Insects from the Late Carboniferous Coal Measures in the UK are well known from studies by pioneering researchers such as Herbert Bolton, Samuel H. Scudder and Henry Woodward, amongst others, (e.g., see Woodward Reference Woodward1876; Scudder Reference Scudder1881, Reference Scudder1883; Bolton Reference Bolton1921, Reference Bolton1922; Ross Reference Ross2010). The fossil insects were mainly collected from several famous localities associated with coal mining activity in the late 19th and early 20th centuries, such as Coseley, Staffordshire, in the West Midlands. However, some old mine spoil heaps, such as Lower Writhlington near Radstock, Bath & NE Somerset, or the Crock Hey opencast pit near Manchester in Lancashire, have yielded new discoveries (Jarzembowski Reference Jarzembowski1989; Anderson et al. Reference Anderson, Dunlop, Eagar, Horrocks and Wilson1999; Prokop et al. Reference Prokop, Nel and Tenny2010).
Palaeodictyoptera appear as one of the first groups of pterygote insects in the Early Pennsylvanian, attaining considerable diversity in the Late Carboniferous and Permian ecosystems, and disappeared at the end of the Permian in the PT mass extinction (Engel et al. Reference Engel, Davis, Prokop, Minelli, Boxshall and Fusco2013).
1. Material and methods
The fossil specimens were observed under Meiji Techno RZ, Leica MZ6, Nikon SMZ646 and Leica MZ 12.5 stereomicroscopes in a dry state and rarely under a film layer of ethyl alcohol. Scanning electron micrograph of uncoated specimen GLAHM A.2680a was obtained using an environmental electron microscope Hitachi S-3700N (Hitachi Ltd, Chiyoda, Tokyo, Japan), at an accelerating voltage of 15kV with a turntable sample holder, at the Department of Paleontology, National Museum in Prague. Line drawings of the patterns of wing venation were made with the aid of a camera lucida attachment. Photographs were taken on a Leica S8APO stereomicroscope with DC170HD camera, Canon D550 digital camera coupled with lenses MP-E 65 mm or EF 50 mm, Nikon Coolpix 4500, or a Wild Makroscope M420 with Nikon D700 camera. The photographs were processed using Adobe Photoshop CS4, and for some images, the focus-stacking software Helicon Focus Pro was used.
The wing venation nomenclature follows Kukalová-Peck (Reference Kukalová-Peck and Naumann1991), with the following symbols used for wing veins (symbols in capitals denote the longitudinal veins): CuA/CuP = cubitus anterior/posterior; MA/MP = median anterior/posterior; RA/RP = radius anterior/posterior; ScA/ScP = subcosta anterior/posterior; 1A = first anal vein.
Institutional repositories. BMB, Booth Museum of Natural History, Brighton, UK; GLAHM, Hunterian Museum, University of Glasgow, UK; NHMUK, Natural History Museum, London, UK; NMS, National Museums of Scotland, Edinburgh, UK; TM, Teylers Museum, Haarlem, The Netherlands.
2. Systematic palaeontology
Order Palaeodictyoptera Goldenberg, Reference Goldenberg1877
Family Breyeriidae Handlirsch, Reference Handlirsch and Englemann1906
Genus Vernooijia gen. nov.
Type species. Vernooijia sassoonae sp. nov.
Etymology. Named after the Dutch collector of fossil insects Bart J. M. Vernooij, who found and donated the holotype of Vernooijia harlemensis (Brauckmann & Gröning, Reference Brauckmann and Gröning1996) comb. nov. to the Teylers Museum in Haarlem.
Diagnosis. The genus differs from all other Breyeriidae in the combination of ScP ending near the apex of C, RP with at least four branches, MP deeply forked and both main branches forked secondarily and CuP ending with two branches.
Vernooijia sassoonae sp. nov.

Figure 1 Vernooijia sassoonae gen. et sp. nov. (Breyeriidae): drawing of holotype, specimen NHMUK II.3092a, b. Abbreviations: 1A = first anal vein; CuA/CuP = cubitus anterior/posterior; MA/MP = media anterior/posterior; RA/RP = radius anterior/posterior; ScP = subcosta posterior. Scale bar = 5 mm.
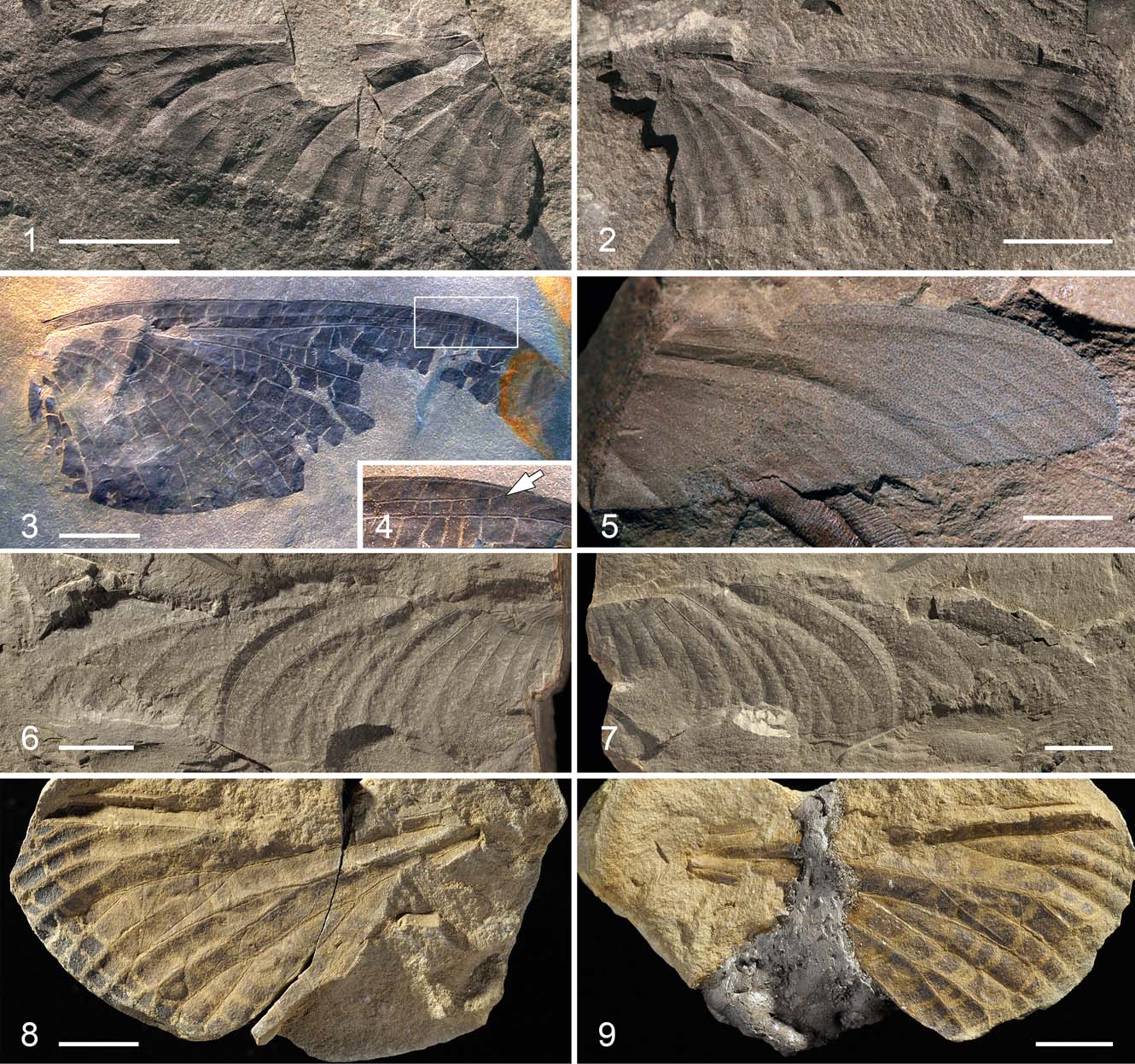
Plate 1 (1–2) Vernooijia sassoonae gen. et sp. nov. (Breyeriidae), photographs of holotype, part and counterpart, NHMUK II.3092a, b. (3–4) Vernooijia harlemensis (Brackmann & Gröning, 1996) comb. nov. (Breyeriidae): (3) photograph of holotype hindwing, specimen TM 24254; (4) photograph of detailed ending of vein ScP. arrow indicates end of subcosta posterior. (5) ?Lycocercidae gen. et sp. indet. (Palaeodictyoptera), photograph of specimen NHMUK II.3093. (6–7) Mazonopterum cooperi sp. nov. (Homoiopteridae), photographs of holotype, part and counterpart, BMB 018818/018819. (8–9) Homaloneura sp. (Spilapteridae), photographs of part and counterpart, NMS G.1911.6.6. Scale bars = 5 mm (1, 2, 5, 8, 9); 10 mm (3, 6, 7).
Etymology. The specific epithet honours the palaeontologist Dr Judyth Sassoon (Bristol University) for her care of the collection.
Diagnosis. The first branch of the anal vein is simple without terminal offshoots.
Holotype. NHMUK II.3092a, b (formerly W1169 in the private coll. of EAJ) part & counter-part.
Type locality. Writhlington Geological Nature Reserve, Lower Writhlington, near Radstock, Bath & NE Somerset, old mine spoil heap; roof shale with Cyperites and Lepidostrobophyllum.
Type strata. Middle Pennsylvanian, Westphalian D/late Asturian (Moscovian).
Description. Hindwing with incomplete wing base, estimated length about 22 mm, maximum width 8.7 mm close to wing base, wing membrane with five dark pigmented oblique stripes merging along the posterior wing margin; numerous simple, faint cross-veins rarely forming smaller cells; simple ScP nearly straight reaching costal margin close to wing apex; simple RA distally parallel to ScP reaching costal margin close to apex; area between RA and RP with few simple oblique cross-veins; RP diverging from RA 3.3 mm distal of the division between CuA and CuP, distally ending with five branches connected by few simple, faint cross-veins; division of veins MA and MP at same level as RA and RP, convex MA simple, widely separated from MP; concave MP deeply forked and both main branches secondarily forked, ending with four branches on posterior wing margin; numerous simple, faint cross-veins between branches of MA and MP; division of CuA and CuP near wing base; convex CuA simple, slightly undulating; concave CuP forked; anal area formed by five long branches connected by faint cross-veins.
Discussion. This fossil has a wing venation pattern characteristic of Breyeriidae: veins RA/RP and stem of M basally very close, RP ending with five terminal branches, MA and CuA both simple, CuP distally forked, and a faint network of irregular cross-veins (Carpenter Reference Carpenter, Moore and Kaesler1992). However, the long ScP ending close to the wing apex is a trait rarely present in Breyeridae, only found in Aviobreyeria (A. gracilis Prokop, Tippeltová, Roques & Nel, 2013), Hasala (H. inferiorsaxonica Brauckmann, Reference Brauckmann1995) and one species of Breyeria (B. harlemensis Brauckmann & Gröning, Reference Brauckmann and Gröning1996). However, the long ScP ending on the costa is a character present in members of the little known Graphiptilidae. According to Kukalová (Reference Kukalová1969b, pp 458–59), this family shares a number of traits in venation and colour markings with Breyeriidae; differing mainly in the denser pattern of the cross-veins, the branches of main veins being obliquely orientated and closer together, instead of almost perpendicular to the posterior wing margin, and widely separated branches. Based on these diagnostic characters, we can exclude the placement of this fossil in Graphiptilidae and support the assignment to Breyeriidae. Kukalová (Reference Kukalová1969b, p. 459) considered the possible attribution of Stobbsia (see below) to Graphiptilidae, but this suggestion was not followed by others, particularly in that S. woodwardiana Handlirsch, Reference Handlirsch and Englemann1908 has only a few terminal branches of the main veins, and therefore the genus remains in Breyeriidae (see Carpenter Reference Carpenter, Moore and Kaesler1992).
On the basis of the partially preserved basal part of a narrow costal area and markedly broad anal area, we consider this specimen to be a hindwing. Prokop et al. (Reference Prokop, Tippeltová, Roques and Nel2013) provided the most recent summary of the Breyeriidae, listing six genera based on wing venation (Aviobreyeria Prokop Prokop, Tippeltová, Roques & Nel, Reference Prokop, Tippeltová, Roques and Nel2013; Breyeria Borre, Reference Borre1875; Hasala Brauckmann, Reference Brauckmann1995; Jugobreyeria Brauckmann, Koch & Kemper, Reference Brauckmann, Koch and Kemper1985; Megaptiloides Handlirsch, Reference Handlirsch and Englemann1906; and Stobbsia Handlirsch, Reference Handlirsch and Englemann1908) and the description of a few immature wing pads. Records are only known from Euramerica and the chronostratigraphical range of this family is from Bashkirian to Gzhelian (Prokop et al. Reference Prokop, Tippeltová, Roques and Nel2013).
Among the genera of Breyeriidae, only Stobbsia and Hasala have ScP ending on the costal margin (Carpenter Reference Carpenter, Moore and Kaesler1992; Brauckmann Reference Brauckmann1995). However, in Stobbsia this ends slightly behind the midwing, unlike in Vernooijia. Further comparison shows marked differences between Stobbsia and Vernooijia in the arrangement of RP, MP and the anal area. Hasala shares with Vernooijia the termination of ScP in the distal part of the wing, but is strikingly different in the more prominent branching of RP, with nine branches and a different arrangement of MP. Thus, the above-mentioned difference supports the referral of this new species to Vernooijia.
Brauckmann & Gröning (Reference Brauckmann and Gröning1996) described Breyeria harlemensis from the Langsettian of South Limbourg, The Netherlands, which shares a long ScP and very similar pattern of branching to V. sassoonae gen. et sp. nov., with the exception of a less developed anal area. Moreover, re-examination of the holotype reveals the presence of ScP ending on C, which excludes placement in the genus Breyeria (see Pl. 1, fig. 3). Therefore, we propose to transfer Breyeria harlemensis to the genus Vernooijia as V. harlemensis comb. nov. This assignment is supported by the branches of the median and cubital veins, which are oblique in V. harlemensis and V. sassoonae, instead of almost perpendicular to the posterior wing margin as in members of Breyeria. However, the comparison of the hindwings between V. sassoonae and V. harlemensis is limited, due to the missing posterior medioapical part of the wing. In the latter species, the pattern of the branches of the media and cubitus can be seen. Differences between both species can be found in the arrangement of the first anal vein being deeply bifurcate in S. sassoonae and with three anterior branches in V. harlemensis. Both species also markedly differ in wing length, with that of V. harlemensis being about three times longer than that of V. sassoonae.
Family Homoiopteridae Handlirsch, Reference Handlirsch and Englemann1906
Genus Mazonopterum Kukalová-Peck & Richardson, Reference Kukalová-Peck and Richardson1983
Type species. Mazonopterum wolfforum Kukalová-Peck & Richardson, Reference Kukalová-Peck and Richardson1983.
Mazonopterum cooperi sp. nov.

Figure 2 Mazonopterum cooperi sp. nov. (Homoiopteridae): drawing of holotype, specimen BMB 018818/018819. Abbreviations: 1A = first anal vein; CuA/CuP = cubitus anterior/posterior; MA/MP = media anterior/posterior; RA/RP = radius anterior/posterior; ScP = subcosta posterior. Scale bar = 10 mm.
Etymology. The specific epithet honours John Cooper (Booth Museum of Natural History, Brighton) on his retirement.
Diagnosis. CuP deeply bifurcated with both branches secondarily forked posteriorly, first anal vein (A1) ending with two main branches on posterior wing margin.
Holotype. Part and counterpart BMB 018818/ 018819, collected by AJR (his first fossil insect find, collected 1987).
Type locality. Writhlington Geological Nature Reserve, Lower Writhlington, near Radstock, Bath & Northeast Somerset, England.
Type strata. Middle Pennsylvanian, Westphalian D/late Asturian (Moscovian).
Description. Large wing, incomplete in anterior and apical parts, estimated length 105 mm, estimated maximum width 27 mm, venation originally hyaline, with dense pattern of cross-veins forming small cells (so called archedictyon); simple ScP preserved in distal part only running parallel and very close to costal margin; convex RA simple, reaching costal margin close to apex, area between ScP and RA with one row of cross-veins; area between RP and RA distally broad, 3.3 mm wide (4–6 archedictyon cells); RP ending with at least eight branches; convex MA, fragmentary and only preserved in mid-wing, may be simple or terminally forked; concave MP well developed, deeply forked, with anterior fork pectinate ending with four branches on posterior wing margin and posterior branch reaching posterior wing margin with five distal pectinate branches; division of CuP and CuA 13.9 mm basad of main fork of MP; simple CuA strongly curved distally reaching posterior wing margin close to proximal branch of MP, CuP deeply forked 5.0 mm from fork of CuA and CuP, both branches secondarily forked posteriorly; anal area formed by seven anal veins, basally connected by strong anal brace (anal bar) in form of a ridge, first anal vein deeply forked, second and third branch terminally forked.
Discussion. This wing is probably a hindwing, due to the very broad anal area. However, we cannot be certain because of the incompletely preserved posterior wing margin close to the wing base, and the fossil also lacks part of the anterior wing margin, which is commonly broader in forewings. The wing can be attributed to Homoiopteridae, due to the combination of the following venation characters: RA and ScP running very close to wing apex; MA simple or just terminally forked; MP deeply forked, main anterior and posterior branches pectinate with four or more terminal branches; anal veins connected by strongly developed anal brace (anal bar); and, finally, considerably large size of the wing. An attribution to Eugereonidae can be excluded, as the fossil possesses a broad anal area with secondarily bifurcated veins, a wide anal bar, MA relatively widely spaced from MP and a broad area between RA and RP, all unknown in members of this family (Carpenter Reference Carpenter, Moore and Kaesler1992; Prokop & Ren Reference Prokop and Ren2007). For further generic placement, we followed the matrix of characters as proposed for genera within Homoiopteroidea by Prokop & Nel (Reference Prokop and Nel2004), supplemented by Prokop et al. (Reference Prokop, Smith, Jarzembowski and Nel2006) and Beckemeyer & Engel (Reference Beckemeyer and Engel2011). One of these important characters is the simple CuA terminating close to MP, which can be seen in Mazonopterum Kukalová-Peck & Richardson, Reference Kukalová-Peck and Richardson1983 and Anglopterum Prokop et al., Reference Prokop, Smith, Jarzembowski and Nel2006. Other genera, such as Boltopruvostia Strand, Reference Strand1929, Scepasma Handlirsch, Reference Handlirsch1911 and Ostrava Kukalová, Reference Kukalová1960, have fewer branches of RP and MP and are not widely spaced, with CuA usually terminally forked, unlike in this fossil.
Pharciphyzelus Beckemeyer & Engel, Reference Beckemeyer and Engel2011 share with the fossil a simple CuA, with rather narrow area between CuA and MP; differing from Mazonopterum in having a narrower region with fewer archedictyon cells between MA and MP and a different pattern of branching of MP (Beckemeyer & Engel Reference Beckemeyer and Engel2011).
Anglopterum, based on A. magnificum Prokop, Smith, Jarzembowski & Nel, Reference Prokop, Smith, Jarzembowski and Nel2006 from the Langsettian of Crock Hey opencast pit, Greater Manchester, Lancashire (UK), shares several characters, particularly vein CuA simple, broadly spaced MA from MP and deep branching of first anal vein; though differs mainly by CuA not being strongly curved and the area between CuA and MP being broader, with three or four rows of archedictyon cells in the widest part; also, it has anal vein with three terminal branches, unlike in this fossil.
Mazonopterum, based on M. wolfforum from the Moscovian of Mazon Creek in Illinois, USA, shares the generic diagnostic characters of widely spaced MA and MP, similar branching pattern of MP, CuA simple and CuP with two main branches secondarily forked.
Therefore, we proposed to assign our fossil to Mazonopterum, with M. cooperi differing from M. wolfforum mainly by the arrangement of the first anal vein with only two terminal branches instead of four or more branches in M. wolfforum, as can be observed on the holotype and paratype specimens. Both species differ markedly in wing length, which is estimated in M. cooperi as about 105 mm, instead of about 160 mm in the holotype of M. wolfforum and 173 mm in the paratype.
Prokop et al. (Reference Prokop, Smith, Jarzembowski and Nel2006) described an incomplete posterior-basal part of a hindwing attributed to Mazonopterum cf. wolfforum from the Upper Asturian of Kilmersdon Colliery tip near Radstock, Bath & NE Somerset, UK. This fragmentary fossil is much larger than M. cooperi, it has a more deeply bifurcated first branch of the anal area and CuP has its posterior branch with at least three branches. Prokop et al. (Reference Prokop, Roques and Nel2014) described an incomplete homoiopterid wing base from the Moscovian of the Avion locality, Pas-de-Calais (France), which shares the first anal vein being bifurcate as in M. cooperi, but diverging more distally as in M. wolfforum. However, the interpretation of the veins was erroneous and needed correcting, starting from CuA anteriorly (see Prokop et al. Reference Prokop, Roques and Nel2014, fig. 2); thus, the division of CuA and CuP is closer to the wing base, ‘MP' is actually CuA, CuP is deeply bifurcated (the anterior branch was erroneously indicated as ‘CuA'), and both secondary branches similar to M. cooperi emerge at the same level as the branching of the first anal vein.
Brauckmann et al. (Reference Brauckmann, Herd and Leipner2015) reported M. wolfforum from localities in northwest and southwest Germany at Piesberg near Osnabrück in Lower Saxony (Westphalian C/D), Ibbenbüren, North Rhine–Westphalia (Westphalian C–D) and Ludweiler, Saarland (probably Westphalian C–Bolsovian).
Family Spilapteridae Brongniart, Reference Brongniart1893
Genus Homaloneura Brongniart, Reference Brongniart1893
Type species. Homaloneura elegans Brongniart, Reference Brongniart1885.
Homaloneura sp.

Figure 3 Homaloneura sp. (Spilapteridae): drawing of specimen NMS G.1911.6.6. Abbreviations: CuA = cubitus anterior; MA/MP = media anterior/posterior; RA/RP = radius anterior/posterior; ScP = subcosta posterior. Scale bar = 5 mm.
1914. Aedoephasma anglica Scudder. Peach, p. 142.
Description. Wing originally with darkly pigmented membrane; simple cross-veins arranged in rows forming large cells; wing fragment length 29.6 mm, estimated total length about 55 mm, estimated width about 16 mm; costal margin markedly serrate and reinforced; ScP running distally parallel to costal margin probably ending close to wing apex; convex RA simple and straight probably reaching wing apex; RP with at least four main branches probably covering whole wing apex area, first branch of RP secondarily forked emerging approximately level with first branch of MA; M forking into MA and MP close to wing base; convex MA anterior branch with three branches, posterior one probably simple; concave MP deeply branched 11.5 mm beyond division of MA and MP with three branches, anterior one probably simple; division of CuA and CuP probably very close to wing base, but only partially preserved, CuA pectinate posteriorly with three simple branches and forth possibly emerging behind level of division of MA and MP.
Material. Part and counterpart, NMS G.1911.6.6. J.S. Neil Coll.
Age and horizon. Middle Coal Measures. Langsettian to Duckmantian, Coseley?, Staffordshire, England.
Discussion. This specimen was originally misidentified (and misspelt) by Peach (Reference Peach1914) as ‘Aedoephasma' anglica Scudder, Reference Scudder1885 (correct spelling Aedoeophasma). Peach listed specimens in the Neil collection and although he did not provide specimen numbers in his paper, his identification is in the Museum register under this specimen number. The specimens are listed in the register as coming from ‘South Staffordshire' and although a locality is not provided, they most likely came from Coseley or, less likely, Dudley (Tipton).
This wing can be attributed to Spilapteridae, due to the following combination of characters in wing venation: RP ending with four or more main branches; simple, straight cross-veins between RA and RP; MA and MP branched; CuA with four terminal branches (three complete and fourth partially preserved), plus wing with colour pattern.
This family is represented by 20 genera from the Late Carboniferous and Permian deposits of Euramerica, Asian Russia and China (Carpenter Reference Carpenter, Moore and Kaesler1992; Sinitshenkova Reference Sinitshenkova, Rasnitsyn and Quicke2002; Li et al. Reference Li, Ren, Pecharová and Prokop2013; Liu et al. Reference Liu, Béthoux, Yin and Ren2015; Prokop et al. Reference Prokop, Nel, Engel, Pecharová and Hörnschemeyer2016a). Furthermore, our fossil shares the diagnostic characters of the genus Homaloneura Brongniart, Reference Brongniart1885, in the presence of a nearly straight or slightly concave anterior wing margin (partially preserved in distal part) and simple cross-veins in the area between RA and RP; but other diagnostic characters, e.g., the form of the convex ridge, are not preserved. However, the pattern of coloration with dark spots filling the cells is found in species belonging to the genus Spilaptera (e.g., Spilaptera packardi Brongniart, Reference Brongniart1885) and some Homaloneura (e.g., H. punctata Brongniart, Reference Brongniart1894). Kukalová (Reference Kukalová1969a), followed by Carpenter (Reference Carpenter, Moore and Kaesler1992), proposed to separate both genera based on straight versus sigmoidal cross-veins in the area between RA and RP and the form of the anal brace as a convex ridge (not present in this fossil). This supports the placement of the fossil in Homaloneura rather than Spilaptera.
This fossil is too incomplete to identify or name to species, so we prefer to keep this fossil as Homaloneura sp. to avoid future taxonomic problems. Based on the very narrow area between ScP and the costal margin preserved in the distal part, it is considered to be a hindwing. The pattern of venation and wing length is similar to H. dabasinskasi from the Moscovian of Mazon Creek in Illinois, USA. This first discovery of Homaloneura sp. (Spilapteridae) from the Langsettian to Duckmantian of Staffordshire in England supplements previous records from France, Germany and the USA.
Lycocercidae gen. et sp. indet.

Figure 4 ?Lycocercidae gen. et sp. indet. (Palaeodictyoptera): drawing of specimen NHMUK II.3093. Abbreviations: CuA = cubitus anterior; MA/MP = media anterior/posterior; RA/RP = radius anterior/posterior; ScP = subcosta posterior. Scale bar = 5 mm.
Material. Specimen NHMUK II.3093, wing apex with associated seed fern Alethopteris leaflet.
Age and horizon. Middle Pennsylvanian, late Westphalian D/early Cantabrian; Forest of Dean, Gloucestershire, England; disused mine spoil heap.
Description. Slightly pointed wing with apical part narrow, thick membrane originally dark coloured, main veins with prominent corrugation, only a few simple cross-veins preserved; ScP running parallel to the anterior wing margin ending at the costa well before the wing apex, simple RA reaching anterior wing margin close to apex; concave RP pectinate with nine posterior branches reaching wing apex and distal posterior margin; simple MA slightly curved distally; concave pectinate MP ending with nine posterior branches; simple convex CuA; terminal fork of branch CuP present.
Remarks. This incomplete wing shows the prominent corrugation of the main longitudinal veins, which is typical for members of Palaeodictyopterida, Odonatoptera and Ephemeropterida. The latter two groups can be excluded, due to the markedly different pattern with prominent intercalary veins. Amongst Palaeodictyopterida, two potential groups are Palaeodictyoptera and Diaphanopterodea, sharing a pattern of numerous branching longitudinal veins. Members of Diphanopterodea (Diaphanopteridae) differ in ScP ending on RA and fewer branches of RP and MP. However, in the case of Namurodiaphidae, such as Camptodiapha atkinsoni Beckemeyer & Engel, Reference Beckemeyer and Engel2011, ScP ends on C and the terminal branches of these veins can be more numerous, but differently arranged instead of having a regular pectination posteriorly (Beckemeyer & Engel Reference Beckemeyer and Engel2011). Thus, the pattern of venation in this fossil fits the Palaeodictyoptera in having a simple MA and CuA and, together with the richly posteriorly branched RP and MP, it suggests several palaeodictyopteran families, such as Eugereonidae, Lithomanteidae and, particularly, Lycocercidae (Carpenter Reference Carpenter, Moore and Kaesler1992). The apex of the genus Apopappus guernei (Brongniart, Reference Brongniart1894) from the Moscovian of Commentry in France is similar to that in this specimen. However, we cannot be sure about the placement because of a lack of other diagnostic characters. Therefore, this specimen is only tentatively placed in the family Lycocercidae.
Palaeodictyoptera family incertae sedis
Idoptilus sp.
(Figs 5, 6, Plate 2, figs 1–4)

Figure 5 Idoptilus sp. nymphal exuvia (Palaeodictyoptera): habitus drawing of specimen GLAHM A.2680a, dorsal view (banded pattern of light and dark transversal stripes on wing pads omitted). Scale bar = 5 mm.

Figure 6 Idoptilus sp. nymphal exuvia (Palaeodictyoptera): scanning electron micrograph of caudal appendages, GLAHM A.2680a, dorsal view.

Plate 2 Idoptilus sp. nymphal exuvia, specimen GLAHM A.2680a: (1) photograph of habitus, immersed in ethanol, dorsal view; (2) photograph of habitus, dorsal view; (3) photograph of habitus, dorso-lateral view; (4) detailed photograph of wing pad tracheation. Abbreviations: ak = anterior keel; cla = caudal abdominal appendages with lamellae; CuA = cubitus anterior; MA = media anterior; msw = mesothoracic wing pad; mtw = metathoracic wing pad; ptw = prothoracic wing pad; RA = radius anterior. Scale bars = 5 mm (1, 2, 3); 2 mm (4).
1967. unidentified cockroach? nymph. Rolfe, p. 308, pl. 50, fig. 8, text-fig. 2C
Material. GLAHM A.2680 (part & counterpart) nymphal exuvia preserved dorsally in siderite nodule.
Age and horizon. Middle Coal Measures, Bolsovian (= Westphalian B), Middle Coal Measures, Stainborough, Barnsley, South Yorkshire, England.
Description. Exuvia of onisciform nymph, total length 19.5 mm. Small hypognathous head surrounded by emarginated prothoracic wing pads, prominent lateral globular eyes, prothorax slightly shorter than meso- and metathorax, prothoracic wing pads distinctly shorter than meso- and metathoracic ones, metathoracic wing pads basally broader covering first abdominal segment. Wing pads with faint banded pattern of light and dark transversal stripes (Pl. 2, fig. 1; more visible in Rolfe Reference Rolfe1967, pl. 50, fig. 8). Prominent anterior keel present on mesothoracic wing pad, position of probably original tracheae and lacunal channels on wing pads partly discernible as corresponding ridges, particularly convex tracheae as RA, MA and CuA markedly elevated. Abdomen with ten discernable tergal segments and prominent laterotergites tapering distally, terminal segment with a pair of appendages in form of triangular lamellae 3.8 mm long, maximum width 0.6 mm, apically folded for short distance, presumably arranged horizontally, and single arched median appendage present between these lateral lamellae.
Discussion. Rolfe (Reference Rolfe1967) tentatively identified this fossil as a cockroach (Blattodea) nymph and considered that “a large oval process projecting from one of the posterior abdominal tergites might conceivably have had a branchial function”. The presence of prothoracic wing pads surrounding the small head (instead of a pronotal disc as is known in Blattodea) and, particularly, the presence of tracheation and lacunal channels of meso- and metathoracic wing pads with three posteriorly curved convex ridges, which probably correspond to the tracheae as RA, MA and CuA (Pl. 2, fig. 4) (as opposed to being straight in the hindwing buds of Blattodea), support the placement of this specimen in Palaeodictyoptera, in contrast to the previous study.
The onisciform shape and form and tracheation of the wing pads are very similar to those of Idoptilus peachii (Woodward, Reference Woodward1887) (see Ross Reference Ross2010). The only differences are that the head appears to be shorter and the prothoracic lobes are more pointed posteriorly in this specimen (the terminal appendages of I. peachii are not known). It could be argued that this nymphal exuvia belongs to a new species, but given that it is not possible to place it in a family and it is a juvenile, then formal description and naming does not seem necessary.
We concur with the interpretation by Rolfe (Reference Rolfe1967) that the form of the terminal appendages could indicate a branchial function. Our re-examination supports the presence of three triangular caudal appendages bearing prominent lateral lamellae, emerging from terminal abdominal segment X (Figs 5, 6, Pl. 2, figs 1–3). We suspect that these lamellae were originally covered with setae, as they bear a dense pattern of fine tubercles and represent tracheal gills. Similarly modified caudal appendages in the form of tracheal gills are known in nymphs of damselflies (Odonata: Zygoptera), and their triangular shape can be found, for instance, in Austrocnemis splendida (Martin, Reference Martin1901) (Tillyard Reference Tillyard1917, text-fig. 26, pl. II, figs 18, 19). However, the state of preservation of the fossil prevents further detailed comparison of these delicate structures.
Whilst other structures, such as the triangular head and prominent abdominal laterotergites, confirm the attribution to Palaeodictyoptera, the presence of caudal appendages in the form of putative tracheal gills was previously unknown in this group (the terminalia of I. peachii are not known; however, they could have been similar to those of I. sp.). Other available nymphs of this order bear only a pair of markedly long and stout cerci, comparable to adults (Wootton Reference Wootton1972; Prokop et al. Reference Prokop, Nel, Engel, Pecharová and Hörnschemeyer2016a, Reference Prokop, Pecharová, Nel, Hörnschemeyer, Krzemińska, Krzemiński and Engel2017). The lifestyle of these nymphs was initially considered as aquatic by some authors (Brongniart Reference Brongniart1885, Reference Brongniart1893; Handlirsh Reference Handlirsch and Englemann1906), but further re-evaluation of available taxa was unable to confirm aquatic adaptations and, thus, rather supported a terrestrial mode of life for these insects (Wootton Reference Wootton1972; Shear & Kukalová-Peck Reference Shear and Kukalová-Peck1989). Another argument supporting the co-existence of nymphs and adults in the same habitats is the presence of specialised mouthparts in the form of a rostrum, which would be difficult to imagine having the same piercing and sucking function in aquatic and terrestrial environments; however, the rostrum is not well known in onisciform nymphs (Prokop et al. Reference Prokop, Pecharová and Ren2016b). Nevertheless, our knowledge of this type of mouthpart is still rather limited, as is that of the palaeodictyopteran diet, which is mostly considered as phytophagy based on a few discovered examples of piercing and sucking damage as trace fossils (e.g., Labandeira & Phillips Reference Labandeira and Phillips1996); and at Writhlington (see Jarzembowski Reference Jarzembowski2004).
Thus, the palaeodictyopteran nymphal exuvia possesses a unique modification of caudal abdominal appendages, here interpreted as tracheal gills. Nevertheless, the results of experimental studies by the removal of caudal gills in zygopterous nymphs demonstrated mainly their function in the locomotion as rudders and of only marginal significance for respiration (see Bodine Reference Bodine1918). These structures presumably represent aquatic adaptations and support an aquatic lifestyle for at least some members of immature Palaeodictyoptera. This raises the question of whether early instars could have been aquatic and later instars terrestrial. Only further discoveries could help answer this.
3. Conclusions
The Carboniferous Coal Measure deposits from the UK are well known for several palaeodictyopteran species described by classic authors. Despite the fact that the golden era of coal mining activity is over, the collection and interpretation of interesting insect fossils continues on old spoil heaps and from examining specimens in museums and private depositories.
The present study includes descriptions of two new taxa: Vernooijia sassoonae gen. et sp. nov. (Breyeriidae) and Mazonopterum cooperi sp. nov. (Homoiopteridae), both from the Middle Pennsylvanian (Westphalian D/Late Asturian) of Writhlington near Radstock. The latter species represents the second known species of Mazonopterum described (the first one was described from Mazon Creek in Illinois, USA) and supplements the previous record based on a fragmentary fossil (Prokop et al. Reference Prokop, Smith, Jarzembowski and Nel2006). Furthermore, our detailed re-examination of venation traits in Breyeria harlemensis allows its transfer to the genus Vernooijia as V. harlemensis (Brauckmann & Gröning, Reference Brauckmann and Gröning1996) comb. nov., and also supports a close relationship to V. sassoonae.
We describe the first record of Homaloneura sp. (Spilapteridae) from the UK, otherwise known from coeval deposits in France, Germany and the USA. A peculiar wing apex, tentatively attributed to Lycocercidae, is reported from the Middle Pennsylvanian of the Forest of Dean in Gloucestershire. Finally, our re-examination of a putative blattodean nymph, figured by Rolfe (Reference Rolfe1967), reveals a corrugated pattern of probably original tracheation on the wing pads, which supports placement within Palaeodictyoptera, and the insect is identified as Idoptilus sp.
Moreover, our detailed study of this nymphal exuvia documents the presence of abdominal caudal appendages with lateral lamellae, previously unknown in nymphs of this group. We assume that these lamellae were originally covered with dense setae and possibly functioned for locomotion and as tracheal gills, such as can occur in the nymphs of damselflies (Odonata: Zygoptera). Thus, the scenario of a possible aquatic lifestyle for nymphs of at least some members of the Palaeodictyoptera, as considered by Brongniart (Reference Brongniart1885, Reference Brongniart1893) and Handlirsch (Reference Handlirsch and Englemann1906), cannot be ruled out.
4. Acknowledgements
We are grateful to Robin Wootton (Exeter University, UK) and Carsten Brauckmann (TU Clausthal, Germany) for their reviews and helpful suggestions. We would also like to thank Claire Mellish (NHM, London, UK) for the prompt registration of two herein described specimens; and John Cooper (Booth Museum of Natural History, Brighton, UK) and Neil Clark (Hunterian Museum, Glasgow, UK) for the loan of specimens. We are grateful for the use of photographs taken by Bill Crighton (NMS) of M. cooperi, Homaloneura sp. and Idoptilus sp. We are grateful to Lenka Váchová (National Museum in Praha) for her assistance with the ESEM. The first author (JP) acknowledges the financial support of the Grant Agency of the Czech Republic (No. 14-03847J). The second author (MP) was supported by the Institutional Research Support grant of the Charles University, Prague (No. SVV 260 313/ 2016). The third author (EAJ) was partly supported by the Chinese Academy of Sciences President's International Fellowship Initiative (2011T2Z04).










