Toxic elements such as cadmium (Cd) and arsenic (As) often co-contaminate paddy soils (Wang et al. Reference Wang, Wen, Chen, Zhang, Cen and Sun2016) and pose a risk to human health through the soil–plant–food chain. Rice (Oryza sativa L.) is the world's dominant staple food, especially in some Asian countries like China (Sun et al. Reference Sun, Van deWiele, Alava, Tack and Du Laing2012). A large area of Chinese paddy soil has been contaminated with heavy metals, especially in Southern China, due to mining and/or irrigation with polluted water (Williams et al. Reference Williams, Lei, Sun, Huang, Lu, Deacon, Meharg and Zhu2009). Human exposure to As and Cd through daily rice consumption has been of great concern due to rice grown in heavy metal contaminated fields (Williams et al. Reference Williams, Lei, Sun, Huang, Lu, Deacon, Meharg and Zhu2009; Sun et al. Reference Sun, Van deWiele, Alava, Tack and Du Laing2012).
In comparison with other remediation methods such as chemical washing (Abumaizar & Smith Reference Abumaizar and Smith1999; Makino et al. Reference Makino, Kamiya, Takano, Itou, Sekiya, Sasaki, Maejima and Sugahara2007), the in-situ immobilisation of heavy metals in contaminated arable fields is an effective and cost-effective remediation technology (Beesley & Marmiroli Reference Beesley and Marmiroli2011), especially for large areas of contaminated agricultural land (Bian et al. Reference Bian, Joseph, Cui, Pan, Li, Liu, Zhang, Rutlidge, Wong, Chia, Marjo, Gong, Munroe and Donne2014; Zheng et al. Reference Zheng, Chen, Cai, Tie, Liu, Reid, Huang, Lei, Sun and Baltrėnaitė2015; Li et al. Reference Li, Ye, Geng, Zhou, Guo, Zhang, Zhao and Wang2016). Biochar has been proved to be particularly effective for the sequestration of heavy metals in soils and their accumulation in edible parts of plants (Zheng et al. Reference Zheng, Cai, Liang, Huang, Chen, Huang, Arp and Sun2012, Reference Zheng, Chen, Cai, Wang, Huang, Xiao and Sun2013; Khan et al. Reference Khan, Reid, Li and Zhu2014). However, the long-term effects of different biochars on the immobilisation of different heavy metals in fields have not been well studied (Cui et al. Reference Cui, Li, Zhang, Pan, Bao and Chang2011; Bian et al. Reference Bian, Joseph, Cui, Pan, Li, Liu, Zhang, Rutlidge, Wong, Chia, Marjo, Gong, Munroe and Donne2014; Li et al. Reference Li, Ye, Geng, Zhou, Guo, Zhang, Zhao and Wang2016). Wheat-straw-derived biochar reduced the Cd content in rice plant tissues (Cui et al. Reference Cui, Li, Zhang, Pan, Bao and Chang2011; Bian et al. Reference Bian, Joseph, Cui, Pan, Li, Liu, Zhang, Rutlidge, Wong, Chia, Marjo, Gong, Munroe and Donne2014) and the lead (Pb) content in plant roots in a three-year field experiment (Bian et al. Reference Bian, Joseph, Cui, Pan, Li, Liu, Zhang, Rutlidge, Wong, Chia, Marjo, Gong, Munroe and Donne2014). The three-year pot-culture experiment showed that hardwood-derived biochar substantially reduced the concentration of Cd and copper (Cu) in rice tissues in the first year, whereas these values increased in the following two years. Corn-straw-derived biochar decreased these values steadily year by year (Li et al. Reference Li, Ye, Geng, Zhou, Guo, Zhang, Zhao and Wang2016). A field experiment is necessary to confirm the effects of different biochars on heavy metal immobilisation for a long period, especially in paddy fields with many contaminants.
In this study, two biochars (rice-straw-derived biochar (RB) and a coconut-by-product-derived biochar (CB)) were chosen to investigate their capacities for reducing Cd and As concentrations in rice. The aims of the present study were to: (1) research the effect of biochars in reducing Cd and As concentrations in rice over two years (four growth seasons); and (2) determine the effects of two biochars in reducing Cd and As concentrations using different amendment rates.
1. Materials and methods
1.1. Experiment site and rice
A two-year field experiment was conducted in the spring of 2014 at Beiyue village, Shaoguan, Guangdong Province, S China, where the cropping regime is dominated by the double rice-cropping systems. Early and late rice were both grown in the same field each growth season (early rice from early April to July and late rice from July to late October). Most paddy fields at Beiyue village had been contaminated with heavy metals (especially Cd). The average concentrations of Cd and As were 0.42±0.06 and 35.4±1.2mgkg–1, respectively, which can be considered as slight pollution based on the Chinese environmental quality standards for soils (GB15618–1995, Grade II). The pH value was 4.76±0.2. A local eugenic rice cultivar (Oryza sativa L.), was chosen for the early (spring) and late (summer) rice cropping in the experiment.
1.2. Experiment treatments
In this two-year field experiment, a randomised block experiment with three replicates was established, with four different levels of biochar treatments (2.25, 6.75, 11.25, 22.5tha–1). No biochar application was set as a control. Each field plot was 4.0×5.0m2.
To understand the role of biochar type on heavy metal stabilisation, two types of biochar – RB and CB – were purchased from Qinfeng Straw Technology Co. Ltd., Nanjing, and utilised in the experiment for their sequestration of Cd and As in soils.
Applied basal fertilisers in each season were urea for nitrogen (N) (225kgha–1), calcium superphosphate for phosphous (P) (225kgha–1), and potassium chloride for kalium (K) (150kgha–1). The basal fertiliser, together with biochar, were applied two days before rice transplanting in the early season of 2014 (first growth season), and was well incorporated into the soil by manual ploughing to a depth of 10–20cm. In the following three growth seasons the biochar treatments were ploughed by machine. The basal fertiliser was applied but no biochar was amended.
1.3. Sample preparation
The early and late rice-cropping seasons were from early April (planted) to July (harvested) and from July (planted) to October (harvested), respectively. Three grains of rice were randomly collected from each plot during harvesting. These were air-dried in the laboratory until constant weight was reached. The grain was dehulled in a motorised dehusker (JLGJ4.5, TZYQ, Zhejiang, China), and the resultant brown rice was milled to fine powder using a blender (Langjia, China). GBW 07602 (GSV-1) rice flour was used as the standard reference material (SRM) for the rice samples. The recoveries of Cd and As from rice grains were 104–121% and 106–118%, respectively. The recoveries of Cd and As in soil were 115% and 103%, respectively.
1.4. Digestion and analysis
The digestion procedure for all rice samples followed the method of Sun et al. (Reference Sun, Van deWiele, Alava, Tack and Du Laing2010). Subsamples of rice powder (0.2g) were weighed into pre-acid-washed 50mL polyethylene centrifuge tubes with HNO3 (2mL) added, and then the tubes were kept overnight at room temperature (∼25°C). Three SRM samples of rice flour (GSV-1) and three blanks were prepared simultaneously for quality control. All samples were microwave-digested (MARS, Matthew Inc., USA) using the same temperature program (Sun et al. Reference Sun, Van deWiele, Alava, Tack and Du Laing2010). The temperature was increased to 55°C within 5min and maintained for 10min, then increased to 75°C within 5min and maintained for 10min, and finally increased to 95°C within 5min and maintained for 30min. After digestion, the samples were cooled and then diluted to 50mL with Millipore ultrapure water (Millipore Milli-Q).
The samples were analysed for total Cd and As concentrations using inductively coupled plasma mass spectrometry (ICP-MS, 7500, Agilent Technologies). Cd isotopes Cd111 and Cd114, as well as indium (In) as an internal standard, were measured simultaneously. All samples were randomised prior to analysis.
1.5. The pH value and dissolved organic matter (DOC) in soil and biochar
Soil pH was measured following Wang et al. (Reference Wang, Wen, Chen, Zhang, Cen and Sun2016). Soil samples (2g) and Millipore ultrapure water (5mL) were mixed in a 10mL centrifuge tube. After agitation (150rpm) at room temperature for 20min, tubes were left to stand for 30min. The pH was tested using a Mettler Toledo 320-S pH meter (Mettler Toledo Instruments Co. Ltd., Shanghai, China). Biochar pH was measured with the modified method (GB/T 12496.7-1999). Biochar samples (0.25g) were weighed into a 10mL centrifuge tube, added to carbon dioxide-free water (5mL) and kept for half an hour. The supernatant was obtained by filtering the mixture and tested with the pH meter. The pH for RB and CB were 9.3±0.1 and 10.4±0.1, respectively.
A total of 4g of soil was weighed into acid pre-washed 50mL polypropylene centrifuge tubes and 20mL of extracting solution was added (1:5 weight/volume ratio). All tubes were shaken for 15min at 200rpm at 20°C, the soil suspensions were then centrifuged for 10min at 7900 g at a temperature of 20°C. Supernatants were filtered through a 0.45μm filter, and DOC was determined using a total organic carbon (TOC) analyser (Liquic TOC, Elementar, Germany). The contents of organic matter for RB and CB were 22.1% and 38.6%, respectively.
1.6. Statistical analysis and quality control
All statistical analyses were conducted using SPSS 17.0 software. One-way analysis of variance (ANOVA) was used to identify significant differences (P<0.05) between Cd or As concentrations.
2. Results and discussion
2.1. Rice yields
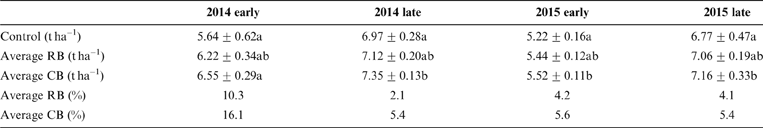
No significant differences in grain yields were observed between different biochar amendments and their dosages (Fig. 1). The average grain yields among different amendment levels were calculated, and a weak but significant increase in the rice grain yields was observed in the presence of CB compared to the corresponding control (P<0.05) for all four growth seasons (Table 1). The amendment with RB increased the average rice yield as well, but not significantly (Table 1). The greatest improvement in rice yield was exhibited in the first growth season: up to 10.3–16.1% higher than that in other seasons (2.1–5.6%) (Table 1). The nutrients from amended biochars were probably utilised at the first growth season and fewer nutrients were derived from biochars in the following ones. It has been reported that some biochars derived from various feedstock could improve rice yield. For example, the addition of sewage-sludge-derived biochar significantly increased grain yield in a pot experiment (Khan et al. Reference Khan, Reid, Li and Zhu2014) and rice yield was enhanced under the treatment of wheat straw biochar in a one-year field experiment (Zhang et al. Reference Zhang, Bian, Pan, Cui, Hussain, Li, Zheng, Zheng, Zhang, Han and Yu2012). Some studies have shown that there was no mean effect on rice yield with biochar amendments from wheat straw (Bian et al. Reference Bian, Chen, Liu, Cui, Li, Pan, Xie, Zheng, Zhang, Zheng and Chang2013; Huang et al. Reference Huang, Yang, Qin, Jiang and Zou2013), although some nutrient levels had been substantially improved (Utomo et al. Reference Utomo, Kusuma and Nugroho2011). Many factors can affect the improvement of plant growth, such as nutrients, pH, cation exchange capacity (CEC) of biochar, the biochar amount amended, soil properties and decreased accumulation of toxic metals. It is likely that more nutrients were contained in CB than RB because the large silicon content (20%) contained in rice husks causes less nutrients in this biochar (Kumar et al. Reference Kumar, Sangwan, Dhankhar, Mor and Bidra2013).
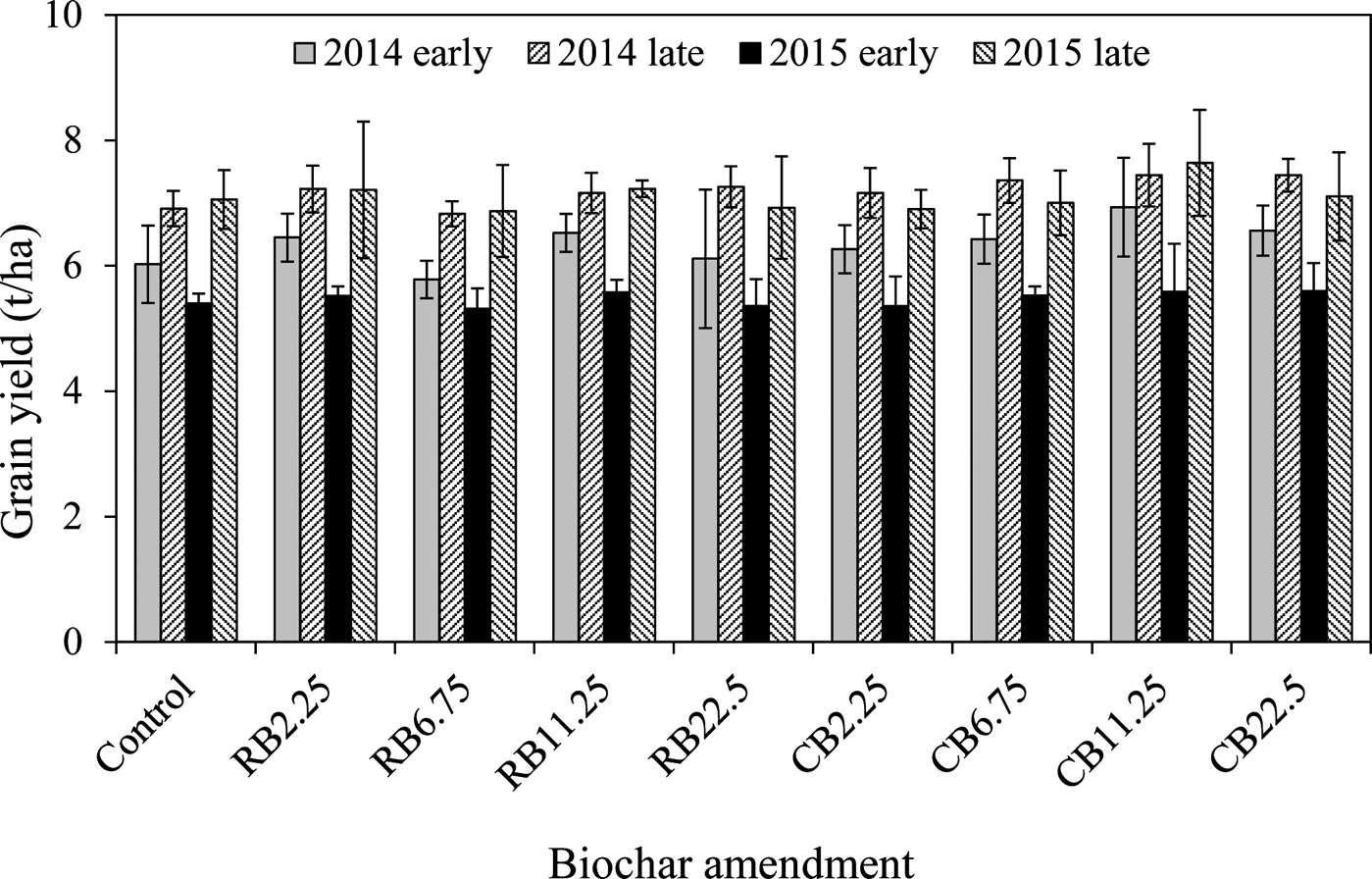
Figure 1 Grain yields of rice with amendments of RB and CB in contaminated paddy soil, respectively, at various levels of biochar treatments (2.25, 6.75, 11.25, 22.5tha–1).
Table 1 The average grain yields (tha–1) for the amendment of different biochars and the percentage (%) for increased average rice yield.
During the two-year experiment, the crop yields in the late season (summer season) were always greater than those in the corresponding early season (spring season), regardless of biochar addition (Fig. 1; Table 1), although the growth period of early rice was longer (110 days) than that of late rice (100 days). It was reported that average temperature and rainfall were the main climatic factors influencing rice yield, and the seasonal average maximum temperature adversely affected rice yield for the two growing seasons (Chung et al. Reference Chung, Jintrawet and Promburom2015), having a strong influence on rice yield from the flowering stage to the full-ripe stage (about 30 days). During this period the local average maximum temperature for early rice was 32°C and higher than that for late rice (28°C). Perhaps this is one of the reasons for a higher rice yield from late rice.
2.2. Cd and As concentrations
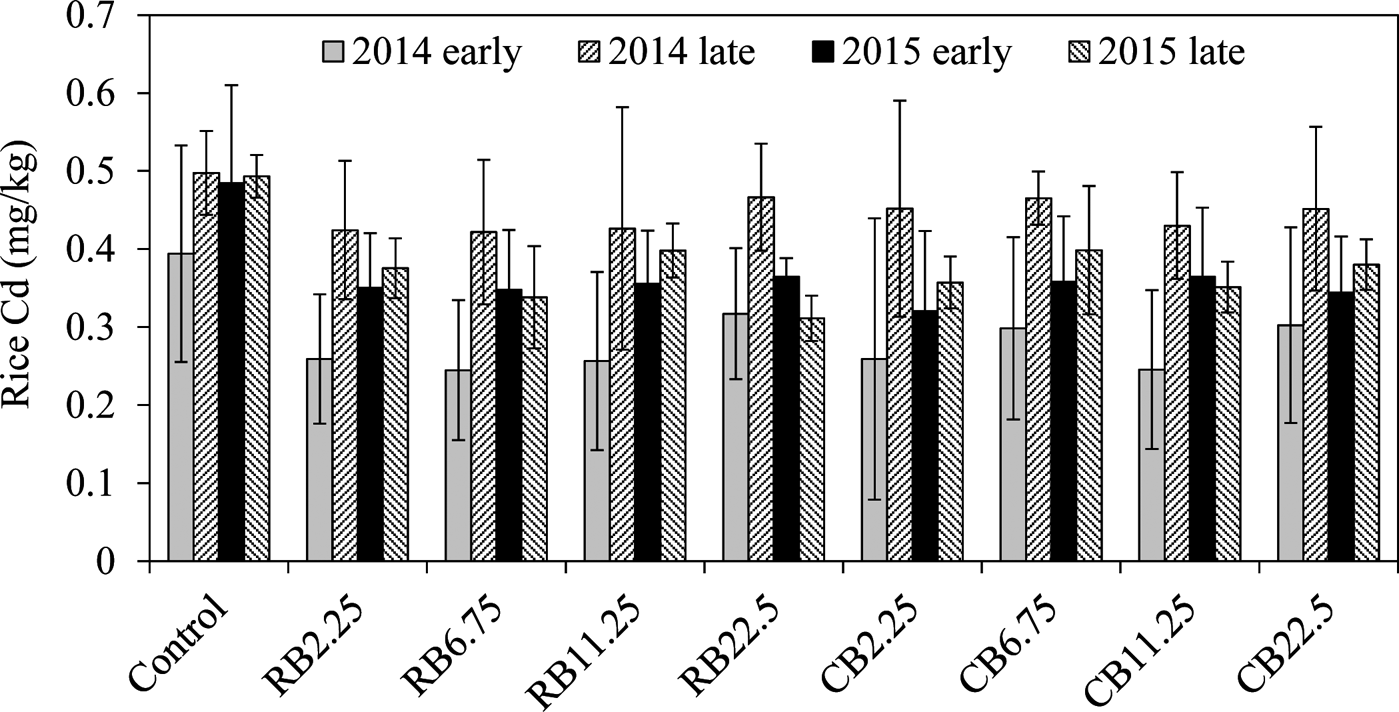
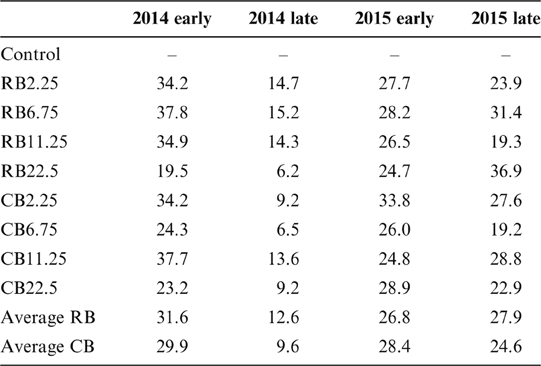
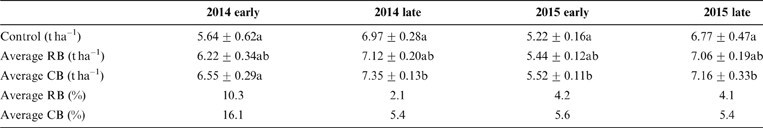
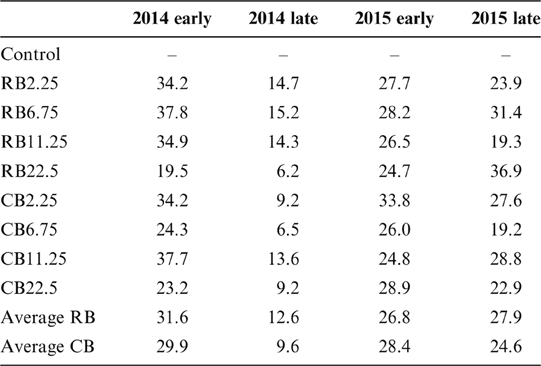
The average Cd content in rice samples of the control was 0.39–0.49mgkg–1 in all four seasons, which markedly exceeded the national food standard limit of 0.2mgkg–1 Cd for rice in China (GB2762-2012). The Cd concentration in rice grain was significantly reduced by amending biochar in the soil, regardless of the biochar types, compared with the corresponding control for the two years (Fig. 2; Table 2). However, the decreases of Cd in rice were not related to the amount of biochar amendments. For example, the addition of biochar at 2.25tha–1 has a similar effect on Cd reduction in rice with the addition of 22.5tha–1 (Fig. 2; Table 2), even for the long-term growth season (2015 late season). No difference was observed in reducing Cd in rice with different biochars and different dosages. The long term effects of the two biochars were similar on Cd immobilisation over the two years (Table 2). A smaller removal of Cd was observed in the second season (2014 late rice), up to 9.6–11.1% of the control (Table 2), and the reasons for this are unclear. In other growing seasons with biochar amendments, rice Cd concentration was reduced by 24.6–31.6% in two years of the experiment, indicating the long-term effect of biochars on the sequestration of Cd in paddy soils.
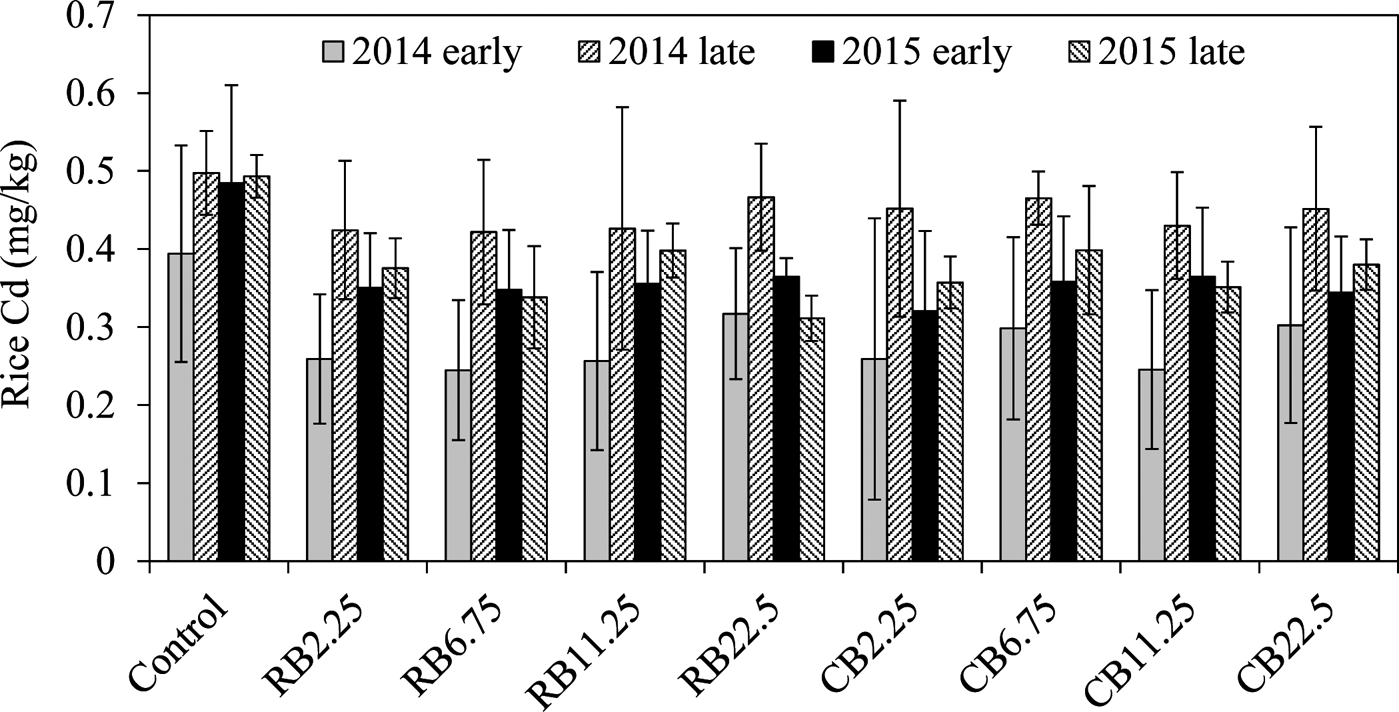
Figure 2 The concentrations of rice Cd with the addition of RB and CB in contaminated paddy soil, respectively, at various levels of biochar treatments (2.25, 6.75, 11.25, 22.5tha–1).
Table 2 The Cd removal (%) in rice grain in the paddy field with the amendment of different biochars at various dosages.
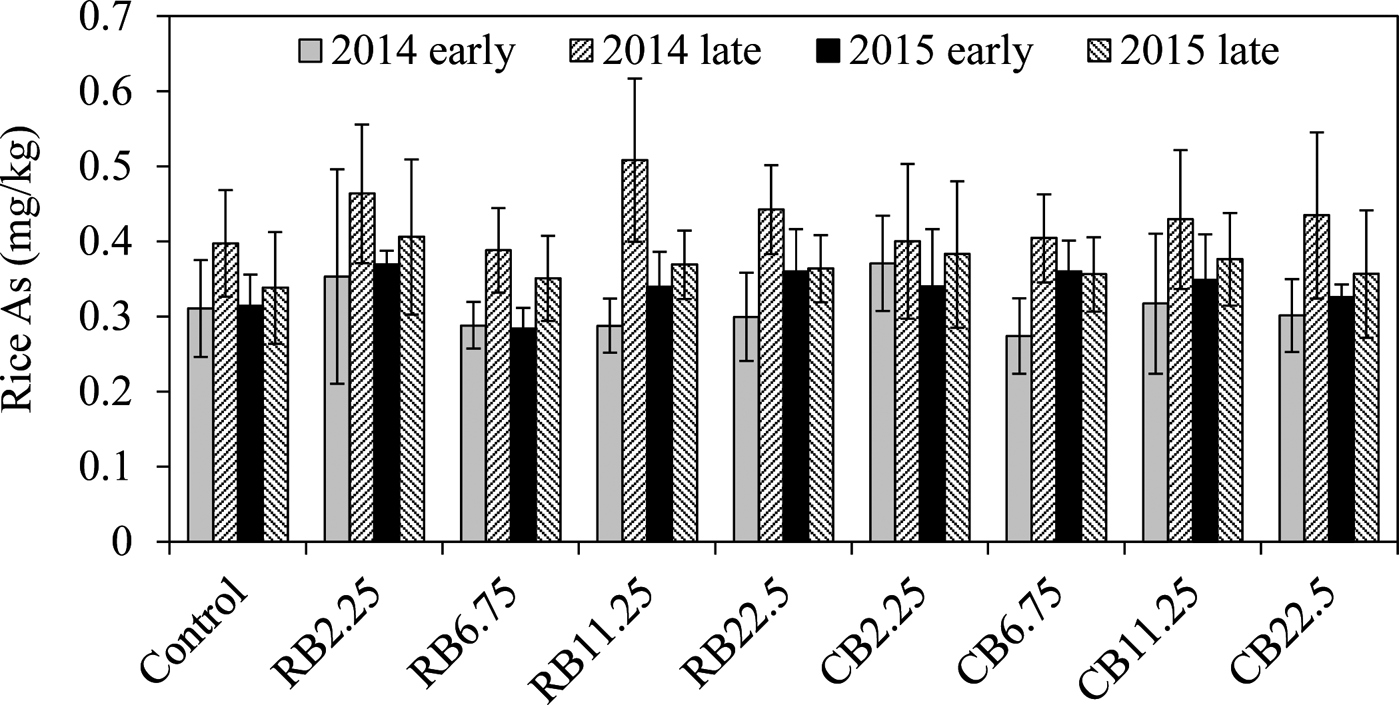
The biochar had no effect in mitigating As in rice (Fig. 3), as reported previously (Zheng et al. Reference Zheng, Cai, Liang, Huang, Chen, Huang, Arp and Sun2012, Reference Zheng, Chen, Cai, Tie, Liu, Reid, Huang, Lei, Sun and Baltrėnaitė2015). Most concentrations of As in rice with biochar treatments were similar or slightly greater than the controls, although no significant difference was observed (Fig. 3). Similar As levels in rice during the four growth seasons after biochar application suggested that biochar amendment did not release As or increase As accumulation in the long-term experiment.
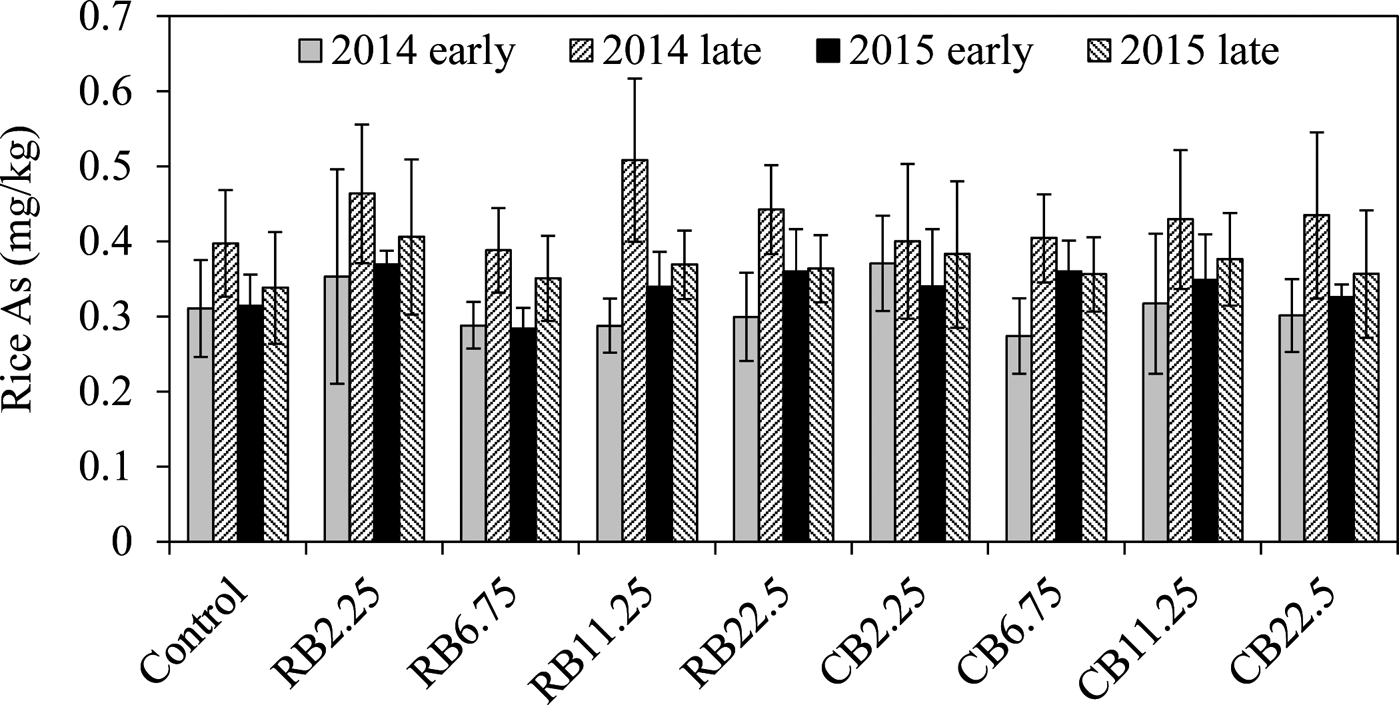
Figure 3 The concentrations of rice As with the addition of RB and CB in contaminated paddy soil, respectively, at various levels of biochar treatments (2.25, 6.75, 11.25, 22.5tha–1).
2.3. pH and DOC in soil
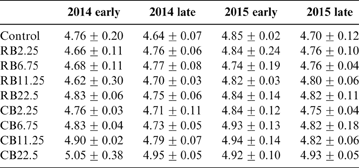
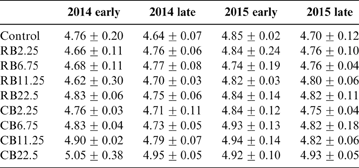
The soil pH in the control treatment remained unchanged throughout the experiment (Table 3). The biochar-amended soil showed no significant difference with control after two years of the experiment. Large amounts of biochar (22.5tha–1) substantially increased soil pH from 4.76 to 4.83 in RB and to 5.05 in CB at the first growth season (P<0.05) (Table 3); this is probably due to the higher pH for CB (10.37) than that for RB (9.30), reflecting the alkaline nature of the biochar. There was little change in soil pH during the four growth seasons. This result is supported by other reports, which evidence the increase of pH with the addition of biochar to paddy soil (Cui et al. Reference Cui, Li, Zhang, Pan, Bao and Chang2011; Li et al. Reference Li, Ye, Geng, Zhou, Guo, Zhang, Zhao and Wang2016). Base cations in biomass were transformed into oxides, hydroxides or carbonates in the biochar during pyrolysis at high temperature (Yuan et al. Reference Yuan, Xu and Zhang2011). The dissolution of these alkaline substances in biochars improved the soil pH (Houben et al. Reference Houben, Evrard and Sonnet2013). With the increase in soil pH, the hydrolysis of metal cations such as Cd2+ promoted precipitates of metal(oxy)hydroxide forms. The enhancement of soil pH facilitated the immobilisation of Cd in soil. However, As existed as a hydro anion in an aquatic environment. The enhancement of pH decreased the number of positively charged sites on soil minerals, which lowered the sorption capacity of negatively charged oxyanions of As and increased As mobilisation and release from soil (Wilson et al. Reference Wilson, Lockwood, Ashley and Tighe2010). Phosphorus and silicon released from biochar competed with As for binding sites at iron oxide surfaces in soils to improve As solubility in pore water (Jain & Loeppert Reference Jain and Loeppert2000; Waltham & Eick Reference Waltham and Eick2002; Zheng et al. Reference Zheng, Cai, Liang, Huang, Chen, Huang, Arp and Sun2012).
Table 3 The soil pH for the amendment of different biochars at different growth seasons.
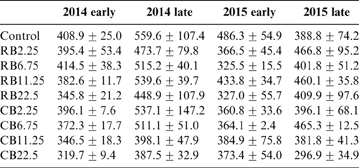
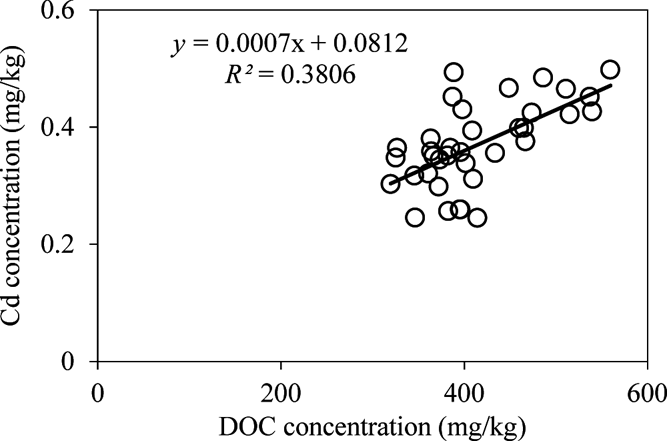
The concentrations of DOC in late rice fields were greater than those in corresponding early rice fields. The roots were left in the field after the early harvest and ploughed directly into the soil. After a week, the late rice was transplanted and the roots were left to decompose during summer growth (Huang et al. Reference Huang, Zhu, Chen, Yin and Sun2012). This suggests that the enhanced DOC was derived from root decomposition. The contents of DOC were not significantly changed in the soil treated with different amounts of RB (P>0.05). The DOC in CB-treated soil decreased with the increase in CB amendments (Table 4). There was a positive relationship between Cd concentrations in rice and DOC content (Fig. 4), indicating that large amounts of DOC in soil enhanced the accumulation Cd in rice. Organic matter can exist in soluble complexes with Cd to reduce metal adsorption onto soil particles and promote metal availability for plants (Antoniadis & Alloway Reference Antoniadis and Alloway2002; Cornu et al. Reference Cornu, Schneider, Jezequel and Denaix2011). It has been reported that high Cd–DOM complexes in the rhizosphere soil solution improve Cd accumulation in plants (Li et al. Reference Li, Liang, Han and Yang2013). This can explain why most average Cd levels in late rice were always greater than those in early rice (Fig. 2). In addition, iron plaque on the root surfaces of rice can also accumulate a variety of heavy metals and metalloids such as Cd and As (Liu et al. Reference Liu, Zhu and Smith2004; Chen et al. Reference Chen, Zhu, Liu and Meharg2005; Hu et al. Reference Hu, Li, Zhu, Huang, Hu and Christie2005). The iron plaque was dissolved during the decay of the rice roots (Wang et al. Reference Wang, Chen, Yang, Wang and Sun2009; Huang et al. Reference Huang, Zhu, Chen, Yin and Sun2012), accompanied by the release of As and Cd during root decomposition and iron plaque reduction, all mediated by microorganisms in the paddy (Zhu et al. Reference Zhu, Duan, Chen, Peng, Chen and Sun2014). The released Cd and As were bioavailable and easily absorbed and accumulated in rice grains. This explains why in late rice the average concentrations of Cd and As were greater than those in the corresponding early rice (Figs 2, 3).
Table 4 The DOC contents (mgkg–1) for the amendment of different biochars at different growth seasons.
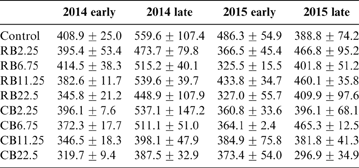

Figure 4 The relationship of rice Cd levels with the DOC contents in soil.
3. Conclusions
Rice yields were significantly increased after the application of CB during the four growth seasons (two-year) experiment, especially during the first growth season. The amendment RB increased the average rice yield but not to a significant level. CB proved superior to RB in improving rice yield over two years. The Cd content in rice was reduced by both biochars, regardless of the biochar application rate, in a long-term contaminated paddy field for at least two years. There were no significant variations in As in rice after biochar addition. Biochar has been shown to have significant and sustainable potential in Cd sequestration for reducing Cd accumulation in rice when applied to paddy soils. Biochar amendment offers a sustainable strategy for mitigating heavy metal pollution in agricultural fields.
4. Acknowledgements
This project was financially supported by the National High Technology Research and Development Program of China (863 Program, 2013AA06A209), the Natural Science Foundation of China (no. 41371459) and the State Key Program of Natural Science Foundation of China (nos 41330853, 41430858).