross-vein sc–r marked.

Figure 4 Helius spiralensis sp. nov., holotype, specimen. MCNA 15078 (male): photographs of the hypopygium in (A) lateral, (B) latero-dorsal and (C) latero-ventral views.
Diagnosis. Palpus approximately 0.25 times longer than rostrum, approximately nine times as long as wide, slightly longer than antenna; rostrum almost twice the length of head; antenna approximately 0.2 times longer than rostrum; hypopygium with gonocoxite comparatively narrow and elongated, almost 2.5 times longer than outer gonostylus, gonocoxite with extra lobe at the apex on dorsal surface, the extra lobe with elongated, strong setae at apex, the extra lobe comparatively wide and elongated, of comparable size to outer and inner gonostylus, only slightly shorter than outer and inner gonostylus; inner gonostylus not very wide, outer gonostylus elongated, narrow; aedeagus very elongated and convolute (spiral).
Etymology: “spira” (Latin)=spiral.
Material examined: Holotype specimen No. MCNA 15078 (male); Peñacerrada I (Moraza) outcrop, Spain, collection, housed in the Museo de Ciencias Naturales de Álava, Vitoria, Spain.
Description. Small species, body brown. Head (Figs 1A, 2B, C): small, 0.26 mm long, with huge eyes; rostrum elongated, narrow, 0.47 mm long, slightly widened at apical part, almost twice the length of head, distinctly shorter than palpus; palpus approximately 0.24 times longer than rostrum, slightly longer than antenna; antenna 0.67 mm long, approximately 0.2 times longer than rostrum (Figs 1A, B; 2A–C), 14-segmented; scape cylindrical, pedicel barrel-like, but narrow, flagellomeres elongated and cylindrical, first flagellomere narrowed at base and widened at apex, flagellomeres 2–14 slightly widened at the base, last flagellomere shorter than penultimate one; all segments of antenna covered by wispy, tiny setae and with elongated setae, on segments 4–10 two elongated setae only slightly longer than segments bearing them, on segments 11–14 three very elongated setae over twice as long as segments bearing them; palpus (Figs 1A; 2B, C) typical for the genus, 4-segmented, 0.58 mm long, very elongated and tiny, segments 1–3 cylindrical, tiny, first palpal segment 0.09 mm long, the second one 0.12 mm, third segment 0.12 mm long, last palpal segment tiny and very elongated, 0.28 mm, but as long as the preceding all taken together; labrum elongated and massive, approximately 0.3 times the length of the rostrum.
Thorax. Wing (Figs 1C; 3A–C) 3.21 mm long, 0.84 mm wide; pterostigma not visible; vein Sc elongated, ending far behind half the length of Rs, opposite approximately 0.25 times length of Rs; cross-vein sc–r ending opposite the end of Sc (Figs 3B, C); R1 ending opposite half of R2+3+4 length; R2+3+4 almost straight, not very elongated, ending opposite approximately 1/2 of vein R5; r-r (R2) atrophied; cross-vein r–m elongated, well expressed equal half the length of cross-vein m–cu; situated approximately once its length from the bifurcation of vein Rs; d-cell closed; M3 1.5 times longer than d-cell, M3 slightly longer than half the length of R5; cross-vein m–cu just behind half the length of d-cell base, A1 almost straight, elongated, A2 slightly waved, not very elongated.
Abdomen. Hypopygium (Figs 1D; 4A–C) 0.57 mm long, with gonocoxite comparatively narrow and elongated, almost 2.5 times longer than outer gonostylus; at the apex of gonocoxite extra lobe on dorsal surface with elongated, strong setae at apex; extra lobe 0.11 mm long, comparable size to gonostyles, slightly shorter than these structures; outer and inner gonostylus of comparable size; outer gonostylus elongated, 0.14 mm long, narrow, directed down along gonocoxite; inner gonostylus 0.13 mm long, not very wide; aedeagus very elongated and convolute.
3. Discussion
The earliest representatives of the genus Helius appear in the fossil record in Early Cretaceous Lebanese amber. The cladistic analysis by Ribeiro (Reference Ribeiro2008) and a new phylogenetic classification by Petersen et al. (Reference Petersen, Bertone, Wiegmann and Courtney2010), based on combined morphological characters (adult, larvae and pupae) and nuclear gene sequence data, confirm the theories of Alexander (Reference Alexander1948a, Reference Alexanderb) and Savchenko (Reference Savchenko1983) about the close relationship between the genera Elephantomyia and Helius. Both groups are characterised by possessing a very elongated rostrum (proboscis), which is usually longer than the head. This structure, in the genus Elephantomyia, is longer than half the body length, whilst in Helius, it is shorter (Savchenko Reference Savchenko1983). There are observed morphological similarities of the adults' thorax and wing venation in the two genera. The wings in both of groups are characterised by their two-branched radial sector (Rs) and atrophy of cross-vein r–r (R2). In respect of the male terminalia features of these genera, the outer gonostylus is more sclerotised than the inner gonostylus in all known Elephantomyia species, and in most species of Helius (Ribeiro Reference Ribeiro2008; Petersen et al. Reference Petersen, Bertone, Wiegmann and Courtney2010). The genus Elephantomyia appears in the fossil record much later than the genus Helius, in the Eocene (Loew Reference Loew1851).
The newly described species, H. spiralensis sp. nov., has a very elongated and convolute aedeagus. This feature is also clearly visible in Elephantomyia; e.g., in the Recent Elephantomyia westwoodi Osten Sacken, Reference Osten Sacken1869. Cretaceous and Eocene Helius species, such as Helius hoffeinsorum Kania, Reference Kania2014, are characterised by having a rather short and thick aedeagus (Fig. 5). However, other characters, such as the very elongated and narrow gonocoxite, the elongated rostrum shorter than half the body length and the extremely elongated last segment of the palpus, allow the placement of the newly described species in the genus Helius.

Figure 5 Hypopygium of representatives of the genus Helius, species from different periods: (A) Helius lebanensis Kania, Krzemiński & Azar, Reference Kania, Krzemiński and Azar2013, holotype, specimen T-7A (after Kania et al. Reference Kania, Krzemiński and Azar2013); (B) Helius spiralensis sp. nov., holotype, specimen MCNA 15078; (C) Helius hoffeinsorum Kania, Reference Kania2014 (after Reference KaniaKania 2014); (D) Elephantomyia wetwoodi Osten Sacken, 1869 (after Ribeiro Reference Ribeiro2008). Abbreviation: aed = aedeagus. Not to scale.
H. spiralensis differs from other species known from this period mainly by the relationships of the rostrum, palpus, antenna and head, and also by the wing venation and morphology of the hypopygium. In contrast to Helius alavensis Kania, Krzemiński & Arillo, Reference Kania, Krzemiński and Arillo2016, the rostrum of H. spiralensis is much longer than the head (twice as long) and is approximately nine times as long as wide, whilst in H. alavensis it is only slightly longer than the head, approximately five times as long as wide. In H. spiralensis, the palpus is very narrow, about 0.25 times longer than the rostrum, whilst the antenna is 0.2 times longer than the rostrum and only slightly longer than the palpus (Figs 1A, 2B, C). The antenna in H. alavensis is approximately 1.3 times as long as the rostrum and about 1.3 times as long as the palpus. The palpus in H. spiralensis is very narrow, but not so narrow as in H. alavensis.
The newly described species distinctly differs from the Barremian and Aptian representatives of the Helius. This small species is characterised by having a closed discal cell (d-cell), in contrast to H. lebanensis Kania, Krzemiński & Azar, Reference Kania, Krzemiński and Azar2013, where the discal cell is open. Moreover, the new species differs from both H. lebanensis and H. ewa Krzemiński, Kania & Azar, Reference Krzemiński, Kania and Azar2014 by the relationship of the rostrum, palpus, antenna and head, as well as by the morphology of the hypopygium. In both species, the aedeagus is not so elongated and there are no additional lobes on the gonocoxite.
This finding of a peculiar new species with a characteristic morphology of the hypopygium, whose aedeagus is similar to that of Elephantomyia, additionally confirms the relationship of these two genera. The first representatives of Elephantomyia are known from the Eocene; we have no data on the occurrence of this genus in older periods. The oldest representatives of Helius are known from the Early Cretaceous fossil record (Rayner & Waters Reference Rayner and Waters1990; Ribeiro Reference Ribeiro2002; Kania et al. Reference Kania, Krzemiński and Azar2013, Reference Kania, Krzemiński and Arillo2016; Krzemiński et al. Reference Krzemiński, Kania and Azar2014). Species such as Helius ewa, H. lebanensis or H. alavensis, with their elongated rostrum, existed just after the time when the Angiospermae appeared. The elongated rostrum, such as in H. spiralensis, was probably an adaptation of these insects to a new food source, such as nectar or pollen. We have no data about the occurrence of Helius in the Jurassic; it is still unknown from which evolutionary line of Jurassic Limoniidae the genus Helius derives. The existence of the representatives of Helius with an elongated rostrum in the Early Cretaceous suggests that the elongated rostrum was probably an adaptation to a new food source and the radiation of these crane-flies was probably rapid (Kania et al. Reference Kania, Krzemiński and Azar2013; Krzemiński et al. Reference Krzemiński, Kania and Azar2014).
4. Acknowledgements
This study is a contribution the project CGL2014-52163 “Iberian amber: An exceptional record of Cretaceous forests at the rise of modern terrestrial ecosystems” of the Spanish Ministry of Economy and Competitiveness.
We would like to acknowledge the reviewers, Professor Dany Azar and Dr. Guilherme Ribeiro, for their corrections and very valuable comments.








