Tristychius arcuatus (Agassiz Reference Agassiz1837) is a medium-sized shark known from skeletal remains that are mostly preserved in ironstone nodules of the Lower Carboniferous Scottish Upper and Lower Oil Shale Groups. Our current understanding of this fish is built largely on Stan Wood's collections of new specimens, assembled in the early 1970s (Wood Reference Wood1975). These fossils provided most of the material that John Dick (Reference Dick1978) used for his monographic description, from which Tristychius emerged as one of the most completely known chondrichthyans from the entire Palaeozoic. It should be acknowledged from the outset that Tristychius is one of the few early chondrichthyans for which the cranial and postcranial skeleton has been described from multiple specimens. However, this motherlode of data has notably failed to deliver corresponding phylogenetic clarity. For the past couple of decades, Tristychius has loitered near to the base of the chondrichthyan crown as a ‘problematic' taxon (Maisey Reference Maisey2011), because its unusual morphology fails to align easily with other grades or clades of Palaeozoic sharks. Although Dick's (Reference Dick1978) reconstruction was included in Zangerl's (Reference Zangerl and Schultze1981) Handbook of Paleoichthyology ‘Chondrichthyes I', Janvier's (Reference Janvier1996) ‘Early Vertebrates' provides only a passing reference to the genus. Thus far, published adjustments to Dick's benchmark description have been minor, limited to notes on the pectoral fin obtained from slightly younger material from the Manse Burn Formation of Bearsden (Coates & Gess Reference Coates and Gess2007). The aim of the present work is to re-describe the neurocranium in the light of new results obtained from computed tomographic (CT) survey of several Tristychius specimens. Some, but not all, of these were included in Dick's (Reference Dick1978) monograph, and all except one resulted from Stan Wood's dedicated field work, whether on the shore of Granton Harbour, Edinburgh, or checking nodule-bearing horizons exposed in nearby road works.
Neurocrania are rich sources of morphological data, and the material presented here both complements and makes extensive use of detailed CT-based descriptions of Cladodoides (Maisey Reference Maisey2005), “Cobelodus” (Maisey Reference Maisey2007), Dwykaselachus (Coates et al. Reference Coates, Gess, Flynn, Criswell and Tietjen2017), Tribodus (Lane Reference Lane2010), sibyrhinchid iniopterygians (Pradel Reference Pradel2010), Kawichthyes (Pradel et al. Reference Pradel2011) and, of particular relevance to the present work, Acronemus (Maisey Reference Maisey2011; see also Rieppel Reference Rieppel1982). Notable pre-CT descriptions of fossil chondrichthyan braincases include those of Orthacanthus and Tamiobatis (Schaeffer Reference Schaeffer1981), Egertonodus (Maisey Reference Maisey1983; revised and augmented in Lane Reference Lane2010), Akmonistion (Coates & Sequeira Reference Coates and Sequeira1998) and Pucapampella (Maisey Reference Maisey and Ahlberg2001a). Notably, all of these publications post-date Dick's (Reference Dick1978) Tristychius monograph.
Tristychius has been long been presented as a likely early hybodont (Dick Reference Dick1978; Dick & Maisey Reference Dick and Maisey1980; Coates & Gess Reference Coates and Gess2007; Maisey Reference Maisey2011), initially identified as such on the basis of finspine details. However, Maisey's (Reference Maisey1987) emended diagnosis of the Hybodontidae established a more restrictive definition, including finspine characteristics that Tristychius unambiguously lacks. The phylogenetic position of Tristychius thus became less certain, alongside its value as a plausible exemplar of early euselachian (sensu Maisey Reference Maisey2011) conditions. Coates & Gess (Reference Coates and Gess2007), Lane (Reference Lane2010) and Pradel et al. (Reference Pradel, Tafforeau, Maisey and Janvier2011) each provided more-or-less consistent discussions of the likely affinities of Tristychius, in which its cranial morphology figured more prominently than finspine characteristics. These studies identified Tristychius as an early branch from the base of the hybodont lineage. However, against this background, Davis et al. (Reference Davis, Finarelli and Coates2012), Dupret et al. (Reference Dupret, Sanchez, Goujet, Tafforeau and Ahlberg2014) and Long et al. (Reference Long, Mark-Kurik, Johanson, Lee, Young, Min, Ahlberg, Newman, Jones, den Blaauwen, Choo and Trinjastic2015) delivered a contrasting result in which Tristychius was excluded from the crown because of its braincase plesiomorphies.
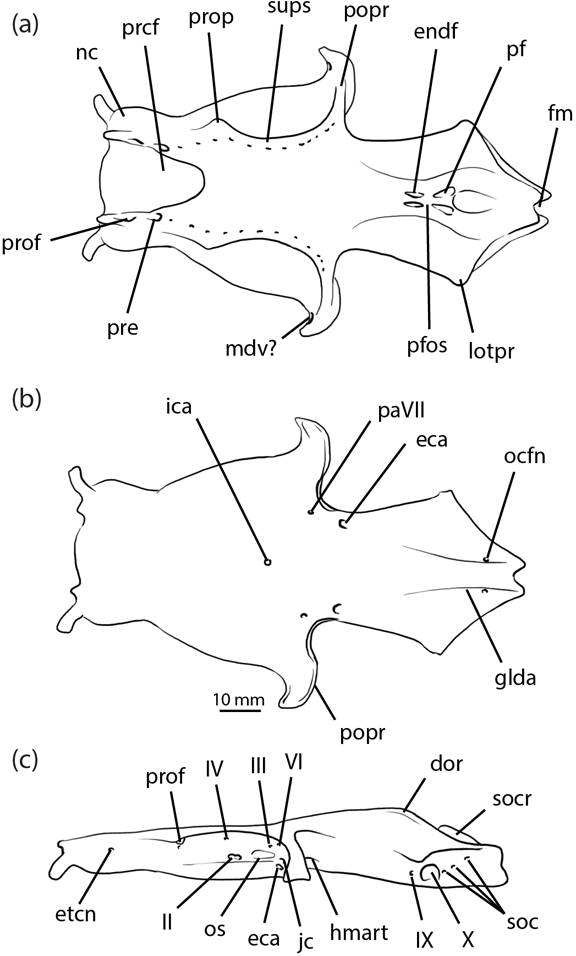
Until now, no new data have been available to test the accuracy of the existing reconstruction and these conflicting phylogenetic hypotheses. It should be noted that none of the neurocrania described in the present work is especially well preserved relative to almost intact and uncrushed specimens such as Cladodoides (Maisey Reference Maisey2005). Nevertheless, the combined results from scanned examples when added to Dick's (Reference Dick1978) restoration (Fig. 1) present an informatively detailed record of the anatomy. Perhaps most significantly, the CT survey revealed the ‘hidden' side of specimen NMS 1972.27.481D (Fig. 2), which is now known to contain the only laterally compressed neurocranium of Tristychius discovered thus far. This specimen, more than any other, transformed the shape of the braincase restoration, and provides an improved estimate of its dorsoventral height in life. The general proportions are similar to those estimated by Dick (Reference Dick1978, text-fig. 9), reproduced here in Figure 1. But, in the new restoration (Fig. 3) details of the orbit and postorbital process are clearer, the attachment and articulation areas for parts of the mandibular and hyoid arches are revealed in new detail, some features are moved to new locations and several endocranial features are exposed for the first time.

Figure 1 Tristychius arcuatus, neurocranium restoration from Dick Reference Dick1978: (a) dorsal view; (b) ventral view; (c) lateral view. Abbreviations: dor = dorsal otic ridge; eca = external carotid artery foramen; endf = endolymphatic foramen; etcn = ethmoid canal; fm = foramen magnum; glda = groove for lateral dorsal aorta (common carotid artery); hmart = hyomandibula articulation area; ica = internal carotid artery foramen; jc = jugular canal; lotpr = lateral otic process; mdv? = mandibular vein foramen; nc = nasal capsule; ocfn = occipital artery foramen; os = optic stalk attachment; paVII = palatine branch of facial nerve foramen; pf = perilymphatic fenestra; pfos = parietal fossa; popr = postorbital process; prcf = precerebral fontanelle; pre = preorbital canal; prof = profundus nerve foramen; prop = preorbital process; soc = spino-occipital nerve foramina; socr = supraoccipital crest; sups = supraorbital shelf; II = optic nerve foramen; III = oculomotor nerve foramen; IV = trochlear nerve foramen; VI = abducens nerve foramen; IX = glossopharyngeal nerve foramen; X = vagus nerve foramen.

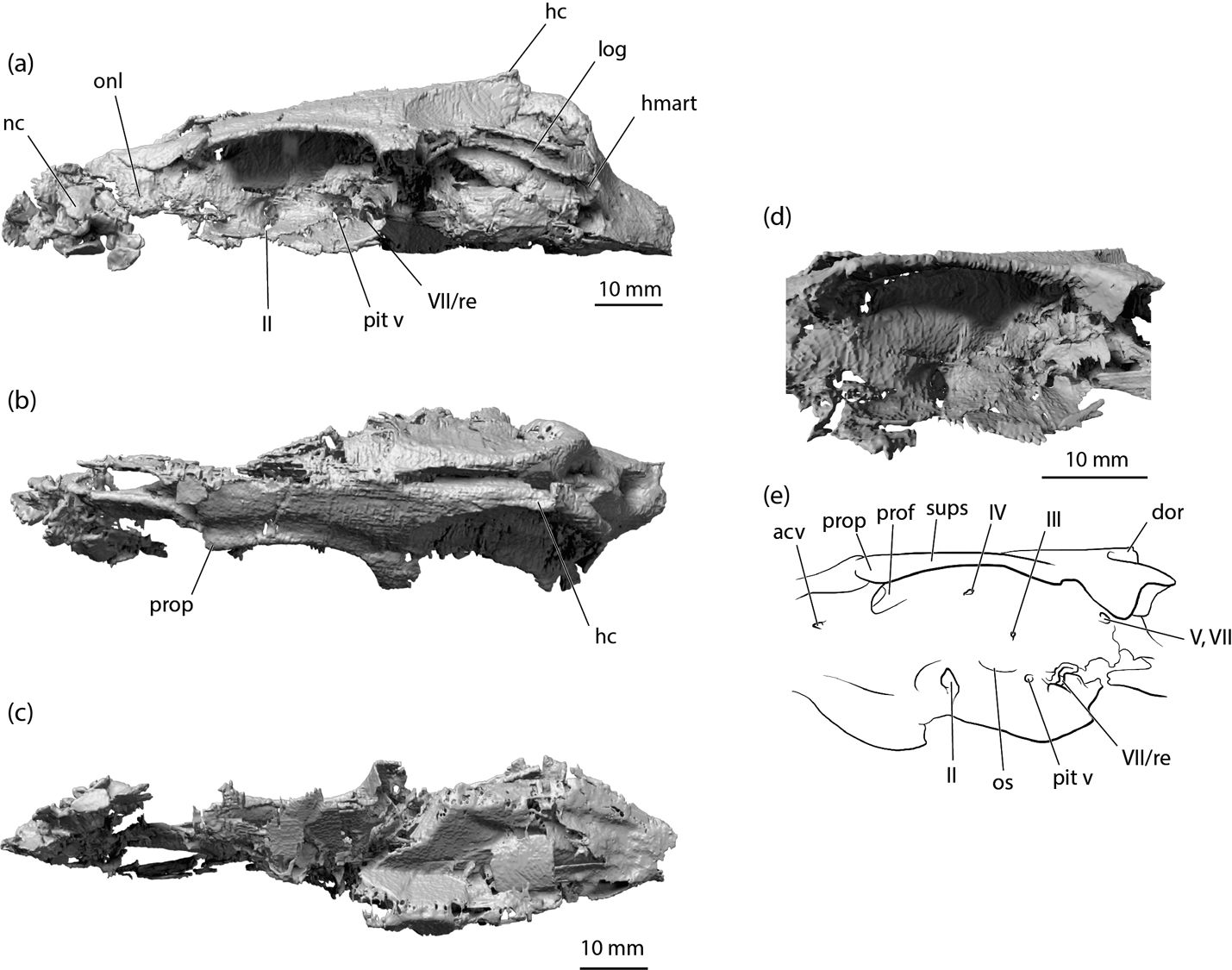
Figure 2 Tristychius arcuatus, NMS 1972.27.481D, rendering of CT scan showing complete, laterally compressed neurocranium: (a) left lateral view; (b) dorsal view; (c) ventral view; (d) detail of left orbit; (e) line drawing of rendering shown in (d). Abbreviations: acv = anterior cerebral vein foramen; dor = dorsal otic ridge; hc = horizontal crest; hmart = hyomandibula articulation area; log = lateral otic groove; nc = nasal capsule; onl = orbitonasal lamina; os = optic stalk base; pit v = pituitary vein foramen; prof = profundus nerve foramen; prop = preorbital process; re = insertion for external rectus muscle; sups = supraorbital shelf; II = optic nerve foramen; III = oculomotor nerve foramen; IV = trochlear nerve foramen; V,VII = trigeminofacial foramen; VII = foramen for anterior branch of facial nerve.

Figure 3 Tristychius arcuatus, neurocranium, new restoration: (a) dorsal; (b) ventral; (c) lateral; (d) occipital; (e) anterolateral views. Abbreviations: acv = anterior cerebral vein foramen; cb = C-bout; dor = dorsal otic ridge; endf = endolymphatic foramen; fm = foramen magnum; fora = orbital artery foramen; glda = groove for lateral dorsal aorta (common carotid artery); hc = horizontal crest; hf = bucco hypophyseal fenestra; hmart = hyomandibula articulation area; ica = internal carotid artery foramen; jc = jugular canal; log = lateral otic groove; nc = nasal capsule; oc cot = occipital cotylus; onl = orbitonasal lamina; os = optic stalk base; pf = perilymphatic fenestra; pfos = parietal fossa; pit v = pituitary vein foramen; poart = postorbital articulation; popr = postorbital process; prcf = precerebral fontanelle; pre = preorbital canal; prof = profundus nerve foramen; prop = preorbital process; re = insertion for external rectus muscle; sbos = suborbital shelf; soc = spino-occipital nerve canals; socr = supraoccipital crest; sos = supraotic shelf; sups = supraorbital shelf; II = optic nerve foramen; III = oculomotor nerve foramen; IV = trochlear nerve foramen; Vmd = mandibular branch of trigeminal nerve foramen; VII = foramen for anterior branch of facial nerve; VIIpa = foramen for posterior ramus of palatine branch of facial nerve; IX = glossopharyngeal nerve foramen; X = vagus nerve foramen.
Here, close comparisons are drawn with hybodontid (Maisey Reference Maisey1983; Lane Reference Lane2010) as well as non-hybodontid braincases (Schaeffer Reference Schaeffer1981; Maisey Reference Maisey2005). Particular comparisons are also made with the incompletely preserved braincase of the Middle Triassic Acronemus (Maisey Reference Maisey2011), another chondrichthyan revealed in new detail by means of CT scanning but placed, thus far, like Tristychius with uncertainty close to the hybodontiforms and early members of the neoselachian clade.
1. Materials
1.1. Image preparation
Scans were completed by the high-resolution X-ray computed tomography facility at the University of Texas at Austin (UTCT; www.digimorph.org). Anatomical reconstructions were completed using Mimics v. 17 (biomedical.materialise.com/mimics; Materialise, Leuven, Belgium) for the three-dimensional modelling, including segmentation, three-dimensional object rendering and STL polygon creation. 3D Studio Max (Autodesk.com/products/3ds-max; Autodesk, San Rafael, USA) was used for further editing of the STLs (color, texture, lighting) and mirroring for the final restoration.
1.2. Abbreviations
Explanations of anatomical abbreviations are provided in figure captions. Terminology follows Dick (Reference Dick1978), Maisey (Reference Maisey1983, 2005) and Lane (Reference Lane2010).
Institutional abbreviation. NMS, National Museum of Scotland, Edinburgh, Scotland.
1.3. Specimens and geological context
All seven specimens used in this study were found on Wardie Beach, Edinburgh (Dineley & Metcalf Reference Dineley and Metcalf1999). Each braincase (and other associated skeletal remains) is preserved in a siderite nodule, and the fossil cartilage is often laced with pyrite. Dick (Reference Dick1998) described these nodules as extremely refractory, and noted the poor preservation of scales, teeth and spines. Calcified cartilage, however, has survived somewhat better, but it is often difficult to recognise on fractured surfaces of broken concretions, and difficult to prepare from the surrounding matrix. Unlike the actinopterygians from Wardie, which are usually flattened, the shark fossils have been described as ‘almost uncrushed' (Dineley & Metcalf Reference Dineley and Metcalf1999). In fact, as is apparent from the present description, all specimens are crushed to some degree, and most have lost an estimated 70 % of their height or width along the axis of compaction.
Fossil fishes have been collected from Wardie since the 1830s. In addition to Dick's (Reference Dick1978, Reference Dick1981, Reference Dick1998; Dick & Maisey Reference Dick and Maisey1980) descriptions of the fossil sharks, classic reviews of the fishes include those by Traquair (Reference Traquair1903) and Wood (Reference Wood1975). Descriptions of the site geology have been presented by Peach et al. (Reference Peach, Gunn, Clough, Hinxman, Grant Wilson, Crampton, Maufe and Bailey1910), Waterston (Reference Mitchel, Walton and Grant1962), Mitchell & Mykura (Reference Mitchell and Mykura1962), Wood (Reference Wood1975) and Clarkson (Reference Clarkson, McAdam and Clarkson1986). The shark assemblage from which the Tristychius material derives, named the Diplodoselache fauna (Dick Reference Dick1981), is characteristic of the nodules of the Oil Shale Group, the Burdiehouse Limestone, and the roof shales of the Lower Limestone coal seams, the Limestone Coal and the Upper Limestone groups (Dineley & Metcalf Reference Dineley and Metcalf1999). This places the age somewhere between the uppermost Viséan and lowermost Serpukhovian (see also Dick Reference Dick1998).
Six of the specimens were collected by Stan Wood in the early 1970s, and the seventh (NMS G. 2015.30.1A, B) (Fig. 4) was discovered a century earlier by another remarkable and largely self-taught palaeontologist, Charles Peach (Taylor & Anderson Reference Taylor and Anderson2015). All of these specimens are deposited in the collections of the National Museums of Scotland, Edinburgh. Besides Tristychius, at least three other chondrichthyan genera are known from the Diplodoselache fauna: the eponymous Diplodoselache (Dick Reference Dick1981); Onychoselache (Dick Reference Dick1978; Dick & Maisey Reference Dick and Maisey1980; Coates & Gess Reference Coates and Gess2007); and Sphenacanthus (Dick Reference Dick1998).

Figure 4 Tristychius arcuatus, NMS G. 2015.30.1A & B, Peach specimen, dorsoventrally compressed otic and occipital region: (a) left lateral view; (b) dorsal view; (c) ventral view; (d) anterolateral view of orbit posterior and trigeminofacial recess; (e) line drawing of rendering in (d). Abbreviations: cb = C-bout; hc = horizontal crest; hmart = hyomandibula articulation area; jc = jugular canal; log = lateral otic groove; pfos = parietal fossa; pit v = pituitary vein canal; poart = postorbital articulation; popr = postorbital process; re = insertion for external rectus muscle; sbos = suborbital shelf; socr = supraoccipital crest; sos = supraotic shelf; sups = supraorbital shelf; Vmd = mandibular branch of trigeminal nerve foramen; V,VII = trigeminofacial foramen; VII = foramen for anterior branch of facial nerve.
2. Systematic palaeontology
Class Chondrichthyes (Huxley Reference Huxley1880)
Cohort Euselachii (Hay Reference Hay1902; sensu Maisey Reference Maisey2011)
Incertae sedis Genus Tristychius (Agassiz Reference Agassiz1837)
Emended diagnosis. Euselachian shark distinguishable from other euselachian genera by the structure of the postorbital attachment of the upper jaw, in that the otic process of the palatoquadrate articulates with single, broad ridge and groove on the ventral surface of the postorbital process. The suborbital shelf is exceptionally wide, and the narrower supraorbital shelf terminates anteriorly with a prominent preorbital process. Dorsal otic ridges flanking the parietal fossa each bear a narrow horizontal crest; a short, acute, supraoccipital crest is present. Finspines with many (11 to 15) costae dorsally, decreasing in number ventrally towards the spine's insertion, to between three and eight costae which lie near its anterior border; posterior surface of finspine flat or concave.
Species Tristychius arcuatus (Agassiz Reference Agassiz1837)
Diagnosis. As for genus
Referred specimens. See Dick (Reference Dick1978) for list of Tristychius arcuatus specimens; an additional specimen is listed in Coates & Gess (Reference Coates and Gess2007).
NMS 1972.27.461A & B (Dick Reference Dick1978, text-fig. 11): head of fish preserved in the round, of negligible value for CT scan because little of the original skeletal structure is preserved, Wardie seashore ‘locality B'; collected by S. P. Wood.
NMS 1972.27.481D (Figs 2, 7a, 8, 9a, 11a–d); Dick Reference Dick1978, text-fig. 8A): laterally compressed braincase, as such thus far unique among all Tristychius specimens, with associated but dismembered jaws, hyoid and gill arches, Wardie seashore ‘locality B'; collected by S. P. Wood.
NMS 1974.23.6 Fig. 5): dorsoventrally compressed braincase, jaws, hyoid and gill arches, with parts of the pectoral girdle and first dorsal fin spine, from Wardie seashore (not listed by Dick Reference Dick1978); collected by S. P. Wood.

Figure 5 Tristychius arcuatus, NMS 1974.23.6, rendering of CT data showing dorsoventrally compressed neurocranium: (a) left lateral view; (b) dorsal view. Abbreviations: inp = internasal plate; nc = nasal capsule; onl = orbitonasal lamina; prcf = precerebral fontanelle; Vmd = mandibular branch of trigeminal nerve foramen.
NMS 1974.23.19 (Dick Reference Dick1978, text-fig. 10): dorsoventrally compressed braincase with disarticulated jaws and other visceral cartilages, from temporary exposure (road works), Granton Crescent, Edinburgh; collected by S. P. Wood.
NMS 1974.23.30A & B (Fig. 6a–c): dorsoventrally compressed braincase, with jaws, hyoid arch and gill skeleton, probably from Wardie seashore ‘locality B' (not listed by Dick Reference Dick1978); collected by S. P. Wood.
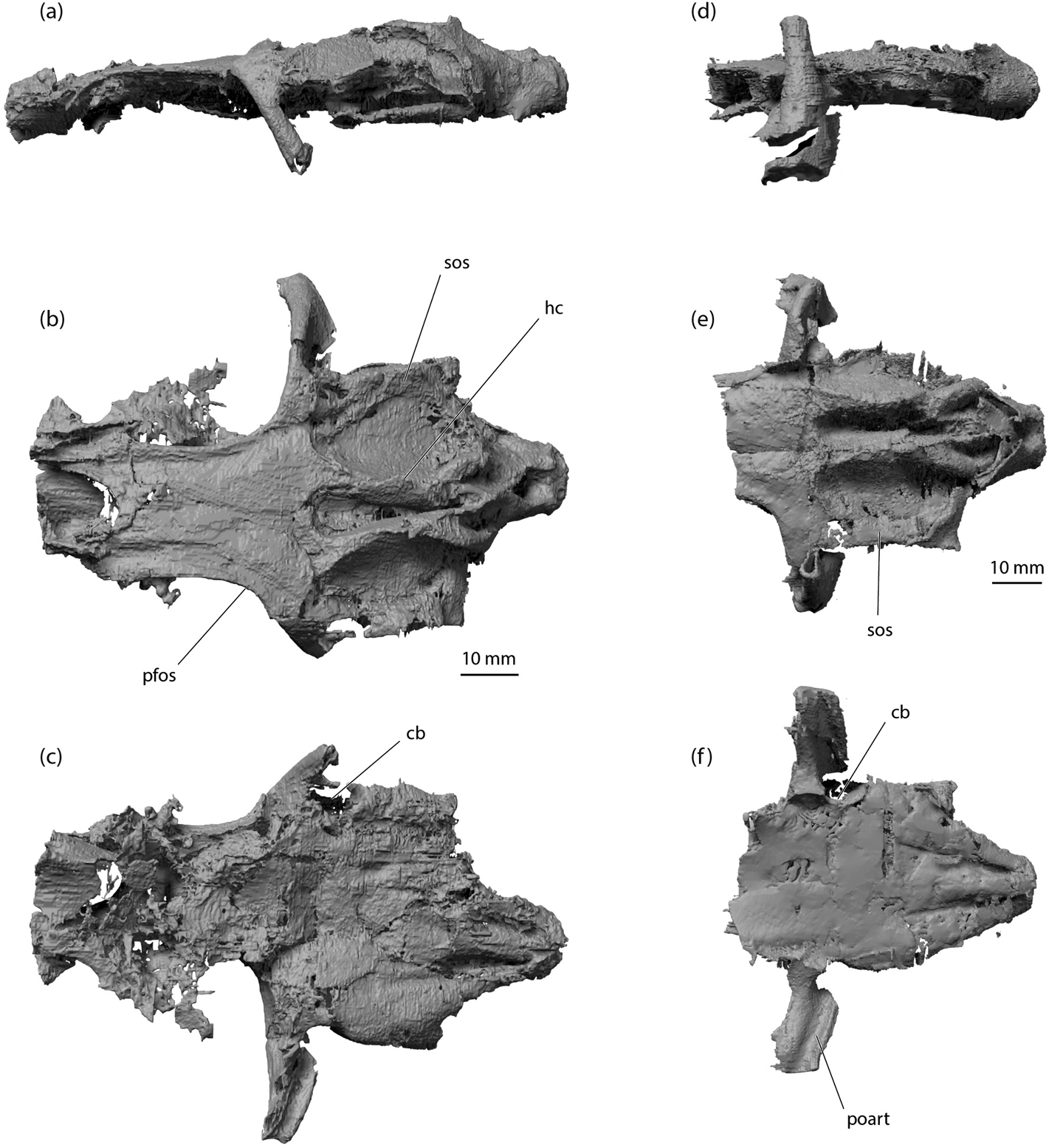
Figure 6 Tristychius arcuatus. (a–c) NMS 1974.23.30A & B, rendering of CT data showing complete, dorsoventrally compressed neurocranium: (a) left lateral view; (b) dorsal view; (c) ventral view. (d–f) NMS 1974.51.2A & B, rendering of CT data showing dorsoventrally compressed otic and occipital region: (d) left lateral view; (e) dorsal view; (f) ventral view. Abbreviations: cb = C-bout; hc = horizontal crest; pfos = parietal fossa; poart = postorbital articulation; sos = supraotic shelf.
NMS 1974.51.2A & B (Fig. 6d–f): dorsoventrally compressed braincase from Wardie seashore (not listed by Dick Reference Dick1978); collected by S. P. Wood.
NMS G. 2015.30.1A & B (Figs 4, 7b, 10, 11e–h): dorsoventrally compressed braincase from Wardie seashore (not listed by Dick Reference Dick1978); collected C. W. Peach in 1870.
3. Description of neurocranium
3.1. Nasal region
The nasal region is most completely preserved in specimens NMS 1972.27.481D, NMS 1974.23.6 and, to a lesser extent, NMS 1974.23.30. The nasal capsules are smaller and more terminally positioned than in Dick's (Reference Dick1978) restoration. It appears that the ‘accessory cartilage' noted by Dick is no more than a fragment of the posterolateral wall of a capsule. Thus, the orbits and nasal capsules are separated by a substantial length of preorbital or postnasal wall, resembling the orbitonasal lamina of chimaeroids (De Beer 1937) or the extended pre-orbital region in Heterodontus (Daniel Reference Daniel1922). The most completely preserved nasal capsule is from the left side of NMS 1972.27.481D (Fig. 2a) and although laterally crushed, it indicates that the nasal openings were directed anterolaterally. In Tristychius the nasal capsules are separated ventrally by an internasal plate, and dorsally by a broad precerebral fontanelle (Fig. 5b, NMS 1974.23.6). There is no clear evidence of a rostral bar on the ventral surface of the internasal plate, as found in hybodontids (Maisey Reference Maisey1983), but the preservation of this area in Tristychius is poor.
The precerebral fontanelle is well formed, large, and extends back to the anterior margin of the supraorbital shelves. The general proportions and relation to flanking grooves and foramina for the lateral line nerve component of the superficial ophthalmic trunk closely match Dick's (Reference Dick1978) interpretation.
3.2. Orbit
Specimens preserving the most complete and most informative sections of the orbit are NMS 1972.27.481D and NMS G. 2015.30.1. The orbit is floored with a broad suborbital shelf and roofed by a narrower supraorbital shelf, in agreement with Dick's (Reference Dick1978) description. In CT scans, the suborbital shelf tends to be poorly resolved, because this wide cartilage sheet usually forms a breakage plane between part and counterpart of a nodule, and calcified material flakes away from the exposed surfaces. Importantly, the suborbital shelf includes no evidence of palatobasal flange-like articulation process such as those present in symmoriids (notably apparent in Ozarcus (Pradel et al. Reference Pradel2014).
The supraorbital shelf has a gently concave lateral edge that extends posterolaterally to blend smoothly into the descending postorbital processes. A shallow groove on the dorsal surface parallels the lateral margin, and within this lies the small foramina for twigs of the superficial ophthalmic trunk, as noted by Dick (Reference Dick1978). Similar features are present in Egertonodus (Maisey Reference Maisey1983; Lane Reference Lane2010). Anteriorly, the supraorbital shelf terminates as a small but robust preorbital process, the dorsal position of which distinguishes it clearly from the ectethmoid process of Egertonodus (Maisey Reference Maisey1983).
The orbit wall is most complete in NMS 1972.27.481D (Fig. 2), but this is broken around the perimeter because of lateral compression. Consequently, a deep recess leading to a profundus foramen/preorbital canal is preserved, but this is situated within a damaged (compression fractured) area of cartilage. At the anterior margin of the orbit, where it blends smoothly with the lateral face of the orbitonasal lamina, a small foramen resembling the anterior cerebral vein foramen in Cladodoides is situated anteroventrally to the preorbital process. The optic nerve foramen lies within a recess at the centre and base of the orbit wall, where it meets the proximal margin of the suborbital shelf. The trochlear nerve foramen (in NMS 1972.27.481D) is well preserved, close to the orbit roof, dorsal but slightly posterior to the anteroposterior level of the optic nerve foramen (Fig. 2d, e). This conflicts with Dick's restoration, which places the trochlear foramen anterior to the optic nerve foramen, but is more consistent with his illustration of NMS 1973.24.41 (Dick Reference Dick1978, text-fig. 4) which shows the trochlear and optic foramina at the same level.
The oculomotor nerve foramen is preserved in NMS 1972.27.481, but concealed in lateral view by a displaced cartilage plate. There is no outgrowth in the orbit wall indicating the presence or base of an optic pedicel. However, a small depression and horizontal groove posterior to the optic nerve foramen resembles Dick's estimate of an optic stalk base, and the feature that Schaeffer (Reference Schaeffer1981) identified as the optic stalk depression in Tamiobatis.
The posterior portion of the orbit resembles the arrangement of foramina and canals in Tamiobatis (Schaeffer Reference Schaeffer1981, fig. 7) or Cladodoides (Maisey Reference Maisey2005, fig. 17B) rather more than conditions in Egertonodus (Maisey Reference Maisey1983; Lane Reference Lane2010). A broad trigeminofacial recess is continuous with the anterior opening of the jugular canal. This is preserved best in the dorsoventrally compressed specimen NMS G. 2015.30.1 (Fig. 4d, e). The medial wall of the recess includes a broad, laterally directed foramen for the trigeminal nerve and anterodorsal lateral line nerves. Anteroventral to this, preserved in NMS 1972.27.481D (Fig. 2a, d, e) there is a large, flared opening, which is also preserved, although less offset ventrally in NMS G. 2015.30.1 (Fig. 4d, e). This distinctive feature might represent the exit of the anterior branch of the facial nerve. However, this places the nerve foramen in a more dorsal location than the far-better-preserved example in Cladodoides (Maisey Reference Maisey2005). Alternatively, this recess could mark the insertion of the external rectus muscle, thereby unexpectedly resembling the paired posterior myodome of early actinopterygians (Gardiner Reference Gardiner1984). Once again, Cladodoides provides a plausible template for this interpretation (Maisey Reference Maisey2005, fig. 16A). Other foramina and structures from this region in Tristychius are poorly preserved, because orbit wall is variously crushed and shattered, but the pituitary vein canal appears to be preserved, located just anterior to the larger recess for nerve or muscle, in both NMS 1972.27.481D and NMS G. 2015.30.
3.3. Postorbital process
The postorbital process and wall is most completely preserved in specimens NMS 1974.23.30, NMS 1974.51.2. and NMS G. 2015.30.1. Like those of Acronemus (Maisey Reference Maisey2011), the postorbital processes of Tristychius have been less affected by dorsoventral compaction than other neurocranial regions (cf. Figs 4a, 5a, 6d). The general form of the process, anteroposteriorly narrow, proximally, and downturned laterally and more substantially structured distally, resembles those depicted and restored by Dick (Reference Dick1978, text-fig. 6). Each is thoroughly mineralised, probably to act as a buttress against forces conveyed from the jaws and jaw musculature. The postorbital wall (the anterior face of the process) is extensive and perforated by the jugular vein in the ventromedial corner. In lateral view, the process is narrow dorsally, but flares and thickens ventrally to form an anteroposteriorly broad ‘foot'. In this regard it differs from the parallel sided postorbital process of Acronemus (Rieppel Reference Rieppel1982, text-fig. 1), but like Acronemus there is an anhedral angle between the base of the process and the basicranium (Figs 3d, 4d). Therefore, contra Coates & Gess (Reference Coates and Gess2007), the Tristychius postorbital process can, in fact, be characterised as extending a short distance below the rest of the basicranium (see also note in Maisey Reference Maisey2011). The articular area is confined to the ventral surface, and therefore occupies that part of the postorbital process that is inferred as derived from the embryonic lateral commissure (de Beer Reference de Beer1937). The location of this surface is in marked contrast to the more general condition of early chondrichthyans, in which a groove extends dorsoventrally across the posterior of the postorbital process, as shown especially clearly in Dwykaselachus (Coates et al. Reference Coates, Gess, Flynn, Criswell and Tietjen2017). In Tristychius, the articulation surface consists of a single, slightly anterolaterally directed, gently curved broad ridge and groove (Figs 4c, 6f). Unlike Acronemus, no part of the articular area extends onto the posterior surface. The simplicity of the articular surface shape suggests that the palatoquadrates were capable of some postero-medial to antero-lateral movement.
In agreement with Dick (Reference Dick1978), aside from the jugular canal, the only foramen present in the anterior surface of the process is positioned ventrally and distally (Figs 4e, 5a). This is the anterior opening of a canal resembling a similarly located channel through the postorbital process of Cladodoides, interpreted as carrying the mandibular branch of the trigeminal nerve (Maisey Reference Maisey2005). The proximal opening of the canal is positioned opposite the likely foramen of the exit of the trigeminal nerve in the anteriormost part of the otic wall.
3.4. Basicranium
The basicranial region is most completely preserved in specimens NMS 1974.23.30, NMS 1974.51.2, and NMS G. 2015.30.1. These examples add little to Dick's (Reference Dick1978) description, but it is now apparent that the grooves for the lateral dorsal aortae are arranged very like those of Acronemus (Maisey Reference Maisey2011, text-fig. 2A). In ventral view, the posterior profile of the junction between the otic wall and the base of the postorbital process is markedly concave, resembling the C-bout of a violin waist (Figs 4c, 6c, f). This feature, once again, is shared with Acronemus, and might signal the location of the spiracular pouch, although chondrichthyan neurocrania are not known to bear a clear morphological marker of pouch location. The floor of the otic region is broad, and although preservation is of insufficient quality to show separate contributory cartilage layers, it is very likely formed by a hypotic lamina. The occipital region projects posteriorly beyond the level of the otic capsules, but, unlike Dick's (Reference Dick1978) restoration of Tristychius (Fig. 1) and unlike Acronemus, the occipital cotylus is not recessed deeply. Furthermore, in lateral view the occipital cotylus is offset ventrally relative to the rest of the basicranial floor, resembling hybodonts such as Tribodus and Egertonodus (Lane Reference Lane2010).
3.5. Otic region
The otic region is well preserved in NMS 1974.23.30, NMS 1974.51.2, and NMS G. 2015.30.1. As described by Dick (Reference Dick1978), a well-formed ridge overlies anterior and posterior semicircular canals on each side of the dorsal midline, and these ridges converge to flank an elongate parietal fossa with a chondrified floor (Dick's ‘median supraotic fossa'). Unexpectedly, these ridges exhibit horizontal crests (Figs 2b, 4a, b, 6b), like those of Orthacanthus and especially Tamiobatis (Schaeffer Reference Schaeffer1981), rendered indistinctly in Cladodoides (Maisey Reference Maisey2005), and identified in an apparently reduced form in Akmonistion (Coates & Sequeira Reference Coates and Sequeira1998). In Tristychius, the posterior extremity of this horizontal crest appears to overlie the posterior boundary of the anterior semicircular canal. The posterior portion of the ridge is smooth, rounded and, in lateral view, conforms closely to the arc of the enclosed canal (cf. Dick, Reference Dick1978, text-fig. 8A).
The parietal fossa lacks a distinct anterior boundary, unlike the fossa in Egertonodus and Tribodus, and the floor is continuous with the cranial roof between the orbits (Figs 4b, 6b). As identified by Dick (Reference Dick1978), separate endolymphatic openings and perilymphatic fenestrae perforate the fossa walls. The posterior of the fossa rises to form the anterior face of a subtriangular platform from which extends posteriorly and dorsally a short, acute supraoccipital crest, best preserved in NMS G. 2015.30.1 (Figs 4b, 7b). The posterior apex of the crest (and platform) extends posteroventrally to the roof of the foramen magnum. Ridges overlying left and right posterior semicircular canals converge anteriorly to meet the platform so that this complex forms a ‘W' shape in dorsal view, with a ‘V'-shaped fossa on either side of the base of the occipital crest (Figs 4b, 5b, 6b, e). In this respect, Tristychius exhibits a mixture of conditions in Egertonodus (Maisey Reference Maisey1983, fig. 8; Lane Reference Lane2010, fig. 29A) and Orthacanthus (Schaeffer Reference Schaeffer1981, figs 5, 6). Notably, like Acronemus, but unlike the xenacanths, Tamiobatis and Cladodoides, the posterior semicircular canal is oriented posterolaterally to flank the dorsal part of the occipital unit. The canal is not enclosed by or directed towards a lateral otic process (cf. Maisey Reference Maisey2011).

Figure 7 Tristychius arcuatus, occipital views of (a) NMS 1972.27.481D, (b) NMS G. 2015.30.1A & B; each with line drawings. Abbreviations: dor = dorsal ridge; fm = foramen magnum; hc = horizontal crest; log = lateral otic groove; oc cot = occipital cotylus; socr = supraoccipital crest; sos = supraotic shelf; IX = glossopharyngeal foramen; X = vagus foramen.
Behind the postorbital process, the otic roof forms a very broad supraotic shelf, once more resembling conditions in Acronemus (Maisey Reference Maisey2011). This shelf is thinly calcified but complete, unlike the shelf in Tamiobatis vetustus (Schaeffer Reference Schaeffer1981). The lateral extremity of the shelf projects as a slender horizontal flange beyond the main body of the lateral otic ridge. The anteriormost part of the flange terminates at a C-bout, more-or-less matching the corresponding profile on the basicranial surface. Behind the C-bout, the extreme lateral rims of these shelves or flanges are nearly parallel on left and right sides. Thus, in dorsal and ventral views, the otic region (posterior to the postorbital processes and anterior to occiput) is nearly rectangular (unlike the kite-shaped restoration in Dick Reference Dick1978). This flange terminates posteriorly, anterior to the posterolateral extremity of a second, lower otic flange (or shelf) (Figs 4b, 6b, e). Between these otic outgrowths, a lateral otic groove resembles the jugular groove of Pucapampella (Maisey Reference Maisey and Ahlberg2001). However, in Tristychius this groove (Figs 2a, 4a) is situated dorsal to the jugular canal exit. Posteriorly, the shelf forming the groove floor flares laterally and curves ventrally to scroll over the hyomandibula attachment area (Figs 2a, 4a). Dick (Reference Dick1978) identified this region as the ‘otic process', and it lies directly anterior and dorsal to the separate foramina for the glossopharyngeal and vagus nerves (cf. Dick Reference Dick1978). Maisey (Reference Maisey2011) identified a corresponding posterolateral extremity in Acronemus as the ‘periotic process', but this term is not used here in order to avoid confusion with the unrelated periotic process of “Cobelodus” (Maisey Reference Maisey2007). Notably, in Tristychius there is no lateral otic process in the sense of the term as used by Schaeffer (Reference Schaeffer1981) for Orthacanthus and Tamiobatis.
3.6. Occipital region
The occipital region is reasonably well preserved in all specimens in the present study, and the proportions are much as described by Dick (Reference Dick1978). In dorsal and ventral views, although the otic regions are exceptionally similar in Tristychius and Acronemus, the occiput projects posteriorly to a far greater degree in Tristychius than in Acronemus. In lateral view, the occipital wall is punctured by a series of three foramina for spinooccipital nerves. In posterior view the large foramen for the vagus nerve is clearly visible, medial to the smaller glossopharyngeal nerve foramen. In occipital view (Fig. 7), the cotylus is slightly wider than the foramen magnum. Dorsally, the occipital crest is flanked by fossae which are themselves limited laterally by the posterior of the dorsal otic ridge. A similar arrangement is manifest in Egertonodus (Maisey Reference Maisey1983, fig. 9E) and Tribodus (Lane Reference Lane2010, fig. 12B). Likewise, the occipital view shows the relation of the lateral extremities of the otic capsule and lateral otic groove to the occiput, and the similarity of this arrangement to the so-called lateral otic process of Egertonodus (Maisey Reference Maisey1983, fig. 9E) and the hyomandibular articulation in Tribodus (Lane Reference Lane2010, fig. 12B).
3.7. Endocranium and endocasts
The walls of the endocranial spaces are preserved best in NMS 1972.27.481D (Fig. 8). The condition of the walls is poor and far worse than in barely distorted neurocrania such as those of Egertonodus (Lane Reference Lane2010) or Dwykaselachus (Coates et al. Reference Coates, Gess, Flynn, Criswell and Tietjen2017). Nevertheless, some general and fairly conservative observations are possible.

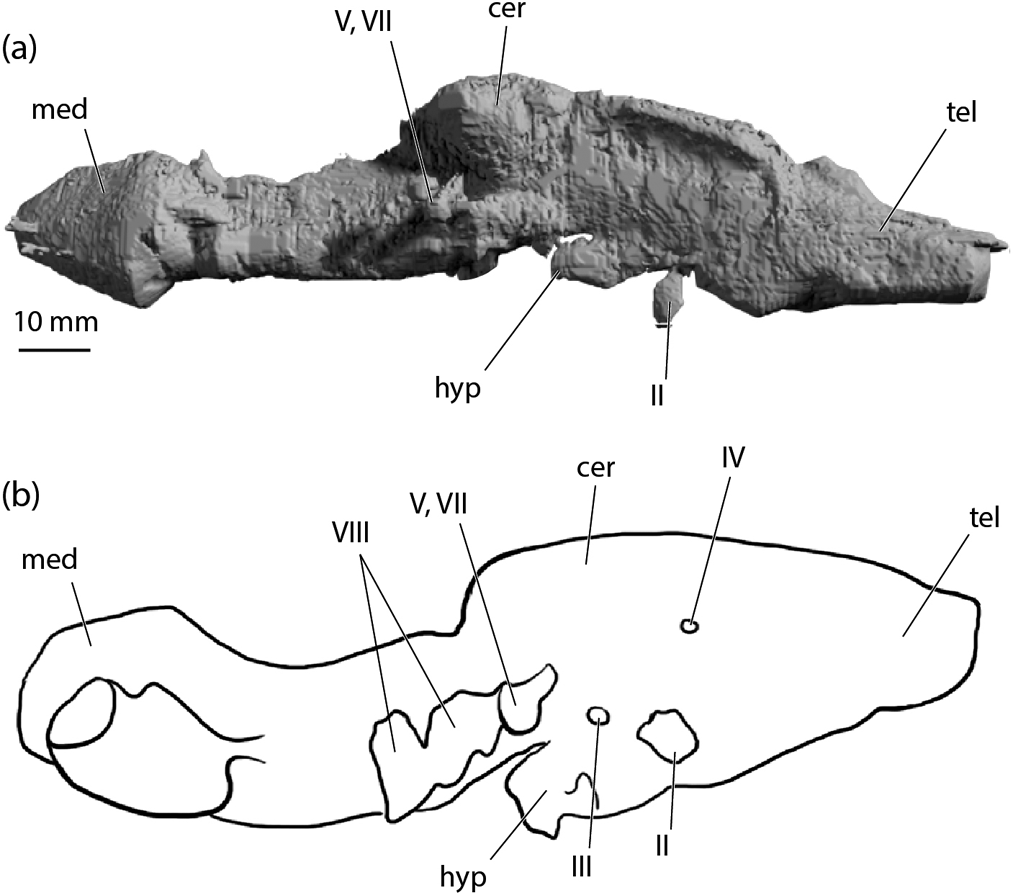
Figure 8 Tristychius arcuatus, NMS 1972.27.481D, medial view of left side in sagittal section: (a) rendering from CT data; (b) line drawing showing major features of the endocranium, including the fractured sections of the floor. Grey shading signifies sectioned cartilage, including displaced laminae obscuring anatomical landmarks such as the optic nerve foramen. Abbreviations: acv = anterior cerebral vein foramen; cer = cerebellar region; ds = dorsum sellae; endf = endolymphatic foramen; fm = foramen magnum; hc = horizontal crest; med = medullary region; oc cot = occipital cotylus; pf = perilymphatic fenestra; pfos = parietal fossa; pit v = pituitary vein foramen; prcf = precerebral fontanelle; prof = profundus nerve foramn; re = insertion for external rectus muscle; soc = spino-occipital nerve canals; socr = supraoccipital crest; tel = telencephalon region; I = olfactory tract canal; II = optic nerve foramen; III = occulomotor foramen; IV = trochlear foramen; VII = foramen for anterior branch of facial nerve; VIII = octaval nerve foramen; IX = glossopharyngeal nerve foramen; X = vagus nerve foramen.
In medial view (sagittal section), similarities with Egertonodus (Lane Reference Lane2010, fig. 34) are more numerous than with Cladodoides (Maisey Reference Maisey2005, fig. 7), the most significant of which is the presence of a medial capsular wall. Ventrally, this is pierced by a large opening that appears to have been for the octaval nerve. Notably, the opening is single and not divided into two, as in Tribodus and Egertonodus (Maisey Reference Maisey, Arratia, Wilson and Cloutier2004; Lane Reference Lane2010). In NMS 1972.27.481D, this communicates between the main cranial cavity and extracranial space instead of the expected interior of the otic capsule. This a side-effect of post mortem damage to the ventral part of the external, otic capsule wall.
Anterior to the otic region, the dorsum sellae is low and projects anteriorly above the basicranial fenestra for the internal carotid arteries. Although poorly shown in cross-section (Fig. 8), this forms a complete shelf spanning the endocranial cavity above the hypophyseal space. Note that the ventrally facing notch shown in Figure 8 is more likely a displaced part of the pituitary vein canal (pit v) in the concertinaed basicranium of NMS 1972.27.481D. The exit for the optic nerve is obscured by a displaced piece of cartilage. Further anteriorly, the exit for the olfactory nerve is visible. The posterior part of the endocranial, sagittal section cuts through the depressed floor of the parietal fossa, dorsal to exits of glossopharyngeal nerve and vagus nerve. This is markedly similar to conditions in Egertonodus (Lane Reference Lane2010, fig. 34).
A virtual endocast of NMS 1972.27.481 (Fig. 9a) provides a limited (in terms of detail) but general comparison with other early chondrichthyan examples. The gross proportions most closely resemble Egertonodus (Lane Reference Lane2010) (Fig. 9b). The space for the telencephalon blends smoothly with the cerebellar space, and the relative size of the hypophyseal chamber is smaller and shallower than those of Cladodoides (Maisey Reference Maisey2005) and “Cobelodus” (Maisey Reference Maisey2007). The infill of the optic nerve exit is displaced ventrally. This (again) is an artefact of laterally compressed preservation and concertinaed folding of the basicranial cartilage. The posterior part of the cerebellar chamber roof drops significantly as it passes beneath the floor of the parietal fossa. The most distinctive feature is the enlarged roof of the chamber of the medullary region in Tristychius. This is apparently absent in non-neoselachian examples, but present and strikingly similar in Egertonodus and Tribodus (Lane Reference Lane2010); described by Maisey (Reference Maisey, Arratia, Wilson and Cloutier2004) as a ‘distinct dome' and also noted as present some extant elasmobranchs; e.g., Notorynchus.

Figure 9 Euselachian cranial cavity endocasts: (a) Tristychius arcuatus, NMS 1972.27.481D, endocast in right, lateral view; (b) Egertonodus basanus, redrawn after Lane (Reference Lane2010, fig. 35), reversed for ease of comparison. Abbreviations: cer = cerebellar region; hyp = hypophyseal recess; med = medullary region; tel = telencephalon region; II = optic nerve foramen; III = occulomotor foramen; IV = trochlear foramen; V, VII = trigeminofacial foramen; VIII = octaval nerve foramen.
Virtual endocasts of the partly crushed skeletal labyrinth have been obtained from specimens NMS G. 2015.30.1 (dorsoventrally compressed) (Figs 10, 11e–h) and NMS 1972.27.481D (laterally compressed) (Fig. 11a–d). Dick (Reference Dick1978) noted that parts of the labyrinth canals are also visible in NMS 1973.23.44; the same is true for NMS 1974.23.19, which includes a break through the occipital region exposing the posterior semicircular canals. Both of these specimens are dorsoventrally compressed (the usual condition of preservation).

Figure 10 Tristychius arcuatus, NMS G. 2015.30.1. A & B, neurocranium rendered semitransparent showing infill of otic skeletal labyrinths in dorsal view. Abbreviations: cb = C-bout; ea = external canal ampulla; esc = external canal; fm = foramen magnum; pfos = parietal fossa; popr = postorbital process; psc = posterior canal; sac = saccular chamber.

Figure 11 Tristychius arcuatus, otic skeletal labyrinths shown as line drawings flanked by rendered virtual endocasts. (a–d) NMS 1972.27.481D, right otic capsule, shown in (a) dorsal, (b) posterior, (c) lateral and (d) medial view. (e–h) NMS G. 2015.30.1, left side otic capsule (reversed) shown in (e) dorsal, (f) posterior, (g) lateral and (h) medial view. Abbreviations: aa = anterior ampulla; asc = anterior canal; ea = external ampulla; esc = external canal; lag = lagenar recess; pa = posterior ampulla; pac = preampullary canal; pf = perilymphatic fenestra; psc = posterior canal; sac = saccular chamber; ur = utricular recess; IX, X = common canal for glossopharyngeal and vagus nerves.
In dorsal view, the saccular chamber of Tristychius is broad (Figs 10, 11e) but not wide enough to meet the external (horizontal) semicircular canal, as in Egertonodus and Tribodus (Lane Reference Lane2010). Furthermore, the semitransparent CT reconstruction of NMS G. 2015.30.1 shows that the otic capsules do not project anteriorly between the postorbital processes. The anteriormost extremities, the anterior ampulla and utricular recess, are located just behind the processes, as in many Palaeozoic sharks but unlike Acronemus (Maisey Reference Maisey2011).
In lateral view, the saccular chamber is partly divided from a posterior lagenar chamber (Fig. 11c). The anterior and posterior semicircular canals arise directly from the saccular chamber. Dick's (Reference Dick1978) interpretation of NMS 1974.23.44 implied that a sinus superior and a crus commune were present, but conditions in the differently compressed specimens studied here indicate otherwise. Notably, the left side posterior semicircular canal of NMS G. 2015.30.1 (Fig. 11h) achieves an almost complete independent arc that passes through the space occupied by a crus commune in other gnathostome clades. A dorsal perspective of the same specimen shows overlap but clear separation between anterior and posterior canals. Thus, like hybodonts and neoselachians, there is no evidence of union between anterior and posterior canals (Maisey Reference Maisey2001b); but unlike hybodonts, the anterior and posterior canals of Tristychius are not widely separated. Moreover, the radius of the arc of the anterior semicircular canal must have been significantly larger than the radius of the posterior canal. This, again, is unlike hybodonts, in which anterior and posterior canals are similarly sized. In medial view, both NMS 1972.27.481D and NMS G. 2015.30.1 show that the posterior canal includes a significant length of ascending preampullary canal. NMS 1972.27.481D preserves this preampullary canal in clear association with the aperture of the perilymphatic fenestra. Finally, from a dorsal perspective, the oblique angle between anterior and posterior canals is unusual, as is the almost anteroposterior orientation of the anterior canal. Comparable conditions are otherwise unique to Acronemus (Maisey Reference Maisey2011, text-fig. 4)
The unusually broad sweep of the external semicircular canal is preserved best in the left side labyrinth of NMS G. 2015.30 (Fig. 10). When scaled to the same anteroposterior labyrinth length as Egertonodus and Tribodus (Lane Reference Lane2010) it is possible to confirm that the external semicircular canal has a correspondingly larger radius. Anteriorly, the canal terminates in a laterally crushed ampulla which connects with a small utricular recess.
Finally, it is noteworthy that although the calcified walls of the skeletal labyrinth record details of the membranous labyrinth, little is preserved of the canals for vagus and glossopharyngeal nerves.
4. Discussion
4.1. Comparative morphology
The new restoration of the Tristychius neurocranium (Fig. 3) builds on Dick's (Reference Dick1978) version (Fig. 1) and allows a better assessment of its place in early chondrichthyan phylogeny. Consistent with Pradel et al.'s (Reference Pradel, Tafforeau, Maisey and Janvier2011) analysis of early sharks, Tristychius is a crown chondrichthyan because it exhibits a suite of derived conditions: a posterior tectum bridging the otic roof; closed otico-occipital and metotic fissures (in adults); an occipital arch wedged between the otic capsules; grooves rather than canals for dorsal lateral aortae in the basicranial cartilage. Further to this, Tristychius is a euselachian in the sense of Maisey's (Reference Maisey2011) definition, meaning that it joins a monophyletic group including the hybodonts plus neoselachians (elasmobranchs and their immediate fossil relatives). Tristychius euselachian synapomorphies include a perilymphatic fenestra; a glossopharyngeal canal that was very likely floored by the hypotic lamina (although the canal itself is barely preserved, the exit of the canal suggests that an alternative arrangement would be very unlikely); a chondrified medial capsular wall; and the absence of a crus commune joining the anterior and posterior semicircular canals. In addition to these features, the newly revealed presence of a pre-ampullary section of the posterior semicircular canal extending dorsally to the perilymphatic fenestra (Fig. 11) represents a further derived characteristic that can be added to the euselachian synapomorphy list (Maisey et al. Reference Maisey, Arratia, Wilson and Cloutier2004; Coates & Gess Reference Coates and Gess2007).
However, this is where the straightforward diagnosis of Tristychius affinities peters out. Despite the abundance of new detail, further evidence linking Tristychius to either of the primary euselachian lineages, the Neoselachii or the Hybodontiformes, is difficult to find. This is important because the evolutionary split between neoselachians and hybodonts likely dates back ∼340 my (Coates & Gess Reference Coates and Gess2007; Maisey Reference Maisey2011) on the basis of the hybodont characteristics of the contemporary genus Onychoselache (Dick Reference Dick1978; Dick & Maisey Reference Dick and Maisey1980; Coates & Gess Reference Coates and Gess2007). However, Tristychius is preserved in far more detail, and more precise and accurate placement of this genus will introduce considerably more character data to phylogenetic hypotheses concerned with the elasmobranch stem lineage origin.
Pradel et al. (Reference Pradel, Tafforeau, Maisey and Janvier2011) joined Tristychius to the hybodonts on the basis of the trochlear nerve foramen situated anterior to the optic nerve foramen (Coates & Sequeira Reference Coates and Sequeira1998; Coates & Gess Reference Coates and Gess2007). But this characteristic is now absent (Figs 2d, e, 3), and the revised pattern of orbit foramina resembles the less specialised arrangements of outgroups such as Tamiobatis vetustus (Schaeffer Reference Schaeffer1981, fig. 19C). Lane's (Reference Lane2010) comparisons of Tribodus and Egertonodus identified a further series of hybodont neurocranial specialisations, but these, too, are absent in Tristychius. Candidate synapomorphies include the presence of a single large foramen for the combined exit of the glossopharyngeal and vagus nerves; in contrast, Tristychius retains separate foramina (Fig. 7c, d). In Tribodus and Egertonodus, two large openings, probably for anterior and posterior octaval nerve branches, connect the cranial cavity to the otic capsule. In Tristychius and neoselachians, this octaval nerve foramen is single. Anterior and posterior semicircular canals in Egertonodus and Tribodus are widely separated, but in Tristychius, and in crown gnathostomes in general, these canals are closely spaced. In Egertonodus and Tribodus, the anterior and posterior semicircular canals are similarly sized (radius of curvature). Once again, Tristychius exhibits the general gnathostome condition, in which the proportions of these canals differ.
Tristychius is further distanced from the base of the conventional hybodont lineage by the absence of cephalic spines, the absence of a large downturned postorbital process, and the presence (i.e., phylogenetic persistence) of a postorbital articulation for the palatoquadrate. Added to these, hybodontids and neoselachians are united (to the exclusion of Tristychius) by the position of the anterior ampulla between the postorbital processes (Maisey Reference Maisey1989, Reference Maisey2011; Maisey et al. Reference Maisey, Arratia, Wilson and Cloutier2004; Coates & Gess Reference Coates and Gess2007).
Tristychius thus occupies an incertae sedis location outside of the main euselachian cluster. However, this solution fails to account for the hybodont-like postorbital process profile and the neurocranial features, noted in the description, that it shares with Acronemus. Furthermore, although the Tristychius postorbital process is anteroposteriorly narrow, like those of hybodonts and Acronemus, the process is downturned (Figs 3, 4) with an anhedral angle to the basicranium (Maisey Reference Maisey2011). This is markedly unlike those of xenacanths, Tamiobatis, Cladodoides and symmoriids. Anteroposterior enlargement of the process and related jugular canal changes are likely hybodont specialisations exclusive of Tristychius. The presence of a palatoquadrate articulation surface on the process is plesiomorphic, but a partly or completely ventral articulation surface is unusual (cf. Maisey's (Reference Maisey2008) comments on Dick's (Reference Dick1978) reconstruction). It follows that the shared configuration of the ventral articulation surface, consisting of one or more ridges and grooves curving laterally and anteriorly, represents a likely synapomorphy linking Acronemus and Tristychius (Fig. 12).

Figure 12 Basioccipital, hypotic lamina and postorbital process positions compared in (a) Tristychius and (b) Acronemus (after Maisey Reference Maisey2011, text-fig. 2). Abbreviations: cb = C-bout; glda = grooves for lateral dorsal aortae; hmart = hyomandibula articulation area; oc cot = occipital cotylus; poart = postorbital articulation; popr = postorbital process; sos = supraotic shelf. Elliptical areas in grey indicate areas occupied by anterior ampulla.
In Tristychius, the articulation surface on the palatoquadrate dorsal rim (Dick Reference Dick1978) consists of a transverse groove immediately anterior to the extremely reduced otic process, and presents a precise match to the ventral surface of the postorbital process. In Acronemus, only the lateral surface of the palatoquadrate is revealed (Rieppel Reference Rieppel1982, text-figs 1, 2), thus no part of the articulation is visible. However, an unnamed Lower Triassic shark from Madagascar includes an extremely Acronemus-like three-dimensionally preserved palatoquadrate, Meckel's cartilage and dentition (Thomson Reference Thomson1982). Significantly, this palatoquadrate, with a characteristically reduced otic process, displays a well-formed transverse and slightly anterolaterally oriented articulation surface resembling that of Tristychius. A Tristychius-style postorbital process and articulation seems also to be present in the Lower Triassic Homalodontus (Mutter et al. Reference Mutter, de Blanger and Neuman2007, Reference Mutter, Neuman and de Blanger2008) of British Columbia, Canada. Homalodontus, already compared with Hybodus and Egertonodus, is known only from dorsolaterally flattened material. But, its neurocranium, palate, lower jaw and fragmented gill skeleton in many respects resemble intermediates between outgroups such as Tamiobatis and the kinds of conditions now emerging in Tristychius.
The breadth of the otic region in Tristychius reflects the span of the otic capsules, rather than increased width of the main cranial cavity (Figs 4b, 10). The shape and size of the supraotic shelves relative to the rest of the preserved neurocranium is, once again, a distinctive characteristic shared with Acronemus. The C-bout separating the anterior limit of the otic shelf from the postorbital process is also shared with Acronemus. None of these features is known in hybodonts, Tamiobatis, xenacanths or symmoriids. Differences between Tristychius and Acronemus crania (posterior to the postorbital processes) seem to relate mostly to the extent of occipital arch intrusion between the posterior semicircular canals. A view of the ventral surfaces reinforces this interpretation (Fig. 12). The basioccipital cartilages and hypotic laminae of Tristychius and Acronemus are more-or-less interchangeable, each with similarly divergent, deep, linear grooves for the lateral dorsal aortae. A key difference concerns the anteroposterior position of this unit (lamina plus occipital arch) relative to the otic capsules and postorbital processes. In Tristychius, the occipital arch and hypotic lamina are located posteriorly, but in Acronemus (Maisey Reference Maisey2011), the entire structure appears to have been shunted forwards.
Two further features of the otic region in Tristychius are of likely phylogenetic significance: the Tamiobatis-like horizonal crests on the dorsal ridge; and the sub-shelf location of the hyomandibular attachment to the otic wall. Elongate horizontal crests of the dorsal ridge are completely unknown in hybodonts, and their discovery in Tristychius was unexpected. Unfortunately, the dorsal surface of Acronemus is too poorly preserved to reveal crest presence or absence. The generally Tamiobatis-like (or Orthacanthus-like) shape of the dorsal ridge and crest in Tristychius suggests that this is simply a conserved plesiomorphy. If so, then the presence of a broad, smooth and crestless dorsal ridge emerges as a derived condition and possible synapomorphy of neoselachians and hybodonts, further weakening the Tristychius–hybodont connection.
The significance of a lateral otic shelf, and relation to possibly homologous otic shelves in modern selachians is explored by Maisey (Reference Maisey2011) in his discussion of Acronemus. Here, the similarity with modern selachian conditions is noted, alongside similarities with the shelf-like ‘postorbital process' (Maisey Reference Maisey1983, 1985) of Egertonodus and Synechodus. It appears that the Tristychius hyoid attachment area shares characteristics with euselachians in general. Notably, none of these taxa exhibits the classic lateral otic process of early chondrichthyan crania (Schaeffer Reference Schaeffer1981) which, to a greater or lesser extent, is an extension of the posterior semicircular canal wall.
4.2. Phylogenetic relationships
Cladograms of hybodont sharks started with Maisey's (Reference Maisey1989, fig. 35) discussion of the likely relationships of the Pennsylvanian genus Hamiltonichthys. In this, Tristychius is the sister group to all other Hybodontiformes. A modified version of this tree in Maisey et al. (Reference Maisey, Arratia, Wilson and Cloutier2004, fig. 2) accommodated Tribodus, but Tristychius and its contemporary, Onychoselache (Dick & Maisey Reference Dick and Maisey1980), were excluded because of uncertainties about the accuracy of available descriptions and the seeming primitiveness of these genera. Coates & Gess (Reference Coates and Gess2007, text-fig. 10), as part of their revision of Onychoselache, re-examined these data and used a modestly expanded character set to reinstall both Onychoselache and Tristychius within the Hybodontiformes, exclusive of the Neoselachii.
In contrast to these previous hypotheses, the present cladogram (Fig. 13) is much simpler, and restricted to a handful of more anatomically disparate genera. Furthermore, the character set is concerned only with neurocranial features or other aspects of cranial skeletal anatomy connected directly to the neurocranium. The branching pattern offered here will be tested in future publications, with substantial additions of characters and taxa, and subjected to a variety of numerical analyses. For the purposes of the present discussion, this proposal offers new groups and characterisations that deserve consideration.

Figure 13 Cladistic hypothesis of neurocranial character distribution among a small sample of early euselachians and the extant spiny dogfish, Squalus. Homoplasic character marked with asterisk. Sources of character information: Squalus – Schaeffer Reference Schaeffer1981, Gans & Parsons Reference Gans and Parsons1964, Marinelli & Strenger Reference Marinelli and Strenger1959; Synechodus – Maisey Reference Maisey1985; Egertonodus – Maisey Reference Maisey1983, Lane Reference Lane2010; Tribodus – Lane Reference Lane2010; Acronemus – Maisey Reference Maisey2011.
Here, in addition to the standard list of synapormorphies (discussed previously), the Euselachii is further supported by the following derived conditions: a postorbital process with a downturned (anhedral) angle; a hyoid articulation located on the posterior part of the otic capsule lateral wall beneath a laterally projecting shelf; and grooves rather than canals in the basicranial cartilage for the dorsal lateral aortae. This last condition depends upon outgroup choice, which in this instance is Cladodoides (Maisey Reference Maisey2005) Tamiobatis or Orthacanthus (Schaeffer Reference Schaeffer1981). If, on the other hand, these genera are replaced with fossil chimaeroids, then the presence of such grooves rather than canals for the lateral dorsal aortae becomes a synapomorphy of crown chondrichthyans.
Euselachians are divided into two clades (Fig. 13), one of which is named provisionally the Tristychiidae (acknowledging that this is a further use of the notional family coined by Moy-Thomas (Reference Moy-Thomas1936), Dick (Reference Dick1978) and Ginter et al. (Reference Ginter, Hampe, Duffin and Schultze2010)). Here, the Tristychiidae includes, at minimum, Tristychius and Acronemus, and their defining synapomorphies include: a postorbital process articulation for the palatoquadrate located mostly on the ventral surface of the postorbital process; a wide and almost parallel-sided (in dorsal view) otic region; a C-bout shaped notch dividing the proximal part of the postorbital process from the anterolateral extremity of supraotic shelf.
The other primary division of the euselachian clade consists of the Neoselachii and Hybodontiformes. Synapomorphies supporting this unnamed group (informally: neoselachimorphs) include: the absence (loss) of the postorbital articulation with the palatoquadrate; a broad, smooth and crestless dorsal ridge; and the anterior projection of the otic skeletal labyrinth so that the anterior ampulla is level with the postorbital process. This last condition either occurs independently in Acronemus, or it represents a euselachian synapmorphy, secondarily absent in Tristychius.
Hybodontiforms are defined by the presence of cephalic spines; an enlarged postorbital process; a trochlear nerve (IV) foramen anterior to the optic nerve (II) foramen; a double octaval nerve (VIII) foramen; a shared exit for glossopharyngeal (IX) and vagus (X) nerves; widely separated anterior and posterior semicircular canals; and similar radius of curvature anterior and posterior semicircular canals. Neoselachians, in turn, are defined by the loss of the lateral commissure, with the consequent absence of an anhedral angle with the basicranium; the presence of a postotic process containing the glossopharyngeal nerve (IX) exit; and an otic capsule that projects posterior to occipital arch.
There is nothing in the branching pattern and character distribution offered here that conflicts with the broader hypothesis assembled by Coates & Gess (Reference Coates and Gess2007). Once again, the introduction of additional genera that seem likely to be linked to the early elasmobranch radiation will provide the most effective test of this scheme. Key taxa are likely to include Wodnika (Schaumberg Reference Schaumberg1977; Zangerl Reference Zangerl and Schultze1981), Hopleacanthus (Schaumberg Reference Schaumberg1982), Sphenacanthus (Dick Reference Dick1998), Homalodontus (Mutter et al. Reference Mutter, de Blanger and Neuman2007, Reference Mutter, Neuman and de Blanger2008) and a more complete description of Thomson's (Reference Thomson1982) unnamed hybodontid from the Triassic of Madagascar. Of these, Sphenacanthus (Dick Reference Dick1998) is the most feasible target for further research. It already appears that the palatoquadrate otic process is reduced dorsoventrally but not anteroposteriorly, and shows no sign of articulation with the postorbital process. Thus, its upper jaw resembles that of Onychoselache and other hybodontids, rather than Tristychius and Acronemus. Visible parts of the Sphenacanthus basicranium are consistent with euselachian conditions, revealing grooves rather than canals for the lateral dorsal aortae. The new cladogram (Fig. 13), therefore, offers the possibility that Sphenacanthus is sister to other members of the neoselachimorph lineage. If so, and the branching pattern rests on very few character states, then the fossil fauna of the Carboniferous Oil Shales Groups of the Edinburgh region (Dick Reference Dick1978, Reference Dick1981, Reference Dick1998; Dick & Maisey Reference Dick and Maisey1980; Coates & Gess Reference Coates and Gess2007) captures an exemplary suite of likely early members of the early elasmobranch radiation: Tristychius, Onychoselache, Sphenacanthus and Diplodoselache.
4.3. The otic labyrinth and suggestions of palaeoecology
The various configurations of the otic labyrinth in fossil (Maisey Reference Maisey2001b) and Recent chondrichthyans, especially those of elasmobranchs (Evangelista et al. Reference Evangelista, Mills, Siebeck and Collin2010; Lisney Reference Lisney2010), have been the focus of sporadic research interest. Aside from providing phylogenetically informative characters, skeletal signatures of labyrinth morphologies offer some indication of inner ear functional biology. Several features of modern elasmobranchs (see also Schaeffer Reference Schaeffer1981) associated with semi-directional low frequency phonoreception are clearly present in Tristychius: a medial capsular wall; an almost circular posterior canal; the separation of the anterior from the posterior canals (absence of a crus); a perilymphatic fenestra communicating with the parietal fossa; and a large saccular chamber. These features mark Tristychius as the earliest chondrichthyan yet known to show direct evidence of specialised adaptations for sound detection. In terms of the conjectured outline of elasmobranch phylogeny (Fig. 13), the acoustic adaptations of the Euselachii could reasonably be described the most characteristic, unifying feature of the clade (similarly, the neoselachimorphs might be summarised as ‘loose-jawed', given the loss of a skeletal articulation between the palate and postorbital process). This inference about the general biology of euselachians begs the question of what they were listening to. Unlike the Chondrichthyes, adaptations for phonoreception in Osteichthyes are linked to vocalisation, and osteichthyan vocalisation is sufficiently widespread, taxonomically, to suggest that vocal-acoustic mechanisms and behaviours are likely ancestral for the entire clade (Bass & Chagnaud Reference Bass and Chagnaud2012). Perhaps these early euselachians were paying attention to noises emitted by osteichthyans, as both likely predators and prey.
The inner ear of Tristychius is further distinguished by the broad sweep of the external canal (Fig. 10). Young (Reference Young1981), noted by Maisey (Reference Maisey2001b), associated large external (horizontal) canals (radius of canal curvature) with fishes with slow turning speeds, and vice-versa for rapid swimmers (and canals with a narrow internal radius). However, recent studies challenge the utility of canal radii measurements as accurate predictors of head movement, orientation, manoeuvrability and swimming behaviour (Evangelista et al. Reference Evangelista, Mills, Siebeck and Collin2010). Nevertheless, of the various elasmobranch labyrinths surveyed by Evangelista et al. (Reference Evangelista, Mills, Siebeck and Collin2010), those with almost comparably large external canals relative to anterior and posterior canals, include Carcharhinus leucas (bull shark) and Orectolobus maculatus (wobbegong). Notably, anadromous bull sharks frequently enter and forage in shallow, non-marine waters, and wobbegongs are mostly benthic. Dick (Reference Dick1981, Reference Dick1998) has repeatedly pointed out that Tristychius was very likely the normal inhabitant of a large fresh or brackish water lagoon. There is, at least, some consistency here, in terms of inferred swimming habits.
Last of all, the laterally compressed saccular chamber of NMS 1972.27.481D (Fig. 11c, d) is damaged: its fragmented walls are driven inwards around a near-conical depression, and a matching, although less well preserved, depression punctures the opposite side of the specimen. It appears that the lateral compression of this particular braincase resulted from a crushing bite. Furthermore, unlike dorsoventrally compressed specimens such as NMS 1974.23.6 and NMS 1974.23.30A & B, which are preserved in articulation with their visceral arches, the jaws, hyoid arch and gill skeleton of NMS 1972.27.481D are present but dismembered. Of the fishes recorded from the Wardie fauna (Dineley & Metcalf Reference Dineley and Metcalf1999), only one is known to have had teeth of sufficient size and spacing to inflict this damage, the rhizodont Rhizodus hibberti.
5. Acknowledgements
This project could not have been undertaken and completed without the dedication, talent and genius of Stan Wood. For access to specimens, we thank N. Fraser and S. Walsh of the National Museums of Scotland. Isaac Krone assisted with the rendering of CT scans. We thank J. R. F. Dick for discussion of the original collection and description of Stan Wood's material; M. Taylor for insight concerning C. W. Peach; and Andrew Bass for discussion of fish vocal-acoustic systems. Z. Johanson and M. Brazeau are thanked for insightful reviews of the manuscript. This project and, in particular, the CT scanning of specimens, was supported by National Science Foundation grants DEB-0917922 and DEB-1541491.