Extinct crocodyliforms exhibited a wider range of forms than their surviving relatives. The most divergent of these were probably the Mesozoic marine clades, but in the Cenozoic, the ziphodont clades were the most striking. These groups are characterised by their laterally flattened, serrate (ziphodont) teeth and often, elevated, laterally flattened (oreinirostral) snouts. Prominent among these ziphodont crocodyliforms was Sebecus icaeorhinus Simpson, Reference Simpson1937. Until recently represented by three species, the genus Sebecus is now restricted to the type species (Paolillo & Linares Reference Paolillo and Linares2007).
In spite of the obvious differences in cranial form from living crocodilians, few papers have been devoted to S. icaeorhinus. The initial description by Simpson (Reference Simpson1937) was short and unillustrated. A later descriptive monograph was commenced by Simpson and completed by Colbert (Reference Colbert1946). Gasparini (Reference Gasparini1972) described the third specimen. This material includes little of the postcranial skeleton, but recently Pol and colleagues (Reference Pol, Leardi, Lecuona and Krause2012) described more complete postcranial remains. Sebecus, belonging to the most widely-distributed Cenozoic ziphodont clade (cf. Turner & Calvo Reference Turner and Calvo2005), derives from the Sarmiento Formation of Late Eocene age (e.g. Madden et al. Reference Madden, Carlini, Vucetich and Kay2010) of Chubut, Argentina. The holotype was recovered from a small bentonite pocket that did not contain the characteristic Notostylops fauna, often found in the Sarmiento Formation (Simpson Reference Simpson1937; Molnar Reference Molnar2010; Pol et al. Reference Pol, Leardi, Lecuona and Krause2012). The sediments were apparently laid down in a shallow lake or pond (Howard Reference Howard1955).
The phylogenetic position of Sebecus is controversial; some consider Sebecus to belong to the sebecosuchian clade of ziphosuchian notosuchian mesoeucrocodylians (Iori & Carvalho Reference Iori and Carvalho2011; Pol & Powell Reference Pol and Powell2011; Pol et al. Reference Pol, Leardi, Lecuona and Krause2012; and the supertree of Bronzati et al. Reference Bronzati, Montefeltro and Langer2012). The alternative view (Holliday & Gardner Reference Holliday and Gardner2012; Riff & Kellner Reference Riff and Kellner2011), separates it from baurusuchid notosuchians following Walker (Reference Walker1968), and places it in the mesoeucrocodylian clade Sebecia (Larsson & Sues Reference Larsson and Sues2007), more closely related to Hamadasuchus, Stolokrosuchus and Peirosaurus. Analysis of postcranial character states however, supports monophyly of the Sebecosuchia (Pol et al. Reference Pol, Leardi, Lecuona and Krause2012).
The differences of the snout and teeth of S. icaeorhinus from those of living crocodilians and perceived similarities to those of some non-avian theropods prompted this study of its jaw musculature and mechanics, the aim of which was to reconstruct the jaw adductor and depressor musculature and their relationships to mandibular kinematics, much as in Ösi & Weishampel (Reference Ösi and Weishampel2009). The analysis presented here is an updated version of part of a thesis submitted as part of the requirements for the degree of Master of Arts at the University of Texas, Austin in 1969; the remainder of that study was published as Molnar (Reference Molnar2010). The paucity of attention given to this taxon makes the study still pertinent (but see Henderson & Weishampel Reference Henderson and Weishampel2002).
Institutional abbreviations. AMNH, American Museum of Natural History, New York City, USA; MMP, Museo Municipal de Mar del Plata, Mar del Plata, Argentina; MPEF, Museo Paleontológico Egidio Feruglio, Trelew, Argentina.
1. Materials and methods
Sebecus icaeorhinus is currently known from seven specimens, all derived from a restricted region of Patagonia, Cañadon Hondo and Cañadon Vaca, in Chubut, Argentina. Cranial material is represented in the holotype, AMNH 3160, and three referred specimens, AMNH 3159 (Colbert Reference Colbert1946), MMP 235 (Gasparini Reference Gasparini1972), and MPEF-PV 1776 (Pol et al. Reference Pol, Leardi, Lecuona and Krause2012) of which the holotype remains the most complete. The other three specimens, MPEF-PV 3970, MPEF-PV 3971 and MPEF-PV 3972, along with MPEF-PV 1776, consist of (mostly) postcranial material. The holotype cranium was disarticulated, but because none of the elements were duplicated nor scattered over a large area and were consistent in size, they probably belong to a single individual. This study was carried out on the holotype, with some observations from Gasparini (Reference Gasparini1972).
The musculature of modern organisms can be observed by dissection, but that of fossil organisms must be inferred usually from bony remains. For fossil taxa with modern relatives, these relatives can provide guidance. Muscle (and tendon) attachments on the fossils are located with reference to selected points or ‘landmarks’ of the morphology of the appropriate elements. Muscle scars are the best landmarks, where they can be discerned. Where they cannot, the muscles are assumed to attach to corresponding parts of homologous elements. If, for example, a muscle extended from the quadrate to the surangular in the modern analogue, then this is reconstructed as its course in the fossil. Further comments on this technique are given by Ostrom (Reference Ostrom1961), Barghusen (Reference Barghusen1973), Molnar (Reference Molnar, Larson and Carpenter2008), Holliday (Reference Holliday2009) and Dilkes et al. (Reference Dilkes, Hutchinson, Holliday, Witmer, Brett-Surman, Holtz and Farlow2012).
Selection of appropriate analogues is critical to the process. The lineage of mesoeucrocodylians leading to the living crocodilians has been noted for being conservative in cranial structure, especially when compared to other archosaurian lineages, such as the theropod–avian clade. However, this is not generally the case for many extinct lineages of crocodyliforms, particularly thalattosuchians and notosuchians. The adductor chamber, as far as known to me, seems basically conservative in form and bony composition, thus it is interesting to ascertain the extent to which the muscle structure of modern taxa, here Alligator mississippiensis Daudin, Reference Daudin1802 and Paleosuchus trigonatus Schneider, Reference Schneider1801, can be extrapolated to their extinct relatives. Where the muscle scars are indistinct, imperceptible or substantially different in form, arbitrary decisions are involved, however, this is not the case here. For Sebecus icaeorhinus and modern crocodilians, similar structural features include the arrangement of cranial elements and openings, diapsid postorbital cranial structure, snouts long relative to the postorbital part of the skull and acuminate teeth.
In the living crocodilians, as in other tetrapods, the jaw musculature sometimes leaves characteristic indications on bones – muscle scars. Fleshy attachments are generally onto smooth, flat or nearly flat surfaces of bones. Such scars are sometimes set off from the general surface by a distinct rim or angulation (as in the case of the M. adductor mandibulae externus superficialis et medialis insertion) and sometimes not (as with the M. adductor mandibulae posterior origin). Tendinous attachments usually arise from ridges along the surface of the bone (as with the crocodilian A-tendon: Iordansky Reference Iordansky1964) or from sharp edges of the element (as with the surface tendon of the M. pterygoideus ventralis).
Having chosen a modern analogue, regions of the muscle attachments are located and the presence of muscle scars ascertained. Muscle scars may often be recognised by touch, i.e. by changes in the surface texture of bone. If scars are not obvious, if the surface texture of the bone is uniform across the expected area of attachment and continues without change into regions where other muscles or tissues attach, then nearby ‘landmarks’ must by used to approximately locate the attachment site. If the modern analogue is well chosen, those scars seen on the fossil should be similar in position and form to those of the analogue. Generally, not all of the attachments will have easily recognisable scars so approximation of the location of the attachments by other landmarks will be necessary.
McGowan (Reference McGowan1986, and citations therein) found considerable variation in the pattern of muscles in the wings and hind limbs of birds. Examination of lacertilian cranial material then located in the Northern Arizona University Quaternary Studies Program collection revealed that muscle scars were present in comparable positions across a range of species (Calumma parsonii (Cuvier Reference Cuvier1825), Chamaeleo calyptratus Duméril & Duméril (Reference Duméril and Duméril1851), Ctenosaura pectinata (Wiegmann Reference Wiegmann1834), Furcifer oustaleti (Mocquard Reference Mocquard1894), Furcifer pardalis (Cuvier Reference Cuvier1829), Hydrosaurus amboinensis (Shaw Reference Shaw1802), Iguana iguana Linnaeus (Reference Linnaeus1758), Polychrus gutturosus Berthold (Reference Berthold1846), Trioceros melleri (Gray Reference Gray1865) and Tupinambis teguixin (Linnaeus Reference Linnaeus1758). The scars, similar to one another in form in each species, but varying in details of form and degree of development, are more prominent on larger specimens, and not seen on specimens smaller than 50 mm in length. Inspection of skulls of A. mississippiensis and Crocodylus porosus Schneider (Reference Schneider1801) gave the similar results, agreeing with the observations of Schumacher (Reference Schumacher, Gans and Parsons1973). This study is based on a single specimen of Sebecus, thus potential differences of the muscle attachments as a result of individual variation were not assessed.
Reconstructing the musculature of an extinct tetrapod is an exercise in anatomy and logic of limited interest. It is more interesting to take the reconstruction a step further and infer properties and behaviours of the once-living organism (cf. Snively & Russell Reference Snively and Russell2007). However, confidence decreases as the number of inferences based on other inferences grows. Although the conclusions presented here are considered reliable, they are based on several levels of inference. Further analysis and explanation of the inferential structure may be found in Molnar (Reference Molnar, Parrish, Molnar, Currie and Koppelhus2013). The reconstruction of the musculature is based largely on dissections of A. mississipiensis and P. trigonatus and descriptions of crocodilian jaw musculature in the literature (Poglayen-Neuwall Reference Poglayen-Neuwall1953, and particularly Iordansky, Reference Iordansky1964). More recent descriptions of crocodilian jaw musculature and tendons may be found in Schumacher (Reference Schumacher, Gans and Parsons1973), van Drongelen & Dullemeijer (1982), Busbey (Reference Busbey1989), Iordansky (Reference Iordansky2000), Holliday & Witmer (Reference Holliday and Witmer2007) and Bona & Desojo (Reference Bona and Desojo2011), and of the tendons in Shimada et al. (Reference Shimada, Sato and Ezure1993).
Motions of bones produced by actions of muscles are physical, rather than biological, effects and, thus, the interpretation of the muscular actions is an exercise in mechanics. The lever arms and lengths of the various muscles were estimated from a physical model of a lateral projection of the reconstructed skull and jaws of AMNH 3160. The modeled jaw was separately mounted and hinged so that it could be freely rotated relative to the skull. Details of this process are given in Molnar (Reference Molnar, Parrish, Molnar, Currie and Koppelhus2013, fig. 1). Measurements were made for angles of depression at intervals of ten degrees to a maximum of 40° for Sebecus. Comparable measurements were made in lateral projection from a skull of Crocodylus niloticus Schneider, Reference Schneider1801 but measuring at 10° increments proved impractical and unnecessary to establishing the curve. The C. niloticus skull was used for lever arm measurements because, scaled to equal length from the premaxilla to the quadrate condyles in lateral view, the skull of C. niloticus more closely matches the reconstructed skull of Sebecus icaeorhinus than does that of A. mississippiensis in the relative height of the skull deck, position of the orbit and postorbital bar, and inclination of the quadrate. The skull of A. mississippiensis has a relatively lower skull deck, more anteriorly placed orbit, and (slightly) more nearly horizontally orientated quadrate. Whereas the differences in the proportions of the skulls of A. mississippiensis and P. trigonatus do not much affect the structures of their jaw musculatures, so A. mississippiensis can be as easily compared to Sebecus as can C. niloticus, differences in the proportions of the skulls of A. mississippiensis and C. niloticus will be expected to more affect the lever arms. The C. niloticus skull was mounted against a base so that it could be photographed repeatedly in the same position. Black thread was run from the estimated center of the area of origin to the estimated center of the area of insertion and was used to represent the lines of actions of the various muscles. Photographs were then made with the jaw open to various angles. The centre of rotation of the joint was established on the photographs as the centre of curvature of the quadrate condyle in lateral view. The lever arm was then measured as the perpendicular distance from the thread to the centre of rotation. A computer input card was used to simulate the zwischensehne (the largest aponeurosis of the adductor muscles) of C. niloticus. A ruler placed with the skull at the level of the quadrate condyle supplied the scale. The percentage extension of the muscles has been calculated from the rest length – the length of the muscle when not generating force, in this case when the mouth was closed at rest, not exerting any force against prey – and extension measurements for both Sebecus and C. niloticus. Shimada et al. (Reference Shimada, Sato and Ezure1993) suggested that, in A. mississippiensis, the tendons attaching to the M. pseudotemporalis and M. intramandibularis may be sufficiently elastic to store potential energy during the opening of the jaws, to be released during closing. This aspect was not modelled here.
The length of each muscle at each stage of jaw depression was estimated by measuring the distance between the inferred centers of the areas of origin and insertion with the model jaw of Sebecus and the actual of C. niloticus rotated to the requisite angle. This length was then divided by the length of the muscle with the jaw closed and multiplied by one hundred to give the percentage extension. The uncertainty in the measurements was approximately two millimetres, hence, the values are accurate to two figures. Manipulation of the surangular piece on the quadrate indicates that this would have restricted the jaw depression to less than 50°. Thus measurements were made for angles of 0° to 40° of gape for Sebecus, zero being taken as that gape for which the tips of the dentary teeth reach the ventral maxillary margin, and to 50° for C. niloticus. Hence, only relative percentage extensions are used here, and the maximum opening of the mouth of Sebecus is assumed to have been determined by the surangular lip or crest.
The advantage of the physical model is its transparency. It depends on the accuracy of the cranial reconstruction. These taxa were chosen for the availability of specimens of taxa phylogenetically closer to Sebecus than birds or lepidosaurs. Bona & Desojo (Reference Bona and Desojo2011) describe differences in muscular attachments and anatomy and in the position of the crest for the A-tendon among species of Caiman. Thus, differences in the reconstructed muscles may result from using different extant taxa as models. However, identification of muscle scars on the fossil specimen should minimise such error. A limitation is that it is a two dimensional model of a three dimensional system. Thus, for example, the medially-directed components of the muscular forces on the mandible (cf. Porro et al. Reference Porro, Holliday, Anapol, Ontiveros, Ontiveros and Ross2011) are neglected as they do not contribute to closing the mouth. Discoveries of preserved soft tissues in older archosaurian material (e.g. Schweitzer Reference Schweitzer2011) raise the possibility of new techniques, such as being able to determine which bone surfaces show evidence of Sharpey's fibres that would permit methods of discerning muscle scars without reference to phylogenetic information. Such techniques might affect the results given here.
Terminology. The muscle terminology used is that of Iordansky (Reference Iordansky1964) for crocodilians, but with slight modifications. The present author was unable to distinguish between the M. adductor mandibulae externus superficialis and the M. adductor mandibulae externus medialis in dissections of Alligator mississippiensis and Paleosuchus trigonatus. Nor do these muscles appear to have separate origin and insertion scars in Sebecus. This difficulty in discerning these muscles appears to be of more general occurrence; the M. add. mand. ext. sup. and M. add. mand. ext. med. fuse and are difficult to distinguish in lizards (Oelrich Reference Oelrich1956; Fisher & Tanner Reference Fisher and Tanner1970) and appear not to have had separate attachment scars in Tyrannosaurus rex Osborn, Reference Osborn1905 (Molnar Reference Molnar, Larson and Carpenter2008) or other derived tyrannosaurs (see also comments of Holliday Reference Holliday2009). Hence, this muscle will be referred to as the M. adductor mandibulae externus superficialis et medialis. Busbey (Reference Busbey1989), however, was able to distinguish the M. add. mand. ext. sup. and M. add. mand. ext. med. in A. mississippiensis, and Holliday & Witmer (Reference Holliday and Witmer2009) also reported distinguishing these muscles. The two portions of the M. pseudotemporalis (Holliday & Witmer Reference Holliday and Witmer2009) are not distinguished here, and Holliday (Reference Holliday2009) notes that the M. pseudotemporalis profundus is vestigial in crocodylians, so ‘M. pseudotemporalis’ as used here is likely the M. pseudotemporalis superficialis. Tsai & Holliday (Reference Tsai and Holliday2011) argue that the M. pseudotemporalis superficialis and M. intramandibularis are actually two parts of the same muscle with the cartilago transiliens a sesamoid, an interpretation adopted here. Because of the incomplete preservation of the known skulls of Sebecus (the pterygoid is incomplete or not preserved) it seems superfluous to distinguish between the pterygoideii A, B, and C, and this group is referred to as the M. pterygoideus ventralis. The pterygoideus D, is referred to as the M. pterygoideus dorsalis. In modern crocodilians, both Holliday & Witmer (Reference Holliday and Witmer2007) and Bona & Desojo (Reference Bona and Desojo2011) distinguished only these two portions.
The terminology used here for the jaw adductors is (abbreviations used follow the full name):
M. adductor mandibulae externus
M. adductor mandibulae externus superficialis et medialis
(M. add. mand. ext. sup. med.)
M. adductor mandibulae externus profundus (M. add. mand. ext. prof.)
M. adductor mandibulae posterior (M. add. mand. post.)
M. adductor mandibulae internus
M. pseudotemporalis (and M. intramandibularis)
M. pterygoideus dorsalis
M. pterygoideus ventralis.
Iordansky (Reference Iordansky1964) published a thorough description of the jaw musculature and the associated tendons of the Crocodylia, later extended by Schumacher (Reference Schumacher, Gans and Parsons1973). For this study of a fossil form, the present author found it practical to follow Iordansky's terminology with the exception of Lakjer's (Reference Lakjer1926) term ‘zwischensehne’.
2. Reconstruction of tendons and comparison with Alligator mississippiensis
The tendinous structure of the adductors of Alligator mississippiensis is shown in Figures 1 and 2 and described by Iordansky (Reference Iordansky1964), Schumacher (Reference Schumacher, Gans and Parsons1973), Busbey (Reference Busbey1989), Shimada et al. (Reference Shimada, Sato and Ezure1993) and Iordansky (Reference Iordansky2000).

Figure 1 Adductor tendons of Alligator mississippiensis Daudin, Reference Daudin1802: (a) ventro-lateral view of posterior part of right side of skull showing locations of A-, B-, and ls-tendons (A, B and LS respectively); (b) Lateral view of right side of skull and mandible, shown as transparent, showing position and orientation of the zwischensehne; (c) ventro-medial view of posterior third of left mandible showing pterygoideus ventralis muscle mass in dashed outline (PP) and semi-ring and U-tendons (SRT and U respectively); (d) medial view of posterior part of left mandible showing location of X-tendon (X). Abbreviations: A= A-tendon; ART=articular; AZ=anterior portion of the main sheet of the zwischensehne; B=B-tendon; CT=cartilago transiliens; IZ=lateral sheet of the zwischensehne embedded within the intramandibularis; LS=ls-tendon; MG=mandibular glenoid; PP=pterygoideus ventralis; PW=pterygoid wing; PZ=posterior portion of the main sheet of the zwischensehne; SRT=Semi-ring tendon; TZ=strap tendon within pseudotemporalis attaching to zwischensehne; U=U-tendon; V=trigeminal foramen; X=X-tendon.

Figure 2 Position and orientation of the zwischensehne (main shaded area) in Alligator mississippiensis Daudin, Reference Daudin1802: (a) ventral view of right posterior quarter of skull and mandible showing zwischensehne. Posterior part of palate shown as if transparent; (b) posterior view of right side of skull and mandible showing zwischensehne. Skull and mandible shown as if transparent. Abbreviations: C=choanae; CT=cartilago transiliens; FM=foramen magnum; IZ=lateral sheet of the zwischensehne within the intramandibularis; TZ=strap tendon within pseudotemporalis attaching to zwischensehne; Z=main sheet of zwischensehne.
The ridges of insertions for the A- and B-tendons are clearly marked on the quadrate of Sebecus icaeorhinus in approximately the same regions as in living forms (Fig. 3b), but the ridge of the A-tendon is more pronounced. The ridge of the ls-tendon (Fig. 3b), is small and confluent with that of the A-tendon, as in adult Alligator mississippiensis (but not in the juvenile examples examined). These ridges were identified by analogy with those of A. mississippiensis.

Figure 3 Ventral view of left quadratojugal, jugal and exoccipital of Sebecus icaeorhinus Simpson, Reference Simpson1937 (b) compared with that region of the skull of Alligator mississippiensis Daudin, Reference Daudin1802 (a), showing ridges of origin of A-, B- and ls-tendons. Inset in (d) shows position of these elements in the skull of S. icaeorhinus. The excavation for the area of origin of the adductor mandibulae posterior is clearly visible in (b) just posterior to the ridge for attachment of the A-tendon. Features of skull of (c) A. mississippiensis and (d) S. icaeorhinus. Abbreviations: A=ridge of attachment for A-tendon; B=ridge of attachment for B-tendon; CAN=opening of canal in ridge for attachment of A-tendon (see text); EPT=epipterygoid; EX=excavation in quadrate referred to in text; J=jugal; LS=ridge of attachment for ls-tendon; LTF=laterotemporal fenestra; O=orbit; PT=pterygoid; Q=quadrate; QJ=quadratojugal; STF=supratemporal fenestra.
The only preserved element of the mandible of Sebecus that contacted the zwischensehne is a portion of the angular that bears the ventral margin of the Meckelian fossa. The inner rim of the trough bears an obliquely-angled V-shaped crest (Fig. 4c). This is presumably homologous with the shallow sigmoid ‘knob’ of the medial rim in A. mississippiensis (Fig. 4a, b), which marks the posteriormost insertion of the main sheet of the zwischensehne. This crest (Fig. 4c) is neither knoblike nor as pitted as in adult A. mississippiensis, and is more anteriorly placed. None of the areas in which the U-tendon inserted are preserved. The main sheet of zwischensehne and the cartilago transiliens are reconstructed by analogy with A. mississippiensis and Paleosuchus trigonatus. Although these tendons are believed to be homologous in all three taxa, the difference in form and relative position of the crest of S. icaeorhinus from the ‘knob’ of living crocodilians and the dependence of the reconstruction of the tendons on that of the muscles, initially indicated reconstruction by analogy, rather than homology, in the interest of minimising the number of assumptions. This is consistent with the caveats of Holliday & Witmer (Reference Holliday and Witmer2007) to recognising homology in extinct forms.

Figure 4 Medial view of posterior part of left dentary and angular in articulation of Alligator mississippiensis Daudin, Reference Daudin1802 in (a) dorso-medial and (b) medial views compared with that of Sebecus icaeorhinus Simpson, Reference Simpson1937 (c) (medial view, reversed), showing knob (A. mississippiensis) and crest (S. icaeorhinus) on angular for attachment on zwischensehne. Inset in (c) shows position of the elements figured in the mandible. Abbreviation: ANG=angular; ART=articular; C=coronoid; D=dentary; SPL=splenial; SRA=surangular; ZA=ridge of attachment of zwischensehne. Scale bars=10 mm.
3. Reconstruction of the jaw musculature of Sebecus icaeorhinus and comparison with those of Alligator mississippiensis and Paleosuchus trigonatus
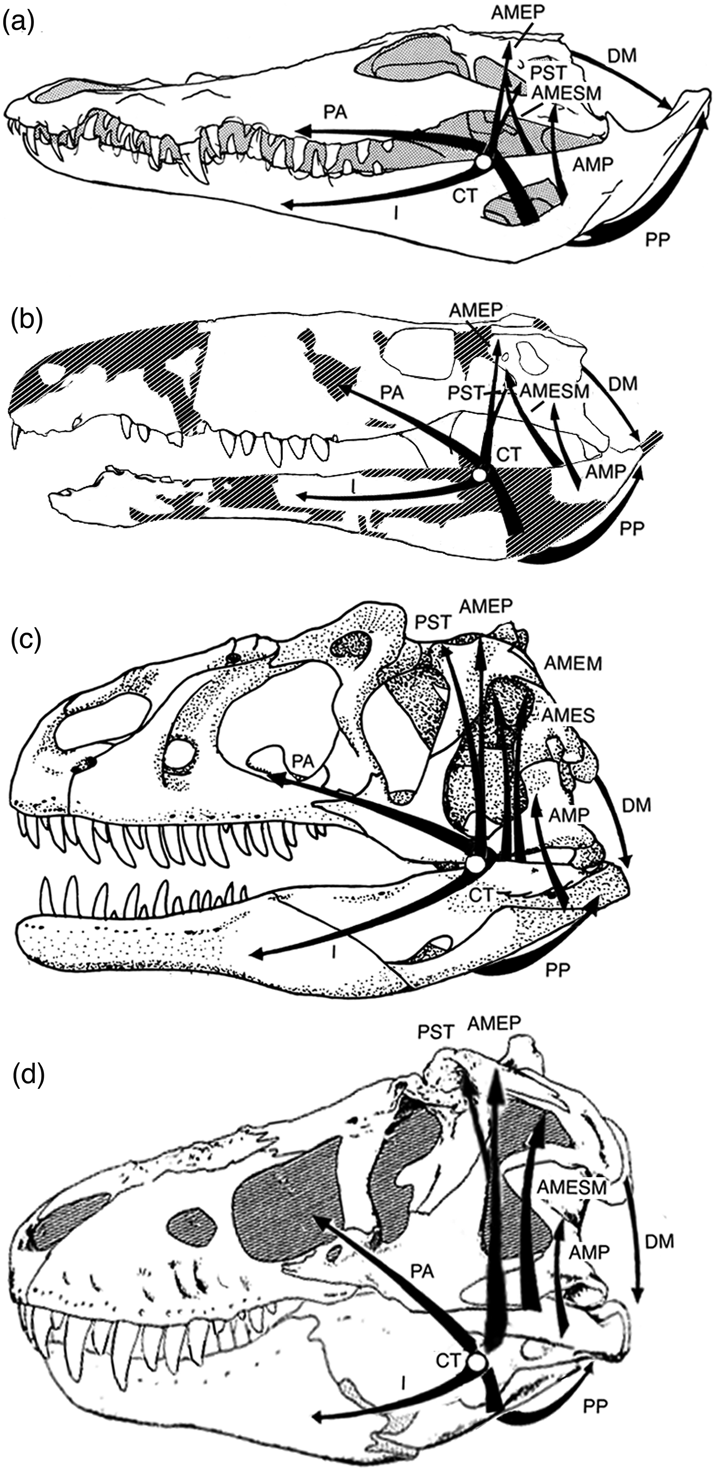
A reconstruction of the cranial and some cervical muscles was carried out by Colbert (Reference Colbert1946), in which the muscles were reconstructed largely by analogy with existing relatives. This study was based on those of Lakjer (Reference Lakjer1926) and Anderson (Reference Anderson1936), thus prior to the new results and insights into the tendinous structures of Iordansky (Reference Iordansky1964). Locations and lines of action of the muscles reconstructed here are given in Figure 5. Images of the attachment areas of the muscles are presented in duplicate in the figures, with and without hatching, because the hatching obscures the form of the attachment areas. Form is critical, with surface texture, to distinguishing muscle scars from surrounding bony surfaces.

Figure 5 Outline drawing of skull and jaws of Sebecus icaeorhinus Simpson, Reference Simpson1937 in lateral view, showing reconstructed positions and lines of action of the jaw muscles. Restored areas indicated by hatching. Abbreviations: AMEP=adductor mandibulae externus profundus; AMESM=adductor mandibulae externus superficialis et medialis; AMP=adductor mandibulae posterior; CT=cartilago transiliens; DM=depressor mandibulae; I=intramandibularis; PA=pterygoideus dorsalis; PP=pterygoideus ventralis; PST=pseudotemporalis.
3.1. M. adductor mandibulae externus superficialis et medialis
This muscle, in both Alligator mississippiensis (Fig. 6a) and Paleosuchus trigonatus, originates from the ventral face of the quadratojugal just above the dorsal edge of the lateral temporal fenestra, with a portion arising from the posterior wall of the channel leading dorsally to the superior temporal fenestra. The area of origin of the latter portion is delimited dorsally by a distinct ridge on the quadrate. The corresponding area in Sebecus icaeorhinus (Fig. 6b) is bounded medially by an antero-posterior ridge along the ventral surface of the quadrate, carrying a canal of uncertain function (Fig. 3d), that opens posteriorly. This sharp ridge, bounding about two-thirds of the presumed area of origin of this muscle, probably served as the origin of the A-tendon. It is confluent with a low ridge that extends posteriorly towards the jaw articulation. There is no noticeable difference in surface texture of the bone from the anterior edge of the lateral temporal fenestra almost to the articulation, hence the muscle probably originated from this whole area as it does in the living forms.

Figure 6 Ventral view of left quadratojugal, jugal and exoccipital of Sebecus icaeorhinus Simpson, Reference Simpson1937 (b) compared with that region of the skull of Alligator mississippiensis Daudin, Reference Daudin1802 (a), showing regions of origin (white hatching) of adductor mandibulae posterior and adductor mandibulae externus superficialis et medialis. Inset in (b) shows position of these elements in the skull of S. icaeorhinus. Abbreviations: AMESM=area of origin of adductor mandibulae externus superficialis et medialis; AMP=area of origin of adductor mandibulae posterior.
The add. mand. ext. sup. med. inserts onto the flat dorsal face of the surangular (Fig. 7) in the two living forms examined. In A. mississippiensis, some of the fibres ‘spill over’ to insert on the internal surface of the surangular, in P. trigonatus they do not. Somewhat less than the posterior half of the surangular of the holotype of Sebecus is preserved and this does not have a comparable facet, but instead the dorsal edge is a ridge. The muscle may have inserted on the sides of this dorsal ridge, or further anteriorly than the part preserved. MMP 235 includes the more anterior part of the surangular, that appears to show a flattened facet along its dorsal edge (Gasparini Reference Gasparini1972, lam. 1B), suggesting that the muscle may have attached along much of the dorsal margin anterior to the articular glenoid.

Figure 7 Dorsal view of posterior half of right mandible of Alligator mississippiensis Daudin, Reference Daudin1802 showing area of attachment (white hatching) of adductor mandibulae externus superficialis et medialis: (a) position of attachment; (b) flat surface for insertion. Note that the angular was broken in this specimen. Abbreviations: AMESM=area of origin of adductor mandibulae externus superficialis et medialis; ART=articular; SRA=surangular.
This muscle in Sebecus was reconstructed from preserved muscle scars.
3.2. M. adductor mandibulae externus profundus
The adductor mandibulae externus profundus (Figs 8, 9d) originates from around and within the superior temporal fossa, from the squamosal, postorbital, and quadrate, with a small portion arising from the lateral surface of the laterosphenoid. A relatively large portion of the fibres originated from a posterior parietal-squamosal shelf within the supratemporal fossa (Fig. 8a, c). This region is of similar form and surface texture in Sebecus icaeorhinus, so the muscle presumably arose from the corresponding parts of the same elements. The parietal-squamosal shelf is relatively larger in Sebecus than in Alligator mississippiensis (Fig. 8a, c) or Paleosuchus trigonatus but is smaller than in Crocodylus. As Colbert (Reference Colbert1946) noted, the postero-lateral borders of the fenestra rise above the general level of the skull roof. Taken together, the relatively large internal shelf and the elevated borders form a relatively larger area of origin than is present in either A. mississippiensis or P. trigonatus.

Figure 8 Dorsal view of posterior third of skull of Alligator mississippiensis Daudin, Reference Daudin1802 (a, b), and skull table and quadrate-squamosal-exoccipital piece of Sebecus icaeorhinus Simpson, Reference Simpson1937 (c, d) and showing area of origin (white hatching) of adductor mandibulae externus profundus from the parietal-squamosal shelf within the superior temporal fossa. The gap between the squamosal and the parietals in S. icaeorhinus resulted from breakage of the dorsal surface of the squamosal. The images are scaled to show equal width of the skull roof. Inset in (d) shows position of these elements in the skull of S. icaeorhinus. (e) Section through postorbital region of skull of generalised alligatorid showing position of shelf within supratemporal fossa from which add. mand. ext. prof. takes origin in part. Section at line in (b), endocranial cavity not shown. Abbreviations: AMEP=dorsal part of area of origin of adductor mandibulae externus profundus; F=frontal; J=jugal; O=orbit; P=parietal; PW=pterygoid wing; Q=quadrate; QJ=quadratojugal; SH=dorsal shelf within supratemporal fossa; SQ=squamosal; V=trigeminal foramen.

Figure 9 (a, b) Lateral view of quadrate-squamosal-exoccipital piece in articulation with skull roof and laterosphenoid of Sebecus icaeorhinus Simpson, Reference Simpson1937, showing areas of origin (white hatching) of the adductor mandibulae posterior and pseudotemporalis are shown. (c, d) Ventro-lateral view of skull of Alligator mississippiensis Daudin, Reference Daudin1802 showing areas of origin of adductor mandibulae posterior and pseudotemporalis for comparison. Area of origin of adductor mandibulae externus profundus also shown. Abbreviations: AMEP=area of origin within channel to supratemporal fossa of adductor mandibulae externus profundus; AMP=area of origin of adductor mandibulae posterior; EPT=epipterygoid; F=frontal; P=parietal; PS=area of origin of pseudotemporalis; PT=pterygoid; Q=quadrate; SQ=squamosal; V=trigeminal foramen.
In both A. mississippiensis and P. trigonatus, and hence presumably also in Sebecus, the add. mand. ext. prof. inserts both bipinnately into a tendon originating from the zwischensehne, and directly into the zwischensehne itself. Both Lakjer (Reference Lakjer1926) and Anderson (Reference Anderson1936) report that in living forms this muscle has a slip that inserts upon the mandible, however my dissections confirm Iordansky's (Reference Iordansky1964) observation that this slip is actually a portion of the adductor mandibulae posterior.
The add. mand. ext. prof. was reconstructed from the identification of the origo area and by homology with the condition in living taxa (cf. Holliday & Witmer Reference Holliday and Witmer2009).
3.3. M. adductor mandibulae posterior
In Alligator mississippiensis and Paleosuchus trigonatus, this muscle originates from the ventral surface of the quadrate posterior to the ridge for the ls-tendon and behind the trigeminal foramen, and also from the B- and internal surface of the A-tendon (Figs 6, 9). In Sebecus icaeorhinus the ridge interpreted as that of the origin of the A-tendon lies between the presumed areas of origin of the add. mand. ext. sup. med. and the add. mand. post. In modern crocodilians, this muscle includes within its body the B-tendon and is immediately adjacent to the A-tendon. By analogy in Sebecus, the add. mand. post. probably also arose in part from the internal surface of the A-tendon. On the ventro-anterior surface of the quadrate, immediately dorsal to the jaw articulation, there is a distinct elliptical excavation in the surface of the bone, about 25 mm long in a longitudinal direction, within the supposed area of origin of the add. mand. post. (Fig. 3d). Ostrom (Reference Ostrom1961) considered similar excavations of the quadrates of hadrosaurian dinosaurs as the complete areas of origin of the add. mand. post. Because the ridges of origin of these two tendons that partly bound the area of origin are far removed from the excavation in Sebecus, it is presumed here that the area of origin was probably somewhat larger than just the excavation. The function of the excavation is unknown.
In living forms, the add mand. post. inserts onto the ventral border of the Meckelian fossa of the angular and the anterior face of the articular. Also, it inserts onto the X-tendon and the zwischensehne. In Sebecus, the anterior face of the articular is smoothly concave (Fig. 10), consistent with a muscle attachment, so the muscle presumably inserted in a similar fashion.

Figure 10 Right articular of Sebecus icaeorhinus Simpson, Reference Simpson1937 showing concave anterior face for insertion of adductor mandibulae posterior and pterygoideus dorsalis: (a) anterior view; (b) antero-medial view. Abbreviations: AMP=area of attachment of adductor mandibulae posterior and pterygoideus dorsalis; MG=mandibular glenoid; RAP=retroarticular process.
In Sebecus, the add. mand. post. was reconstructed from the identification of the area of origin and by homology with the condition in living taxa.
3.4. M. pseudotemporalis
In both Alligator mississippiensis and Paleosuchus trigonatus, the pseudotemporalis arises from the ventrolateral surface of the laterosphenoid, just anterior to the trigeminal foramen, and from the external surface of the adjacent medial ascending portion of the pterygoid, antero-ventral to the trigeminal foramen (Fig. 9c, d). This portion of the pterygoid is not preserved or not accessible in specimens of Sebecus icaeorhinus; the laterosphenoid is, however, and the corresponding portion bears a slight excavation similar to those marking the origin of the pseudotemporalis in A. mississippiensis and P. trigonatus (Fig. 9b). Thus the pseudotemporalis probably had the same origin in all three genera. Holliday & Witmer (Reference Holliday and Witmer2009) found that the origin of the pseudotemporalis superficialis altered from the anterior part of the supratemporal fossa to the laterosphenoid sometime during mesoeucrocodylian evolution. Holliday & Gardner (Reference Holliday and Gardner2012) regard thalattosuchians as having attachments of several muscles within the supratemporal fossa, and find thalattosuchians more derived than Sebecus, as do Pol et al. (Reference Pol, Leardi, Lecuona and Krause2012). Thus, it is possible that the pseudotemporalis also took origin from the well-developed rostral shelf within the supratemporal fossa of S. icaeorhinus.
In the living forms, as presumably in Sebecus, the pseudotemporalis inserts onto the dorsal surface of the zwischensehne at the cartilago transiliens.
The intramandibularis portion (Fig. 5) extends from the tendon arising from the cartilago transiliens at the lateral edge of the zwischensehne and attaches to the internal surface of the Meckelian canal of the jaw. The relevant portions of the jaws of the AMNH specimens of Sebecus are very imperfectly preserved and the bone is much thinner than the corresponding bone of living forms. Presumably Sebecus had an intramandibularis, but nothing further may be deduced about it.
As with the previous two muscles, this muscle was reconstructed from the identification of the origo area and by homology with the condition in living taxa
3.5. M. pterygoideus dorsalis
This muscle (Fig. 5) originated from the internal surface of the maxilla, the dorsal surface of the palatine and the pterygoid, the posterior surface of the connective tissue partition separating the orbit from the nasal capsule, and from the interorbital septum in both A. mississippiensis and P. trigonatus. In Sebecus icaeorhinus, the origin was probably similar; the internal surfaces of the maxilla, palatine and pterygoid are smooth, consistent with having given origin to a muscle. The palatine bears a thin partition on the dorsal surface, extending diagonally in an antero-lateral-postero-medial direction, which probably served to separate the muscle external to the partition from the nasal passage or capsule medial to the partition. The relatively greater height of the maxilla in Sebecus potentially provided a relatively greater origo area than is present in living crocodilians.
This muscle in modern forms and, hence, presumably in Sebecus, inserts chiefly onto the zwischensehne, and to a lesser extent fuses with the add. mand. post. and the pterygoideus ventralis to insert onto the anterior face of the articular. In Sebecus this face is strongly concave (Fig. 10), unlike that of living forms, and thus potentially provided a somewhat larger area for insertion.
This muscle was reconstructed partly by homology with the condition in living forms and partly from identification of presumed attachment areas in Sebecus.
3.6. M. pterygoideus ventralis
The pterygoideus ventralis (Fig. 5) originates from the posterior edge of the pterygoid and the ectopterygoid. Neither area is preserved in the specimens of Sebecus icaeorhinus.
The muscle inserts onto the postero-medial surface of the lower jaw, extending onto the external surface in Alligator mississippiensis and Crocodylus spp., but not in Paleosuchus trigonatus. In Sebecus, most of the articular and parts of the surangular and angular are known, and have smooth surfaces, so it is likely that the muscle inserted on these bones as in A. mississippiensis. It probably extended slightly onto the external surface of the surangular.
The pterygoideus ventralis was reconstructed by homology with the condition in living forms.
3.7. M. depressor mandibulae
In both Alligator mississippiensis and Paleosuchus trigonatus, the depressor mandibulae takes its origin from the ventral portion of the posterior surface of the squamosal, the postero-lateral corner of the parietal, and from along the external edge of the exoccipital ventral to the exoccipital-quadrate contact (Fig. 11c). The area of origin is broad, flat and delimited by low ridges. A. mississippiensis has a tendon that originates from the externo-ventral corner of the exoccipital and extends approximately two-thirds of the distance to the insertion, from which fibres originate in a bipinnate fashion. The posterior surface of the squamosal of Sebecus icaeorhinus bears a low ridge running from the externo-ventral portion of the surface to the medio-dorsal portion. This ridge is approximately in the same position as that delimiting the lower part of the depressor mandibulae attachment in A. mississippiensis and, hence, likely marks the ventral border of the area of origin in Sebecus. The extreme lateral part of the posterior surface of the exoccipital is pitted, as in living crocodilians, and presumably forms the rest of the area of origin of this muscle. The area of origin is about twice as large relative to the area of the posterior surface of the skull in Sebecus as in A. mississippiensis (Fig. 11a, c) and P. trigonatus.

Figure 11 (a, b) Posterior view of quadrate-squamosal-exoccipital piece of Sebecus icaeorhinus Simpson, Reference Simpson1937 showing area of origin (white hatching) of depressor mandibulae. (c, d) Posterior view of skull of Alligator mississippiensis Daudin, Reference Daudin1802 showing the same region for comparison with that of S. icaeorhinus. (a, c) Area of origin. (b, d) Facet for origin. The images are scaled to show equal width of the skull roof. Abbreviations: BO=basioccipital; EO=exoccipital; FM=foramen magnum; Q=quadrate; SO=supraoccipital; SQ=squamosal.
The depressor mandibulae in A. mississippiensis and P. trigonatus inserts over the entire dorsal surface of the retroarticular process, on both the articular and surangular portions. The anterior part of the process of the surangular of Sebecus, bears a distinct facet for the insertion of the depressor (Fig. 12a, b). The smooth dorsal face of the articular is also similar to those in modern forms, hence, the depressor probably inserted over much, or all, of the dorsal surface of the retroarticular process as in living forms.

Figure 12 (a, b) Dorsal view of left surangular and articular of Sebecus icaeorhinus Simpson, Reference Simpson1937 showing part of facet for insertion of depressor mandibulae, and medial inclination of the lateral margin of the retroarticular process. (c, d) Dorsal view of posterior portion of mandible and retroarticular process of Alligator mississippiensis Daudin, Reference Daudin1802 showing facet for insertion of depressor mandibulae. In (a, b), the surangular has been reversed photographically. Abbreviations: ART=articular; SRA=surangular.
The depressor mandibulae of Sebecus was reconstructed from the preserved muscle scars.
4. Functional analysis of the jaw musculature
4.1. Lever arm of the muscles of Sebecus compared with those of Crocodylus
Given the ginglymous form of the cranio-mandibular joint, the mandibles of Sebecus are taken to have rotated rigidly about those joints as in modern crocodilians, but not as in all extinct forms (e.g. Ösi & Weishampel Reference Ösi and Weishampel2009). Thus, the strength of the ‘forces’ exerted by the teeth and the ability to handle food depends on the rotational analogue of force, termed ‘torque’ in physics or ‘moment’ in engineering and biomechanics. The magnitude of torque or moment is proportional to the perpendicular distance between the line of action of the moment and the center of rotation, here the jaw joint. This distance is the lever arm or moment arm (for more on this see, e.g. Fowles Reference Fowles1962; Hendricks et al. Reference Hendricks, Subramony and van Blerk1999; Kane & Levinson Reference Kane and Levinson2005). For a given jaw adductor strength, the greater its lever arm, the more moment may be exerted in closing the jaws or the faster the jaws may be closed.
The results of these measurements are presented in a series of graphs (Fig. 13). The measurements from Sebecus icaeorhinus have been corrected to correspond to a skull equal in length to the Crocodylus niloticus skull used. Each muscle is considered individually with reference to its function. Given that opening the jaw was a simple rotation, its rotational or angular acceleration is the result of an imposed moment, just as linear acceleration is the result of an imposed force. Since the jaw is analogous to a lever with the fulcrum at the cranio-mandibular joint, the force generated at any point along the mandible multiplied by its lever arm is equal to the sum of the products of the forces exerted by the adductors each multiplied by its respective lever arm. If the forces generated by the muscles are equal, the contribution of each to the moment will depend only upon the lever arm. Since the forces generated by a muscle of an extinct organism cannot be directly determined, one way to approach an understanding of its contribution to the moment is by considering its lever arm. This, however, gives only a rough approximation without some idea of the relative strengths of the muscles. In part, because of the incompleteness of the specimens, attempts at assessing the adductor strengths (as, for example, in van Drongelen & Dullemeijer Reference Van Drongelen and Dullemeijer1982; Wroe et al. Reference Wroe, McHenry and Thomason2005) were not carried out.

Figure 13 Graphs of lever arm against gape for Sebecus icaeorhinus Simpson, Reference Simpson1937 and Crocodylus niloticus Schneider, Reference Schneider1801. Abscissa is gape in degrees and ordinate is lever arm in centimetres: (a) adductor mandibulae externus superficialis et medialis. Note that the ordinate is to a different scale from other graphs here; (b) adductor mandibulae externus profundus; (c) pseudotemporalis; (d) adductor mandibulae posterior; (e) pterygoideus dorsalis; (f) depressor mandibulae.
The vertical distance to which the jaw may be depressed is largely determined by two factors: the amount of rotation possible at the jaw joint (in turn determined by the forms of the joint surfaces of the involved elements and by the ligaments and joint capsule present at the joint), and the amount by which the jaw adductors can be extended beyond their rest length (that obtained when the mouth is closed). Although ligaments and joint capsule were doubtless present, the amount by which they restricted movement cannot be directly determined. The articulation is ginglymous, permitting only a rotational movement in the vertical plane, as in living crocodilians.
Immediately posterior to the glenoid, there is a strong crest or lip on the surangular, inclined anteriorly at an angle of approximately 30° (Colbert Reference Colbert1946, fig. 19). This crest presumably would have limited the gape. The orientation of the crest with respect both to the rest of the mandible and the quadrate region of the skull is unknown, because the surangular is incomplete (Colbert Reference Colbert1946). Thus the angles of opening of the jaw for estimating the lever arm were limited to 40°.
4.2. M. adductor mandibulae externus superficialis et medialis
The lever arm of this muscle is about 14% greater in Sebecus icaeorhinus than in Crocodylus niloticus at all gapes measured (Fig. 13a).
4.3. Mm. pseudotemporalis, adductor mandibulae externus profundus and pterygoideus dorsalis
These muscles (Fig. 13c, b, e) are discussed together as all insert chiefly or wholly upon the zwischensehne. The muscles do not have the same lines of action, so the contraction of one will alter the lever arms of the others (with the exception of the pars intramandibularis that affects the lever arms of the other muscles, but because of its location within the mandible, would apparently have its lever arm unaffected by their actions).
For example, in Crocodylus niloticus measurements were made of the lever arms of the pseudotemporalis and the add. mand. ext. prof. The zwischensehne was simulated with a data input punch (Hollerith) card then used in mainframe computers, that was first appressed to the dorsal face of the pterygoid wing, as if the pterygoideus dorsalis was contracted. It was then allowed to rise with the simulated contractions of the pseudotemporalis and the add. mand. ext. prof., as would occur when the pterygoideus dorsalis were relaxed. The pseudotemporalis, which is attached to the main sheet of the zwischensehne by the cartilago transiliens, comes to the same position in both cases as the jaw is opened and, hence, has the same lever arm in either situation when the gape in large. When the gape is about 20°, the position of the main sheet can change the lever arm by about 10% of its maximum value. The add. mand. ext prof. inserts upon the zwischensehne and apparently may occupy more than one position when the jaw is opened to 40°; the difference in lever arm between the extreme positions measured can be approximately 10% of the maximum lever arm. The minimum lever arm occurs when the sheet of the zwischensehne is allowed to rise with the contraction of the pseudotemporalis and add. mand. ext. prof. This minimum is greater in Sebecus icaeorhinus for the pseudotemporalis, add. mand. ext. prof. and pterygoideus dorsalis than in C. niloticus. Van Drongelen & Dullemeijer (Reference Van Drongelen and Dullemeijer1982) observed the position of the cartilago transiliens in Caiman crocodilus with X-ray photography. They found the cartilago, and by inference the attached zwischensehne, could be positioned so as to ‘lock’ the mandible in an open or closed position, as hypothesised by Iordansky (Reference Iordansky1964). Thus, whether or not such ‘locking’ occurred in Sebecus icaeorhinus, it seems reasonable to assume that the position of the cartilago transiliens and the zwischensehne could be altered by muscular action, as in C. crocodilus.
The portion of the pterygoideus dorsalis that inserts into the jaw consists of fibres parallel to those in sheet of the zwischensehne (upon which most of the fibres insert). Therefore, the direction of the main sheet relative to the jaw is taken as the direction of action of the pterygoideus dorsalis. The ridge for insertion of the zwischensehne onto the angular is relatively more anterior in Sebecus than in C. niloticus, and this apparently has the result that when the jaws are closed, the pterygoideus dorsalis lever arm in Sebecus is about 30% less than in C. niloticus. But as the jaw is opened, the lever arm becomes slightly greater than in C. niloticus as the pseudotemporalis and the add. mand. ext. prof. are relaxed. In both genera, the pseudotemporalis and add. mand. ext. prof. pull the zwischensehne dorsally and slightly posteriorly and hence decrease its lever arm.
4.4. M. adductor mandibulae posterior
This is the only muscle in Sebecus that has a lever arm less than in Crocodylus niloticus. The lever arm of this muscle in Sebecus icaeorhinus is about 80% that of C. niloticus for all angles measured (Fig. 13d).
4.5. M. pterygoideus ventralis
The lever arm of this muscle was not estimated for Sebecus icaeorhinus since none of the areas of attachment are preserved.
4.6. M. depressor mandibulae
The lever arm of this muscle is about 10% greater in Sebecus icaeorhinus than in Crocodylus niloticus, at angles of 10° and greater (Fig. 13f).
5. Measurements of muscle length and extension
Length was measured and extension calculated for the adductor mandibulae externus superficialis et medialis, adductor mandibulae externus profundus, adductor mandibulae posterior, pseudotemporalis and depressor mandibulae. These muscles, with the exception of the depressor, all have relatively greater rest length in Sebecus icaeorhinus than in Crocodylus niloticus. The rest length and contracted length at various angles of jaw opening of the depressor mandibulae are about the same in the two genera. Because of the incomplete preservation of AMNH 3160, measurements were not feasible for the other jaw muscles.
With the exception of the add. mand. ext. prof. (Fig. 14b), the contracted lengths of the various muscles are greater in Sebecus than in C. niloticus. This may be the result of the slightly greater relative height of the skull in Sebecus than in Crocodylus (Fig. 15a, b). The contracted length of the add. mand. ext. prof. of C. niloticus tends to approach that of Sebecus as the jaw is opened even when the main sheet of the zwischensehne is depressed.

Figure 14 Graphs of muscle length against gape for Sebecus icaeorhinus Simpson, Reference Simpson1937 and Crocodylus niloticus Schneider, Reference Schneider1801. Abscissa is gape in degrees and ordinate is estimated muscle length in centimetres: (a) adductor mandibulae externus superficialis et medialis; (b) adductor mandibulae externus profundus; (c) pseudotemporalis; (d) adductor mandibulae posterior; (e) depressor mandibulae.

Figure 15 Comparison of reconstructed positions and lines of action of jaw musculatures of Sebecus icaeorhinus (b), Allosaurus fragilis (c) and Tyrannosaurus rex (d) with those of Crocodylus (a). Images scaled to equal premaxilla-to-quadrate condyle length. The existence of a cartilago transiliens in the theropods is uncertain. Abbreviations: AMEM=adductor mandibulae externus medialis; AMEP=adductor mandibulae externus profundus; AMES=adductor mandibulae externus superficialis; AMESM=adductor mandibulae externus superficialis et medialis; AMP=adductor mandibulae posterior; CT=cartilago transiliens; DM=depressor mandibulae; I=pars intramandibularis; PA=pterygoideus dorsalis; PP=pterygoideus ventralis; PST=pseudotemporalis. Not to scale. (Note: (a) modified after Schumacher Reference Schumacher, Gans and Parsons1973; (b) modified after Molnar Reference Molnar2010; (d) modified after Osborn Reference Osborn1912).
The percentage extension data show that for gapes of approximately 30° and 40°, the muscles of Sebecus exhibit less percentage extension than those of C. niloticus. Hence, if the maximum percentage extension were the same in both, Sebecus would have a slightly greater gape.
6. Functional aspects of the skull morphology
The relatively greater length of the muscles of Sebecus icaeorhinus over those of Crocodylus niloticus probably correlates with the relatively greater height of the skull in Sebecus (Fig. 15a, b), as well as an apparently more elongate quadrate (Fig. 3a, b). Some attachment areas were relatively larger in Sebecus than in Alligator mississippiensis. The origo area of the adductor mandibulae externus profundus in the dorsal portion of the supratemporal fossa was relatively larger than that of A. mississippiensis (Fig. 8a, c). Furthermore, as recognised by Colbert (Reference Colbert1946), the margins of the fossa are elevated and, hence, would have afforded more area for attachment of muscle fibres. However, the area of that part of the origin within the channel leading to the fenestra could not be assessed, so it seems likely that the total area of origin was larger than in A. mississippiensis, but this has not been conclusively demonstrated. The elevated snout (Fig. 15a, b) implies that more area was available for attachment of the pterygoideus dorsalis, but in the absence of any clear indication of the origo site, this, too, cannot be demonstrated. The insertion area for the pterygoid muscles and the adductor mandibulae posterior on the anterior face of the articular is concave (Fig. 10), whereas in the modern specimens examined, it was nearly planar. For equal perimeters, the area of a concave surface is greater than that of a more nearly planar surface, hence the potential here for attachment of relatively more muscle fibres. Furthermore, the area of origin of the depressor mandibulae was also relatively greater in Sebecus than in A. mississippiensis (Fig. 11a, c). On the other hand, the attachment areas of the adductor mandibulae externus superficialis et medialis were not relatively larger in Sebecus than in the modern forms examined, and other attachment areas could not be assessed. Taken together, these considerations suggest that the adductor muscles of Sebecus were relatively as large, and sometimes larger, than in A. mississippiensis and P. trigonatus, but further work on this point would be welcome. No evidence was found that any of the areas for muscle attachment were relatively smaller than in modern forms.
The jaw articulation of Sebecus is double, in the sense that both the quadrate and quadratojugal are involved in the cranial part of the cranio-mandibular joint (Buffetaut Reference Buffetaut1975). The articulation is strictly ginglymous, permitting only a rotational movement in the vertical plane. The surangular lip at the back of the mandibular glenoid (Colbert Reference Colbert1946, fig. 19) probably acted both to prevent disarticulation of the jaws, as a result of their being drawn forward by the pterygoideus muscles, as well as to limit the amount of gape.
One of the most obvious characteristics of Sebecus is its laterally flattened teeth (Colbert Reference Colbert1946; Langston Reference Langston1965). The teeth of the holotype exhibit no discernible wear, either as facets or rounding of the tip (both seen in theropod teeth). However, only the one specimen was examined, and most teeth are broken at the tip (e.g. Colbert Reference Colbert1946, fig. 21). The teeth came into close approximation when the mouth was shut, as in theropods. Unlike teeth of living crocodilians, which do not appear to have a slicing action, sebecosuchian teeth may have acted as much to cut as to hold (cf. Abler Reference Abler1992; D'Amore Reference D'Amore2009).
The ectopterygoid of C. niloticus makes an angle with the skull of just over 60°, while that of Sebecus apparently made a shallower angle of about 55° (Molnar Reference Molnar2010); the posterior portion of the pterygoid is not known in Sebecus. As the mandible is reconstructed, the crest of insertion of the zwischensehne lies just anterior to the ventral end of the ectopterygoid, unlike conditions in existing crocodilians. Any re-adjustment of the position of the angular with respect to the ectopterygoid to bring this crest posterior to the ectopterygoid, results in displacing the large caniniform tooth of the dentary from its corresponding notch in the premaxilla. The close approximation between the angular and the ectopterygoid when the jaw is closed may, therefore, explain the small size of the crest of insertion of the zwischensehne. Presumably some portion of the main sheet of the zwischensehne itself wrapped anteriorly around the distal end of the pterygoid-ectopterygoid plate to insert on the angular. This permits a relatively more anterior insertion than in C. niloticus and, hence, the greater mechanical efficiency, as well as increasing the area available for the pterygoideus dorsalis and add. mand. post. to insert into the lateral wall of the mandible.
7. Function of the muscles and tendons in Sebecus icaeorhinus
Van Drongelen & Dullemeijer (Reference Van Drongelen and Dullemeijer1982) and Busbey (Reference Busbey1989) discussed the functions of the snout and jaws of Alligator mississippiensis in feeding and other activities, as well as recording the activity of the muscles in feeding. Busbey found, like van Drongelen & Dullemeijer, that all adductors functioned during closing of the mouth and crushing by the jaws.
7.1. M. adductor mandibulae externus superficialis et medialis
Both in Alligator mississippiensis and Paleosuchus trigonatus, and presumably in Sebecus icaeorhinus, the muscle is long, thin and consists of parallel fibres. It was found that the cross section of the muscle in A. mississippiensis was larger only than those of the pseudotemporalis and add. mand. ext. prof., approximately the situation reported by Schumacher (Reference Schumacher, Gans and Parsons1973), but Busbey (Reference Busbey1989) found the cross section to be rather greater. This discrepancy could be a result of individual variation if the specimen dissected (origin not known) was a captive, since those used by Busbey were wild. There seems no reason to suppose this muscle was relatively more powerful in Sebecus than in the specimen studied. Busbey found this adductor to act during adduction and crushing, it probably acted similarly in Sebecus as well. As Gans & Bock (Reference Gans and Bock1965) remark, parallel-fibred muscles are those capable of the greatest extension and this muscle shows the greatest percentage extension at any given gape in both Crocodylus niloticus and Sebecus. Thus, the add. mand. ext. super. med. may have been the critical limiting factor of the gape in these animals, if the gape was not limited by the surangular crest.
7.2. M. adductor mandibulae posterior
The add. mand. post. is complexly pinnate in both Alligator mississippiensis and Paleosuchus trigonatus and contains several tendon sheets, including the B-tendon. The relationships of the ridge for the B-tendon are much the same in A. mississippiensis, P. trigonatus and Sebecus icaeorhinus, so it may be presumed that the structure of the add. mand. post. was also similar. In the living forms, the medial part of this muscle is mostly parallel-fibred, while the lateral portion is ‘tetrapinnate’ with fibres inserting into the B-tendon and linking the B-tendon to two of its subsheets. Thus, in sagittal section a W-shaped pattern of muscle fibres is formed, with tendon sheets at each vertex (Fig. 16). The medial parallel-fibred part inserts onto the ventral portion of the area of insertion, while the ‘tetrapinnate’ portion inserts onto the dorsal part, much nearer the area of origin. The ‘tetrapinnate’ part will generate a greater force with a lesser contraction than a parallel-fibred muscle of similar size (Gans & Bock Reference Gans and Bock1963, Reference Gans and Bock1965), and, thus, is more mechanically efficient in a position close to the articulation than a parallel-fibered muscle. Busbey (Reference Busbey1989) indicated that this muscle functioned to close the jaws, hold them shut (against prey) and may also have acted to prevent dislocation at the jaw joint.

Figure 16 Sketch of a parasagittal section of the adductor mass in Paleosuchus trigonatus, showing the ‘tetrapinnate’ structure of the adductor mandibulae posterior. Curved lines indicate direction of fibres, cross-hatching indicates fibres approximately perpendicular to the page. The pterygoid wing is shown in section. Abbreviations: AMP=adductor mandibulae posterior; AMP/PA=mingled fibres of the adductor mandibulae posterior and pterygoideus dorsalis; B=B-tendon; PA=pterygoideus dorsalis; PP=pterygoideus ventralis (fibres not shown); PST= pseudotemporalis; PW=pterygoid wing (in section).
In Sebecus, this muscle has the lowest lever arm of the jaw muscles and the only one that is lower than any of C. niloticus. This muscle also has a low rate of percentage extension, being extended to only 115% of rest length at a gape of 40° in Sebecus. This muscle appears to have functioned in closing of the mouth, but it would also tend to prevent disarticulation of the jaw joint when the more mechanically efficient adductors close the jaw against resistance. The placement of its line of action close to the jaw articulation would result in the resistance (prey) acting as a fulcrum, and the muscle would then act to rotate the mandible about the resistance so that the mandible would remain in articulation. This muscle in A. mississippiensis has a smaller cross section than either of the two pterygoid muscles, as was presumably the case in Sebecus. Its area of insertion in Sebecus is relatively as large as in A. mississippiensis, hence, the muscle was probably as powerful in Sebecus as in living forms.
7.3. Mm. adductor mandibulae externus profundus, pterygoideus dorsalis and pseudotemporalis
The pseudotemporalis and add. mand. ext. prof. are both parallel-fibred muscles of small cross section in Alligator mississippiensis and Paleosuchus trigonatus, but they are the most mechanically efficient of the adductors. While undoubtedly exerting a pull upon the mandible, they are of smaller cross section and, thus, presumably weaker than the other adductors. As discussed previously, both muscles (including the intramandibularis) can exert an effect on the lever arm of the powerful pterygoideus dorsalis. Taken together, they act as the antagonist for the intramandibularis portion of the pseudotemporalis. The pseudotemporalis and add. mand. ext. prof. rotate the zwischensehne dorso-posteriorly and reduce the lever arm of the pterygoideus dorsalis. The intramandibularis acts in the opposite sense, to rotate the zwischensehne antero-ventrally and increase the lever arm of the pterygoideus dorsalis. The three also act in different directions in the frontal plane in living crocodilians, probably more so than they may have done in Sebecus icaeorhinus because there they are more vertically-orientated in that plane. Busbey (Reference Busbey1989) found these muscles in A. mississippiensis to act during crushing and the adductor mandibulae externus muscles to act in holding prey. The intramandibularis was active both during opening and closing of the mouth, during opening stretching fibres of the add. mand. ext. prof. and pterygoideus dorsalis, and during closing possibly modifying the force or speed of closure. As previously mentioned, van Drongelen & Dullemeijer (1982) found that the position of the cartilago transiliens in Caiman crocodilus could be altered by the attached muscles as suggested here for Sebecus.
The pterygoideus dorsalis is the largest and presumably the most powerful jaw adductor in both the living forms and probably in Sebecus. It has one of the most advantageous lever arms. This muscle is mostly parallel-fibred in modern forms with unipinnate structure where it inserts onto the zwischensehne. The lever arm in living forms is second in magnitude only to that of the add. mand. ext. prof. and decreases with increasing gape at about the same rate. This was likely also true of Sebecus. The pterygoideus dorsalis is the only adductor that apparently does not attain its maximum lever arm when the jaw is shut. The lever arm is greatest in Crocodylus niloticus and Sebecus at a gape of about 20°, owing probably to the effects of the add. mand. ext. prof. and the pseudotemporalis described in the preceding paragraph. These muscles likely acted in Sebecus, much as seen by Busbey (Reference Busbey1989) in A. mississippiensis.
7.4. M. pterygoideus ventralis
This muscle in Alligator mississippiensis and Paleosuchus trigonatus seems to be the second most powerful adductor. This may reasonably be assumed to have been the case also with Sebecus icaeorhinus. But as neither the area of origin nor insertion is known, nothing more can be said, except that Busbey (Reference Busbey1989) found this muscle to adduct the mandible and enhance joint stability in A. mississippiensis. It presumably acted similarly in Sebecus.
7.5. M. depressor mandibulae
This muscle was found to be active by van Drongelen & Dullemeijer (1982) and Busbey (Reference Busbey1989) during closing of the mouth. The former authors also found it active when the mouth was closed, but only rarely when the mouth was being opened. They suggested that the depressor acts to reduce strain during the bite. If Sebecus were fully terrestrial, the mouth could have been opened simply by relaxing the adductors, and reduction of strain at the jaw joint and in adjacent elements may have been the primary function of the depressor.
7.6. Zwischensehne
The chief function of this tendon seems to be to transmit forces generated by the pterygoideus dorsalis to the mandible. It also ties together the pterygoideus dorsalis, adductor mandibulae externus profundus, pseudotemporalis, and pars intramandibularis to allow the last three to adjust the lever arm and direction of pull of the pterygoideus dorsalis.
7.7. A-tendon, B-tendon and ls-tendon
These tendons are associated with the adductor mandibulae posterior. Iordansky (Reference Iordansky1964) interpreted them as allowing a greater number of muscle fibres to exist in the restricted volume between the pterygoideus dorsalis and the jaw (cf. Holliday & Witmer Reference Holliday and Witmer2007). This is a reasonable interpretation of their function in Sebecus icaeorhinus.
7.8. U-tendon, X-tendon and Semi-ring tendon
The areas of attachment for the U-, X-, and Semi-ring tendons are so incompletely known in Sebecus icaeorhinus that no comment is feasible.
8. Discussion
The similarities of the trophic structures, specifically tooth and snout form, of sebecosuchians to those of large theropods, usually unspecified, are common knowledge (Langston Reference Langston1956; Pol et al. Reference Pol, Leardi, Lecuona and Krause2012). However, the implications of these similarities have been little explored, except for Henderson & Weishampel (Reference Henderson and Weishampel2002) and Riff & Kellner (Reference Riff and Kellner2011). Part of the motivation for this study was the similarity of the skulls of sebecosuchians to those of certain theropods (specifically Allosaurus, Ceratosaurus and albertosaurs), as well as of their teeth, described as similar to those of dinosaurs or megalosaurs (Langston Reference Langston1956). Specific similarities to Sebecus are akinetic skulls (Molnar Reference Molnar1991; Holliday & Witmer Reference Holliday and Witmer2008; but see Rayfield et al. Reference Rayfield, Norman and Upchurch2002 for a different view), elevated, narrow, laterally compressed snouts, and laterally flattened, serrate cheek teeth, with teeth of more rounded section anteriorly in both jaws. Some (other) large theropods also have secondary palates (Molnar Reference Molnar1991; Rayfield et al. Reference Rayfield, Milner, Xuan and Young2007). These cranial trophic similarities are not shared with other, more distantly related reptiles with similar teeth, such as varanoid lizards and Phoboscincus, nor with oviraptorosaurs, therizinosaurs, ornithomimosaurs or dromaeosaurs among theropods. Given the other differences in rostral structure, such as antorbital fenestrae, at issue was to what extent the adductor muscular structure was also similar. At least some of these similarities seem to be a result of similar trophic selective ‘forces’, such as the oreinirostral snout being better suited to resisting dorsoventrally-directed forces during feeding than the platyrostral form (Busbey Reference Busbey and Thomason1995; McHenry et al. Reference McHenry, Clausen, Daniel, Meers and Pendharkar2006; Rayfield & Milner Reference Rayfield and Milner2008). The distribution of tooth form inferred for both the phylogenies given by Riff & Kellner (Reference Riff and Kellner2011) and Pol et al. (Reference Pol, Leardi, Lecuona and Krause2012), suggests that ziphodont teeth, as well as other similarities of the trophic apparatus (Henderson & Weishampel Reference Henderson and Weishampel2002), may have evolved independently. However, the narrow oreinirostral snout is now recognised as plesiomorphic for archosauromorphs (Rayfield & Milner Reference Rayfield and Milner2008), and further discoveries may show that the ziphodont dentition is also plesiomorphic.
The skulls of only two theropods, Tyrannosaurus rex and Allosaurus fragilis, have been analysed in sufficient detail (Molnar Reference Molnar, Larson and Carpenter2008, Reference Molnar, Parrish, Molnar, Currie and Koppelhus2013) for preliminary comparison with the reconstructed mandibular muscular structure of Sebecus icaeorhinus (Fig. 15). The tendon attachments and, hence, presumably the tendons seen in living crocodilians, are not found in the two theropods examined. This suggests a different tendinous architecture in the theropod jaw adductors from sebecosuchians. Specifically, there is little indication in these theropods of the complex pinnate architecture seen in some crocodilian adductors. The two theropod snouts examined are relatively deeper than in Sebecus or modern crocodilians (Fig. 15), and the largest adductors are the vertical adductors of the postorbital region (Molnar Reference Molnar, Larson and Carpenter2008). In living crocodilians, and probably Sebecus, the largest adductors are the pterygoideus muscles. In both sebecosuchians and theropods (Molnar Reference Molnar, Parrish, Molnar, Currie and Koppelhus2013), the maximum lever arms occur when the mandible is nearly shut. A similar trophic bony architecture need not imply similar associated muscular architecture.
The reconstruction of the muscles presented here largely confirms that of Colbert (Reference Colbert1946). Most of the jaw muscles of Sebecus icaeorhinus have a lever arm about 10% greater than those of the corresponding lever arms in Crocodylus niloticus, the adductor mandibulae externus superficialis et medialis having a lever arm 14% greater in Sebecus than in C. niloticus. The pterygoideus dorsalis has a lever arm in Sebecus greater than in C. niloticus when the mouth is open. The adductor mandibulae posterior has a lever arm in Sebecus only 80% that in C. niloticus. In both the extinct and existing forms, the lever arms of most of the adductors decrease with increasing gape of the jaw. An exception is the pterygoideus dorsalis, whose lever arm may be altered by contraction of the pseudotemporalis, the add. mand. ext. prof., or the intramandibularis (or any combination of these). The depressor mandibulae of Sebecus also has a lever arm about 10% greater than that of C. niloticus.
The relatively narrow snout of Sebecus suggests comparison with crocodylomorphs with elongate tubular and, hence, also narrow snouts. In Sebecus, most of the jaw adductors appear to have been relatively more powerful than in Alligator mississippiensis. In the extant long-snouted crocodilians, the add. mand. ext. prof. is relatively larger than in shorter snouted forms (Gadow Reference Gadow, Harmer and Shipley1901; Busbey Reference Busbey1989). These longirostrine forms, however, have a smaller and relatively weaker pterygoideus dorsalis (cf. Iordansky Reference Iordansky1964). The relatively large size of the supratemporal fenestra suggests that this adductor structure also occurred in the extinct long-snouted forms (metriorhynchids, teleosaurs, etc.); it is not found in Sebecus. Hence, it follows that the greater development of the jaw adductors of Sebecus is not the same kind of phenomenon as the greater development of jaw adductors of the long-snouted forms. This result might be expected from considering the oreinirostral rather than tubular platyrostral snout of Sebecus.
Apparently, Sebecus could achieve a greater gape than C. niloticus for the same degree of muscle extension. The more efficient and apparently larger adductors, the possibly greater gape, and the theropod-like snout and tooth forms suggest that Sebecus may have had different habits from living crocodilians, a conclusion consistent with study of the postcranial skeleton (Pol et al. Reference Pol, Leardi, Lecuona and Krause2012). Snively & Russell (Reference Snively and Russell2007) point out that inertial feeding is known both in living crocodilians and living palaeognathous birds, so may be considered likely for Sebecus. Alligator mississippiensis commonly feeds upon rather small animals, although not hesitating to tackle large mammals, such as pigs or deer, when given the chance (McIhenny Reference McIhenny1935, p. 48 ff.). Rolling to dismember large prey is used by some living platyrostral crocodilians (Dereniyagala Reference Dereniyagala1939) but is not the only feeding technique used (cf. van Drongelen & Dullemeijer Reference Van Drongelen and Dullemeijer1982; Cleuren & De Vree Reference Cleuren, De Vree and Schwenk2000; Westaway et al. Reference Westaway, Thompson, Wood and Njau2011). With its cutting dentition, more efficient jaw and likely stronger adductors, it might be suggested that Sebecus habitually fed upon larger prey relative to its size than Alligator (cf. Henderson & Weishampel Reference Henderson and Weishampel2002), possibly large mammals, and was capable of cutting pieces of food for swallowing without recourse to rolling.
These functional/behavioural inferences are based on deduction from osteology and reconstructed myology and, thus, are regarded as plausible. However, it is widely realised that animals do not always behave in ways that seem reasonable to human observers, and uncertainty regarding the amount to which the opening of the mouth was restricted by the surangular limits confidence in these behavioural inferences. Further work along the lines of extant behavioural interpolation (Snively & Russell Reference Snively and Russell2007) might shed more light on feeding behaviour of Sebecus.
9. Conclusions
The mandibular adductor and depressor muscles were reconstructed from identification of muscle and tendon attachments, and by analogy with the attachments and positions of the muscles in living crocodilians. The results are similar in most respects to those of Colbert (Reference Colbert1946). Lever arms of the depressor mandibulae and mandibular adductors, except the adductor mandibulae posterior, of Sebecus icaeorhinus are relatively greater than those of Crocodylus niloticus. The percentage extensions of the muscles when the mouth is open are similar to those of C. niloticus, but greater for the adductor mandibulae externus superficialis et medialis and pseudotemporalis. As indicated by the areas of the muscle attachments, some of the adductors and the depressor mandibulae were relatively larger than in Alligator mississippiensis and, hence, more fibres could potentially attach at these sites. This, in turn, suggests that those muscles may have been relatively larger in Sebecus. The greater lever arms suggest that Sebecus may have had a relatively stronger bite than the modern forms examined, and the evidence that some of the jaw muscles may have been relatively larger than in A. mississippiensis and Paleosuchus trigonatus also suggests a relatively stronger bite in Sebecus. The laterally compressed, serrate nature of the teeth suggests that they were primarily used in cutting. These points, taken together if the inferences are correct, suggest in turn that the dismemberment rolling used by living crocodilians may have been unnecessary in Sebecus, where, instead, dismemberment of prey may have been accomplished by slicing.
Skulls of many theropod dinosaurs are generally similar to those of sebecosuchians in the possession of a relatively deep, relatively narrow snout and laterally-compressed, serrate (ziphodont) teeth. Subsequent work on Tyrannosaurus rex (Molnar Reference Molnar, Larson and Carpenter2008, Reference Molnar, Parrish, Molnar, Currie and Koppelhus2013) and Allosaurus fragilis permits comparison of the reconstructed adductor structure. This structure was probably substantially different in these theropods from Sebecus. There is no osteological indication, in the theropods examined, for the complex tendinous architecture of the adductors found in modern crocodilians, and likely present in Sebecus. The vertically-orientated adductors, add. mand. ext. sup. med., adductor mandibulae externus profundus, pseudotemporalis and adductor mandibulae posterior, seem to have been larger in cross section than the pterygoideii, the reverse of the situation in Sebecus, and probably in crocodyliforms generally. Differences in the size and form of the supratemporal fenestra in longirostrine aquatic and marine mesoeucrocodylians suggest differences in the structure of the adductor muscles in these forms from those of Sebecus.
The appearance of muscle scars for the add. mand. ext. sup. med., add. mand. ext. prof., pseudotemporalis and depressor mandibulae and tendon attachments for the A-, B- and ls-tendons in Sebecus strongly suggests that the adductor structure of mesoeucrocodylians is conservative and was established before the divergence of the sebecosuchian and modern crocodilian lineages. Thus, with care, modern crocodilians may be used as models for reconstructing the jaw musculature of other extinct mesoeucrocodylians.
None of these results contradicts the notion of sebecosuchian mesoeucrocodylians as fully land-dwelling predators.
10. Acknowledgements
The original work was done under the supervision of Wann Langston, Jr., so it seems appropriate that it (finally!) appears here. The kind and helpful assistance of Herbert Barghusen, Chris Brochu, Edwin H. Colbert, Zulma Gasparini, David D. Gillette, B. J. K. Molnar, J. H. Ostrom, R. Pascual, Bryan Patterson and W. Sill, as well as that of two anonymous referees, is much appreciated.